Abstract
Catheter ablation is an increasingly used and successful treatment choice for right ventricular outflow tract (RVOT) arrhythmias. While the role of endocavitary structures and the regional morphology of the ventricular inflow tract and the right atrium as a cause for difficulty with successful ablation are well described, similar issues within the RVOT are not well understood. It is also not commonly appreciated that one of the papillary muscles is located within the proximal right ventricular outflow tract. We report 3 patients in which ventricular arrhythmia was targeted and ablated in the conus papillary muscle. The anatomic features, potential role of the fascicular conduction system, and unique challenges with mapping arrhythmia arising from this structure are discussed.
Keywords: Lancisi bundle, Purkinje fiber, premature ventricular complex, right ventricular outflow tract, ventricular tachycardia, conus papillary muscle, endocavitary structures, ventricular fibrillation
Introduction
Right ventricular outflow tract (RVOT) arrhythmia is the most common ventricular arrhythmia arising in structurally normal hearts.1, 2 Catheter ablation is an increasingly used and successful treatment choice for patients with RVOT arrhythmia and drug-refractory symptoms. Procedural failure, however, occurs in up to 25% to 30% of attempted ablations.3, 4
Several predictors for ablation failure have been identified, including difficulty with initiating arrhythmia, origin of arrhythmia at or above the semi-lunar valves, and discrete myocardial sleeves within the great arteries.5-9
While the role of endocavitary structures (papillary muscles, moderator band, and pectinate-like trabeculations) causing difficulty with ablation in the right ventricular inflow portions have been well described,10, 11 their role has not been well appreciated for right ventricular outflow tract ablation.
Specific difficulty with mapping and ablating papillary muscles of the inflow tract caused by variable exit, catheter stability, and related conduction tissue is known.10 While the potential arrhythmogenic target related to papillary muscles in the right ventricle has been reported,12 technical and mapping problems presented by the conus papillary muscle (CPM), given its origin in the outflow tract and adjacent parahisian conduction tissue, are unknown.
In this manuscript, we report 3 patients where ventricular arrhythmia was eliminated by visualizing, mapping, and targeting the conus papillary muscle. We describe the regional anatomy, the utility of intracardiac echocardiography (ICE),13 and the unique electrograms and exit sites mapped on this structure.
Case 1
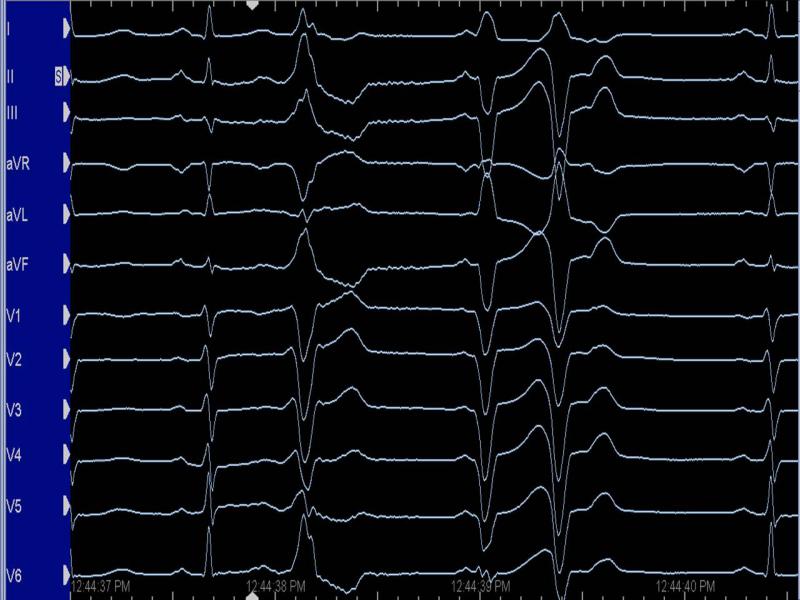
A 47-year-old female with symptomatic premature ventricular complexes (PVCs) was referred for ablation. Her symptoms were refractory to medical therapy, and two prior attempts at PVC ablation were unsuccessful. Holter monitor showed brief runs of nonsustained, polymorphic ventricular tachycardia (VT), triggered by frequent PVCs of two principal morphologies. At EP study, frequent PVCs of the two morphologies noted previously on Holter were repeatedly observed (Figure 1). Both PVCs had an identical coupling interval and showed a left bundle branch block inferior axis pattern. AVL was isoelectric in one PVC morphology, while positive in the second PVC.
Figure 1. PVCs of Patient 1 recorded in the EP laboratory.
