Abstract
Frataxin (Yfh1 in yeast) is a conserved protein and deficiency leads to the neurodegenerative disease Friedreich’s ataxia. Frataxin is a critical protein for Fe-S cluster assembly in mitochondria, interacting with other components of the Fe-S cluster machinery, including cysteine desulfurase Nfs1, Isd11 and the Isu1 scaffold protein. Yeast Isu1 with the methionine to isoleucine substitution (M141I), in which the E. coli amino acid is inserted at this position, corrected most of the phenotypes that result from lack of Yfh1 in yeast. This suppressor Isu1 behaved as a genetic dominant. Furthermore frataxin-bypass activity required a completely functional Nfs1 and correlated with the presence of efficient scaffold function. A screen of random Isu1 mutations for frataxin-bypass activity identified only M141 substitutions, including Ile, Cys, Leu, or Val. In each case, mitochondrial Nfs1 persulfide formation was enhanced, and mitochondrial Fe-S cluster assembly was improved in the absence of frataxin. Direct targeting of the entire E. coli IscU to ∆yfh1 mitochondria also ameliorated the mutant phenotypes. In contrast, expression of IscU with the reverse substitution i.e. IscU with Ile to Met change led to worsening of the ∆yfh1 phenotypes, including severely compromised growth, increased sensitivity to oxygen, deficiency in Fe-S clusters and heme, and impaired iron homeostasis. A bioinformatic survey of eukaryotic Isu1/prokaryotic IscU database entries sorted on the amino acid utilized at the M141 position identified unique groupings, with virtually all of the eukaryotic scaffolds using Met, and the preponderance of prokaryotic scaffolds using other amino acids. The frataxin-bypassing amino acids Cys, Ile, Leu, or Val, were found predominantly in prokaryotes. This amino acid position 141 is unique in Isu1, and the frataxin-bypass effect likely mimics a conserved and ancient feature of the prokaryotic Fe-S cluster assembly machinery.
Author Summary
Frataxin was discovered because mutations in the corresponding gene cause the neurodegenerative disease Friedreich’s ataxia. The finding that frataxin protein physically associates with scaffold proteins Isu1/IscU places it squarely in the pathway of Fe-S cluster assembly. Fe-S clusters are essential cofactors for many proteins involved in cellular respiration, DNA repair, translation and other processes. Frataxin is conserved throughout evolution, being present in eukaryotes such as yeast and human and in some prokaryotes including E. coli. However, differences exist between the eukaryotic and prokaryotic forms of frataxin. The eukaryotic forms are critical for Fe-S cluster assembly whereas prokaryotic forms are more dispensable. We found that a key to this difference is a single amino acid in the scaffold protein Isu1 at position 141. Changes of the eukaryotic amino acid, Met, to prokaryotic amino acids, Ile, Leu, Cys, or Val, rendered mitochondria more frataxin-independent. No other changes were able to replicate this effect. Thus, Isu1 containing Met at position 141 may have coevolved with frataxin in eukaryotes, conferring frataxin-dependence. In contrast, the appearance of other amino acids at this position may have rendered prokaryotic cells less dependent on frataxin.
Introduction
Frataxin is a highly conserved protein that is found in both prokaryotic and eukaryotic organisms [1]. The protein was originally identified based on its connection to Friedreich’s ataxia, which is an inherited neurodegenerative and cardiodegenerative disease resulting from a deficiency of frataxin [2]. Recently a mitochondrial Fe-S cluster assembly protein complex was identified consisting of frataxin in association with the cysteine desulfurase Nfs1, the small eukaryote-specific protein Isd11, and the scaffold protein Isu1 [3,4,5]. This protein complex serves to synthesize Fe-S cluster intermediates on Isu1 for subsequent transfer to the myriad proteins that use Fe-S cluster cofactors [6,7]. Iron-sulfur cluster intermediates contain iron and sulfur bound to the Isu1 scaffold in a [Fe2S2] configuration [8]. Although the precise function of frataxin has not been defined, it probably plays a role in sulfur and/or iron donation to Fe-S cluster intermediates [9,10].
The entire process of Fe-S cluster biogenesis is highly conserved between eukaryotic mitochondria and prokaryotic organisms [6,7]. Similar components are present, and in many cases the corresponding proteins function as orthologs. Sulfur for Fe-S cluster synthesis is derived from cysteine via the action of a cysteine desulfurase (Nfs1 in yeast, IscS in bacteria). This enzyme binds the amino acid cysteine in a substrate-binding site via the pyridoxal phosphate (PLP) cofactor [8]. The bound substrate is subjected to nucleophilic attack by an active site cysteine present on a moveable loop of the protein, forming a persulfide (e.g. Nfs1-S-SH in yeast). The persulfide sulfur is then transferred to recipients including Isu1 and used in building Fe-S clusters [11]. Frataxin (Yfh1 in yeast, CyaY in E. coli) interacts with the cysteine desulfurase and may be involved in regulating the enzyme activity. Whereas a positive regulatory effect has been observed for the yeast or human proteins [12,13], a negative regulatory effect has been observed for the E. coli homolog [14], and this difference has still not been explained [15]. Isd11 is a small accessory subunit that interacts with the eukaryotic Nfs1 and is necessary for its cysteine desulfurase activity [16]. However, Isd11 is eukaryote specific, being entirely absent from prokaryotic lineages [17]. Iron combines with sulfur on the scaffold protein to form Fe-S cluster intermediates. The scaffolds (Isu1 in yeast, IscU in E. coli) are highly homologous proteins [18]. The iron donation step is poorly characterized, and frataxin has also been implicated in this step. Both yeast and E. coli frataxins bind iron with low affinity on acidic residues in vitro and interact with their respective scaffold proteins in vitro and in vivo, and thus they may participate in iron donation [19,20]. Electrons provided by ferredoxin (Yah1 in yeast, Fdx in E. coli) are needed for reduction of iron or sulfur during Fe-S cluster intermediate formation [21]. Following formation of the intermediate on Isu1 or IscU, coordinated by three critical cysteines in the protein backbone, Hsp70 chaperones and cochaperones (Ssq1 and Jac1 in yeast, HscA and HscB in E. coli) interact with the scaffolds, mediating Fe-S cluster transfer in an ATP-dependent manner [22].
In terms of phenotypes resulting from frataxin deficiency, however, eukaryotes and prokaryotes show major differences. Total lack of frataxin is lethal in humans and metazoans [4]. Deletion of the YFH1 gene in yeast is associated with extremely deleterious effects, including slow growth, oxidant sensitivity, heme deficiency and lack of Fe-S clusters [23,24]. In addition, frataxin deficiency is associated with a curious iron homeostatic phenotype characterized by constitutive and unregulated cellular iron uptake. Within the cell iron accumulates in mitochondria in the form of biologically unavailable ferric phosphate nanoparticles. This constellation of findings apparently results from defective Fe-S proteins in the iron-sensing machinery [25,26]. In contrast to the yeast mutants, the effects of frataxin deletion in E. coli are mild. The bacterial deletion strain shows normal growth and does not exhibit iron homeostatic abnormalities or sensitivity to oxidative stress, although in one report the protein level for respiratory complex I was reduced [27].
A spontaneously occurring mutation in a frataxin-deleted yeast strain was found to effectively bypass the severe Δyfh1 phenotypes, restoring normal growth, Fe-S cluster protein levels, iron homeostasis, heme synthesis, and oxidative stress resistance. The effect was conferred by the Met to Ile change of amino acid 141 in the scaffold protein Isu1 [28]. The altered Isu1 was able to bind and activate the Nfs1 cysteine desulfurase in the absence of frataxin, thus providing a possible explanation for the bypass activity [29,30]. Interestingly, isoleucine is the amino acid utilized by E. coli in the homologous position of IscU. Thus in yeast lacking frataxin, the Met to Ile change in Isu1, by substituting the E. coli amino acid at this position, effectively rendered yeast more frataxin independent and more "prokaryote like".
