Abstract
Pancreatic cancer is a malignant cancer common worldwide having poor prognosis, even when diagnosed at its early stage. Cell adhesion plays a critical role in cancer invasion and metastasis. Integrins are major mediators of cell adhesion and play an important role in invasion and metastatic growth of human pancreatic cancer cells. Snake disintegrins are the most potent ligands of several integrins and have potential therapeutic applications for cancers. We have previously cloned and expressed a new recombinant RGD-disintegrin from Crotalus adamanteus (r-Cam-dis). This recently published r-Cam-dis has an extra nine amino acids derived from the vector (SPGARGSEF) at the N-terminus end and has strong anti-platelet activity. However, this r-Cam-dis contains the contamination of the cleavage of the N-terminal end of the pET-43.1a cloning vector. In this study, we have cloned r-Cam-dis in a different cloning vector (pGEX-4T-1) showing five different amino acids (GSPEF) at the N-terminal part. This new r-Cam-dis was expressed and tested for inhibition of platelet aggregation, specific binding activity with seven different integrins, and inhibition of adhesion of three different pancreatic cancer cell lines on laminin-1 and vitronectin. The r-Cam-dis showed potent binding to αvβ3 integrin, but was moderate to weak with αvβ5, αvβ6, α2β1, and α6β1. Interestingly, the inhibition of r-Cam-dis on pancreatic cancer cell lines adhesion to laminin-1 was more effective than that to vitronectin. Based on our binding results to integrin receptors and previous adhesion studies using function-blocking monoclonal antibodies, it is suggested that r-Cam-dis could be inhibiting adhesion of pancreatic cancer cell lines through integrins α2β1, α6β1, αvβ5, and αvβ6.
Keywords: Binding activity, Crotalus adamanteus, cell adhesion, disintegrins, integrins, pancreatic cancer
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related deaths in the United States (Siegel et al, 2014). The overall 5-year survival rate (2003–2009) is 6% (Siegel et al, 2014) and median survival period is 3–6 months (Spinelli et al, 2006). The prognosis of pancreatic cancer patients remains poor due to difficulties in early detection, rapid tumor growth, extensive invasion and metastasis, and a high resistance to treatment (Stathis and Moore, 2010; Wolfgang et al, 2013). Therefore, identification and development of new therapeutic agents against this malignancy is needed.
Cell adhesion is critical for many biological processes such as hemostasis, wound healing, angiogenesis, and also cancer progression and metastasis (Zigler et al, 2010; Eke and Cordes, 2014). Cell adhesion is mediated by the specific interactions of cell surface receptors, integrins, with extracellular matrix molecules such as collagens, fibronectin, laminins, and fibrinogen. Integrins are the main cell adhesion receptor molecules that modulate a variety of cellular functions such as tumor metastasis (Desgrosellier and Cheresh, 2010).
Integrins are heterodimers (α- and β-subunits) on the surface of cells that mediate cell-cell and cell-extracellular matrix interactions, as well as signal transduction. There are 18 α and 8 β known subunits, which generate at least 24 distinct integrin heterodimers (Hynes, 2002). There are at least 11 different integrins containing the β1 subunit and mediates the interaction of most extracellular matrix proteins with tumor cells (Felding-Habermann, 2003). Specific integrins preferentially bind to distinct extracellular matrix proteins including fibrinogen, collagens, fibronectin, laminins, vitronectin, and cellular receptor through short peptide sequences such as Arg-Gly-Asp (RGD), Glu-Ile-Leu-Asp-Val (EILDV), or Arg-Glu-Asp-Val (REDV) (Plow et al, 2000; Hynes, 2002). Pancreatic cancer cell lines express several integrins that allow these cells to bind extracellular matrix proteins such as collagen, vitronectin, laminins, and fibronectin and promote the invasive phenotype of pancreatic cancer. However, it has been shown that laminin-1, a major extracellular matrix protein in the basement membrane, is involved in the proliferation, differentiation, and survival of pancreatic precursor cells (Jiang et al, 1999; Jiang et al, 2001). In pancreatic cancer cells, the expression of an integrin profile is modulated on cancer cells in accordance with the extracellular matrix modification. Integrin subunits α2, α3, α5, α6, αv, and β1 are expressed on most pancreatic cancer cell lines including BxPC-3, AsPC-1, and Panc-1 (Löhr et al, 1996; Grzesiak and Bouvet, 2006; Lee et al, 2011; Zhu et al, 2011); whereas the expression of integrins α6β1 and αvβ3 in pancreactic cancer cell lines and tissues are associated with invasion (Vogelmann et al, 1999; Hosotani et al, 2002). Integrin α2β1 has also been described to mediate malignant phenotypes by increasing adhesion, proliferation, and migration in pancreatic cancer cells (Grzesiak and Bouvet, 2006). Although integrin α2β1 is known to be a primarily collagen receptor, it has been shown that the α2β1 integrin can interact with different ligands including type I collagen, type IV collagen, and mouse laminin (laminin-1) on fast-growing Colo-357 (FG-RFP) pancreatic cancer cells (Grzesiak et al, 2011). Integrin α6β1 and α3β1 have been reported to be overexpressed and functionally active in metastatic formation through binding to laminin-1 (Vogelmann et al, 1999; Sawai et al, 2003; Binkley et al, 2004; Grzesiak et al, 2007). In addition, integrin α5β1, a fibronectin receptor, plays key roles in invasion by irradiated pancreatic cancer cell lines including Panc-1, BxPC-3, and MiaPaCa-2 (Yao et al, 2011). Moreover, Zhou et al (2013) demonstrated that abnormal expression of integrin β1 subunit is related to the poor differentiation, rapid progress, easy metastasis, and poor prognosis of pancreatic cancer suggesting that the expression of integrin β1 mRNA and protein expression in blood may serve as a biomarker for the development and metastasis of pancreatic cancer and as a prognosis indicator for pancreatic cancer.
Integrins αvβ3 and αvβ5 have also been reported to play an important role in tumor cell adhesion and migration and is functionally involved in metastasis and angiogenesis of various tumor types (Weis and Cheresh, 2011). Cirulli et al (2000) demonstrated that both integrins αvβ3 and αvβ5 (major vitronectin receptors) are highly expressed in pancreatic ductal cells and clusters of undifferentiated cells emerging from the ductal epithelium. The integrin αvβ6 is an epithelial-specific integrin that is a receptor for fibronectin, vitronectin, and tenascin. Integrin αvβ6 has also reported to be expressed in many types of cancers including pancreatic, cervical, lung, and colon cancers (Van Aarsen et al, 2008; Bandyopadhyay and Raghavan, 2009), whereas its expression in corresponding normal tissue is low or undetectable. Pancreatic ductal adenocarcinomas exhibit the highest integrin αvβ6 expression among gastroenteropancreatic adenocarcinomas (Sipos et al, 2004). In addition, integrins αvβ3 and αvβ6 have recently been identified as target biomarkers in the detection of pancreatic cancer in vivo using imaging systems (Liu et al, 2014; Trajkovic-Arsic et al, 2014).