(Left panel: PVC 1; Right panel: PVC 2)
During point-to-point mapping, the earliest electrograms for both PVCs were close to each other and were located in the posteromedial proximal RVOT (Figure 2). The local electrogram was 35 ms ahead of both QRS morphologies. With further mapping, a pre-potential suggestive of a fascicular signal was noted between these two breakout sites, and pacing at low output reproduced both principal PVC morphologies (Figure 2). Fluoroscopy (Figure 3) and ICE imaging (Figure 4) showed that the mapping catheter was positioned on the mid-portion of the conus papillary muscle. Radiofrequency energy delivery in this region simultaneously eliminated both PVCs with runs of polymorphic VT seen during ablation. There was no evidence of recurrence at 6 months follow-up.
Figure 2. Activation and pacemapping of PVCs in Case 1.
Left panel: Intracardiac electrogram near ablation site shows a pre-potential (marked by red arrow) between the breakout sites of both PVCs, and was 95 ms ahead of the surface QRS. The ventricular electrogram was 20 ms after the onset of the surface QRS.
Right panel: Pacemapping at the site of ablation with low output capture reproduced both PVC morphologies.
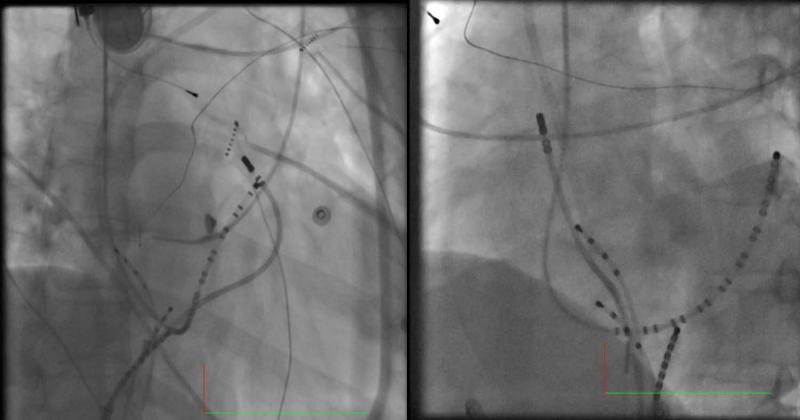
Figure 3. Ablation catheter at the ablation site in Case 1.
(Left: LAO view; Right: RAO view)
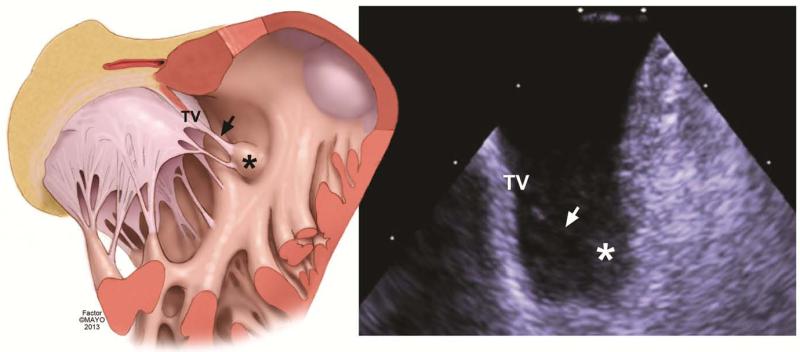
Figure 4. Conus Papillary Muscle was the site of PVC ablation.
Left panel is an illustration showing correlative structures seen on the ICE image (right panel). The body of the conus papillary muscle is marked with an asterisk in both images. The body of the conus papillary muscle is seen with an attached tendinous chord (marked by arrow) that is part of the tricuspid valve (TV).
Case 2
A 63-year-old with prior history of non-ST elevation myocardial infarction from coronary vasospasm was referred for ablation of medically refractory and highly symptomatic PVCs. A 48-hour Holter monitor showed 33,000 PVCs and brief 6- to 8-beat runs of VT. At EP study, two PVCs were noted (Supplemental figure 1). Mapping of the first PVC found earliest activation just above the septal parietal trabeculation. The earliest site of activation for the second PVC was at the posteromedial wall of the proximal RVOT where a sharp Purkinje potential was seen ahead of the local ventricular electrogram. ICE imaging confirmed that the ablation catheter was located on the conus papillary muscle. Ablation at this site successfully eliminated the PVCs with no recurrence or symptoms at approximately one-year follow-up.