Here we have delved more into the genetics of this frataxin-bypass phenomenon, finding more prokaryotic features of Isu1 bypass mutants. Randomly selected Isu1 bypass mutants were confined to a single amino acid position, and the amino acids conferring bypass were all present in homologous prokaryotic proteins. The prokaryotic homologs were identified both in organisms with frataxin and in organisms without frataxin, underscoring the frataxin-independence associated with these particular scaffold mutants. The entire E. coli IscU (targeted to mitochondria with a leader sequence) conferred more frataxin-independence, whereas a reverse substitution, in which the eukaryotic amino acid Met was introduced at the same position, conferred more frataxin-dependence. Examination of the set of Isu1/IscU sequences in available databases also suggests that Isu1-Met appears almost exclusively in eukaryotes and likely coevolved with frataxin. The substituted Isu1-Ile probably mimics a conserved and ancient feature of the prokaryotic Fe-S cluster assembly complex, relieving the frataxin requirement.
Results
Genetic dominance of the M141I Isu1 or Isu2 substitution conferring frataxin-bypass
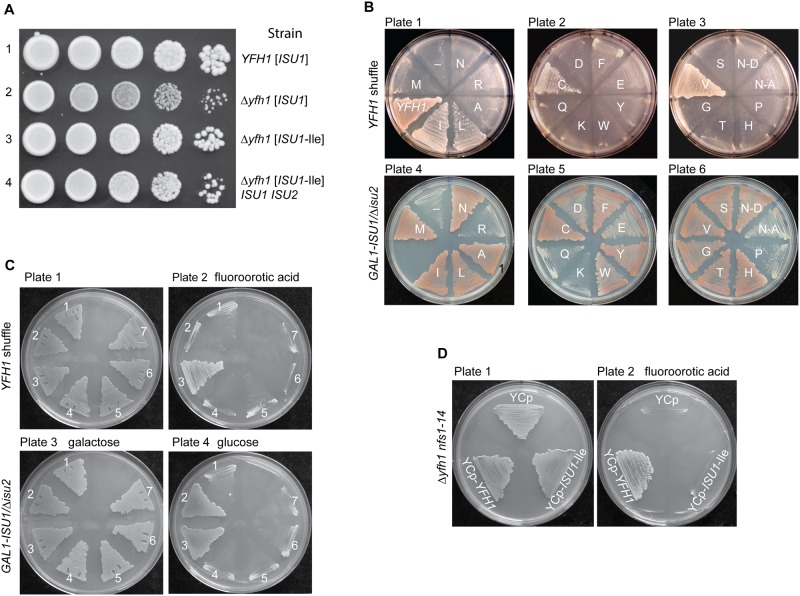
The frataxin-bypass mutant was isolated as a spontaneously arising clone of more rapidly growing cells in a background of a frataxin-deleted haploid yeast strain (Δyfh1) [28]. The bypass activity was conferred by a single point mutation (ATG to ATA), changing the amino acid of codon 141 of ISU1 from Met to Ile. However yeast S. cerevisiae carries two redundant genes coding for Fe-S cluster scaffolds, ISU1 and ISU2 [31]. The initial presentation of the suppressor or bypass mutant suggested that it was genetically dominant, because it was able to bypass Δyfh1 even in the presence of a normal copy of ISU2.
In order to evaluate this genetic dominance further, matched Δyfh1 strains were compared, one expressing a single copy of substituted ISU1 and deleted ISU2, called Δyfh1 [ISU1-Ile] (Table 1) and another expressing both ISU1 and ISU2 in addition to the plasmid-borne substituted Isu1-M141I, called Δyfh1 [ISU1-Ile] ISU1 ISU2 (Table 1). As assessed by colony formation, both strains showed improved growth compared with the Δyfh1 control (Fig 1A, compare rows 3 and 4 to row 2), although the single copy [ISU1-Ile] showed slightly better Δyfh1 suppression activity as assessed by growth (Fig 1A, compare rows 3 and 4), and other phenotypes such as iron uptake and Fe-S cluster levels. Thus, a high degree of genetic dominance of the Isu1 Met to Ile substitution was observed in producing reversal of Δyfh1 phenotypes.
Table 1. List of yeast strains.
| Strain number | Name | Genotype | Source |
|---|---|---|---|
| 70–31 | YFH1 shuffle | MATa ura3-52 lys2-801(amber) ade2-101(ochre) trp1-Δ63 his3-Δ200 leu2-Δ1 Δyfh1::HIS3 ISU1 ISU2 [pGAL1-YFH1, URA3] | [28] |
| 109–9 | YFH1 (Δisu1) shuffle | MATa ura3-52 lys2-801(amber) ade2-101(ochre) cyh2 Δyfh1::TRP1 Δisu1::HIS3MX6 ISU2 [pRS318- YFH1-CYH2-LEU2] | This work |
| 110–66 | MATa ura3-52 lys2-801(amber) ade2-101(ochre) trp1-Δ63 his3-Δ200 leu2-Δ1 Δyfh1::HIS3 ISU1 ISU2 [pGAL1-YFH1-URA3][YCplac22-ISU1-M141I] | [28] | |
| 108–54 | Δyfh1 ISU1 ISU2 [ISU1-Ile] | MATa ura3-52 lys2-801(amber) ade2-101(ochre) trp1-Δ63 his3-Δ200 leu2-Δ1 Δyfh1::HIS3 ISU1 ISU2 [YCplac22-ISU1-M141I] | This work |
| 113–26 | MAT? ura3-52 lys2-801(amber) ade2-101(ochre) trp1-Δ63 leu2-Δ1 cyh2 nfs1-14::LEU2 Δyfh1::HIS3 ISU1 ISU2 [pRS416-YFH1] | This work | |
| 115–10 | GAL1-ISU1/Δisu2 (Parent) | MATa lys2-801(amber) ade2-101(ochre) trp1-Δ63 leu2-Δ1 YFH1 Δisu2::URA3 His3MX6:GAL1-ISU1 | This work |
| 115–26 | Δyfh1 [ISU1] | Parent with [YCplac22-ISU1] Δyfh1::LEU2 | [30] |
| 115–28 | Δyfh1 [ISU1-Ile] | Parent with [YCplac22-ISU1-M141I] Δyfh1::LEU2 | [30] |
| 116–51 | Δyfh1 [ISU1-Val] | Parent with [YCplac22-ISU1-M141V] Δyfh1::LEU2 | This work |
| 116–53 | Δyfh1 [ISU1-Leu] | Parent with [YCplac22-ISU1-M141L] Δyfh1::LEU2 | This work |
| 116–54 | Δyfh1 [ISU1-Cys] | Parent with [YCplac22-ISU1-M141C] Δyfh1::LEU2 | This work |
| 117–36 | YFH1 [iscU] | Parent with [YCplac22-iscU E. coli] | This work |
| 117–37 | YFH1 [iscU-Met] | Parent with [YCplac22-iscU-I108M E. coli] | This work |
| 117–40 117–42 | Δyfh1 [iscU] | Parent with Δyfh1::LEU2 [YCplac22-iscU E.coli] | This work |
| 117–44 117–46 | Δyfh1 [iscU-Met] | Parent with Δyfh1::LEU2 [YCplac22-iscU-I108M E. coli] | This work |
| 114–58 | YFH1 [ISU1] | Parent with [YCplac22-ISU1] | This work |
Fig 1. Genetic manipulations of ISU1-Ile and other ISU1 alleles.