Disintegrins bind to and block many biological functions of integrins on cell surfaces. These proteins are mainly found in snake venoms from the Viperidae and Crotalidae families (Juárez et al, 2008). Disintegrins specificity depend on a tripeptide motif located in a loop that is formed by the pairing of cysteine residues. Most disintegrins contain a tripeptide, the RGD motif, which bind to the integrin αIIbβ3 on the platelet surface and inhibit platelets (Calvete, 2013). Some of RGD-disintegrins are able to bind to integrins αvβ3 and αvβ5 on the cell surface of some tumor cells and inhibit cell migration and metastasis in various tumor cell types such as lung, breast, and bone cancers (Yang et al, 2005; Oliva et al, 2007; Swenson et al, 2007; Calvete, 2013). Recombinant disintegrins that bind to αvβ3 and/or αvβ5 receptors have also been reported to have anti-angiogenic properties (Ramos et al, 2008; Montenegro et al, 2012; Lucena et al, 2012; Lucena et al, 2014). However, studies on the specific interaction of recombinant disintegrins to several other integrins have been rare.
We have previously reported that r-Cam-dis, recombinant RGD-disintegrin derived from Crotalus adamanteus, showed strong anti-platelet effects (Suntravat et al, 2013). However, the r-Cam-dis was partially purified and contained the N-terminal part of the cleaved Nus tag (∼14kDa) as a contaminant (Suntravat et al, 2013). In the present study, we cloned a P-II class snake venom metalloproteinase (CamVMPII)-derived RGD-disintegrin using a different cloning vector (pGEX-4T-1 vector) to improve the purity of r-Cam-dis. (CamVMPII, Genebank accession no. JX457344). We show that this new r-Cam-dis inhibits platelet aggregation, binds to soluble integrins αvβ3, αvβ5, αvβ6, α2β1, α6β1, and demonstrates the inhibition of adhesion on three different human pancreatic cancer cell lines (AsPC-1, Panc-1, and BxPC-3) to laminin-1 and vitronectin.
Materials and Methods
PCR amplification and cDNA cloning of r-Cam-dis
A full-length cDNA encoding a Crotalus adamanteus venom metalloproteinase II was used (GenBank accession no. JX457344) as a PCR template to subclone its disintegrin domain. PCR was used to generate double stranded cDNA, with the following disintegrin-specific primers (a forward primer 5′-CGCGAATTCGAGGTGGGAGAAGATTGTGACTG-3′ and a reverse primer 5′-GACTCGAGTTAGCCATAGAGGCCATTTCTGGGA-3′, two restriction enzyme sites (underlined): EcoRI in forward primer and XhoI in reverse primer) as previously described (Suntravat et al, 2013). PCR amplification consisted of a cycle of 94°C (3min), 40 cycles of 94°C (30sec), 60°C (30sec), and 72°C (1min). A final extension step was performed for 10min, at 72°C. The PCR product was digested with EcoRI and XhoI and gel purified. The PCR product was ligated into EcoRI and XhoI sites of pGEX-4T-1 expression vector (GE Healthcare Lifesciences, Uppsala, Sweden), which was a different vector as previously described in Suntravat et al (2013). The ligated plasmid was transformed into E. coli Top10 competent cells (Invitrogen, CA, USA). Plasmid was extracted using the GenElute plasmid miniprep kit (Sigma-Aldrich, MO, USA). Plasmids containing inserts of the predicted size for Cam-dis were performed by PCR and further confirmed by sequencing for construction of in-frame.
Expression and purification of r-Cam-dis
Once the sequence was obtained, in-frame r-Cam-dis-pGEX-4T-1 plasmid containing an extra five amino acids from this cloning vector was transformed into E. coli BL21 (DE3) star cells (Invitrogen). BL21 cells harboring recombinant plasmid DNA was first cultured in 100ml fresh Luria-Bertani (LB) medium overnight at 37°C with shaking at 225rpm on an Innova® 43 incubator shaker (New Brunswick Scientific, CT, USA). After inoculation of the overnight culture into 2l of fresh LB medium, the culture cells were grown at 37 °C with shaking at 225rpm on an Innova® 43 incubator shaker (New Brunswick Scientific) until the absorbance at 600nm (OD600) reached 0.6. The culture was induced with a final concentration of 0.1mM isopropyl β-D-thiogalactopyranoside (IPTG) for 5hr to induce expression of recombinant proteins. Bacterial cells were collected by centrifugation at 10000xg for 10min and resuspended in 1x BugBuster Protein Extraction reagent (Novagen CA, USA) by gentle vortexing, using 5ml reagent per gram of wet cell paste. Cells were resuspended and incubated on a shaking platform for 20min at room temperature. The lysate was centrifuged at 16000xg for 20min at 4°C. The soluble supernatant was purified using a glutathione S-transferase (GST)-binding resin (Novagen) in Econo-Column chromatography column (BIO-RAD, CA, USA), which was previously equilibrated with 1x phosphate buffer saline (PBS), pH 7.4. r-Cam-dis proteins were cleaved and eluted from GST bound to GST-binding resin by thrombin cleavage. Thrombin was removed from r-Cam-dis using a 1ml HiTrapTM Benzamidine FF (high sub) column (Amersham Biosciences, NJ, USA) according to the manufacturer’s instruction. The column was equilibrated with 5 column volumes of binding buffer (20mM sodium phosphate, 0.15M NaCl, pH 7.5). One milliliter of the sample was loaded into the column and r-Cam-dis protein was obtained by washing the column with a high salt buffer (20mM sodium phosphate, 1M NaCl, pH 7.5). The column was finally washed with 10 column volumes of elution buffer (10mM HCl, 0.5M NaCl, pH 2.0) to remove the thrombin bound to the column. r-Cam-dis was dialyzed in 1x PBS and concentrated using a 3kDa AmiconUltra-15 centrifugal filter (Millipore, Carrigtwohill, Ireland), electrophoresed on SDS-PAGE under non-reducing condition. Protein concentration was estimated from the absorbance at 280nm.
N-terminal sequencing
r-Cam-dis (4μg) was transferred from an SDS-PAGE onto an Immobilon®-P Membrane, polyvinylidene fluoride (PVDF) (Millipore Corporation, MA, USA) using a Semi-Dry Transblot Cell (BIO-RAD) at 125mA for 1hr. The membrane was stained with Coomassie blue R-250 for 5min and distained with 50% (v/v) methanol for 5min. The sample membrane was sent out for N-terminal amino acid sequencing at the Protein Facility, Office of Biotechnology, Iowa State University, Iowa.
