Abstract
Stimulating the brain to drive its adaptive plastic potential is promising to accelerate rehabilitative outcomes in stroke. Ipsilesional Primary Motor Cortex (M1) is invariably facilitated. However, evidence supporting its efficacy is divided, indicating we may have over-generalized its potential. Since M1 and its corticospinal output are frequently damaged, in patients with serious lesions and impairments, ipsilesional premotor areas (PMA) could be useful alternates instead. We base our premise on their higher probability of survival, greater descending projections, and an adaptive potential, which is causal for recovery across the seriously impaired. Using a conceptual model, we describe how chronically stimulating PMA would strongly affect key mechanisms of stroke motor recovery, such as facilitating plasticity of alternate descending output, restoring inter-hemispheric balance, and establishing widespread connectivity. Although at this time it is difficult to predict whether PMA would be ‘better’, it is important to at least investigate whether they are reasonable substitutes for M1. Even if stimulation of M1 may benefit those with maximum recovery potential, while that of PMA may only help the more disadvantaged, it may still be reasonable to achieve some recovery across the majority rather than stimulate a single locus fated to be inconsistently effective across all.
Keywords: Stroke, Rehabilitation, Transcranial Direct Current Stimulation (tDCS), Transcranial Magnetic Stimulation (TMS), Brain Stimulation, Diffusion Tensor Imaging (DTI), functional Magnetic Resonance Imaging (fMRI), Premotor, Corticospinal, Transcallossal
Introduction
Stoke is the leading cause of serious long-term adult disability. More than 60% of survivors experience chronic deficits of paretic upper limb/hand (Broeks and others 1999). Although some motor abilities can return spontaneously, complete recovery is rare. Patients rate dysfunction of the upper limb as one of the most serious impediments to their quality of life (Ones and others 2005). While rehabilitation carries promise, gains are modest with standard treatment (French and others 2007), and access to rehabilitation diminishes as reimbursement becomes limited. Therefore, the impetus has been to maximize the therapeutic potential of neuro-rehabilitation. One of the most popular methods involves stimulating the brain.
The classical approach involves stimulating the Primary Motor Cortex (M1) (Adkins-Muir and Jones 2003; Plautz and others 2003), which is known to be the ‘final common pathway’ of control of movement. Evidence that its adaptive plasticity is intimately associated with the return of upper extremity skill makes it an even more attractive locus. Animal studies show that with retraining of skill at the paretic distal forelimb, peri-infarct M1 reorganizes; surviving representations of trained distal limb amplify and re-surface (figure 1) (Nudo and others 1996). This evidence forms the foundation for the original approach to brain stimulation; it is believed that the adaptive potential of peri-infarct M1 would serve as a high-yielding substrate to target in stroke rehabilitation. Indeed, animal models show augmented functional benefits when peri-infarct M1 is stimulated during rehabilitation. Spared representations in peri-infarct cortex expand and re-emerge via increases in dendritic density, synaptogenesis, and long-term potentiation (LTP)-like synaptic efficacy (Adkins-Muir and Jones 2003; Plautz and others 2003). Clinical studies have similarly witnessed an adjunctive advantage of stimulation, related to increased excitability of ipsilesional M1 and its improved ability to counteract exaggerated inhibition exerted by its homologue (Bolognini and others 2011; Edwards and others 2009). Table 1 lists early clinical studies where the effect of adjunctive stimulation in rehabilitation was positive when compared to rehabilitation delivered alone. For instance, phase I and II trials of epidural motor cortical stimulation showed augmented rehabilitative outcomes, as did pilot studies employing noninvasive stimulation via magnetically induced (transcranial magnetic stimulation, TMS) or direct electrical currents (transcranial direct current stimulation, tDCS) (citations in Table 1).
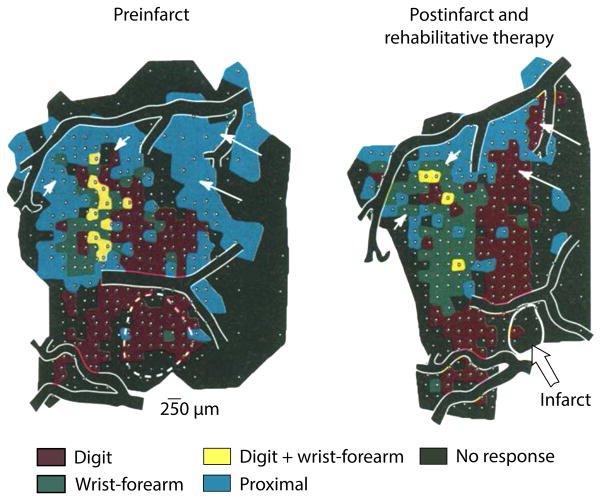
Fig. 1. Why Ipsilesional Primary Motor Cortex (M1) was first targeted with stimulation in rehabilitation.
Adapted from Nudo and others (1996). Ipsilesional M1 was first targeted with stimulation in stroke rehabilitation because pioneering work in animal models had suggested that the region shows adaptive plasticity with return of skill in recovery. Here, Nudo and others (1996) illustrate how ipsilesional M1 reorganizes with rehabilitative training in a non-human primate model of stroke. The infarct (dashed circle) has destroyed ~22% of the digit (red) representations and 4% of the wrist-forearm (green) representations in the region of M1. With rehabilitative training involving the distal forelimb, the animal exhibits reorganization of representations within peri-infarct M1 (right). The spared digit representation expands by ~15% while the wrist-forearm representations increase by 58.5%. Nudo and others conclude that with skill relearning, surviving representations of trained distal forelimb expand in the peri-infarct territory, as well as occupy territory previously claimed by proximal segments (indicated by white arrows denoting areas in blue). These pivotal findings emphasized two important themes in stroke recovery: skill-based rehabilitative training invokes adaptive plasticity in the stroke brain and peri-infarct M1 lies at the center of this process. Thus, subsequent studies chose peri-infract M1 as a target for stimulation in rehabilitation.
Table 1. Summary of Studies stimulating M1 in conjunction with Stroke Rehabilitation.
The table summarizes studies that paired rehabilitation/training of paretic upper limb in stroke with stimulation of the brain. While the first portion discusses promise of stimulation across pilot and initial efficacy trials, a later section shows synopsis of studies that found variable effects across patients. A uniform theme can be deduced across the latter. Studies including larger numbers of participants, particularly those with cortical damage and/or loss of viable corticospinal output, seem to lack demonstration of an advantage of facilitating excitability of ipsilesional M1. Even when excitability of ipsilesional M1 may be augmented, there may still be limited benefit for function. The table serves to support our premise- motor areas that are viable and projecting viable corticospinal output would be stronger substrates to target with stimulation.