(A) Genetic dominance of ISU1-Ile conferring frataxin-bypass. Strains YFH1 [ISU1], Δyfh1 [ISU1], Δyfh1 [ISU1-Ile], and Δyfh1 [ISU1-Ile] ISU1 ISU2 (Table 1) were compared by spotting serial 5-fold dilutions of 105 cells on YPAD plates and photographing three days later. (B) Frataxin-bypass function and scaffold function of ISU1 alleles. A series of plasmids was constructed in the YCplac22 backbone. These plasmids carried native ISU1 or ISU1 alleles with substitutions of residue M141 to all 19 other standard amino acids. N123D or N123A substitutions were also constructed, predicted to give disordered or structured conformations, respectively [34]. Plasmid-borne YFH1 and empty vector (-) were included as controls. To test frataxin-bypass function the ISU1 alleles were transformed into the YFH1 shuffle strain, and the transformed cells were transferred to fluoroorotic acid (FOA) plates to remove the covering YFH1-URA3 plasmid and expose the Δyfh1 phenotype (plates 1–3). To test scaffold function the ISU1 alleles were transformed into the GAL1-ISU1/Δisu2 strain and plated on raffinose carbon source to repress genomic GAL1-ISU1. (C) Effects of cysteine mutations. Plasmids with various cysteine substitutions in Isu1 were tested for frataxin-bypass function by introducing them into the YFH1 shuffle strain and counterselecting on FOA (plates 1 and 2). The Isu1 substitutions were also tested for scaffold function by introducing them into the GAL1-ISU1/Δisu2 strain and shifting the carbon source from galactose to glucose (plates 3 and 4). Key to transformants. 1. Empty plasmid YCplac22, 2. ISU1, 3. ISU1-M141I, 4. ISU1-C69A-M141I, 5. ISU1-C96A-M141I, 6. ISU1-C139A-M141I, 7. ISU1-C139A-M141C. (D) Δyfh1 nfs1-14 double mutant does not support frataxin-bypass by ISU1-M141I. Shuffle strain 113–26 (Δyfh1 nfs1-14 [pRS416-YFH1]) was transformed with YCplac22, YCplac22-YFH1, or YCplac22-ISU1-Ile. Transformants were patched onto tryptophan drop-out medium without (plate 1) or with (plate 2) FOA.
ISU1 and ISU2 encode highly homologous proteins, with 83% amino acid identity. Isu1 and Isu2 proteins are functionally redundant, although the endogenous expression level of Isu1 is roughly seven times higher than that of Isu2, and Isu1 represents most of the cellular Isu protein in a wild-type strain [32]. Significantly the critical Met (amino acid 141 in Isu1 or 133 in Isu2) is present in both proteins. The adjacent cysteine that functions as an Fe-S cluster ligand and the adjoining frataxin-binding motif are also present in both proteins [33]. ISU2 and ISU2-Ile, in which the Met-133 was changed to Ile, were experimentally evaluated. Results show that ISU2-Ile conferred frataxin-bypass activity in a YFH1 shuffle strain (S1 Fig). Bypass activity was conferred whether it was expressed from the native ISU2 promoter or from the ISU1 promoter (S1 Fig). Thus, genetic dominance for frataxin-bypass by ISU2-Ile was observed, similar to that of ISU1-Ile. Furthermore, the ISU2 and ISU2-Ile constructs were also able to support growth of the GAL1-ISU1/Δisu2 strain indicating that they were functional as Isu. The reason that only the ISU1-Ile was isolated and ISU2-Ile was not isolated in the original screen for Δyfh1 suppressors is probably due to the lack of saturation of this genetic screen.
Isu1 substitutions at M141: Frataxin-bypass function and scaffold function
All 20 possible amino acids were substituted at position M141 in a single copy plasmid-borne ISU1. Each mutant form of ISU1 was tested for frataxin-bypassing activity by transforming the plasmids into a YFH1 shuffle strain, followed by counterselection with fluoroorotic acid on raffinose medium to remove the covering plasmid (Fig 1B, plates 1–3). After counterselection, robust growth was noted for positive control YCplac22-YFH1, and slow growth was noted for the empty plasmid, YCplac22 (Fig 1B, plate 1, “YFH1” versus “-”), consistent with the important role of frataxin for normal growth. The ISU1-Ile plasmid with the Met to Ile change restored robust growth in the absence of YFH1 (Fig 1B, plate 1, “I”). Similarly, plasmids substituted at the same amino acid position and containing ISU1-Leu, ISU1-Cys, and ISU1-Val, conferred improved growth (Fig 1B, plates 1–3). These ISU1 alleles thus carried frataxin-bypass activity and were genetically dominant, given that wild-type genomic copies of ISU1 and ISU2 were still present.
Fe-S cluster assembly scaffold function was tested separately. The collection of ISU1 alleles was transformed into the GAL1-ISU1/Δisu2 strain. In this strain the redundant ISU2 was deleted and ISU1 was placed under control of GAL1, a galactose-dependent promoter. When the transformants were shifted to a non-inducing carbon source (i.e. raffinose-based medium), only plasmid-borne ISU1 was expressed, allowing scaffold function for each allele to be scored. The wild-type ISU1 (Fig 1B, plate 4, M) supported normal growth, and a large set of ISU1 plasmids with amino acid substitutions also supported normal growth, indicating that these were highly functional ISU1 proteins (Fig 1B, plates 4–6, I, L, A, N, C, F, Y, S, V, G, T and H). Importantly, all of the substitutions conferring frataxin-bypass activity (Fig 1B, plates 1–3, I, L, C, and V) were also functional ISU1 scaffold proteins. Another set of substitutions was partially functional as shown by slowed growth (Fig 1B, plate 5, D, E and W), and these mutants tended to accumulate iron, indicating that they were hypomorphic mutants in the Fe-S cluster assembly pathway. A small set of substituted alleles was completely non-functional and did not grow at all on the raffinose plates (Fig 1B, plates 4–6, R, Q, K and P).
The E. coli ortholog, IscU, was studied by NMR methodology [34] and found to assume interconverting disordered or structured conformations. When a conserved amino acid in the primary sequence, Asn 123 in the Isu1 numbering, was substituted with Asp, the conformation of the mutant protein was shown to be shifted preferentially to the disordered form. Alternatively when Asn 123 was substituted with Ala, the conformation was shifted to the structured form [34]. We wondered if the frataxin-bypassing activity could be related to one or the other of these conformations. The Asp change (N-D; presumably disordered) and the Ala change (N-A; presumably structured) were introduced into YCplac22-ISU1 and the respective plasmids were transformed into the YFH1 shuffle strain. However, neither conferred any bypass activity (Fig 1B, plate 3, N-D and N-A). The Asp 123 form conferred growth to the GAL1-ISU1/Δisu2 strain indicating efficient scaffold function, but the Ala 123 form was poorly functional in this assay (Fig 1B, plate 6, N-D versus N-A).
Substitutions of critical cysteine residues inactivate both frataxin-bypass activity and scaffold activity of ISU1-Ile
Isu1 with the Met to Ile substitution, ISU1-Ile, exhibited both frataxin-bypassing activity and scaffold activity. Therefore the question arose of whether both activities must necessarily reside in the same molecule. Alternatively, the ISU1-Ile might act on wild-type ISU1 protein, stimulating it to perform as the primary scaffold. The existence of a protein complex containing the suppressor ISU1-Ile protein and the wild-type ISU1 protein could provide a physical context for such stimulation to occur [35,36]. To begin to address this question genetically, the three cysteine residues of Isu1, presumed to be Fe-S cluster ligands [31], were individually replaced by alanine. The C69A, C96A and C139A forms of Isu1 were introduced into strain GAL1-ISU1/Δisu2, and they were unable to support growth on their own in non-inducing glucose-containing medium. Double mutants were then constructed in which the critical cysteine substitutions were combined with the suppressor ISU1-Ile change of residue 141 in the same molecule. The mutated ISU1 alleles, C69A-M141I, C96A-M141I and C139A-M141I, were tested for frataxin-bypass activity in the YFH1 shuffle strain, and no bypass activity was observed (Fig 1C, plate 2). Likewise the mutated ISU1 alleles were tested for scaffold activity by expression in the GAL1-ISU1/Δisu2 strain in glucose, and no complementation was observed (Fig 1C, plate 4). The frataxin-bypass activity of the Cys substitution of residue 141 raised the possibility that the cysteine at this location was functioning as an alternative Fe-S cluster ligand instead of Cys 139. In a genetic experiment, Cys 139 was replaced with alanine in the presence of the M141C substitution. However, in this case, neither scaffold activity nor bypass activity was observed (Fig 1C, plates 2 and 4, number 7), making it unlikely that the Cys substituted at position 141 can function as an alternative Fe-S cluster ligand. In summary, substitutions that abrogated Isu1 scaffold function by altering the Fe-S cluster ligands also abrogated frataxin-bypass function.