Inhibition of platelet aggregation
The inhibition of adenosine diphosphate (ADP)-induced platelet aggregation by r-Cam-dis was determined by measuring the impedance of human whole blood in a Chrono-Log Whole Blood Aggregometer (Chrono-Log, PA, USA) as previously described (Suntravat et al, 2013). The percent inhibition of platelet aggregation was calculated using the following equation: [(C-E/C)]x100, where C is the units of platelet aggregation (ohms) for the control, and E is the unit of platelet aggregation (ohms) for the experimental fraction. The extent of the inhibition of platelet aggregation was assessed by comparison with the maximal aggregation induced by the control dose of agonist (ADP). The median inhibitory concentration (IC50) was calculated from a dose-dependent curve using Microsoft Excel 2011.
Binding of soluble integrins to immobilized r-Cam-dis
The interaction of r-Cam-dis with soluble recombinant human integrins was performed as described previously (Lucena et al, 2014). All recombinant human integrins were purchased from R&D Systems (MN, USA) including integrin αvβ3 (3050-AV), αvβ5 (2528-AV), αvβ6 (3817-AV), α2β1 (5698-A2), α3β1 (2840-A3), α5β1 (3230-A5), and α6β1 (7809-A6). Mouse anti-integrins monoclonal antibodies αvβ3 (23C6 clone), αvβ5 (P5H9 clone), α3 (IA3 clone), α5 (612557 clone), α6 (MP4F10 clone), and β6 (437216 clone) were from R&D Systems. Mouse anti-α2β1 (BHA2.1 clone) monoclonal antibody was from Millipore (CA, USA). Briefly, the microtiter plates (96-well) were coated with 100µl of r-Cam-dis at various concentrations (0.005µM–1.2μM for αvβ3, αvβ5, αvβ6, α2β1 integrins, 0.05μM-16μM for α6β1, and 0.01μM-5μM for α3β1 and α5β1 integrins) in PBS, pH 7.4 at 4°C for 18hr. After washing three times with washing buffer (PBS buffer, pH 7.4 containing 0.05%, v/v Tween-20), the remaining sites on the wells were blocked with 1% (w/v) bovine serum albumin (BSA) in PBS containing 0.05% (v/v) Tween (PBS-T) for 1hr at room temperature. The plates were then washed with washing buffer and followed by addition of 100μL of soluble integrins αvβ3, αvβ5, α2β1, α3β1, α5β1, α6β1 (20μg/ml) or αvβ6 (5μg/ml), in 0.5% (w/v) BSA in PBS-T and separately incubated with each integrin at room temperature for 2hr, with the exception of the plate with integrin α6β1, which was incubated at 4°C for 24hr. After incubation and washing step, mouse anti-integrins monoclonal antibodies αvβ3, αvβ5, α3, α5, α6, and α2 (10μg/ml), and β6 (5μg/ml) were added and incubated for 1 hr at room temperature. After the washing step, 100μl/well of 1:1500 diluted horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (KPL, MD, USA was added and incubated for 1hr. A final wash was performed and 100μl/well of TMB substrate solution (0.2g/l 3,3’,5,5’-tetramethylbenzidine and 0.01% (v/v) H2O2 in citric acid buffer; KPL) was added. The reaction was stopped with 100μl/well of TMB stop solution (KPL), and the absorbance was measured in a microplate reader (Beckman Coulter model AD 340) at 450nm. Commercial echistatin (Sigma-Aldrich), a disintegrin that binds with a high affinity to integrin αvβ3, was used as a positive disintegrin control. Wells were coated only with 1mg/ml BSA (no disintegrin) to detect non-specific binding. Data on the graph was net specific binding, which was obtained by subtracting optical density values of the total binding from wells coated only with BSA. The error bars represent the standard deviations.
Cell line and culture conditions
The human pancreatic tumor cell lines (BxPC-3, AsPC-1, and Panc-1) were purchased from American Type Culture Collection (ATCC, VA, USA). BxPC-3 and AsPC-1 were maintained in Roswell Park Memorial Institute medium (RPMI)-1640 with L-glutamine and Phenol Red (ATCC) containing 10% (v/v) fetal bovine serum (Gibco, NY, USA) and antibiotics (50units/ml penicillin and 50μg/ml streptomycin) (ATCC). Panc-1 was maintained in Minimum Essential Medium (MEM) containing 10% (v/v) fetal bovine serum and antibiotics (50units/ml penicillin and 50μg/ml streptomycin). The cells were cultured in a humidified 5% (v/v) CO2 air incubator at 37 °C. All pancreatic cancer cells used in the adhesion assay were from passages 2–6.
Adhesion assay
Since laminin-1 and vitronectin are involved in the malignant phenotype of pancreatic cancer cells as described above and r-Cam-dis was able to bind to integrins αvβ3, αvβ5 (vitronectin receptors), α6β1 (laminin receptor), α2β1 (collagen and laminin receptor), and αvβ6 (fibronectin and vitronectin receptor), we decided to use vitronectin and laminin-1 to determine the specificity of adhesion and the inhibition of adhesion of three different pancreatic cancer cell lines on these extracellular matrix proteins by r-Cam-dis. r-Cam-dis was used to inhibit the binding of human pancreatic tumor cells to extracellular matrix proteins including vitronectin and laminin-1 coated plates using a modified method as described by Lucena et al (2012). Duplicate wells in a 96-well plate (Falcon® Tissue Culture Plate) were coated with 0.1ml of vitronectin or laminin-1 (isolated from mouse Engelbreth-Holm-Swarm tumor, Sigma-Aldrich) at 10μg/ml in 0.01M PBS, pH 7.4, and incubated overnight at 4°C. The plate was blocked with 0.2ml of 5% (w/v) BSA in PBS and incubated at 37°C for 1hr. Cells were harvested, counted, and resuspended in minimum essential medium(MEM) containing 1% BSA at 5x105 cells/ml (BxPC-3) or 7.5x105 cells/ml (AsPC-1 and Panc-1). The r-Cam-dis (0.05ml) was added to the cell suspension (0.45ml) at various concentrations and allowed to incubate at 37°C for 1hr. The blocking solution was aspirated, and the cell/disintegrin suspensions (0.2ml) were added to the wells coated with matrix protein and incubated at 37°C for 1hr for BxPC-3 or 2hr for AsPC-1 and Panc-1. The solution was aspirated and washed three times with PBS-5% (w/v) BSA by filling and aspirating. A total of 0.2ml of MEM medium in 1% (w/v) BSA containing 2.5mg/ml of 3-[4,5-Dimethylthiazol-2-yl] 2,5-diphenltetrazolium bromide (MTT) (5:1, v/v) was added to the wells containing cells and incubated at 37°C for 2hr. The MTT was aspirated and 0.1ml of dimethyl sulfoxide (DMSO) was added to the wells to lyse the cells. The absorbance was read at 570nm using a Beckman Coulter model AD 340 reader. Untreated cells adhere to the matrix was considered as a negative control. The percent inhibition was calculated by the following formula: [(absorbance of negative control - absorbance of cell/r-Cam-dis)/absorbance of negative control]x100.
Statistical analyses
The results were expressed as the mean+standard deviation (SD). Their significance was analyzed by the student’s t-test. The level of significance was at P<0.05.