| Study | Final Target | Type of Stimulation | Study Design | Frequency/duration of Training | Paired Training/Rehab | Findings/remarks |
|---|---|---|---|---|---|---|
| Studies discussing positive effects of brain stimulation as add-on in rehabilitation | ||||||
| (Bolognini and others 2011) | M1 | Transcranial. Up-regulating Ipsilesional while down-regulating Contralesional | Randomized, Sham-controlled in chronic (N=14) | 10 sessions | Constraint-induced movement therapy | Stimulation improves paretic hand function, strength and its perceived use presumably by up-regulating Ipsilesional M1 and reducing callossal inhibition from Contralesional |
| (Brown and others 2006) | M1 | Invasive, epidural for up-regulating Ipsilesional | Randomized controlled multi-center Phase I trial in Chronic (N=8) | 5 days/week X 3 weeks | Occupational therapy | Safe, except for post-surgical infection. Stimulation remarkably improved function of paretic hand vs. therapy. During intra-operative mapping, most patients showed evoked response in paretic limb, confirming viable corticospinal tracts from ipsilesional M1 |
| (Huang and others 2008; Levy and others 2008) | M1 | Invasive, epidural for up-regulating Ipsilesional | Randomized controlled multi-center phase II trial comparing stimulation + therapy vs. therapy alone in Chronic moderate to moderate-severe (N=24) | 26 sessions over 6 weeks | Occupational therapy: task-oriented therapy | Safe, except for post-surgical seizure that occurred prior to stimulation. Both groups improved, but greater and clinically meaningful gains when stimulation was added on to rehabilitation; gains maintained as well. Of stimulation group, 42% showed evoked response in paretic limb, confirming viable corticospinal tracts from ipsilesional M1 |
| (Chang and others 2010; Chang and others 2012) | M1 | Transcranial. Up-regulating Ipsilesional | Longitudinal, pseudo-randomized, sham-controlled in subacute (N=28) | 10 sessions over 2 weeks | Motor practice- reaching and grasping | All improved, but stimulation generated additional benefit for long. Related work (2012) shows greater fMRI activation in ipsilesional supplementary motor, basal ganglia and superior parietal. |
| (Conforto and others 2012) | M1 | Transcranial. Down-regulating Contralesional | Randomized, sham-controlled in acute-to-subacute (N=30) | 10 sessions over 2 weeks | Customary rehabilitation | Tasks related to daily living and strength improved in patients receiving stimulation as add-on. |
| (Edwards and others 2009) | M1 | Transcranial. Up-regulating Ipsilesional | Preliminary in Chronic (N= 6) | 1 session | Robotic training | Functional improvement not witnessed albeit excitability of ipsilesional M1 was up regulated and sustained with robotic training |
| Kakuda and others (2012) | M1 | Transcranial. Down-regulating Contralesional | Single group design, multi-center for chronic stroke (N=204). No Control group | 22 sessions over 15 days | Offline 2-hr Occupational Therapy | Motor function improved. Effects significant despite short duration of constraint-induced therapy. Lack of control group expressed as limitation. |
| (Khedr and others 2005) | M1 | Transcranial. Up-regulating Ipsilesional | Randomized sham-controlled in acute (N=52) | 10 sessions | Standard Physical therapy | Stimulation, as add-on, alleviated functional disability and improved independence more than rehabilitation. Potentially by aiding ipsilesional excitability though effect not significant vs. sham |
| (Kim and others 2006) | M1 | Transcranial. Up-regulating Ipsilesional | Randomized, Sham-controlled, cross-over in Chronic (N=15) | Single session each | Offline motor sequence learning | Stimulation as add-on to motor sequence training improved accuracy and movement time vs. training alone. Excitability of ipsilesional M1 was up regulated in association with accuracy. |
| (Lefebvre and others 2013) | M1 | Transcranial. Up-regulating Ipsilesional while down-regulating Contralesional | Randomized, Sham-controlled, cross-over in Chronic (N=18) | Single session each | Online Motor Skill Learning involving tracking | Add-on stimulation benefitted skill, efficiency and retention vs. learning alone. |
| (Lindenberg and others 2010b; Lindenberg and others 2012b) | M1 | Transcranial. Up-regulating Ipsilesional while down-regulating Contralesional | Single group design for chronic stroke (N=10) | 5 daily sessions (early training) followed by another 5 (late training) | Online Physical and Occupational Therapy | Stimulation as add-on benefitted rehabilitative outcomes more in early than late training. Related study (2010) shows fMRI activation of ipsilesional M1 increased with stimulation |
| (Nair and others 2011) | M1 | Transcranial. Down-regulating Contralesional | Randomized, Sham-controlled in chronic (N=14) | 5 daily sessions | Online Occupational therapy | Stimulation conferred advantage to rehabilitation, improving range and upper limb function. Stimulation associated with reduced fMRI intensity of activation in contralesional M1 |
| (Ochi and others 2013) | M1 | Transcranial. Up-regulating Ipsilesional in one group, down-regulating Contralesional in another | Randomized controlled crossover design in chronic with moderate-to-severe paresis (N= 18) | 5 daily sessions each, with 2-day interval for cross-over | Robotic training involving forearm rotation and wrist flexion/extension | Limited albeit similar improvement for hand function and its perceived use in both groups. Spasticity alleviated with contralesional stimulation, more so in right hemispheric stroke |
| (Sasaki and others 2013) | M1 | Transcranial. Up-regulating Ipsilesional in one group, down-regulating Contralesional in another | Randomized, Sham-controlled. Early after stroke (N= 27) | 5 daily sessions | Offline Conventional Rehabilitation | Both types of stimulation were effective in improving strength and tapping frequency. Although differences between stimulation groups were not significant, benefits of up-regulating ipsilesional M1 outweighed those vs. sham. |
| (Takeuchi and others 2012) | M1 | Transcranial. Up-regulating Ipsilesional in one group, down-regulating Contralesional in another. Both offered concurrently in 3rd group | Randomized, controlled (N= 27) | Single session | Offline pinch training | Down-regulating contralesional M1, and its combination with up-regulating ipsilesional improves motor training. Down-regulating contralesional causes bimanual coordination to deteriorate, but its combination with up-regulating M1 prevents such deterioration |
| (Wu and others 2013) | M1 | Transcranial. Down-regulating Ipsilesional to reduce spasticity | Randomized, Sham-controlled (N= 45) | 5 sessions/week X 4 weeks | Offline Physical Therapy | Stimulation significantly improved tone, motor function, and activities of daily living |
| (Zimerman and others 2012) | M1 | Transcranial. Down-regulating Contralesional | Randomized, sham-controlled, crossover. Subcortical well-recovered (N= 12) | Single session each | Online motor sequence learning | Down-regulating contralesional M1 facilitates early learning and retention. Effects associated with up-regulated intra-cortical excitability of ipsilesional |
| Studies discussing variable success of brain stimulation as add-on in rehabilitation | ||||||
| (Ackerley and others 2010) | M1 | Transcranial. Up-regulating Ipsilesional. Down-regulating Contralesional | Randomized, Sham-controlled, cross-over in Chronic (N= 10) | Single session each | Precision Grip | Up-regulating ipsilesional M1 before training effective. Down-regulating contralesional ineffective at up-regulating excitability of ipsilesional; instead reduced function |
| (Hesse and others 2011) | M1 | Transcranial. Up-regulating Ipsilesional in one group, down-regulating Contralesional in another | Randomized, sham-controlled, multi-center in acute-subacute who were severe (N=96) | 5 sessions/week X 6 weeks | Online bilateral robot training, offline Physical and Occupational Therapy | All improved but no advantage of stimulation. Majority had large infarct with cortical-subcortical, or predominantly cortical damage. Most showed no evoked response in paretic limb, confirming lack of viable corticospinal tracts from ipsilesional M1. When only subcortical analyzed, benefit of down-regulating contralesional tended to be significant. |
| (Malcolm and others 2007) | M1 | Transcranial. Up-regulating Ipsilesional | Randomized Sham-controlled in chronic (N=20) | 10 consecutive weekday sessions plus home exercises | Offline Constraint-induced Movement Therapy | Though stimulating ipsilesional M1 up-regulated its excitability, it failed to translate to advantage in motor function. No added advantage for group receiving stimulation in rehabilitation. |
| (Pomeroy and others 2007) | M1 | Transcranial. Down-regulating Ipsilesional | Randomized, placebo-controlled, 4-group design. Early after stroke (N=24) | 8 daily sessions | Offline Voluntary Muscle Contraction or placebo movement | No additional advantage of stimulation for function, though stimulation as add-on to voluntary movement up-regulated ipsilesional excitability |
| (Seniow and others 2012) | M1 | Transcranial. Down-regulating Contralesional | Randomized, Sham-controlled in moderate impairment (N= 40) | 5 daily sessions X 3 weeks | Offline Physical Therapy | Both groups improved significantly, but there was no greater advantage of additive stimulation |
| (Talelli and others 2012) | M1 | Transcranial. Up-regulating Ipsilesional in one, down-regulating contralesional in another | Semi-randomized, controlled in chronic with mild-to-moderate impairment (N=41) | 10 sessions | Offline Physical Therapy | All patients improved, but addition of stimulation did not augment improvements |
| (Emara and others 2009) | M1 | Transcranial. Up-regulating Ipsilesional in one group and down-regulating Contralesional in another | Randomized, Sham-controlled in acute (N=60) | 10 sessions over 2 weeks | Physical therapy | Improvement in select patients; affecting contralesional M1 failed to benefit individuals with cortical lesions while affecting ipsilesional M1 directly benefitted those with cortical or non-cortical lesions consistently. No in those with improvement total anterior circulation stroke or ones with no residual arm function. |
| (Harvey and Winstein 2009) For design. Results unpublished, discussed in (Nouri and Cramer 2011; Plow and others 2009; Plow and Machado 2013) | M1 | Invasive, epidural for up-regulating Ipsilesional | Randomized controlled multi-center phase III trial comparing stimulation + therapy vs. therapy alone in Chronic moderate to moderate-severe (N=146) | 26 sessions over 6 weeks | Occupational therapy: task-oriented therapy | Add-on stimulation in rehabilitation not any more effective vs. rehabilitation alone. Of stimulation group, only 15% could show evoked response in paretic limb; when this subgroup studies, benefit significant vs. rehabilitation alone (Nouri and Cramer 2011) |
The disconnect
Unfortunately, as later and larger clinical trials failed to replicate early promise of adjunctive stimulation (see Table 1: studies discussing variable success), it became apparent that adaptive potential of peri- and ipsi-lesional M1 could not be harnessed consistently across patients. Even though their restitution may represent the best basis for stroke motor recovery (Cramer 2004; Lotze and others 2006), such an opportunity is more likely in animal than in human models. In animal work, samples tend to be perfectly homogenous with regard to mechanisms of injury, site and size of lesions, post-ictal duration, age of onset, pre-morbid conditions, and pre- and post-stroke activities or training (Plow and others 2009). In contrast, clinical studies suffer from confounding influence of all of these factors. The heterogeneous etiology, infeasibility to stratify according to age, location and profile of lesion, varying post-stroke duration, and varying premorbid as well as post-stroke lifestyle and rehabilitation altogether create extremely inhomogeneous cohorts. Therefore, ‘variable’ success of stimulating M1 in clinical studies, versus in animal work, where the technique originated, is a product of variation across its study samples (Plow and others 2009). Nevertheless, the importance of current evidence is paramount because, for the first time, plasticity of the brain could be directed via methodological advances, such as its stimulation, to safely push therapeutic limits (Fregni and Pascual-Leone 2007; Hummel and Cohen 2006). Meanwhile, the question- what would be an ideal clinical target that is reliable and effective across most patients- remains to be addressed.