Case 3
A 25-year-old female with refractory symptomatic ventricular arrhythmia that included PVCs and short runs of polymorphic VT was referred for ablation. Several PVCs were noted, including one that was successfully targeted and ablated in the vicinity of the pulmonic valve. One of the PVCs had earliest activation on the posterior proximal RVOT and was at the base of the conus papillary muscle visualized with ICE (Supplemental Figure 2). Ablation at this site eliminated this PVC, with runs of polymorphic VT during ablation
Discussion
We report 3 patients that required mapping and ablation on the conus papillary muscle to eliminate ventricular arrhythmia.
Regional anatomy and clinical relevance
Papillary muscles occur at multiple and variable sites in the right and left ventricular inflow chambers.14, 15 In the right ventricle, one of the septal papillary muscles arises from the outflow tract.16-19 This muscle has been referred to as a Lancisi muscle or bundle with clinicians referring to it as the conus papillary muscle in conjunction with the tendinous chord at the medial papillary muscle complex.16, 18, 19 Autopsy studies have demonstrated the presence of the CPM in 82% of human hearts,20 and when present is found at the base of the subpulmonary infundibulum adjacent to the distal bundle of His as it penetrates the membranous septum and proximal right bundle branch (Figure 5). In many cases, the belly of the CPM lies at the posterior extremity of the septal marginal trabeculation, while in others it arises near its root. More anterolaterally, discrete trabeculation without attached chordae may be found.
Figure 5. Conus papillary muscle location and related anatomical structures.
Panel A. Gross anatomical dissection showing anatomical structure of the conus papillary muscle and its inter-relationships with the surrounding structures.
Panel B. Illustration of gross specimen from Panel A showing structures related to the conus papillary muscle (noted with an *). Notice the belly of the muscle situated in between the anterolateral and posterolateral aspects of the septomarginal trabeculation, along with its tendinous chordal connection to the tricuspid valve.
The endocavitary structures (CPMs, prominent trabeculations) may cause difficulty with mapping and ablation. While the supravalvar myocardial sleeves are a recognized cause of difficulty, and techniques for imaging and mapping have been developed, similar site-specific mapping and assessment of contact when ablation may be of value in such cases.
Electrocardiographic and electrogram characteristics
In our patients, variable morphologies of at least two distinct varieties were noted clinically. As has been described with papillary muscle VT in the left ventricle,10 this phenomenon may represent varying exit at the base of the papillary muscle. An important clue when mapping these arrhythmias in our patients was the finding of approximately equally early electrogram sites at the base of the RVOT crux at disparate locations. This suggested a site between these locations which was eventually found and determined with concurrent ICE imaging to be the CPM.
The local electrograms in these patients show the presence of a fascicle-like signal that preceded ventricular activation. The proximity of the His and right bundle to the CPM suggests that some terminal branches from the right bundle lies in close relationship to the CPM similar to what has been described with the left ventricular papillary muscles and the moderator band.10 This possible Purkinje fiber origin may also explain polymorphic ventricular arrhythmia that was observed in some instances with these patients.21, 22 Recently, a malignant form of ventricular arrhythmia in patients with bileaflet mitral valve prolapse has been described.23 Interestingly in these patients, in addition to site of origin for the arrhythmia-inciting PVCs in the LV papillary muscle, a second morphology arising from the right ventricular outflow tract was noted. Whether or not this activity represents fascicular origin in the PCM needs further evaluation. As with papillary muscle mapping and ablation elsewhere, ICE imaging may be critical to identify this structure and monitor ablation. Tricuspid valve function can be simultaneously followed during energy delivery with the ICE probe placed within the inflow portion of the right ventricle and imaging done of the RVOT and tricuspid valve looking above from this distal location.13, 24
Summary
The CPM may represent the site of origin for ventricular arrhythmia and be the cause for difficulty with ablation of patients with RVOT VT. Simultaneous early activation sites at more than one location in the proximal RVOT, varying QRS morphologies with similar coupling intervals, and Purkinje-like signals preceding ventricular activation are clues that should lead the invasive electrophysiologist to consider this possibility and use ICE to determine exact catheter locations on the CPM.
Supplementary Material
Supplemental figure 1. PVCs of Patient 2 recorded in the EP laboratory
Left: Clinical PVC ablated just above the septoparietal trabeculation. This PVC is characterized by its narrow QRS width of 128 ms and very brief initial slurring followed by sharp upstroke. Right: New PVC emerged after ablation of the clinical PVC. Despite having a similar axis, the QRS width of this PVC was 140 ms, and the sharp upstroke of the clinical PVC is not seen. The precordial transition of was noticed at V3 instead of V4.
Supplemental figure 2. Clinical PVC of Patient 3
The PVC had a LBBB pattern, right inferior axis. There was an R wave in V1, and the transition was seen at V4.