Cysteine desulfurase hypomorphic allele nfs1-14 does not permit frataxin-bypass by ISU1-Ile
A mutant allele of NFS1, nfs1-14, was identified because of its effects on iron homeostasis, and it was later found to be associated with decreased cysteine desulfurase activity in mitochondria [16]. The cells carrying this mutant allele were viable and grew well in most media despite the low activity of cysteine desulfurase. However, the combination of nfs1-14 and Δyfh1 was synthetically lethal (Fig 1D, YCp). YFH1 (Fig 1D, YCp-YFH1) introduced into the nfs1-14 Δyfh1 strain restored growth. However, although ISU1-Ile was able to bypass Δyfh1 alone, it was unable to rescue the nfs1-14 Δyfh1 double mutant (Fig 1D, YCp-ISU1-Ile). These data suggest that a threshold level of cysteine desulfurase activity is required for the frataxin-bypass activity of ISU1-Ile. Recent biochemical data indicate that frataxin stimulates the cysteine desulfurase activity of Nfs1 [13]. Furthermore, the suppressor ISU1-Ile acts in a similar fashion to stimulate Nfs1 even in the absence of frataxin, perhaps explaining its bypass activity [29]. Thus it makes sense that in the absence of a sufficiently active Nfs1, bypass of Δyfh1 by the Ile-substituted ISU1 will not occur.
Biochemical characteristics of newly discovered frataxin-bypassing mutant alleles of ISU1
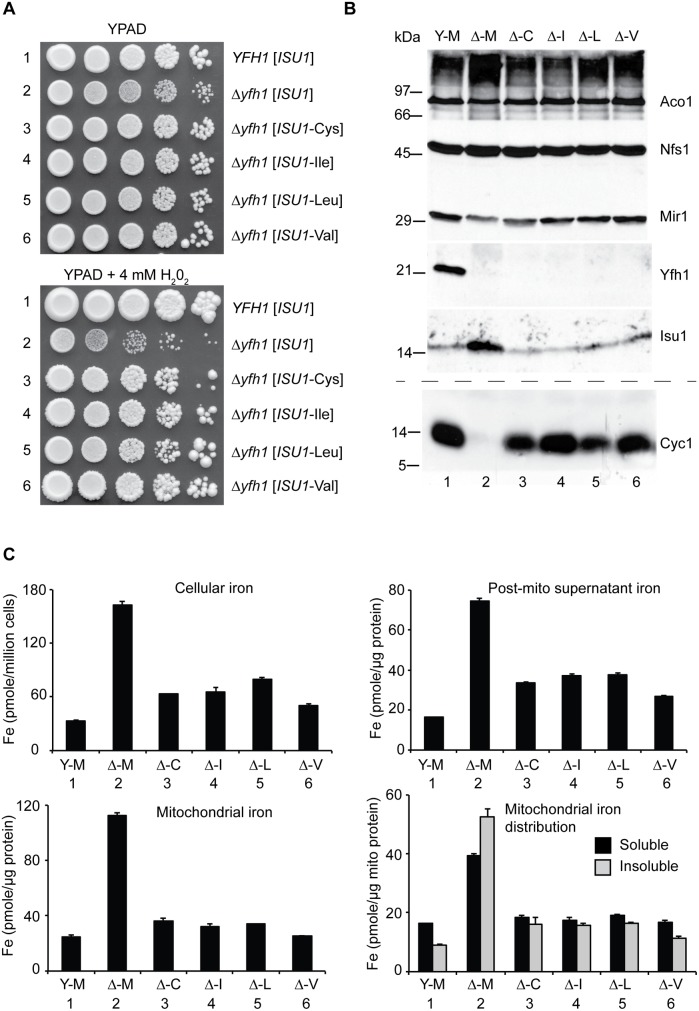
The frataxin-bypassing activity of ISU1 protein with changes of Met 141 to Cys, Ile, Val or Leu was shown by growth enhancement of the YFH1 shuffle strain. In this strain, the chromosomal ISU1 and ISU2 were still intact (Fig 1B). To examine and compare these effects in more detail, matched strains were constructed in which each of the bypassing ISU1 alleles was expressed in the absence ISU2 (Table 1). The starting strain was designated YFH1 [ISU1], indicating a YFH1 positive strain with plasmid carrying wild-type ISU1 (Met at position 141) (Table 1). Another strain was designated Δyfh1 [ISU1], indicating a strain deleted for YFH1 and carrying wild-type ISU1. Similarly, Δyfh1 [ISU1-Cys], Δyfh1 [ISU1-Ile], Δyfh1 [ISU1-Leu], and Δyfh1 [ISU1-Val], were used to designate deletions of YFH1 with the indicated ISU1 mutant alleles (Table 1). The growth of the YFH1 [ISU1] strain was more rapid than the Δyfh1 [ISU1] strain as shown by the larger colony size (Fig 2A, upper panel, rows 1 and 2). Hydrogen peroxide exposure exacerbated the Δyfh1 phenotype [37], further slowing growth and viability as a consequence of increased oxidative stress (Fig 2A, lower panel, rows 1 and 2). The ISU1 alleles with Cys, Leu, or Val at position 141, similar to the ISU1 M141I allele, improved growth of Δyfh1 under these conditions, including in the presence of hydrogen peroxide, although not quite to levels in the frataxin plus strain (Fig 2A, upper and lower panels, rows 1–6).
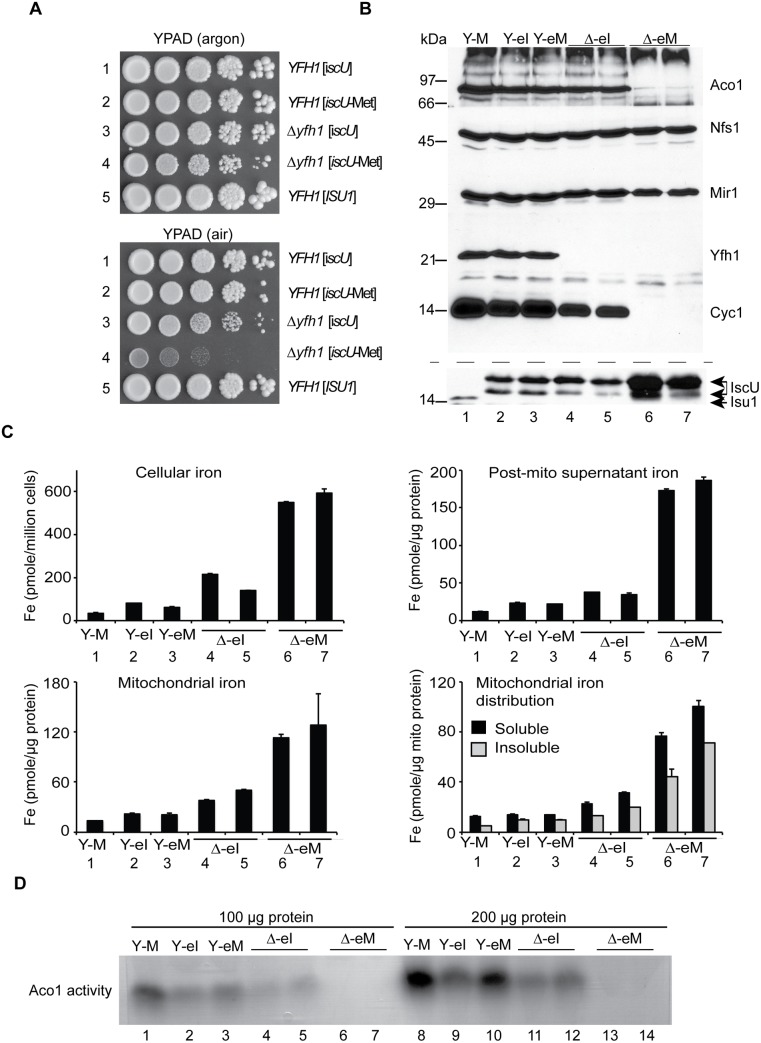
Fig 2. Characterization and comparison of frataxin-bypass substitutions of ISU1 amino acid 141: ISU1-Cys, ISU1-Ile, ISU1-Leu and ISU1-Val.