Alternatives: A novel hypothesis
We hypothesize that ipsilesional premotor areas (PMA) could serve as useful alternatives. For the purposes of this discussion, we use the term PMA to describe areas in frontal lobe that are rostral to M1 as defined by Fulton (Wise 1985). However, in line with clarifications by Morecraft (2002) and Dum and Strick (1991), we restrict the term to only include areas that possess the unique ability to influence motor output at M1 and at the spinal cord. These areas include supplementary motor area (SMA), cingulate motor areas (CMA), and the premotor cortex (PMC) with its dorsal (PMd) and ventral (PMv) components. Consult Fig. 3 (below) for a schematic illustrating the expanse of PMA. In ongoing investigation, it is still unclear whether chronic stimulation of the PMA offers any potential advantage for clinical stroke rehabilitation (Plow and others 2013). Nevertheless, here, we discuss why they would likely be a suitable alternative to target in clinical stroke rehabilitation as opposed to the traditional M1.
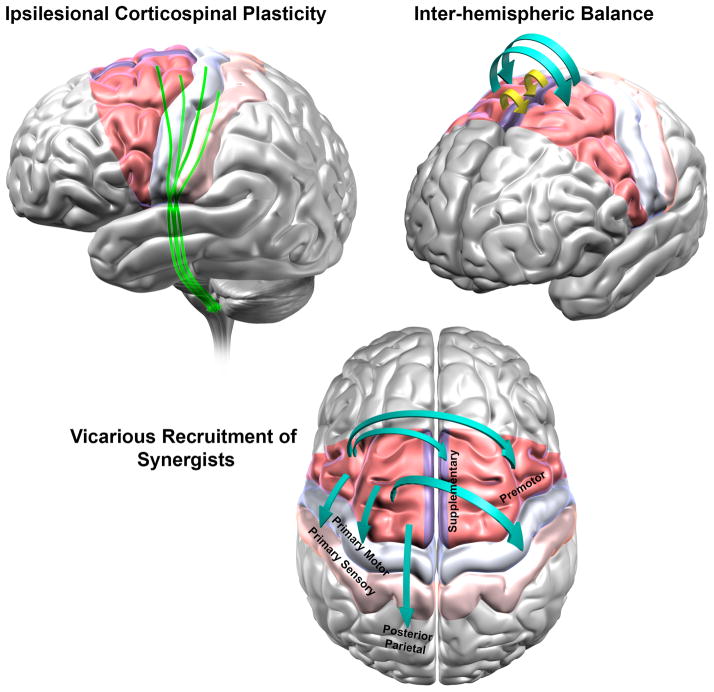
Fig. 3. Why would stimulation of ipsilesional PMA be able to affect most mechanisms of clinical recovery in stroke?
The figure shows the anatomical region of primary motor cortex (M1) and the expanse of PMA [red = Lateral Premotor Areas, purple = Medial Premotor as Supplementary Motor Area, and Cingulate Motor Area, which cannot be shown in this view). PMA constitute more than 60% of the frontal cortex that project to the spinal cord. Medial wall, such as Supplementary Motor Area and medial premotor cortex, receive arterial supply from a source that is different from the commonly infarcted middle cerebral artery that supplies M1. Thus, PMA would have greater probability of survival than M1 and their constituent regions could serve as effective substitutes for M1.
Based on evidence from three of our studies (Carey and others 2007; Cunningham and others 2013b; Plow [nee (Bhatt)] and others 2007) and neuroanatomic, physiologic, and functional evidence from that of several others, we present a hypothetical model of how and why stimulation of ipsilesional PMA would be most effective in modulating majority of mechanisms of human stroke recovery in rehabilitation. Clinical recovery of paretic hand function is believed to occur via at least 3 adaptive processes- corticospinal plasticity (A) return of balance between excitability of ipsilesional and contralesional motor regions (B) and vicarious recruitment of widespread frontal and parietal synergistic regions (C). Stimulating PMA would be able to facilitate (A) because they contribute CST, as extensively as M1, forming direct, parallel output to spinal cord, independent of M1. In stroke, their CST can exhibit plasticity via axonal sprouting and/or cortico-cortical facilitation of CST from M1. Stimulation of PMA would aid (B) because they possess abundant callossal connections, both homotopic and heterotopic, which are far more extensive than between M1 and its homologue. In fact, in stroke, their callossal connectivity enhances i.e. undergoes adaptive plasticity, with restorative therapy. Their stimulation would finally also facilitate (C) because they have extensive functional connectivity with ipsilateral posterior parietal that enhances in recovery, structural connectivity with ipsilateral sensory cortex that rewires following stroke affecting M1, and strong structural connectivity with ipsilateral and contralateral motor areas (M1 and other PMA). With their stimulation, they would be able to help recruit widespread synergists in motor function.
1. PMA have a higher probability of survival
One of the most critical advantages that PMA have is their relatively high chance of survival in typical stroke. SMA residing in the anterior cerebral artery territory is spared in >97% of first time stroke patients (Bogousslavsky and Regli 1990). The high probability of survival of the PMA is linked to their expanse, where neuronal labeling in primate models show that PMA altogether constitute the majority (>60%) of frontal cortex projecting to the spinal cord (Dum and Strick 1991). Probability of survival is critical when defining targets because it can explain why targeting M1 generalized varyingly from animal to clinical models. As discussed earlier, animal models tend to be homogenous with focal and stereotypical lesions, thus majority of peri-infarct M1 is spared (Dancause and Nudo 2011). In humans, however, lesions are diffuse, sparing M1 only partially and variably. Since M1 resides in the territory supplied by branches of the middle cerebral artery, one that is most commonly affected in stroke, it survives mainly in those with small, focal infarcts (Cramer and others 2000). We have witnessed that patients with damaged M1 are more likely to be ‘poorly recovered’, while majority of those who exhibit subcortical strokes or cortical strokes sparing M1 are ‘well-recovered’ (Plow [nee (Bhatt)] and others 2007). Thus, M1’s potential to adapt in recovery and serve as a consistent locus for stimulation is inevitably infrequent in humans by nature of their lesions. As an alternative, higher probability of their survival could make the PMA likely candidates to chronically stimulate for recovery.
Since, in humans, stroke has greater chances of subcortical extension as well, lesions are more likely to damage M1’s output, which is the most important predictor of recovery i.e. the corticospinal tracts (CST) and alternate descending motor tracts (Lindenberg and others 2010a; Lindenberg and others 2012a; Stinear and others 2007). In this case, CST from PMA may act as alternate substrates. Although originally they were believed to contribute only to reticulospinal tracts projecting to axial/proximal muscles (Freund and Hummelsheim 1985), Dum and Strick (1991) showed via retrograde labeling in non-human primates that PMA on the medial wall alone constitute ~40% of CST to the hand. Since this proportion matches or exceeds that from M1 (He and others 1993), with greater probability of their survival in middle cerebral artery stroke, PMA would offer useful alternative pathways for recovery of the hand. Since it has become known that PMA form direct, parallel modules for control of distal forelimb, independent of M1, our classical understanding of M1 as the ‘final common pathway’ is questioned. M1 may simply be a part of parallel processing (Dum and Strick 1991), which means that in injury, other substrates, such as PMA, could offer useful cortical and corticospinal alternatives.
2. PMA can undergo adaptive re-mapping in recovery
Can PMA substitute the role of M1 in injury? As discussed earlier, re-mapping of peri-infarct M1 in post-injury recovery was a finding of ‘paradigmatic’ proportions in neuro-rehabilitation (Nudo and others 1996). Are PMA similarly able to re-map in animals, and in humans?
Neuronal labeling and physiologic mapping in animal models show that even when a majority of hand representation of M1 is destroyed, PMv can re-map its representation by almost 50% (Frost and others 2003). Functional neuroimaging supports evidence for such re-mapping in humans. During movements of the paretic hand, patients exhibit task-related functional MRI (fMRI) activation of ipsilesional PMA (Plow [nee (Bhatt)] and others 2007; Seitz and others 1998; Ward and others 2007; Weiller and others 1992). Their activation increases proportionally with damage to M1 and its CST (Ward and others 2007). We show that with return-of-skill, they are activated linearly (Plow [nee (Bhatt)] and others 2007). With long-term learning, intensity of only PMC reduces, but not that of M1, representing improved efficiency (Carey and others 2007; Plow [nee (Bhatt)] and others 2007). We ascribe their flexibility to re-map to their somatotopic organization. SMA possesses integrated somatotopic representations similar to M1, while PMC differentiates distal and proximal representations similar to the sensory cortex (Cunningham and others 2013b). Their multi-layered structure containing abstract and discrete somatotopic organization may allow them the flexibility to re-map and to be a substitute for primary sensorimotor cortical damage.