Results
Recombinant production of r-Cam-dis
It was previously demonstrated that r-Cam-dis inhibits platelet activities (Suntravat et al, 2013). In the present study, r-Cam-dis was cloned into the pGEX-4T-1 expression vector containing the Glutathione S-transferase (GST) tag for affinity purification, which has been previously reported to express, in high levels, soluble and active recombinant disintegrins in E. coli (Sánchez et al, 2010; Lucena et al, 2012). In addition, this vector allows mild elution conditions for release of fusion proteins from the affinity matrix, thus minimizing effects on functional activity.
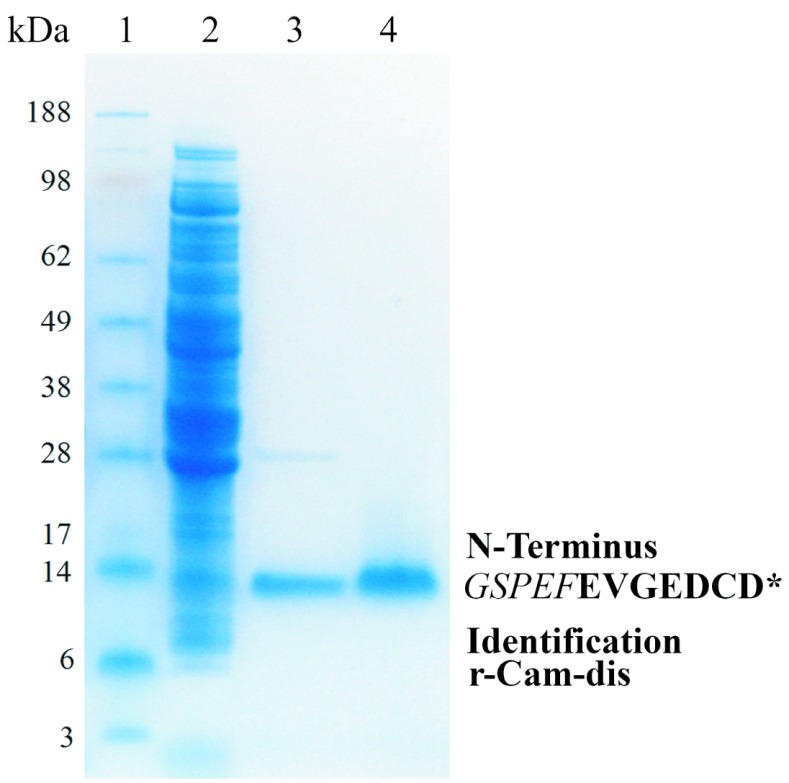
After r-Cam-dis was cleaved from the GST by thrombin treatment, a yield of 1mg of protein per liter of culture was obtained. Purified r-Cam-dis was identified by N-terminal sequence analysis. The r-Cam-dis contained an additional five amino acids from the vector at the N-terminus end (GSPEF), for a total calculated molecular weight of r-Cam-dis with GSPEF of about ∼8.4kDa with a pI 4.36 by Protein Identification and Analysis Tools on the Expasy Server (Figure 1).
Figure 1.
Expression and purification of r-Cam-dis analysis by 4–12% SDS-PAGE gel under non-reducing condition. Samples were run on 4–12% (w/v) Bis-Tris Gel using an Xcell SureLock Mini-Cell at 200V for 30min. The gel was stained with Rapid-Stain. Lane 1: SeeBlue Plus2 Markers; lane 2: soluble fraction of lysates of expressed E. coli BL21 cells by BugBuster reagent (150μg); lane 3: cleaved r-Cam-dis after wash with binding buffer (3μg); lane 4: purified r-Cam-dis after wash with high salt buffer (3 μg). An asterisk (*) represents the N-terminal amino acid sequence of purified r-Cam-dis containing the five amino acids from the vector (italicized) before the disintegrin sequence, which are shown in bold letters.
Inhibition of platelet aggregation
r-Cam-dis was initially tested for the inhibition of ADP-induced platelet aggregation activity. The r-Cam-dis inhibited ADP-induced platelet aggregation in a dose-dependent manner with the IC50 value of 8.88nM (Figure 2).
Figure 2.
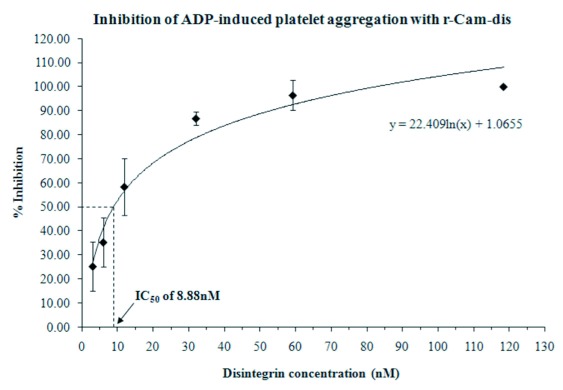
Inhibition of platelet aggregation using whole blood by r-Cam-dis. A Chronolog aggregometer was used to measure ADP-induced platelet aggregation by impedance. A total of 10μl of r-Cam-dis at varying concentrations was added to whole blood and incubated 1min at 37°C prior to adding 10μM of ADP. The error bars represent the standard deviation from three independent experiments with n=3.
Binding of r-Cam-dis to integrins
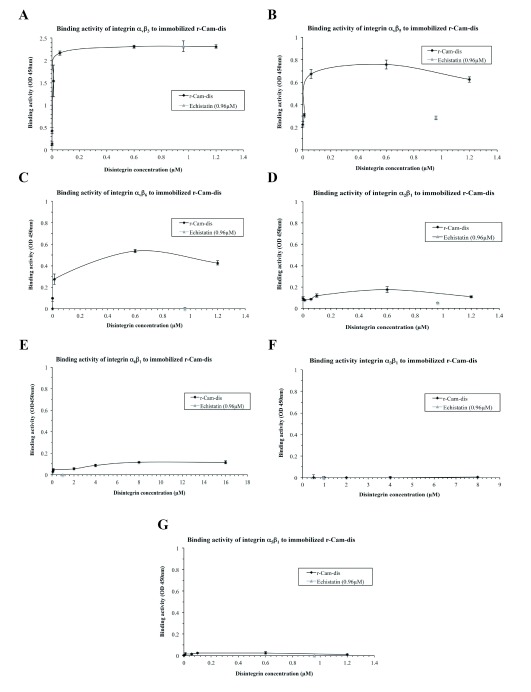
To confirm that r-Cam-dis is capable of direct integrin binding, we employed indirect ELISA assay. As shown in Figure 3, r-Cam-dis was able to bind to integrins αvβ3, αvβ5, αvβ6, α2β1, and α6β1 (Figure 3A-E) but not to α3β1 and α5β1 (Figure 3F and 3G). The binding activity was most potent in the presence of integrin αvβ3. Echistatin, a well-known RGD-disintegrin that bind preferentially to the integrin αvβ3, showed binding specificity to only integrins αvβ3 and αvβ5 and was considerably less effective when compared with r-Cam-dis.