(A) Growth defects in Δyfh1 are corrected by the frataxin-bypass suppressors. The strains YFH1 [ISU1] (Y-M), Δyfh1 [ISU1] (Δ-M), and suppressor strains Δyfh1 [ISU1-Cys] (Δ-C), Δyfh1 [ISU1-Ile] (Δ-I), Δyfh1 [ISU1-Leu] (Δ-L), and Δyfh1 [ISU1-Val] (Δ-V), were grown in an argon-filled chamber, and serial dilutions were spotted on YPAD agar, without or with 4 mM hydrogen peroxide. (B) Mitochondrial protein levels by immunoblotting. Strains were inoculated from the argon-filled chamber into aerobic defined raffinose medium for 16–24 h prior to isolating mitochondria. Mitochondrial proteins (100 μg) were separated by SDS-PAGE and transferred to nitrocellulose. Strips were cut from a single blot and probed with the indicated antibodies against mitochondrial proteins (Aco1, Nfs1, Mir1, Yfh1, and Isu1). Cytochrome c (Cyc1) was analyzed using a duplicate gel. (C) Iron homeostasis in the frataxin-bypass suppressors. The indicated strains were grown in air for six doublings in defined raffinose medium in the presence of 10 μM 55Fe ascorbate. Cells were washed free of radioactive iron and separated into mitochondria and post-mitochondrial supernatant. An aliquot of mitochondria was lysed in the presence of 0.1% Triton X-100 for 10 min at room temperature, and supernatant (soluble) and pellet (insoluble) fractions were separated by centrifugation at 20,000 x g for 30 min at 4°C. Iron content was reported for whole cells (pmol per million cells), or various cellular fractions (pmol per micrograms protein).
In terms of mitochondrial proteins, immunoblotting with anti-frataxin antibody confirmed the correctness of the genetic assignments: the YFH1 strain expressed frataxin (Fig 2B, Y-M, lane 1) and the Δyfh1 strains did not (Fig 2B, lanes 2–6). The ISU1 alleles were all expressed in mitochondria. The abundance of Isu1 protein was increased in the Δyfh1 [ISU1] strain in the presence of the ISU1-Met allele (Fig 2B, Δ-M, lane 2), but in the presence of the bypassing ISU1 alleles, Isu1 expression returned to normal, similar to the YFH1 positive strain (Fig 2B, lanes 3–6). The changes in protein abundance were several fold, and most likely attributed to Aft1/2 transcriptional effects and protein stability effects [32]. Nfs1 protein levels were unchanged in all the strains consistent with the lack of regulation of the protein level (Fig 2B), although cysteine desulfurase activity was strongly regulated (see below).
Cytochrome c, a heme protein, was undetectable in the Δyfh1 [ISU1] strain (Fig 2B, lane 2) compared with the wild-type (Fig 2B, lane 1). The various suppressor strains recovered significant levels of cytochrome c (Fig 2B, lanes 3–6). Cytochrome c is regulated transcriptionally and post-transcriptionally by heme availability [38]. The dramatic changes in protein abundance probably reflect changes in heme synthesis and steady state heme levels.
Iron homeostasis was examined using steady state labeling of growing cells with radioactive 55Fe. In YFH1 [ISU1] strain, cellular iron levels were appropriately regulated, whereas in the mutant Δyfh1 [ISU1] strain, by comparison, excess iron accumulated (Fig 2C, Cellular iron, Y-M versus Δ-M). The presence of the suppressor ISU1 mutant alleles, ISU1-Cys, ISU1-Ile, ISU1-Leu, or ISU1-Val, restored cellular iron levels towards normal (Fig 2C, Cellular iron, Δ-C, Δ-I, Δ-L, Δ-V). The radiolabeled cells were subjected to subcellular fractionation, separating mitochondrial and post-mitochondrial fractions (Fig 2C, Mitochondrial iron and Post-mito supernatant iron). The iron quantitation for these fractions resembled the patterns for whole cell iron. The radiolabeled mitochondrial iron showed features that were dependent on YFH1. In the YFH1 [ISU1] mitochondria, the predominant portion was solubilized by exposure to non-ionic detergents such as Triton X-100, whereas in the Δyfh1 [ISU1] mitochondria, proportionally more iron was recovered in the pellet following centrifugation in the presence of detergent (Fig 2C, Mitochondrial iron distribution, Y-M versus Δ-M). Most likely this effect reflects the accumulation of nanoparticles of ferric phosphate that is a hallmark feature of Δyfh1 mitochondria [25]. In mitochondria from the different suppressor strains the amount of insoluble iron was decreased compared with the control deletion strain Δyfh1 [ISU1], indicating an improvement in the biochemical properties of mitochondrial iron pools (Fig 2C, Mitochondrial iron distribution, Δ-M versus Δ-C, Δ-I, Δ-L, Δ-V).
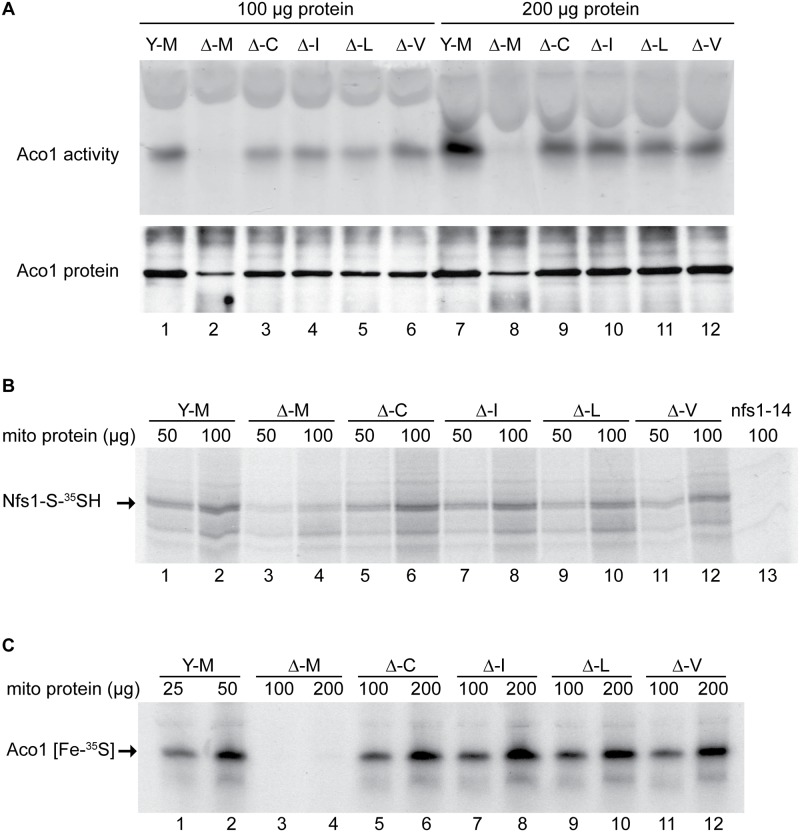
Aconitase (Aco1), an abundant [Fe4S4] protein of mitochondria, was evaluated. An in- gel assay showed the aconitase enzyme activities in mitochondrial lysates. The control strain YFH1 [ISU1] (Fig 3A, upper panel, lanes 1 and 7, Y-M) showed concentration dependent activity. The frataxin minus strain Δyfh1 [ISU1] (Fig 3A, upper panel, lanes 2 and 8, Δ-M) had no detectable activity regardless of the concentration of lysate in the assay. The various suppressors (Fig 3A, upper panel, Δ-C, Δ-I, Δ-L, Δ-V, lanes 3–6 and lanes 9–12) recovered significant activity, although not entirely to wild-type levels. Aconitase protein (Fig 3A, lower panel) as detected by immunoblotting was present in YFH1 [ISU1] control (lanes 1 and 7), and was decreased in the frataxin-minus Δyfh1 [ISU1] (lanes 2 and 8) mitochondria, perhaps because the apoprotein was turned over more rapidly by mitochondrial proteases. Aconitase protein levels were restored in the suppressor strains (Fig 3A, lower panel, lanes 3–6 and lanes 9–12), consistent with recovery of enzyme activity.
Fig 3. Assessment of Fe-S cluster assembly in mitochondria isolated from frataxin-bypass mutants.