Determining whether re-mapping is causal to recovery, and not simply epiphenomenal, would attest to their adaptive potential. Lesion- or inactivation-approaches are great experimental tools to test such hypotheses. In non-human primates, Liu and Rouiller (1999) demonstrated that following a complete lesion to the M1’s hand representation, the PMA’s hand representations, rather than non-hand territories in peri-lesional M1, re-mapped. The re-mapping of the PMA was critical because their subsequent inactivation (using GABA-agonist muscimol) re-instated deficits. In rodents, similarly, Zeiler et al. (2013) confirmed that reorganization of the medial PMA is essential using a double-lesion approach. Virtual inactivation is possible in humans as well, where TMS can suppress cortical activity to link a region to ongoing behavior (Pascual-Leone 2006). In patients with infarcts of M1, or its CST, TMS applied to the ipsilesional PMd delays reaction time of moving the paretic finger (Fridman and others 2004). Delays are even significant when contralesional PMd is inactivated (Johansen-Berg and others 2002) and are particularly longer in those with greater impairment (Takeuchi and others 2007). With inactivation, it is concluded that re-mapping of the PMA is causal to recovery, particularly in those with large lesions to the M1. The adaptive re-mapping of the PMA is believed to be a product of their anatomic substrates, which includes alternate CST (Liu and Rouiller 1999), intra-cortical processes (Zeiler and others 2013), and their flexible somatotopic organization (Cunningham and others 2013b).
The role of re-mapping of M1, on the other hand, is not unequivocal. Even when it survives, its fMRI activation is inconsistent, particularly in the vicinity of the lesion (Cramer and others 2000), and weakly related to recovery (Binkofski and Seitz 2004). We have shown that patients regaining dexterity can show classical plasticity, where there is greater fMRI activation in the ipsilesional as opposed to contralesional M1; however, patients improving to a similar degree via another treatment fail to demonstrate this ‘characteristic’ plasticity (Plow [nee (Bhatt)] and others 2007) (Fig. 2). In fact, we witness the opposite in a different cohort, where, as patients regain function, a precipitous shift occurs in activation from the ipsilesional to the contralesional M1 (Carey and others 2007).
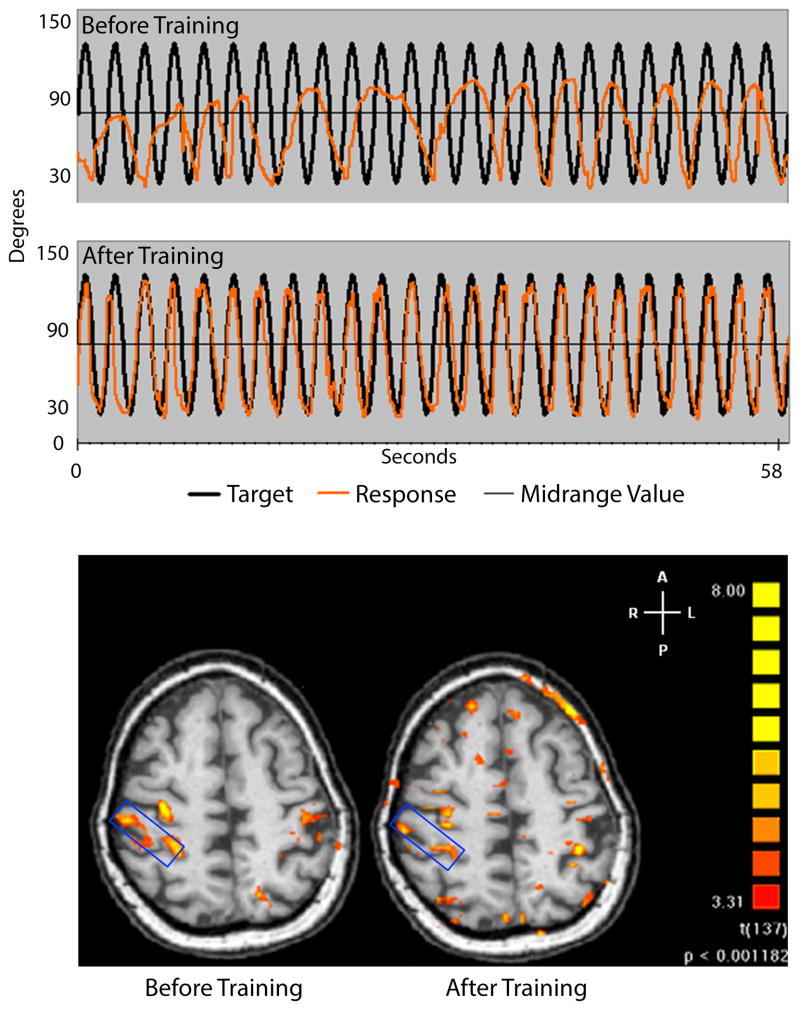
Fig. 2. Variable relation of adaptive potential of ipsilesional M1 in human recovery: Adapted from Plow [nee (Bhatt) and others 2007].
Unlike animals (as in Fig. 1), in humans with stroke, ipsilesional M1 shows a variable relation with recovery. In a clinical study, we showed that patients recover with skill relearning, but activity of ipsilesional M1 does not adapt in the same way as would be expected based on previous work in animals (Fig. 1). Panel A shows pretest (top) and posttest (bottom) for a patient with chronic stroke who underwent skill relearning. Skill task involved visuomotor tracking where patients were required to follow sinusoidal target waveform (black line) using spatially and temporally accurate movements of paretic fingers. Patients could view their response (orange line), which they were asked to align accurately with the target. The patient shown here improves following a month of training; scores improve from −25.79% (panel A, top) to 33.6% (panel A, bottom). Panel B shows corresponding change in fMRI activation. The blue rectangle highlights ipsilesional M1. Before training, patient shows stronger ipsilesional than contralesional activation during tracking with paretic left hand. Even though patient recovers remarkably in tracking skill, fMRI activation becomes less ipsilesional than contralesional at posttest. Therefore, recovery occurred even though activation of ipsilesional versus contralesional M1 weakened, which is opposite of what is known classically in recovery, i.e. greater fMRI activation of ipsilesional than contralesional M1. In fact, patients are also known to recover when contralesional areas are more active as well. Thus, human mechanisms of recovery are extremely variable, and re-mapping of ipsilesional M1 is not as coherent as once classically believed.
Thus, while the relation between re-mapping of the M1 and functional recovery remains variable (Carey and others 2007; Feydy and others 2002; Plow [nee (Bhatt)] and others 2007), the re-mapping of the PMA studied using neuronal labeling and physiologic mapping with fMRI, and confirmed via lesion-induced or virtual inactivation, supports their causative influence. Notably though, their re-mapping is more apparent in patients who have incompletely recovered. Thus, while they may adaptively compensate for injured M1, their potential may not be adequate. Nevertheless, the evidence that PMA could facilitate recovery amongst the most impaired, for whom this prospect would otherwise be impossible (Johansen-Berg and others 2002), creates opportunities for the majority of survivors.
3. PMA can affect processes of adaptive plasticity underlying stroke recovery
We believe that stimulation of the ipsilesional PMA could facilitate those mechanisms of plasticity that are classically known to be associated with stroke recovery (Dancause and Nudo 2011; Plow and Machado 2013). For this, we propose the following theoretical model (Fig. 3).
CST plasticity
With recovery, CST and alternate output from surviving motor cortices amplify (Lindenberg and others 2010a; Lindenberg and others 2012a; Stinear and others 2008), becoming more excitable, eliciting larger motor potentials in the paretic muscles, and involving output from additional, more extensive areas (Wittenberg and others 2003). Since their contribution to CST is extensive and independent of M1, we premise that stimulating PMA would elicit robust CST plasticity. Our premise is strengthened by evidence that with precentral stroke in primates, the recovery of fine motor skills is supported by structural plasticity of the CST from the SMA (McNeal and others 2010). In case of a failing M1, CST from the PMC increase its responsiveness; for instance, following stimulation that inhibits activity of M1, responses from PMC become heightened (Schmidt and others 2013).