Figure 3.
Interaction of immobilized r-Cam-dis with integrins A) αvβ3, B) αvβ5, C) αvβ6, D) α2β1, E) α6β1, F) α3β1, and G) α5β1. Integrin binding was measured by indirect ELISA assay as described in Materials and Methods. Absorbance at 450 nm of the individual well was measured to determine the binding activity. The error bars represent the standard deviation from two independent experiments with n=2.
Inhibition of cell adhesion to vitronectin and laminin-1
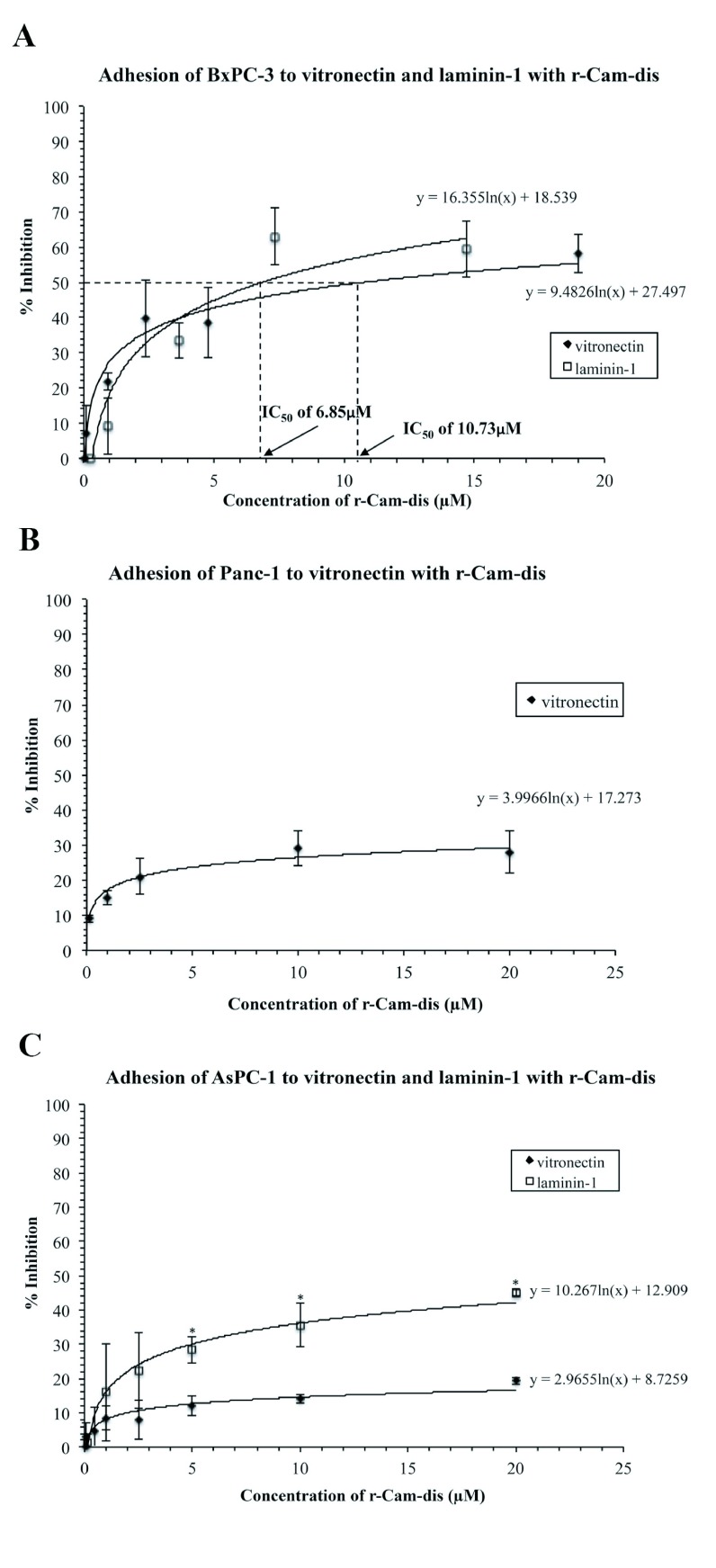
The r-Cam-dis inhibited BxPC-3 adhesion to vitronectin and laminin-1, in a concentration-dependent manner with IC50 values of 10.73μM and 6.85μM, respectively (Figure 4A). Adhesion to vitronectin in Panc-1 was dose-dependently inhibited up to 28.9±5.2% (Figure 4B). The inhibition of Panc-1 binding to laminin-1 was not investigated because untreated Panc-1 control cells did not adhere to laminin-1. The r-Cam-dis also inhibited AsPC-1 adhesion to vitronectin and laminin-1 by 19.3±1% and 45±1%, respectively (Figure 4C).
Figure 4.
Effects of r-Cam-dis on adhesion of BxPC-3, Panc-1, and AsPC-1 pancreatic cancer cell lines on vitronectin and laminin-1. A) BxPC-3, B) Panc-1, and C) AsPC-1 were seeded in 96-well plates, which were pre-coated with vitronectin or laminin-1 in the absence (PBS added), or presence of various concentrations of r-Cam-dis. Cell adhesion was measured by MTT technique and the results were expresses as percent of inhibition. The results are expressed as mean±SD (n=3). An asterisk (*) indicates the significant difference between the inhibition of vitronectin adhesion and laminin-1 adhesion in each cell type by r-Cam-dis at P<0.05.
Discussion
Pancreatic cancer is a leading cause of cancer death throughout the world due to its rapid metastasis rendering late detection. Metastasis and the invasion of tumor cells to both nearby and distant organs are the most critical aspects of cancer (Spano et al, 2012; Alizadeh et al, 2014). Cell adhesion is a critical process for tumor growth as well as tumor metastasis, which is regulated by integrin adhesion molecules. Therefore, integrins have become a target for future anti-cancer drugs (Cayrol et al, 2011; Chen et al, 2013; Shi et al, 2014; van der Horst et al, 2014). Integrin antagonists are currently in clinical trials for cancer therapy including monoclonal antibodies such as etaracizumab, abegrin (McNeel et al, 2005; Hersey et al, 2010), RGD-based antagonists such as cilengitide, a cyclic RGD-peptapeptide antagonist of integrins αvβ3 and αvβ5 (Beekman et al, 2006; Nabors et al, 2007), and non-RGD-based antagonists such as ATN-161 (Cianfrocca et al, 2006).