The same strains as in Fig 2 were assayed as follows: (A) Aconitase activity by in-gel assay. Mitochondria were lysed and 100 μg (lanes 1–6) or 200 μg (lanes 7–12) proteins were separated by native gel and developed using reagents that reflect aconitase activity (upper panel). The same lysates were separated by SDS-PAGE and analyzed by immunoblotting with anti-aconitase antibody to show the level of total aconitase protein (lower panel). (B) Persulfide formation on Nfs1 in mitochondria. Isolated and intact mitochondria were depleted of nucleotides and NADH by incubation at 30°C for 10 min. Either 50 μg or 100 μg protein equivalents were labeled for 15 min with 35S-cysteine. Samples were diluted with buffer, mitochondria were recovered by centrifugation, and total proteins were analyzed by non-reducing SDS-PAGE and autoradiography. The persulfide Nfs1-S-35SH is indicated by an arrow. Mitochondria from the nfs1-14 mutant was included as a negative control (lane 13), because this hypomorphic Nfs1 mutant has negligible persulfide-forming activity [16]. (C) New Fe-S cluster synthesis on apoaconitase in isolated mitochondria. Isolated and intact mitochondria, were labeled with 35S-cysteine in the presence of added 4 mM ATP, 1 mM GTP, 5 mM NADH and 10 μM ferrous ascorbate for 30 min at 30°C. Mitochondria were recovered and membranes were ruptured. After centrifugation, the supernatant fractions containing soluble mitochondrial proteins (corresponding to 25 or 50 μg for YFH1 [ISU1] and 100 or 200 μg for the other strains) were separated by native gel prior to autoradiography. The arrow indicates the aconitase protein containing newly made radiolabeled [Fe-35S] clusters.
The persulfide-forming activity in these mitochondria was tested as an indication of their cysteine desulfurase activity. Isolated and intact mitochondria were depleted of endogenous nucleotides and NADH by incubation at 30°C for 10 min, thereby blocking Fe-S cluster biogenesis without disrupting cysteine desulfurase activity [16]. After labeling with 35S-cysteine, mitochondrial proteins were separated by non-reducing SDS-PAGE, and the persulfide covalently bound to Nfs1 was visualized by autoradiography (Fig 3B, Nfs1-S-35SH). The signal was absent in nfs1-14 mitochondria, which were unable to form significant Nfs1 persulfide because of a hypomorphic mutation in Nfs1 (Fig 3B, lane 13) [16]. The specificity of the Nfs1-persulfide signal was further confirmed by immunoprecipitation with anti-Nfs1 antibody [16]. Several other radiolabeled bands were detected in mitochondria (Fig 3B), some of which were present in the nfs1-14 control and some of which were absent. Thus these background bands could be attributed to direct binding of 35S-cysteine, persulfide transfer from Nfs1 which was incompletely blocked, or binding of other reactive cysteine persulfides to mitochondrial proteins. The YFH1 [ISU1] mitochondria (Fig 3B, lanes 1 and 2, Y-M) had significantly more Nfs1 persulfide than the frataxin-minus mutant Δyfh1 [ISU1] (Fig 3B, lanes 3 and 4, Δ-M). Each of the suppressor mutants (Fig 3B, Δ-C, Δ-I, Δ-L, and Δ-V; lanes 5–12) recovered persulfide-forming activity in mitochondria. Differences among the suppressor alleles were not apparent, and each one provided rescue of the persulfide-forming activity that was deficient in Δyfh1 [ISU1] mitochondria.
Next, 35S-cysteine labeling of isolated intact mitochondria was performed in the presence of added ATP, GTP, NADH and iron, thereby permitting multiple cycles of Fe-S cluster formation to occur [16]. Soluble proteins from these mitochondria were separated on native gels, and autoradiography was used to detect newly synthesized Fe-S clusters on aconitase (Fig 3C, Aco1 [Fe-35S]). In the”wild-type” YFH1 [ISU1] mitochondria (Fig 3C, lanes 1 and 2, Y-M) a strong signal was observed, whereas in the frataxin-minus Δyfh1 [ISU1] (Fig 3C, lanes 3 and 4, Δ-M) no signal was present, consistent with the important role of frataxin in mitochondrial Fe-S cluster assembly. In the various suppressor mutants, although they still lacked frataxin, Fe-S cluster synthesis on Aco1 was restored to 24–31% of the YFH1 control as assessed by densitometry and correction for the total amount of protein loaded (Fig 3C, lanes 5–12).
Random mutagenesis of ISU1 and screening for additional frataxin-bypass suppressor alleles
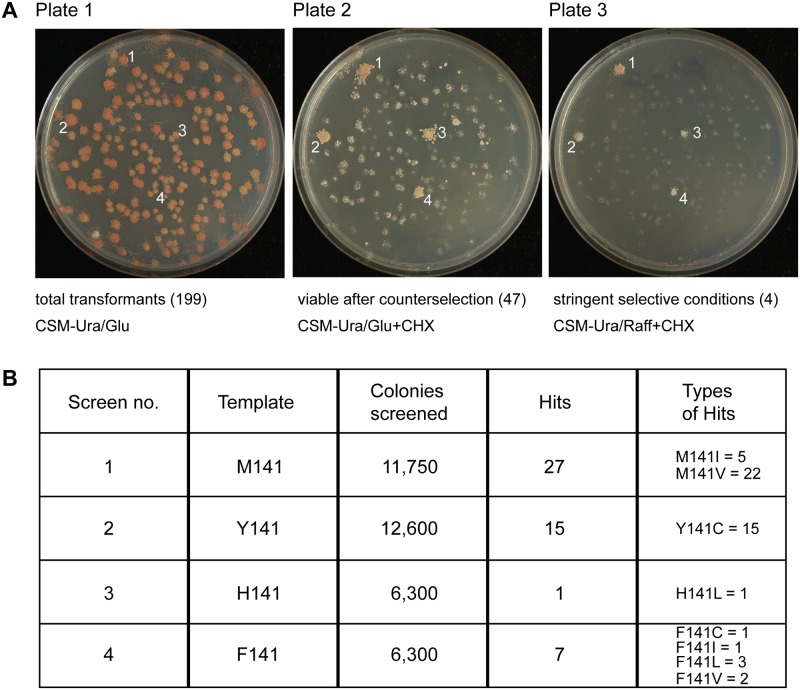
A library of randomly mutated ISU1 plasmids was generated by error-prone PCR and transformed into a YFH1 shuffle strain also deleted for ISU1. The colonies appeared uniform (Fig 4A, plate 1). The diversity of this library was confirmed by sequencing the inserts from randomly selected colonies. Of 42 colonies evaluated in this way, inserts were identified that included 72 amino acid changes in Isu1, which were well distributed throughout the coding region (S1 Table, controls). Transformants were replicated to cycloheximide plates, counterselecting against the YFH1-containing plasmid and uncovering the Δyfh1 phenotype. Most colonies grew slowly on glucose or not at all on raffinose, but a few colonies exhibited robust growth (Fig 4A, plates 2 and 3). Plasmid DNA rescued from these more rapidly growing Δyfh1 colonies was sequenced and in most cases was found to contain single nucleotide changes in ISU1 conferring substitutions of residue 141 of the coding sequence. In some cases, YFH1 sequences were found to have recombined into the ISU1 plasmid and these clones were discarded. The PCR randomization was then repeated with different ISU1 templates starting with Y141, H141 or F141, and a large number of colonies (approximately 36,950 representing 17,588 amino acid changes in Isu1) was screened in this way. The “hits” with frataxin-bypass activity included amino acid changes of M141 to Ile, Cys, Leu, and Val, sometimes in combination with other amino acids changes and sometimes alone (Fig 4B and S1 Table). However, no other amino acid change or combination of changes was able to confer frataxin-bypass activity. All possible amino acid changes in ISU1 were not sampled, and only single nucleotide changes at position 141 were selected. The failure to find amino acid substitutions with bypass activity at other locations in the Isu1 protein may derive from the many constraints on this essential scaffold protein, which must interact with multiple partner proteins and perform multiple functions, such as stimulating Nfs1 activity, coordinating Fe-S clusters and transferring Fe-S clusters [39]. Based on these results, the possibility of finding another ISU1 mutant with frataxin-bypass activity, while not entirely ruled out, seems unlikely.