Understandably, however, some would argue an important caveat. In healthy primates, stimulation of PMA evokes spinal neural responses less frequently and spread across fewer sets of upper limb muscles than M1 (Boudrias and others 2010; Zinger and others 2013). Despite prevalent anatomic connections of their CST (Dum and Strick 1991; He and others 1993), Maier and others (2002) and Zinger and others (2013) discuss that their connections to spinal neurons for distal muscles are less extensive than from M1 (Zinger and others 2013). Although in healthy primates, CST of PMA are unable to directly activate spinal motor neurons dedicated to finger muscles, based on evidence in the injured (see above) (Liu and Rouiller 1999; McNeal and others 2010; Zeiler and others 2013), we still believe CST from PMA may modulate this primary mechanism of plasticity.
Inter-hemispheric balance between the ipsilesional and contralesional motor cortices can return with recovery (Machado and others 2003; Taub and others 2003). Following stroke, this balance is disrupted due to abnormalities of mutual transcallosal inhibition (Murase and others 2004; Taub and others 2003). Inhibition exerted by ipsilesional upon contralesional motor cortices reduces, which leads to unabated activity of the latter. Contralesional areas instead intensify their inhibition upon the already weak ipsilesional, which explains post-stroke dysfunction (Murase and others 2004). Using chronic stimulation, efforts have always focused upon either facilitating ipsilesional M1 or inhibiting contralesional M1 to rectify the imbalance (Table 1). However, since evidence supporting the utility of such approaches is controversial [follow Table 1], mitigating inter-hemispheric imbalance via M1-M1 route is questionable. Here, we argue that M1-M1 route would invariably be challenging because M1 possesses the weakest, patchiest callossal connections with its homologue (Fang and others 2008; Rouiller and others 1994). Instead, chronic stimulation of PMA may be more effective at facilitating return of inter-hemispheric balance since they contain the most abundant callossal connections. Tracer injections in SMA, PMd, and PMv reveal extensive homotopic connections and hetereotopic connections (Boussaoud and others 2005; Dancause and others 2007; Fang and others 2008; Rouiller and others 1994). Extensive callossal connectivity of PMA may help them mediate abstract higher-order movement planning for bilateral movements (Boussaoud and others 2005; Fang and others 2008), while ‘acallossal’ structure of M1 (cf. (Rouiller and others 1994)) may instead be suitable for lateralized movements. Therefore, chronic stimulation of PMA, in contrast to M1, may offer greater opportunities for coordinating and rebalancing inter-hemispheric activity in stroke.
Vicariation and reversal of diaschisis
The initial deficit in stroke stems in part from disconnected influence of higher-order attention systems such as superior parietal cortices upon the motor network (Inman and others 2012). This phenomenon, known as diaschisis, explains neurologic deficits that cannot be explained from loss of function directly attributed to infarcted area. With recovery, areas deafferented from lesion site can become reintegrated, where functional connectivity between frontal-parietal cortices and ipsilesional M1 can improve particularly in moderate-to-severely impaired (Machado and Baker 2012; Park and others 2011), and alternate higher motor ipsilesional and contralesional cortices can be vicariously recruited (Bestmann and others 2010; Dancause and others 2006; Frost and others 2003; Johansen-Berg and others 2002; Lotze and others 2012; Plow [nee (Bhatt)] and others 2007; Seitz and others 1998; Ward and others 2007; Weiller and others 1992).
We believe chronic stimulation of PMA would help restore such connectivity and reverse diaschisis because they share extensive connections with ipsilesional posterior parietal, M1, primary sensory cortices (S1), and contralesional homologous and heterologous cortices (Dancause and others 2007). As an example, Dancause and others (2005) show rewiring between ipsilesional PMv and S1 in primate models. Similarly, Hamadjida and others (2012) illustrate structural callossal plasticity in primate models of stroke, while James and others (2009) discuss functional connectivity between ipsilesional PMC and homologue with behavioral recovery in primates and human models of stroke respectively.
Testing the hypothesis in clinical investigations
It remains unknown still whether chronic stimulation of PMA in stroke motor recovery is effective at augmenting rehabilitative outcomes. While this is being investigated clinically (Plow and others 2013), we discuss how one can test whether they indeed affect mechanisms as we propose here (Fig. 3) to potentially serve as a reasonable and useful substitute across a majority of survivors.
But, first, it is necessary to determine how PMA can be targeted. In the same vein as the parent motor cortical technique, epidural stimulation is feasible with the advantage of spatially specific targeting. Epidural stimulation targeting ventral and lateral PMC is found to carry preliminary efficacy for rehabilitation in aphasia (Cherney and others 2012) and focal dystonia (Lalli and others 2012). The site 2 cm anterior to the M1 hand knob area is chosen (Lalli and others 2012) or is localized using task-related fMRI activation (Cherney and others 2012). Before initiating trials involving epidural techniques, however, noninvasive transcranial stimulation can be critical to create proof-of-principle and validate or refute hypothesized mechanisms. As one of its forms, repetitive TMS (rTMS) can generate electrical currents in the brain via electromagnetic induction to produce lasting changes in cortical excitability. Frequencies ≤ 1 Hz are considered inhibitory, while frequencies ≥ 5 Hz are considered facilitatory for underlying excitability (Fitzgerald and others 2006). As an even simpler analogue, tDCS can apply low-level currents (0–2.5 mA) via surface electrodes to scalp. Despite the use of low current levels, it can depolarize membrane potentials to alter excitability. Although less focal than epidural, targeting PMA with rTMS and tDCS is being tested for its efficacy in Parkinson’s disease (Shirota and others 2013) and gait abnormalities in leukoaraiosis (Kaski and others 2013).
Mechanism: Understanding probability of survival of PMA
Anatomic cortical landmarks of PMA are well outlined based on MRI in our own work and that of others (Dassonville and others 2001; Plow [nee (Bhatt)] and others 2007). Now, deciphering the probability of structural integrity of their CST involves a relatively new application. CST from different regions, whether PMC, SMA, or M1, can be delineated using Diffusion Tensor Imaging (DTI) (Cunningham and others 2013a; Ruber and others 2012; Schulz and others 2012); tracts can be compared with those from homologues in contralesional hemisphere to understand which of the motor areas indeed has the most surviving output.
Mechanism: Studying re-mapping of PMA
Task-related fMRI activation during movement of paretic hand is a useful method to explore re-mapping of the PMA; as discussed earlier, patients with greater impairments recruit ipsilesional and contralesional PMA over the course of recovery since output from lesioned M1 may be inadequate.
However, to confirm what type of influence they exert in recovery, virtual inactivation using TMS has become critical. During movement of paretic hand, it can be applied alone, concurrently with fMRI, or with offline fMRI to study how transiently inactivating a locus affects movement and the regional activation. For example, single pulse TMS targeting PMd induces reaction time delays in the more impaired, indicating its facilitatory potential for recovery. Using online fMRI, Bestmann et. al. (2010) confirm that such targeting is indeed facilitatory because it improves activation in ipsilesional sensorimotor cortex. However, online TMS-fMRI is technically challenging. Therefore, an offline method that we have recently applied for parietal cortices can serve as a reasonable substitute (Plow and others 2014a). PMA activity could first be inhibited using 1 Hz rTMS, and then followed promptly with fMRI during movement of paretic hand. If performance following rTMS suffers in relation to sham, and fMRI activation of target and its synergists alters, then one can validate the causative value of PMA in recovery.
Mechanism: PMA in affecting adaptive plasticity in recovery
To know whether their chronic stimulation invokes plasticity of their CST, one can examine fast output responses from PMA using TMS. Such responses can be evoked at 22 mm to 53 mm anterior from the M1, and as such are considered separate from the M1 (Schmidt and others 2013; Teitti and others 2008). To study these responses, evoked motor responses in the paretic muscles are plotted as cortical maps. Maps describe regional excitability of the CST devoted to the paretic hand. Changes in maps thus can be used to assess whether CST from more anterior and antero-medially located PMC and SMA subtend recovery in stroke (Byrnes and others 2001) and strength and dexterity in healthy (Neva and others 2014; Plow and others 2014b).
These interpretations can be confounded, however, if due to current spread of TMS, CST from M1 instead are excited. Computing electric field values can be confirmatory. When mapping PMA, if remote electric field at M1 is lower than that required for evoking motor responses, then shifts in map towards PMA could be considered their independent plasticity attributed to their own CST rather than those of the M1 (Schmidt and others 2013).
Restoration of inter-hemispheric balance can be captured with fMRI (Plow [nee (Bhatt)] and others 2007; Rehme and others 2011; Ward and others 2003), and TMS can determine whether callossal physiology is the basis of change. For instance, using bi-hemispheric pulses between contralesional PMd and ipsilesional M1, Bestmann and others (2010) concluded that contralesional PMd was less inhibitory in movements of paretic hand, especially in more impaired.