Abbreviations
- CPM
conus papillary muscle
- CIE
intracardiac echocardiography
- PVC
premature ventricular complex
- RVOT
right ventricular outflow tract
- VT
ventricular tachycardia
Footnotes
No disclosures.
References
- [1].Schreiber D, Kottkamp H. Ablation of idiopathic ventricular tachycardia. Curr Cardiol Rep. 2010;12:382–388. doi: 10.1007/s11886-010-0121-x. [DOI] [PubMed] [Google Scholar]
- [2].Lee GK, Klarich KW, Grogan M, Cha YM. Premature ventricular contraction-induced cardiomyopathy: a treatable condition. Circ Arrhythm Electrophysiol. 2012;5:229–236. doi: 10.1161/CIRCEP.111.963348. [DOI] [PubMed] [Google Scholar]
- [3].Stevenson WG, Soejima K. Catheter ablation for ventricular tachycardia. Circulation. 2007;115:2750–2760. doi: 10.1161/CIRCULATIONAHA.106.655720. [DOI] [PubMed] [Google Scholar]
- [4].Calvo N, Jongbloed M, Zeppenfeld K. Radiofrequency catheter ablation of idiopathic right ventricular outflow tract arrhythmias. Indian Pacing Electrophysiol J. 2013;13:14–33. doi: 10.1016/s0972-6292(16)30585-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Asirvatham SJ. Correlative anatomy for the invasive electrophysiologist: outflow tract and supravalvar arrhythmia. J Cardiovasc Electrophysiol. 2009;20:955–968. doi: 10.1111/j.1540-8167.2009.01472.x. [DOI] [PubMed] [Google Scholar]
- [6].Friedman PA, Asirvatham SJ, Grice S, Glikson M, Munger TM, Rea RF, Shen WK, Jahanghir A, Packer DL, Hammill SC. Noncontact mapping to guide ablation of right ventricular outflow tract tachycardia. J Am Coll Cardiol. 2002;39:1808–1812. doi: 10.1016/s0735-1097(02)01864-8. [DOI] [PubMed] [Google Scholar]
- [7].Srivathsan KS, Bunch TJ, Asirvatham SJ, Edwards WD, Friedman PA, Munger TM, Hammill SC, Cha YM, Brady PA, Jahangir A, Bradley DJ, Rea RF, Packer DL, Shen WK. Mechanisms and utility of discrete great arterial potentials in the ablation of outflow tract ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2008;1:30–38. doi: 10.1161/CIRCEP.107.750315. [DOI] [PubMed] [Google Scholar]
- [8].Hasdemir C, Aktas S, Govsa F, Aktas EO, Kocak A, Bozkaya YT, Demirbas MI, Ulucan C, Ozdogan O, Kayikcioglu M, Can LH, Payzin S. Demonstration of ventricular myocardial extensions into the pulmonary artery and aorta beyond the ventriculo-arterial junction. Pacing Clin Electrophysiol. 2007;30:534–539. doi: 10.1111/j.1540-8159.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- [9].De Simone CV, Noheria A, Lachman N, Edwards WD, Gami AS, Maleszewski JJ, Friedman PA, Munger TM, Hammill SC, Packer DL, Asirvatham SJ. Myocardium of the superior vena cava, coronary sinus, vein of Marshall, and the pulmonary vein ostia: gross anatomic studies in 620 hearts. J Cardiovasc Electrophysiol. 2012;23:1304–1309. doi: 10.1111/j.1540-8167.2012.02403.x. [DOI] [PubMed] [Google Scholar]
- [10].Abouezzeddine O, Suleiman M, Buescher T, Kapa S, Friedman PA, Jahangir A, Mears JA, Ladewig DJ, Munger TM, Hammill SC, Packer DL, Asirvatham SJ. Relevance of endocavitary structures in ablation procedures for ventricular tachycardia. J Cardiovasc Electrophysiol. 2010;21:245–254. doi: 10.1111/j.1540-8167.2009.01621.x. [DOI] [PubMed] [Google Scholar]
- [11].Liu XK, Barrett R, Packer DL, Asirvatham SJ. Successful management of recurrent ventricular tachycardia by electrical isolation of anterolateral papillary muscle. Heart Rhythm. 2008;5:479–482. doi: 10.1016/j.hrthm.2007.11.013. [DOI] [PubMed] [Google Scholar]
- [12].Crawford T, Mueller G, Good E, Jongnarangsin K, Chugh A, Pelosi F, Jr, Ebinger M, Oral H, Morady F, Bogun F. Ventricular arrhythmias originating from papillary muscles in the right ventricle. Heart Rhythm. 2010;7:725–730. doi: 10.1016/j.hrthm.2010.01.040. [DOI] [PubMed] [Google Scholar]