Fig 4. Screening of randomized ISU1 in search of new frataxin-bypass suppressors reveals only amino acid substitutions of residue 141.
(A) A library of linear ISU1 sequences with random mutations was created by error prone PCR and co-transformed with a gapped plasmid (pRS416-ISU1) into a YFH1 (Δisu1) shuffle strain. The transformants contained recombined ISU1 sequences with random mutations, and appeared uniform on defined uracil drop-out medium (plate 1). The transformants were replicated to cycloheximide and glucose containing plates, removing the covering pRS318-YFH1 plasmid (plate 2). The transformants were also replicated to cycloheximide and raffinose containing plates (plate 3), selecting against the Δyfh1 phenotype. (B) Summary table of screening results indicating starting template, number of colonies screened, number of hits conferring frataxin-bypass, and types of hits.
E. coli cross-species complementation and frataxin-bypass
The Met to Ile substitution in yeast ISU1 that conferred frataxin-bypass activity did so by altering the methionine at position 141 (107 in the signal-cleaved mature protein) to the amino acid isoleucine used in the E. coli IscU in the corresponding position (I108 in IscU). Interestingly, deletion of the homologous frataxin gene cyaY in E. coli gave milder phenotypes in that organism than in yeast. Slowed growth, iron accumulation, and oxidative stress sensitivity were not observed in the E. coli knockout [27] in contrast to the yeast knockout. A series of species cross-complementation studies was undertaken in order to test the relative frataxin dependence or independence of yeast expressing the entire E. coli IscU protein targeted to mitochondria. For comparison, IscU protein was reverse engineered to place Met at position 108, as in the eukaryotic Isu1. The IscU protein (authentic E. coli protein with Ile) and IscU-Met (substituted E. coli protein with Met) separately were fused to the leader sequence of yeast mitochondrial protein CoxIV. Each of these fusion constructs was transformed into the GAL1-ISU1/Δisu2 strain.
The E. coli IscU proteins, with the authentic isoleucine or with the substituted methionine, were able to function in yeast as indicated by complementation of GAL1-ISU1/Δisu2 cells. Each of these complemented strains grew well, indicating that the E. coli IscU or IscU-Met targeted to yeast mitochondria could function as the only Fe-S cluster assembly scaffold in the cell. No difference between iscU or iscU-Met expressing cells was noted in terms of growth (Fig 5A, rows 1 and 2). Frataxin was deleted in these strains, and a striking growth phenotype was observed. Both the Δyfh1 [iscU] or Δyfh1 [iscU-Met] could be maintained under an argon atmosphere with subtle differences in colony size on agar plates (Fig 5A upper panel). However, following air exposure, Δyfh1 [iscU] continued to grow with a doubling time of 2.5 h, whereas Δyfh1 [iscU-Met] progressively slowed until the doubling time reached 8.5 h in defined raffinose-based medium (Fig 5A, compare top panel for argon growth to bottom panel for aerobic growth in rows 3 and 4). This air/oxygen dependent growth inhibition was much more severe for the Δyfh1 [iscU-Met] strain than for the matched Δyfh1 [ISU1], carrying Δyfh1 and yeast ISU1-Met. Perhaps the hybrid yeast-E. coli Fe-S cluster assembly machinery is particularly oxygen sensitive in the absence of frataxin. One of the functions of frataxin could be to shield the Fe-S cluster assembly machinery from oxygen [10].
Fig 5. E. coli iscU or iscU-Met targeted to yeast mitochondria.
(A) Growth phenotypes in argon or in air. Strains with mitochondrial targeted E. coli IscU proteins indicated in the figure were grown on YPAD in argon or in air for 3 days and photographed. Strain YFH1 [ISU1] was included as a control. (B) Mitochondrial protein levels by immunoblotting. The following strains were evaluated: 1) YFH1 [ISU1] or Y-M, 2) YFH1 [iscU] or Y-eI, 3) YFH1 [iscU-Met] or Y-eM, 4) Δyfh1 [iscU] or Δ-eI clone 1, 5) Δyfh1 [iscU] or Δ-eI clone 2, 6) Δyfh1 [iscU-Met] clone 1 or Δ-eM, and 7) Δyfh1 [iscU-Met] clone 2 or Δ-eM. Cultures were inoculated into defined raffinose medium bubbled with argon. After an initial growth period the cultures were shifted to air. The doubling time of the Δyfh1 [iscU-Met] clones in air started at 2.5 h but prolonged to greater than 8 h, necessitating a longer growth period prior to harvesting these cells. Mitochondrial proteins were separated by SDS-PAGE, and transferred to nitrocellulose, which was cut horizontally into segments and probed with various antibodies against various mitochondrial proteins (Aco1, Nfs1, Mir1, Yfh1, and Cyc1). The segment used for anti-Cyc1 was stripped and reprobed with anti-Isu1. The antibody to the yeast Isu1 was used to detect the E. coli IscU. The two bands attributed to E. coli IscU are indicated by a double arrow, and yeast Isu1 is indicated by a single arrow. (C) Iron homeostasis. The indicated strains were precultured in argon-bubbled raffinose medium and then shifted to air in the presence of 10 μM 55Fe ascorbate. Following growth to a density of 2 x 107 cells/ml, cells were harvested. Total cellular iron and iron in cellular fractions (post-mitochondrial supernatant, mitochondria, and soluble or insoluble fractions of mitochondria) were measured as in Fig 2C. (D) Aconitase activity. Mitochondria from the indicated strains were lysed, and 100 μg (lanes 1–7) or 200 μg (lanes 8–14) of proteins were separated by native gel and analyzed for aconitase activity using the in-gel assay.
Isolated mitochondria were examined for protein expression by immunoblotting. The yeast Isu1 migrated as a single band of about 14 kDa in mitochondria (Fig 5B, lane 1). The CoxIV fusions with the E. coli proteins reacted strongly with antibody raised against the yeast Isu1, giving rise to a slower migrating doublet (Fig 5B, lanes 2–7). The slower migrating band co-migrated with the bacterial expressed and purified IscU at about 17 kDa, and therefore it likely represents the CoxIV signal sequence-cleaved form of the IscU fusion protein. The more rapidly migrating form may be a proteolytic product. The retarded gel mobility of E. coli IscU compared with yeast Isu1 may be explained by its lower pI (4.7 for the E. coli IscU versus 9.3 for the yeast Isu1), which is associated with decreased binding of SDS and slower migration in the gel [40]. We have observed a similarly aberrant slow migration of Yfh1 due to its many acidic residues and failure to bind SDS [41].
In the Δyfh1 [iscU-Met] mitochondria, IscU protein was markedly increased in abundance (Fig 5B, lanes 6 and 7). By contrast, in the Δyfh1 [IscU] mitochondria, the IscU protein level was comparable to that of the frataxin plus strains (Fig 5B, lanes 4 and 5, compare with lanes 2 and 3). The difference may be traced to defective Fe-S cluster assembly in the Δyfh1 [iscU-Met] cells. In cells with impaired Fe-S cluster assembly, Isu1 protein abundance was previously shown to be up-regulated due to increased iron-dependent transcription mediated by the Aft1/2 regulator, and decreased turnover mediated by the Pim1 protease [32]. Furthermore, the E. coli IscU proteins might be poorly recognized by the yeast Pim1, further slowing turnover and increasing abundance (Fig 5B, lane 1 versus lanes 2 and 3). Other mitochondrial proteins were also examined. Nfs1 protein levels were comparable in all cases, consistent with the lack of regulatory changes (Fig 5B). Yfh1 was detected in the cells consistent with the predicted genotypes, being present in YFH1 [ISU1], YFH1 [iscU], and YFH1 [iscU-Met] but absent in Δyfh1 [iscU] and Δyfh1 [iscU-Met] (Fig 5B). Cytochrome c, an indicator of cellular heme status, was present in the Δyfh1 [iscU] mitochondria expressing the authentic E. coli protein but completely undetectable in Δyfh1 [iscU-Met] mitochondria expressing the substituted form of the E. coli protein (Fig 5B, compare lanes 4 and 5 versus lanes 6 and 7).