Vicarious recruitment of alternate ipsilesional (Dancause and others 2006; Frost and others 2003; Plow [nee (Bhatt)] and others 2007; Seitz and others 1998; Ward and others 2007; Weiller and others 1992) and contralesional (Bestmann and others 2010; Johansen-Berg and others 2002; Lotze and others 2012) motor regions and changes in their connectivity with ipsilesional PMA or with M1 can be studied with resting state fMRI. Based on correlated cortical activity in resting state, resting state fMRI defines functional connectivity between regions. However, if regional connectivity and its direction of influence are important, then rTMS and tDCS are reasonable tools to pair with TMS. For instance, rTMS of PMA exerts intensity- and frequency-specific effects (Baumer and others 2003; Rizzo and others 2004), while tDCS of PMA exerts polarity-specific influence (Boros and others 2008). Such regional influences reveal cortico-cortical connectivity between PMA and M1, but more importantly they reveal the direction of influence (facilitation or inhibition). Knowing how PMA and M1 affect each other would be critical to planning targets for stimulation.
Conclusions
After all is considered, although at this time it is difficult to predict whether PMA would be ‘better’ targets across patients, it behooves us to investigate whether they would at least be reasonable substitutes to M1. Stimulation of M1 may be perfectly suited for some, but given the variability of existing experimental data (Table 1), it is less realistic that it is the end-all-be-all for stroke motor recovery. In this regard, PMA with better probability of survival, greater projections to the spinal cord, causative functional potential, excitable alternate CST, stronger callossal connections, and widespread connectivity could instead serve as feasible, alternate targets to chronically stimulate in stroke motor rehabilitation.
A caveat, however, still remains. Since M1 may offer the best basis for recovery, patients who may benefit maximally from stimulation of M1 may include ones with the strongest residual potential, hence they may recover the most. Those who benefit from stimulation of PMA instead may include more disadvantaged patients; hence they may not recover to a similar degree. Nevertheless, it would be more reasonable to achieve at least some recovery across a majority who are more disadvantaged than to stimulate a single locus fated to be inconsistently effective across all.
We understand that choosing one target versus another is less meaningful if ultimately the goal is to “tailor” interventions based on predictors of therapeutic response. Still, our present hypothesis that makes a case for the PMA as alternate targets is a strategic step towards the futuristic goal of tailored stimulation. By identifying who responds and does not respond to stimulation of M1 versus stimulation of PMA, such as in ongoing and recent clinical trials (Nouri and Cramer 2011; Plow and others 2009; Plow and Machado 2013), we would finally be able to stratify candidates for individualized treatments. Lastly, besides clinical significance, chronically stimulating the PMA to drive stroke motor rehabilitation would also serve as the ‘litmus test’ of our longstanding neuroscientific beliefs in their role in human recovery.
Acknowledgments
Grant Support: This work is supported by grants from the National Institutes of Health (1K01HD069504) and the American Heart Association (13BGIA17120055) to EP.
Footnotes
Conflicts of Interest: AM has the following conflicts of interest to disclose: ATI, Enspire and cardionomics (Intellectual property and possible distribution rights); Functional Neurostimulation (consultant), Medtronic (fellowship support).
Contributor Information
Ela B Plow, Email: plowe2@ccf.org.
David Cunningham, Email: cunnind6@ccf.org.
Nicole Varnerin, Email: varnern@ccf.org.
Andre Machado, Email: machada@ccf.org.
References
- Ackerley SJ, Stinear CM, Barber PA, Byblow WD. Combining theta burst stimulation with training after subcortical stroke. Stroke. 2010;41(7):1568–72. doi: 10.1161/STROKEAHA.110.583278. [DOI] [PubMed] [Google Scholar]
- Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003;25(8):780–8. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- Baumer T, Lange R, Liepert J, Weiller C, Siebner HR, Rothwell JC, et al. Repeated premotor rTMS leads to cumulative plastic changes of motor cortex excitability in humans. Neuroimage. 2003;20(1):550–60. doi: 10.1016/s1053-8119(03)00310-0. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Swayne O, Blankenburg F, Ruff CC, Teo J, Weiskopf N, et al. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI. J Neurosci. 2010;30(36):11926–37. doi: 10.1523/JNEUROSCI.5642-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F, Seitz RJ. Modulation of the BOLD-response in early recovery from sensorimotor stroke. Neurology. 2004;63(7):1223–9. doi: 10.1212/01.wnl.0000140468.92212.be. [DOI] [PubMed] [Google Scholar]
- Bogousslavsky J, Regli F. Anterior cerebral artery territory infarction in the Lausanne Stroke Registry. Clinical and etiologic patterns. Arch Neurol. 1990;47(2):144–50. doi: 10.1001/archneur.1990.00530020040012. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Vallar G, Casati C, Latif LA, El-Nazer R, Williams J, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair. 2011;25(9):819–29. doi: 10.1177/1545968311411056. [DOI] [PubMed] [Google Scholar]
- Boros K, Poreisz C, Munchau A, Paulus W, Nitsche MA. Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. Eur J Neurosci. 2008;27(5):1292–300. doi: 10.1111/j.1460-9568.2008.06090.x. [DOI] [PubMed] [Google Scholar]
- Boudrias MH, Lee SP, Svojanovsky S, Cheney PD. Forelimb muscle representations and output properties of motor areas in the mesial wall of rhesus macaques. Cereb Cortex. 2010;20(3):704–19. doi: 10.1093/cercor/bhp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaoud D, Tanne-Gariepy J, Wannier T, Rouiller EM. Callosal connections of dorsal versus ventral premotor areas in the macaque monkey: a multiple retrograde tracing study. BMC Neurosci. 2005;6:67. doi: 10.1186/1471-2202-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeks JG, Lankhorst GJ, Rumping K, Prevo AJ. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil. 1999;21(8):357–64. doi: 10.1080/096382899297459. [DOI] [PubMed] [Google Scholar]
- Brown JA, Lutsep HL, Weinand M, Cramer SC. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery. 2006;58(3):464–73. doi: 10.1227/01.NEU.0000197100.63931.04. [DOI] [PubMed] [Google Scholar]
- Byrnes ML, Thickbroom GW, Phillips BA, Mastaglia FL. Long-term changes in motor cortical organisation after recovery from subcortical stroke. Brain Res. 2001;889(1–2):278–87. doi: 10.1016/s0006-8993(00)03089-4. [DOI] [PubMed] [Google Scholar]
- Carey JR, Durfee WK, Plow [nee (Bhatt)] E, Nagpal A, Weinstein SA, Anderson KM, et al. Comparison of finger tracking versus simple movement training via telerehabilitation to alter hand function and cortical reorganization after stroke. Neurorehabil Neural Repair. 2007;21(3):216–32. doi: 10.1177/1545968306292381. [DOI] [PubMed] [Google Scholar]
- Chang WH, Kim YH, Bang OY, Kim ST, Park YH, Lee PK. Long-term effects of rTMS on motor recovery in patients after subacute stroke. J Rehabil Med. 2010;42(8):758–64. doi: 10.2340/16501977-0590. [DOI] [PubMed] [Google Scholar]
- Chang WH, Kim YH, Yoo WK, Goo KH, Park CH, Kim ST, et al. rTMS with motor training modulates cortico-basal ganglia-thalamocortical circuits in stroke patients. Restor Neurol Neurosci. 2012;30(3):179–89. doi: 10.3233/RNN-2012-110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney LR, Harvey RL, Babbitt EM, Hurwitz R, Kaye RC, Lee JB, et al. Epidural cortical stimulation and aphasia therapy. Aphasiology. 2012;26(9):1192–1217. doi: 10.1080/02687038.2011.603719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforto AB, Anjos SM, Saposnik G, Mello EA, Nagaya EM, Santos W, Jr, et al. Transcranial magnetic stimulation in mild to severe hemiparesis early after stroke: a proof of principle and novel approach to improve motor function. J Neurol. 2012;259(7):1399–405. doi: 10.1007/s00415-011-6364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC. Functional imaging in stroke recovery. Stroke. 2004;35(11 Suppl 1):2695–8. doi: 10.1161/01.STR.0000143326.36847.b0. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Moore CI, Finklestein SP, Rosen BR. A pilot study of somatotopic mapping after cortical infarct. Stroke. 2000;31(3):668–71. doi: 10.1161/01.str.31.3.668. [DOI] [PubMed] [Google Scholar]
- Cunningham DA, Machado A, Rajagopalan V, Lowe MJ, Jones S, Beall E, et al. DTI versus fMRI: accuracy and reliability in predicting response to TMS in Stroke. Annals of Neurology: Special Issue 2013 Annual Meeting. 2013a;74(S17):S1–S120. [Google Scholar]
- Cunningham DA, Machado A, Yue GH, Carey JR, Plow EB. Functional somatotopy revealed across multiple cortical regions using a model of complex motor task. Brain Res. 2013b;1531:25–36. doi: 10.1016/j.brainres.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Mahnken JD, Nudo RJ. Interhemispheric connections of the ventral premotor cortex in a new world primate. J Comp Neurol. 2007;505(6):701–15. doi: 10.1002/cne.21531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25(44):10167–79. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Zoubina EV, Plautz EJ, Mahnken JD, et al. Effects of small ischemic lesions in the primary motor cortex on neurophysiological organization in ventral premotor cortex. J Neurophysiol. 2006;96(6):3506–11. doi: 10.1152/jn.00792.2006. [DOI] [PubMed] [Google Scholar]