We have previously demonstrated that r-Cam-dis (containing extra nine amino acids, SPGARGSEF) is a potent anti-platelet inhibitor. Unexpectedly, the r-Cam-dis was partially purified and contained the N-terminal part of the cleaved Nus tag (∼14kDa) as a contaminant (Suntravat et al, 2013). In this study, we improved the purity by cloning r-Cam-dis into a pGEX-4T-1 vector (N-terminus GST tagged vector) (Figure 1). r-Cam-dis, containing five different amino acids (GSPEF), dose-dependently inhibited ADP-induced platelet aggregation with an IC50 of 8.88nM (Figure 2), which was about 1.5 times less efficient than our previously reported r-Cam-dis (6nM) (Suntravat et al, 2013). This indicated that the addition of amino acids at N-terminus end is thought to cause conformation changes that alter its biological activity. However, this r-Cam-dis is more efficient than other recombinant disintegrins with IC50 values ranging from 34nM to 6μM (Sánchez et al, 2010).
Since snake disintegrins are potent and specific antagonists of several integrins. Grzesiak and Bouvet (2006) reported that pancreatic cancer cells including BxPC-3, Panc-1, and AsPC-1 are expressed in varying degrees of immunoreactivity for the laminin-binding integrins α2, α3, α6, the vitronectin-binding αv together with the β1, β3, β5 subunits. In this study, we showed that r-Cam-dis bound most potently to αvβ3 integrin. While the interaction of r-Cam-dis with integrins αvβ5 and αvβ6 was moderate and weak with integrins α2β1 and α6β1 (Figure 3). We also provide the preliminary experiments of the inhibition of adhesion of BxPC-3, Panc-1, and AsPC-1 cells on vitronectin and laminin-1 by r-Cam-dis. r-Cam-dis inhibited all three different pancreatic cancer cell lines to vitronectin and laminin-1 having the most potent adhesion inhibition effect on laminin-1, except for Panc-1 cells, which do not attach on laminin-1 (Figure 4). Tani et al (1997) reported that pancreatic carcinoma cell lines including BxPC-3, CFPAC-1, and Panc-1 preferably adhere to laminin-5 compared to laminin-1, however, BxPC-3 and Panc-1 cells adhere to laminin-1 to some extent. BxPC-3 cells showed similar adhesion to vitronectin and laminin-1, while Panc-1 cells preferred vitronectin over laminin-1 and fibronectin. In addition, Grzesiak and Bouvet (2008) using inhibition experiments with function-blocking anti-integrin antibodies including anti-β1, anti-β3, anti-β4, anti-β5, showed that β1 integrin plays an important role in promoting adhesion of pancreatic cancer cell lines including AsPC-1, Panc-1, MiaPaCa-2, and BxPC-3 to fibrinogen, laminin-1, and type IV collagen. On type I collagen, cells mediate specifically by the α2β1 integrin (Grzesiak and Bouvet, 2006).
Additionally, in vitro shRNA knockdown studies by Grzesiak et al (2011) demonstrated that knockdown of the β1 and α2 integrin subunits significantly inhibits cell adhesion and migration of fast-growing Colo-357 (FG-RFP) pancreatic cancer cells on type I and type IV collagen as well as laminin-1. By contrast, on vitronectin, cells bind predominantly via the integrin αvβ5, with involvement from β1 integrins as well (Grzesiak and Bouvet, 2006; Grzesiak and Bouvet, 2008). Taken together, it is possible that r-Cam-dis mediates the inhibition of adhesion through the binding of integrins α2β1 and α6β1 and involvement from αvβ5 integrin as well. However, r-Cam-dis was also bound to integrin αvβ6 (vitronectin receptor) and no studies on function-blocking antibody directed against αvβ6 on adhesion of AsPC-1, Panc-1, and BxPC-3 to extracellular matrix proteins have been investigated, therefore, integrin αvβ6 should not be excluded to be a possible target of r-Cam-dis. To verify the specific interaction of r-Cam-dis directly against these integrins on the surface of pancreatic cancer cell lines, the inhibition of cell binding to immobilized monoclonal anti-integrin antibodies by r-Cam-dis should be further investigated.
Interestingly, the adhesion of AsPC-1 cells to laminin-1 and vitronectin and Panc-1 cells to laminin-1 were only partially inhibited by r-Cam-dis, which might be due to the different intensity of expressed integrins on different cell types. It has been previously reported that the expression level of α2 integrin in AsPC-1 is lower than that compared to BxPC-3 cells (Grzesiak and Bouvet, 2006; Ikenaga et al, 2012). Previous studies on αvβ3 and αvβ5 expression during pancreatic islet ontogeny demonstrated that adult islet cells show consistently low levels of both intergrins αvβ3 and αvβ5 expression as compared with fetal cells (Cirrulli et al, 2000). The molecular mechanisms involved in the inhibitory effect of r-Cam-dis on adhesion in pancreatic cancer cell lines remain to be elucidated.
The roles of platelets in tumor stability, growth, and metastasis have been implicated (Menter et al, 2014). Integrin-mediated inside-out and outside-in signaling and/or crosstalk play an essential role in the biologic responses of platelets. Platelet integrins include primarily αIIbβ3 (binds fibrinogen or von Willebrand factor), αvβ3 (binds vitronectin), α2β1 (binds collagen), α5β1 (binds fibronectin), and α6β1 (binds laminin). Platelet integrins and their adhesive ligands that serve as bridging proteins participate in tumor-induced platelet aggregation (McNicol and Israels, 2008). Once activated, platelets release contents such as alpha granules and microparticles that facilitate tumorigenesis including adhesion, proliferation, and metastasis (Bambace and Holmes, 2011). It has been reported that human pancreatic cancer cell lines including PC-3, PC-44, AsPC-1, BxPC-3, Capan-2, Panc-1 are able to induce platelet aggregation in vitro, suggesting that platelet activation might support metastasis in pancreatic cancer (Heinmöller et al, 1995). Recently, the use of the anti-platelet drug, Clopidogel, decreased the size of the tumors and restored hemostasis in an ectopic model of pancreatic cancer and significantly inhibited the development of metastases in a syngeneic orthotopic mice model of pancreatic cancer (Mezouar et al, 2015). Our results showed that r-Cam-dis is a very potent inhibitor of platelet aggregation and is able to bind to several integrins that are found on both platelets and pancreatic cancer cell lines, however, the in vitro adhesion of Panc-1 and AsPC-1cells to laminin-1 and vitronectin were only partially inhibited. It is possible that r-Cam-dis may exert a stronger inhibitory effect if the pancreatic cancer cell lines were co-cultured with activated platelets and r-Cam-dis, a study which deserves further exploration.
Conclusions
We provide preliminary data showing that r-Cam-dis recognizes many integrins including, those involved in many pathological processes such as cell adhesion, migration, tumor invasion and metastasis and also inhibits an adhesion effect of three different pancreatic cancer cell lines. However, further studies on functional inhibition using integrins α2β1, α6β1, αvβ5, and αvβ6 monoclonal antibodies and apoptosis would greatly help in uncovering the exact molecular mechanism of r-Cam-dis in inhibiting adhesion of pancreatic cancer cell lines. r-Cam-dis could have a foundation for the development of targeted therapeutic approaches.