- [13].Asirvatham SJ, Bruce CJ, Friedman PA. Advances in imaging for cardiac electrophysiology. Coron Artery Dis. 2003;14:3–13. doi: 10.1097/00019501-200302000-00002. [DOI] [PubMed] [Google Scholar]
- [14].Aktas EO, Govsa F, Kocak A, Boydak B, Yavuz IC. Variations in the papillary muscles of normal tricuspid valve and their clinical relevance in medicolegal autopsies. Saudi Med J. 2004;25:1176–1185. [PubMed] [Google Scholar]
- [15].Nigri GR, Di Dio LJ, Baptista CA. Papillary muscles and tendinous cords of the right ventricle of the human heart: morphological characteristics. Surg Radiol Anat. 2001;23:45–49. doi: 10.1007/s00276-001-0045-7. [DOI] [PubMed] [Google Scholar]
- [16].Restivo A, Smith A, Wilkinson JL, Anderson RH. The medial papillary muscle complex and its related septomarginal trabeculation. A normal anatomical study on human hearts. J Anat. 1989;163:231–242. [PMC free article] [PubMed] [Google Scholar]
- [17].Wenink AC. Is there a definite papillary muscle of the conus? [proceedings] Acta Morphol Neerl Scand. 1977;15:235–236. [PubMed] [Google Scholar]
- [18].Wenink AC. The medial papillary complex. Br Heart J. 1977;39:1012–1018. doi: 10.1136/hrt.39.9.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xanthos T, Dalivigkas I, Ekmektzoglou KA. Anatomic variations of the cardiac valves and papillary muscles of the right heart. Ital J Anat Embryol. 2011;116:111–126. [PubMed] [Google Scholar]
- [20].Loukas M, Tubbs RS, Louis RG, Jr, Apaydin N, Bartczak A, Huseng V, Alsaiegh N, Fudalej M. An endoscopic and anatomical approach to the septal papillary muscle of the conus. Surg Radiol Anat. 2009;31:701–706. doi: 10.1007/s00276-009-0510-2. [DOI] [PubMed] [Google Scholar]
- [21].Weerasooriya R, Hsu LF, Scavee C, Sanders P, Hocini M, Cabrera JA, Horlitz M, Schley P, Guelker H, Jais P, Haissaguerre M. Catheter Ablation of Ventricular Fibrillation in Structurally Normal Hearts Targeting the RVOT and Purkinje Ectopy. Herz. 2003;28:598–606. doi: 10.1007/s00059-003-2491-y. [DOI] [PubMed] [Google Scholar]
- [22].Srivathsan K, Gami AS, Ackerman MJ, Asirvatham SJ. Treatment of ventricular fibrillation in a patient with prior diagnosis of long QT syndrome: importance of precise electrophysiologic diagnosis to successfully ablate the trigger. Heart Rhythm. 2007;4:1090–1093. doi: 10.1016/j.hrthm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- [23].Sriram CS, Syed FF, Ferguson ME, Johnson JN, Sarano ME, Cetta F, Cannon BC, Asirvatham SJ, Ackerman MJ. Malignant Bileaflet Mitral Valve Prolapse Syndrome in Patients with Otherwise Idiopathic Out of Hospital Cardiac Arrest. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.02.060. [DOI] [PubMed] [Google Scholar]
- [24].Packer DL, Stevens CL, Curley MG, Bruce CJ, Miller FA, Khandheria BK, Oh JK, Sinak LJ, Seward JB. Intracardiac phased-array imaging: methods and initial clinical experience with high resolution, under blood visualization: initial experience with intracardiac phased-array ultrasound. J Am Coll Cardiol. 2002;39:509–516. doi: 10.1016/s0735-1097(01)01764-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. PVCs of Patient 2 recorded in the EP laboratory
Left: Clinical PVC ablated just above the septoparietal trabeculation. This PVC is characterized by its narrow QRS width of 128 ms and very brief initial slurring followed by sharp upstroke. Right: New PVC emerged after ablation of the clinical PVC. Despite having a similar axis, the QRS width of this PVC was 140 ms, and the sharp upstroke of the clinical PVC is not seen. The precordial transition of was noticed at V3 instead of V4.
Supplemental figure 2. Clinical PVC of Patient 3
The PVC had a LBBB pattern, right inferior axis. There was an R wave in V1, and the transition was seen at V4.