- Dancause N, Nudo RJ. Shaping plasticity to enhance recovery after injury. Prog Brain Res. 2011;192:273–95. doi: 10.1016/B978-0-444-53355-5.00015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassonville P, Lewis SM, Zhu XH, Ugurbil K, Kim SG, Ashe J. The effect of stimulus-response compatibility on cortical motor activation. Neuroimage. 2001;13(1):1–14. doi: 10.1006/nimg.2000.0671. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11(3):667–89. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DJ, Krebs HI, Rykman A, Zipse J, Thickbroom GW, Mastaglia FL, et al. Raised corticomotor excitability of M1 forearm area following anodal tDCS is sustained during robotic wrist therapy in chronic stroke. Restor Neurol Neurosci. 2009;27(3):199–207. doi: 10.3233/RNN-2009-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara T, El Nahas N, Elkader HA, Ashour S, El Etrebi A. MRI can Predict the Response to Therapeutic Repetitive Transcranial Magnetic Stimulation (rTMS) in Stroke Patients. J Vasc Interv Neurol. 2009;2(2):163–8. [PMC free article] [PubMed] [Google Scholar]
- Fang PC, Stepniewska I, Kaas JH. Corpus callosum connections of subdivisions of motor and premotor cortex, and frontal eye field in a prosimian primate, Otolemur garnetti. J Comp Neurol. 2008;508(4):565–78. doi: 10.1002/cne.21706. [DOI] [PubMed] [Google Scholar]
- Feydy A, Carlier R, Roby-Brami A, Bussel B, Cazalis F, Pierot L, et al. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33(6):1610–7. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584–96. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3(7):383–93. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- French B, Thomas LH, Leathley MJ, Sutton CJ, McAdam J, Forster A, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev. 2007;(4):CD006073. doi: 10.1002/14651858.CD006073.pub2. [DOI] [PubMed] [Google Scholar]
- Freund HJ, Hummelsheim H. Lesions of premotor cortex in man. Brain. 1985;108 (Pt 3):697–733. doi: 10.1093/brain/108.3.697. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127(Pt 4):747–58. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89(6):3205–14. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- Hamadjida A, Wyss AF, Mir A, Schwab ME, Belhaj-Saif A, Rouiller EM. Influence of anti-Nogo-A antibody treatment on the reorganization of callosal connectivity of the premotor cortical areas following unilateral lesion of primary motor cortex (M1) in adult macaque monkeys. Exp Brain Res. 2012;223(3):321–40. doi: 10.1007/s00221-012-3262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RL, Winstein CJ. Design for the everest randomized trial of cortical stimulation and rehabilitation for arm function following stroke. Neurorehabil Neural Repair. 2009;23(1):32–44. doi: 10.1177/1545968308317532. [DOI] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci. 1993;13(3):952–80. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse S, Waldner A, Mehrholz J, Tomelleri C, Pohl M, Werner C. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: an exploratory, randomized multicenter trial. Neurorehabil Neural Repair. 2011;25(9):838–46. doi: 10.1177/1545968311413906. [DOI] [PubMed] [Google Scholar]
- Huang M, Harvey RL, Stoykov ME, Ruland S, Weinand M, Lowry D, et al. Cortical stimulation for upper limb recovery following ischemic stroke: a small phase II pilot study of a fully implanted stimulator. Top Stroke Rehabil. 2008;15(2):160–72. doi: 10.1310/tsr1502-160. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Non-invasive brain stimulation. Lancet Neurol. 2006;5(8):708–12. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- Inman CS, James GA, Hamann S, Rajendra JK, Pagnoni G, Butler AJ. Altered resting-state effective connectivity of fronto-parietal motor control systems on the primary motor network following stroke. Neuroimage. 2012;59(1):227–37. doi: 10.1016/j.neuroimage.2011.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James GA, Lu ZL, VanMeter JW, Sathian K, Hu XP, Butler AJ. Changes in resting state effective connectivity in the motor network following rehabilitation of upper extremity poststroke paresis. Top Stroke Rehabil. 2009;16(4):270–81. doi: 10.1310/tsr1604-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99(22):14518–23. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaski D, Dominguez RO, Allum JH, Bronstein AM. Improving gait and balance in patients with leukoaraiosis using transcranial direct current stimulation and physical training: an exploratory study. Neurorehabil Neural Repair. 2013;27(9):864–71. doi: 10.1177/1545968313496328. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65(3):466–8. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- Kim YH, You SH, Ko MH, Park JW, Lee KH, Jang SH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37(6):1471–6. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- Lalli S, Piacentini S, Franzini A, Panzacchi A, Cerami C, Messina G, et al. Epidural premotor cortical stimulation in primary focal dystonia: clinical and 18F-fluoro deoxyglucose positron emission tomography open study. Mov Disord. 2012;27(4):533–8. doi: 10.1002/mds.24949. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Thonnard JL, Laloux P, Peeters A, Jamart J, Vandermeeren Y. Single Session of Dual-tDCS Transiently Improves Precision Grip and Dexterity of the Paretic Hand After Stroke. Neurorehabil Neural Repair. 2013 doi: 10.1177/1545968313478485. [DOI] [PubMed] [Google Scholar]
- Levy R, Ruland S, Weinand M, Lowry D, Dafer R, Bakay R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: a multicenter feasibility study of safety and efficacy. J Neurosurg. 2008;108(4):707–14. doi: 10.3171/JNS/2008/108/4/0707. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010a;74(4):280–7. doi: 10.1212/WNL.0b013e3181ccc6d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010b;75(24):2176–84. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Zhu LL, Ruber T, Schlaug G. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum Brain Mapp. 2012a;33(5):1040–51. doi: 10.1002/hbm.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Zhu LL, Schlaug G. Combined central and peripheral stimulation to facilitate motor recovery after stroke: the effect of number of sessions on outcome. Neurorehabil Neural Repair. 2012b;26(5):479–83. doi: 10.1177/1545968311427568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rouiller EM. Mechanisms of recovery of dexterity following unilateral lesion of the sensorimotor cortex in adult monkeys. Exp Brain Res. 1999;128(1–2):149–59. doi: 10.1007/s002210050830. [DOI] [PubMed] [Google Scholar]
- Lotze M, Beutling W, Loibl M, Domin M, Platz T, Schminke U, et al. Contralesional motor cortex activation depends on ipsilesional corticospinal tract integrity in well-recovered subcortical stroke patients. Neurorehabil Neural Repair. 2012;26(6):594–603. doi: 10.1177/1545968311427706. [DOI] [PubMed] [Google Scholar]
- Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. Journal of Neuroscience. 2006;26(22):6096–102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A, Baker KB. Upside down crossed cerebellar diaschisis: proposing chronic stimulation of the dentatothalamocortical pathway for post-stroke motor recovery. Front Integr Neurosci. 2012;6:20. doi: 10.3389/fnint.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado AG, Shoji A, Ballester G, Marino R., Jr Mapping of the rat’s motor area after hemispherectomy: The hemispheres as potentially independent motor brains. Epilepsia. 2003;44(4):500–6. doi: 10.1046/j.1528-1157.2003.37602.x. [DOI] [PubMed] [Google Scholar]
- Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex. 2002;12(3):281–96. doi: 10.1093/cercor/12.3.281. [DOI] [PubMed] [Google Scholar]
- Malcolm MP, Triggs WJ, Light KE, Gonzalez Rothi LJ, Wu S, Reid K, et al. Repetitive transcranial magnetic stimulation as an adjunct to constraint-induced therapy: an exploratory randomized controlled trial. Am J Phys Med Rehabil. 2007;86(9):707–15. doi: 10.1097/PHM.0b013e31813e0de0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal DW, Darling WG, Ge J, Stilwell-Morecraft KS, Solon KM, Hynes SM, et al. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J Comp Neurol. 2010;518(5):586–621. doi: 10.1002/cne.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Herrick JL, Stilwell-Morecraft KS, Louie JL, Schroeder CM, Ottenbacher JG, et al. Localization of arm representation in the corona radiata and internal capsule in the non-human primate. Brain. 2002;125(Pt 1):176–98. doi: 10.1093/brain/awf011. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55(3):400–9. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nair DG, Renga V, Lindenberg R, Zhu L, Schlaug G. Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tDCS. Restor Neurol Neurosci. 2011;29(6):411–20. doi: 10.3233/RNN-2011-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neva JL, Vesia M, Singh AM, Staines WR. Modulation of left primary motor cortex excitability after bimanual training and intermittent theta burst stimulation to left dorsal premotor cortex. Behav Brain Res. 2014;261:289–96. doi: 10.1016/j.bbr.2013.12.029. [DOI] [PubMed] [Google Scholar]