Acknowledgements
Funding for the project was provided by the NIH/Biological Materials Resource Grant, Viper Resource Grant #s 3P40OD010960-10S1 and 2P40OD010960-11A1 (NNTRC, Texas A&M University-Kingsville, Dr EE Sánchez) and Texas A&M University-Kingsville-Research Development Funds (Acct. # 160302 and 160315-00022). We want to thank Nora Diaz DeLeon, Mark Hockmuller, Juan Salinas and the rest of the NNTRC personnel for their assistance.
Abbreviations
- CamVMPII
P-II class snake venom metalloproteinase from Crotalus adamanteus
- cDNA
complementary deoxyribonucleic acid
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Completing Interests
None declared.
References
- Alizadeh AM, Shiri S, Farsinejad S. Metastasis review: from bench to bedside. Tumour Biol. 2014;35:8483–8523. doi: 10.1007/s13277-014-2421-z. [DOI] [PubMed] [Google Scholar]
- Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9:237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Raghavan S. Defining the role of integrin alphavbeta6 in cancer. Curr Drug Targets. 2009;10:645–652. doi: 10.2174/138945009788680374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman KW, Colevas AD, Cooney K, et al. Phase II evaluations of cilengitide in asymptomatic patients with androgen-independent prostate cancer: scientific rationale and study design. Clin Genitourin Cancer. 2006;4:299–302. doi: 10.3816/CGC.2006.n.012. [DOI] [PubMed] [Google Scholar]
- Binkley CE, Zhang L, Greenson JK, et al. The molecular basis of pancreatic fibrosis: common stromal gene expression in chronic pancreatitis and pancreatic adenocarcinoma. Pancreas. 2004;29:254–263. doi: 10.1097/00006676-200411000-00003. [DOI] [PubMed] [Google Scholar]
- Calvete JJ. The continuing saga of snake venom disintegrins. Toxicon. 2013;62:40–49. doi: 10.1016/j.toxicon.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Cayrol C, Bertrand C, Kowalski-Chauvel A, et al. αv integrin: a new gastrin target in human pancreatic cancer cells. World J Gastroenterol. 2011;17:4488–4495. doi: 10.3748/wjg.v17.i40.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Fong YC, Tang CH. Novel strategies for the treatment of chondrosarcomas: targeting integrins. Biomed Res Int. 2013 doi: 10.1155/2013/396839. 2013, 396839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfrocca ME, Kimmel KA, Gallo J, et al. Phase 1 trial of the antiangiogenic peptide ATN-161 (Ac-PHSCN-NH(2)), a beta integrin antagonist, in patients with solid tumours. Br J Cancer. 2006;94:1621–1626. doi: 10.1038/sj.bjc.6603171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli V, Beattie GM, Klier G, et al. Expression and function of alpha(v)beta(3) and alpha(v)beta(5) integrins in the developing pancreas: roles in the adhesion and migration of putative endocrine progenitor cells. J Cell Biol. 2000;150:1445–60. doi: 10.1083/jcb.150.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke I, Cordes N. Focal adhesion signaling and therapy resistance in cancer. Semin Cancer Biol. 2015;31C:65–75. doi: 10.1016/j.semcancer.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–213. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- Grzesiak JJ, Bouvet M. The alpha2beta1 integrin mediates the malignant phenotype on type I collagen in pancreatic cancer cell lines. Br J Cancer. 2006;94:1311–1319. doi: 10.1038/sj.bjc.6603088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiak JJ, Bouvet M. Activation of the alpha2beta1 integrin-mediated malignant phenotype on type I collagen in pancreatic cancer cells by shifts in the concentrations of extracellular Mg2+ and Ca2+ Int J Cancer. 2008;122:2199–2209. doi: 10.1002/ijc.23368. [DOI] [PubMed] [Google Scholar]
- Grzesiak JJ, Smith KC, Burton DW, et al. Integrin-mediated laminin-1 adhesion upregulates CXCR4 and IL-8 expression in pancreatic cancer cells. Surgery. 2007;141:804–814. doi: 10.1016/j.surg.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiak JJ, Tran Cao HS, Burton DW, et al. Knockdown of the β(1) integrin subunit reduces primary tumor growth and inhibits pancreatic cancer metastasis. Int J Cancer. 2011;129:2905–2915. doi: 10.1002/ijc.25942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinmöller E, Schropp T, Kisker O, et al. Tumor cell-induced platelet aggregation in vitro by human pancreatic cancer cell lines. Scand J Gastroenterol. 1995;30:1008–1016. doi: 10.3109/00365529509096346. [DOI] [PubMed] [Google Scholar]
- Hersey P, Sosman J, O’Day S, et al. Etaracizumab Melanoma Study Group. A randomized phase 2 study of etaracizumab, a monoclonal antibody against integrin alpha(v)beta(3), + or - dacarbazine in patients with stage IV metastatic melanoma. Cancer. 2010;116:1526–1534. doi: 10.1002/cncr.24821. [DOI] [PubMed] [Google Scholar]
- Hosotani R, Kawaguchi M, Masui T, et al. Expression of integrin alphaVbeta3 in pancreatic carcinoma: relation to MMP-2 activation and lymph node metastasis. Pancreas. 2002;25:e30–e35. doi: 10.1097/00006676-200208000-00021. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ikenaga N, Ohuchida K, Mizumoto K, et al. Pancreatic cancer cells enhance the ability of collagen internalization during epithelial-mesenchymal transition. PLoS One. 2012;7:e40434. doi: 10.1371/journal.pone.0040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang FX, Georges-Labouesse E, Harrison LC. Regulation of laminin 1-induced pancreatic beta-cell differentiation by alpha6 integrin and alpha-dystroglycan. Mol Med. 2001;7:107–114. [PMC free article] [PubMed] [Google Scholar]
- Juárez P, Comas I, González-Candelas F, et al. Evolution of snake venom disintegrins by positive Darwinian selection. Mol Biol Evol. 2008;25:2391–2407. doi: 10.1093/molbev/msn179. [DOI] [PubMed] [Google Scholar]
- Lee CY, Marzan D, Lin G, et al. α2 Integrin-Dependent Suppression of Pancreatic Adenocarcinoma Cell Invasion Involves Ectodomain Regulation of Kallikrein-Related Peptidase-5. J Oncol. 2011 doi: 10.1155/2011/365651. 2011, 365651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhr M, Trautmann B, Göttler M, et al. Expression and function of receptors for extracellular matrix proteins in human ductal adenocarcinomas of the pancreas. Pancreas. 1996;12:248–259. doi: 10.1097/00006676-199604000-00007. [DOI] [PubMed] [Google Scholar]
- Lucena SE, Jia Y, Soto JG, et al. Anti-invasive and anti-adhesive activities of a recombinant disintegrin, r-viridistatin 2, derived from the Prairie rattlesnake (Crotalus viridis viridis) Toxicon. 2012;60:31–39. doi: 10.1016/j.toxicon.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucena SE, Romo K, Suntravat M, et al. Anti-angiogenic activities of two recombinant disintegrins derived from the Mohave and Prairie rattlesnakes. Toxicon. 2014;78:10–17. doi: 10.1016/j.toxicon.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Liu H, Ma T, et al. Integrin αvβ6-Targeted SPECT Imaging for Pancreatic Cancer Detection. J Nucl Med. 2014;55:989–994. doi: 10.2967/jnumed.113.132969. [DOI] [PubMed] [Google Scholar]