- Nouri S, Cramer SC. Anatomy and physiology predict response to motor cortex stimulation after stroke. Neurology. 2011;77(11):1076–83. doi: 10.1212/WNL.0b013e31822e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272(5269):1791–4. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- Ochi M, Saeki S, Oda T, Matsushima Y, Hachisuka K. Effects of anodal and cathodal transcranial direct current stimulation combined with robotic therapy on severely affected arms in chronic stroke patients. J Rehabil Med. 2013;45(2):137–40. doi: 10.2340/16501977-1099. [DOI] [PubMed] [Google Scholar]
- Ones K, Yilmaz E, Cetinkaya B, Caglar N. Quality of life for patients poststroke and the factors affecting it. J Stroke Cerebrovasc Dis. 2005;14(6):261–6. doi: 10.1016/j.jstrokecerebrovasdis.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Park CH, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, et al. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 2011;42(5):1357–62. doi: 10.1161/STROKEAHA.110.596155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A. Disrupting the brain to guide plasticity and improve behavior. Prog Brain Res. 2006;157:315–16. doi: 10.1016/s0079-6123(06)57019-0. [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25(8):801–10. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- Plow [nee (Bhatt)] E, Nagpal A, Greer KH, Grunewald TK, Steele JL, Wiemiller JW, et al. Effect of finger tracking combined with electrical stimulation on brain reorganization and hand function in subjects with stroke. Exp Brain Res. 2007;182(4):435–47. doi: 10.1007/s00221-007-1001-5. [DOI] [PubMed] [Google Scholar]
- Plow EB, Carey JR, Nudo RJ, Pascual-Leone A. Invasive cortical stimulation to promote recovery of function after stroke. Stroke. 2009;40(5):1926–31. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EB, Cattaneo Z, Carlson T, Alvarez GA, Pascual-Leone A, Battelli L. The compensatory dynamic of inter-hemispheric interactions in visuospatial attention revealed using rTMS and fMRI. Frontiers in Human Neuroscience. 2014a doi: 10.3389/fnhum.2014.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EB, Cunningham DA, Beall E, Jones S, Wyant A, Bonnett C, et al. Effectiveness and neural mechanisms associated with tDCS delivered to premotor cortex in stroke rehabilitation: study protocol for a randomized controlled trial. Trials. 2013;14:331. doi: 10.1186/1745-6215-14-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EB, Machado A. Invasive Neurostimulation in Stroke Rehabilitation. Neurotherapeutics. 2013 doi: 10.1007/s13311-013-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EB, Varnerin N, Cunningham DA, Janini D, Bonnett C, Wyant A, et al. Age-related Weakness of Proximal Muscle studied with Motor Cortical Mapping: A TMS Study. PLoS ONE. 2014b doi: 10.1371/journal.pone.0089371. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy VM, Cloud G, Tallis RC, Donaldson C, Nayak V, Miller S. Transcranial magnetic stimulation and muscle contraction to enhance stroke recovery: a randomized proof-of-principle and feasibility investigation. Neurorehabil Neural Repair. 2007;21(6):509–17. doi: 10.1177/1545968307300418. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, Wang LE, Fink GR, Grefkes C. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. Neuroimage. 2011;55(3):1147–58. doi: 10.1016/j.neuroimage.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo V, Siebner HR, Modugno N, Pesenti A, Munchau A, Gerschlager W, et al. Shaping the excitability of human motor cortex with premotor rTMS. J Physiol. 2004;554(Pt 2):483–95. doi: 10.1113/jphysiol.2003.048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp Brain Res. 1994;102(2):227–43. doi: 10.1007/BF00227511. [DOI] [PubMed] [Google Scholar]
- Ruber T, Schlaug G, Lindenberg R. Compensatory role of the cortico-rubro-spinal tract in motor recovery after stroke. Neurology. 2012;79(6):515–22. doi: 10.1212/WNL.0b013e31826356e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Mizutani S, Kakuda W, Abo M. Comparison of the effects of high- and low-frequency repetitive transcranial magnetic stimulation on upper limb hemiparesis in the early phase of stroke. J Stroke Cerebrovasc Dis. 2013;22(4):413–8. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Fleischmann R, Bathe-Peters R, Irlbacher K, Brandt SA. Evolution of premotor cortical excitability after cathodal inhibition of the primary motor cortex: a sham-controlled serial navigated TMS study. PLoS ONE. 2013;8(2):e57425. doi: 10.1371/journal.pone.0057425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Park CH, Boudrias MH, Gerloff C, Hummel FC, Ward NS. Assessing the Integrity of Corticospinal Pathways From Primary and Secondary Cortical Motor Areas After Stroke. Stroke. 2012 doi: 10.1161/STROKEAHA.112.662619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz RJ, Hoflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch Neurol. 1998;55(8):1081–8. doi: 10.1001/archneur.55.8.1081. [DOI] [PubMed] [Google Scholar]
- Seniow J, Bilik M, Lesniak M, Waldowski K, Iwanski S, Czlonkowska A. Transcranial magnetic stimulation combined with physiotherapy in rehabilitation of poststroke hemiparesis: a randomized, double-blind, placebo-controlled study. Neurorehabil Neural Repair. 2012;26(9):1072–9. doi: 10.1177/1545968312445635. [DOI] [PubMed] [Google Scholar]
- Shirota Y, Ohtsu H, Hamada M, Enomoto H, Ugawa Y. Supplementary motor area stimulation for Parkinson disease: a randomized controlled study. Neurology. 2013;80(15):1400–5. doi: 10.1212/WNL.0b013e31828c2f66. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Coxon JP, Fleming MK, Byblow WD. Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain. 2008;131(Pt 5):1381–90. doi: 10.1093/brain/awn051. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130(Pt 1):170–80. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Tada T, Chuma T, Matsuo Y, Ikoma K. Disinhibition of the premotor cortex contributes to a maladaptive change in the affected hand after stroke. Stroke. 2007;38(5):1551–6. doi: 10.1161/STROKEAHA.106.470187. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Tada T, Matsuo Y, Ikoma K. Low-frequency repetitive TMS plus anodal transcranial DCS prevents transient decline in bimanual movement induced by contralesional inhibitory rTMS after stroke. Neurorehabil Neural Repair. 2012;26(8):988–98. doi: 10.1177/1545968311433295. [DOI] [PubMed] [Google Scholar]
- Talelli P, Wallace A, Dileone M, Hoad D, Cheeran B, Oliver R, et al. Theta burst stimulation in the rehabilitation of the upper limb: a semirandomized, placebo-controlled trial in chronic stroke patients. Neurorehabil Neural Repair. 2012;26(8):976–87. doi: 10.1177/1545968312437940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, Uswatte G, Morris DM. Improved motor recovery after stroke and massive cortical reorganization following Constraint-Induced Movement therapy. Phys Med Rehabil Clin N Am. 2003;14(1 Suppl):S77–91. ix. doi: 10.1016/s1047-9651(02)00052-9. [DOI] [PubMed] [Google Scholar]
- Teitti S, Maatta S, Saisanen L, Kononen M, Vanninen R, Hannula H, et al. Non-primary motor areas in the human frontal lobe are connected directly to hand muscles. Neuroimage. 2008;40(3):1243–50. doi: 10.1016/j.neuroimage.2007.12.065. [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126(Pt 6):1430–48. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Frackowiak RS, Thompson AJ, et al. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur J Neurosci. 2007;25(6):1865–73. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. 1992;31(5):463–72. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- Wise SP. The primate premotor cortex fifty years after Fulton. Behav Brain Res. 1985;18(2):79–88. doi: 10.1016/0166-4328(85)90064-6. [DOI] [PubMed] [Google Scholar]
- Wittenberg GF, Chen R, Ishii K, Bushara KO, Eckloff S, Croarkin E, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17(1):48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- Wu D, Qian L, Zorowitz RD, Zhang L, Qu Y, Yuan Y. Effects on decreasing upper-limb poststroke muscle tone using transcranial direct current stimulation: a randomized sham-controlled study. Arch Phys Med Rehabil. 2013;94(1):1–8. doi: 10.1016/j.apmr.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Zeiler SR, Gibson EM, Hoesch RE, Li MY, Worley PF, O’Brien RJ, et al. Medial premotor cortex shows a reduction in inhibitory markers and mediates recovery in a mouse model of focal stroke. Stroke. 2013;44(2):483–9. doi: 10.1161/STROKEAHA.112.676940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimerman M, Heise KF, Hoppe J, Cohen LG, Gerloff C, Hummel FC. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke. 2012;43(8):2185–91. doi: 10.1161/STROKEAHA.111.645382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinger N, Harel R, Gabler S, Israel Z, Prut Y. Functional organization of information flow in the corticospinal pathway. J Neurosci. 2013;33(3):1190–7. doi: 10.1523/JNEUROSCI.2403-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]