- McNeel DG, Eickhoff J, Lee FT, et al. Phase I trial of a monoclonal antibody specific for alphavbeta3 integrin (MEDI-522) in patients with advanced malignancies, including an assessment of effect on tumor perfusion. Clin Cancer Res. 2005;11:7851–7860. doi: 10.1158/1078-0432.CCR-05-0262. [DOI] [PubMed] [Google Scholar]
- McNicol A, Israels SJ. Beyond hemostasis: the role of platelets in inflammation, malignancy and infection. Cardiovasc Hematol Disord Drug Targets. 2008;8:99–117. doi: 10.2174/187152908784533739. [DOI] [PubMed] [Google Scholar]
- Menter DG, Tucker SC, Kopetz S, et al. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33:231–269. doi: 10.1007/s10555-014-9498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezouar S, Darbousset R, Dignat-George F, et al. Inhibition of platelet activation prevents the P-selectin and integrin-dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo. Int J Cancer. 2015;136:462–475. doi: 10.1002/ijc.28997. [DOI] [PubMed] [Google Scholar]
- Montenegro CF, Salla-Pontes CL, Ribeiro JU, et al. Blocking αvβ3 integrin by a recombinant RGD disintegrin impairs VEGF signaling in endothelial cells. Biochimie. 2012;94:1812–1820. doi: 10.1016/j.biochi.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Nabors LB, Mikkelsen T, Rosenfeld SS, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25:1651–1657. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva IB, Coelho RM, Barcellos GG, et al. Effect of RGD-disintegrins on melanoma cell growth and metastasis: involvement of the actin cytoskeleton, FAK and c-Fos. Toxicon. 2007;50:1053–1063. doi: 10.1016/j.toxicon.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Plow EF, Haas TA, Zhang L, et al. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- Ramos OH, Kauskot A, Cominetti MR, et al. A novel alpha(v)beta (3)-blocking disintegrin containing the RGD motive, DisBa-01, inhibits bFGF-induced angiogenesis and melanoma metastasis. Clin Exp Metastasis. 2008;25:53–64. doi: 10.1007/s10585-007-9101-y. [DOI] [PubMed] [Google Scholar]
- Sánchez EE, Lucena SE, Reyes S, et al. Cloning, expression, and hemostatic activities of a disintegrin, r-mojastin 1, from the mohave rattlesnake (Crotalus scutulatus scutulatus) Thromb Res. 2010;126:e211–e219. doi: 10.1016/j.thromres.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai H, Funahashi H, Yamamoto M, et al. Interleukin-1alpha enhances integrin alpha(6)beta(1) expression and metastatic capability of human pancreatic cancer. Oncology. 2003;65:167–173. doi: 10.1159/000072343. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, et al. Cancer statistics. CA. Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Sipos B, Hahn D, Carceller A, et al. Immunohistochemical screening for beta6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro. Histopathology. 2004;45:226–236. doi: 10.1111/j.1365-2559.2004.01919.x. [DOI] [PubMed] [Google Scholar]
- Shi J, Fan D, Dong C, et al. Anti-tumor effect of integrin targeted (177)Lu-3PRGD2 and combined therapy with Endostar. Theranostics. 2014;4:256–266. doi: 10.7150/thno.7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano D, Heck C, De Antonellis P, et al. Molecular networks that regulate cancer metastasis. Semin. Cancer Biol. 2012;22:234–249. doi: 10.1016/j.semcancer.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Spinelli GP, Zullo A, Romiti A, et al. Long-term survival in metastatic pancreatic cancer. A case report and review of the literature. JOP. 2006;7:486–491. [PubMed] [Google Scholar]
- Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- Suntravat M, Jia Y, Lucena SE, et al. cDNA cloning of a snake venom metalloproteinase from the eastern diamondback rattlesnake (Crotalus adamanteus), and the expression of its disintegrin domain with anti-platelet effects. Toxicon. 2013;64:43–54. doi: 10.1016/j.toxicon.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson S, Ramu S, Markland FS. Anti-angiogenesis and RGD-containing snake venom disintegrins. Curr Pharm Des. 2007;13:2860–2871. doi: 10.2174/138161207782023793. [DOI] [PubMed] [Google Scholar]
- Tani T, Lumme A, Linnala A, et al. Pancreatic carcinomas deposit laminin-5, preferably adhere to laminin-5, and migrate on the newly deposited basement membrane. Am J Pathol. 1997;151:1289–1302. [PMC free article] [PubMed] [Google Scholar]
- Trajkovic-Arsic M, Mohajerani P, Sarantopoulos A, et al. Multimodal molecular imaging of integrin αvβ3 for in vivo detection of pancreatic cancer. J Nucl Med. 2014;55:446–451. doi: 10.2967/jnumed.113.129619. [DOI] [PubMed] [Google Scholar]
- van der Horst G, Bos L, van der Mark M, et al. Targeting of alpha-v integrins reduces malignancy of bladder carcinoma. PLoS One. 2014;9:e108464. doi: 10.1371/journal.pone.0108464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmann R, Kreuser ED, Adler G, et al. Integrin alpha6beta1 role in metastatic behavior of human pancreatic carcinoma cells. Int J Cancer. 1999;80:791–795. doi: 10.1002/(sici)1097-0215(19990301)80:5<791::aid-ijc25>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. αV integrins in angiogenesis and cancer. Cold Spring Harb Perspect Med. 2011;1:a006478. doi: 10.1101/cshperspect.a006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RS, Tang CH, Chuang WJ, et al. Inhibition of tumor formation by snake venom disintegrin. Toxicon. 2005;45:661–669. doi: 10.1016/j.toxicon.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Yao H, Zeng ZZ, Fay KS, et al. Role of α(5)β(1) Integrin Up-regulation in Radiation-Induced Invasion by Human Pancreatic Cancer Cells. Transl Oncol. 2011;4:282–292. doi: 10.1593/tlo.11133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Chiu D, Qin D, et al. Expression of CD44v6 and integrin-β1 for the prognosis evaluation of pancreatic cancer patients after cryosurgery. Diagn Pathol. 2013;8:146. doi: 10.1186/1746-1596-8-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu GH, Huang C, Qiu ZJ, et al. Expression and prognostic significance of CD151, c-Met, and integrin alpha3/alpha6 in pancreatic ductal adenocarcinoma. Dig Dis Sci. 2011;56:1090–1098. doi: 10.1007/s10620-010-1416-x. [DOI] [PubMed] [Google Scholar]
- Zigler M, Dobroff AS, Bar-Eli M. Cell adhesion: implication in tumor progression. Minerva Med. 2010;101:149–162. [PubMed] [Google Scholar]