Iron homeostasis was also evaluated. Two independent clones of Δyfh1 [iscU-Met] cells were tested because of the genetic instability and changeable phenotypes associated with this genotype. Both clones exhibited strongly increased cellular iron uptake compared with the unsubstituted control clones Δyfh1 [iscU] (Fig 5C, Cellular iron, bars 6 and 7). Iron accumulated in the post-mitochondrial supernatant and mitochondria of the Δyfh1 [iscU-Met] strains (Fig 5C, Post-mito supernatant and Mitochondrial iron, bars 6 and 7). The increase in mitochondrial iron levels was about 2–4 fold more than in the matched Δyfh1 [iscU] strains (Fig 5C, Mitochondrial iron, bars 4 and 5). Iron accumulated in both soluble and insoluble forms as assessed by centrifugation in the presence of Triton X-100, probably indicating ferric phosphate nanoparticle accumulation [25,26]. In all cases, the Δyfh1 [iscU-Met] mitochondria accumulated more insoluble iron than the Δyfh1 [iscU] mitochondria [Fig 5C, Mitochondrial iron distribution, bars 6 and 7 versus bars 4 and 5). In the frataxin-plus strains expressing E. coli proteins, YFH1 [iscU] and YFH1 [iscU-Met], iron homeostasis was mostly preserved (Fig 5C, all panels, bars 2 and 3). The most severe loss of iron homeostasis occurred in the Δyfh1 [iscU-Met] strain and correlated with the concurrent severe deficiency of Fe-S cluster proteins. The mechanism by which defective mitochondrial Fe-S cluster assembly perturbs iron homeostasis is still poorly defined. However, it is likely that important roles are played by loss-of-function of Fe-S cluster binding proteins such as Aft1/2 [42] and glutathione reductases [6].
Aconitase activity, measured by the in-gel assay of mitochondria, was present in Δyfh1 [iscU] mitochondria from two independent clones (Fig 5D, lanes 4, 5 and lanes 11, 12) and absent in Δyfh1 [iscU-Met] mitochondria from two independent clones (Fig 5D, lanes 6, 7 and lanes 13, 14). Aco1 protein was present in normal amounts in Δyfh1 [iscU] but markedly decreased in Δyfh1 [iscU-Met] mitochondria, consistent with increased turnover of the apoprotein (Fig 5B). By contrast, in frataxin plus mitochondria, aconitase protein and activity were present in all cases. Aconitase activity was greatest in the frataxin plus ISU1 expressing yeast mitochondria (Fig 5D, lanes 1 and 8), slightly less in the frataxin plus E. coli iscU expressing mitochondria (Fig 5D, lanes 2, 3 and lanes 9, 10), and slightly less again in the frataxin null iscU expressing mitochondria (Fig 5D, lanes 4, 5 and lanes 11, 12). Thus aconitase activity correlated well with other features of these strains, including growth, cytochrome c levels, and iron homeostasis.
Bioinformatic survey of Isu1/IscU
Isu1/IscU entries in the public database RefSeq (6064 in total) were collected and aligned on the highly conserved 12 amino acid sequence LPPVK LH CSX LA, using the Muscle algorithm [43]. The sequences were then sorted according to the amino acid at position X, where X is Met in the yeast Isu1 and Ile in the Isu1 suppressor mutant (S2 Table).
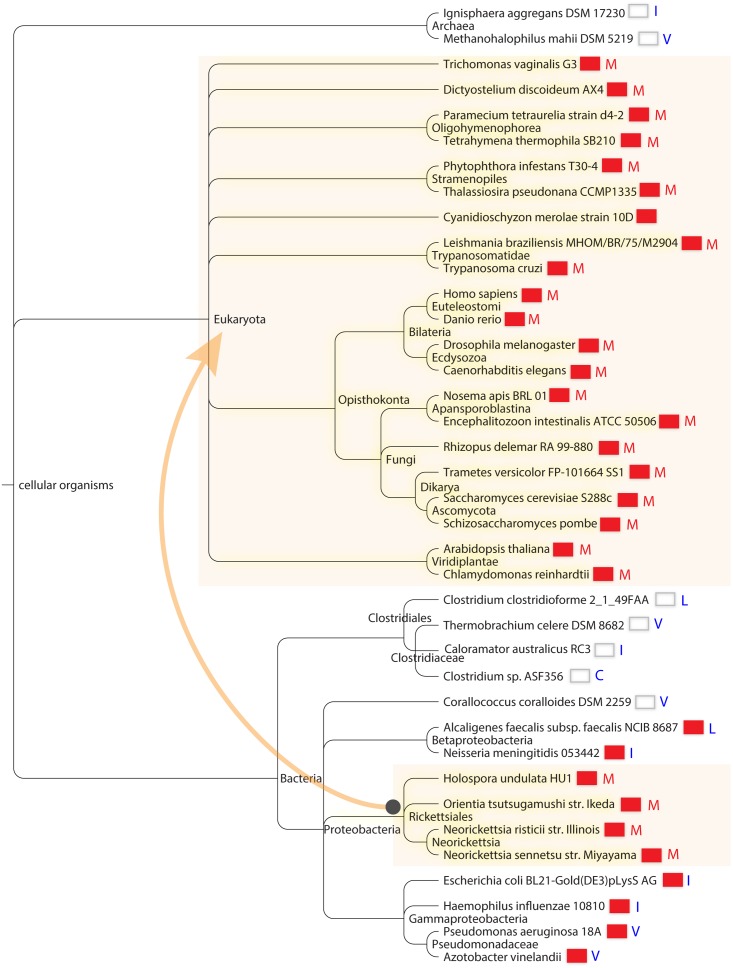
Sorting on this position revealed highly interesting groupings. Firstly, we found that Isu1/IscU sequences with Met were present almost exclusively in eukaryotic species (302 of 307 entries, S2 Table). The converse was also true i.e. eukaryotic species had Isu1/IscU with Met present in almost all cases. The proteins with Met at position X were found in the most diverse branches of eukaryotes, including Excavata that lack classical mitochondria, Chromalveolata, various yeasts including Zygomycota, Basidiomycota, Ascomycota, land plants, photosynthetic single-celled organisms, various metazoans including worms, fish, flies, mice and humans (Fig 6). The only significant exceptions to the rule that Isu1/IscU with Met occurs in eukaryotes were several proteobacterial rickettsial species, including Holospora undulata, Neorickettsia risticii, Neorickettsia sennetsu, and Orientia tsutsugamushi. These organisms are intracellular parasites that are the closest living relatives of mitochondria. Interestingly, all these species have retained frataxin in their genomes [1], suggesting that IscU-Met and frataxin may have been co-inherited with the rest of the Fe-S cluster assembly machinery during the endosymbiotic event that gave rise to mitochondria (Fig 6) [1].
Fig 6. Cosegregation of Isu1/IscU-M and frataxin in global taxonomy.
Distribution of species containing M (red letter) versus C I L V (blue letters) at the X position of the motif LPPVK LH CSX LA and correlation with the presence of frataxin. The presence of frataxin is indicated by a red colored box next to each species in the taxonomic tree. The depicted species were chosen to maximize diversity. The species are displayed using the NCBI Taxonomy browser according to their positions in the NCBI Taxonomy Database. The arrow connecting shaded areas in Proteobacteria and Eukaryota represents the direction of possible horizontal gene transfer occurring during mitochondrial creation.
The lists of Isu1/IscU proteins using Cys, Ile, Leu, and Val at position X, i.e. those amino acid substitutions of yeast Isu1 conferring frataxin-bypass activity, included predominantly prokaryotic organisms. The Cys list had only 6 entries, all from bacteria, including several Clostridium species and an uncultured archeon (S2 Table). For the Ile list, almost all of the species were from prokaryotes (171 of 178 entries). The exceptions, i.e. eukaryotic species on this list, were interesting in that they often possessed more than one Fe-S cluster assembly scaffold gene. For example, the eukaryotic bumble bee, cucumber, armadillo and a single type of Mediterranean fly each carried Isu1 proteins with Ile at position 141, but the same organisms possessed another gene which used Met, as is typical for almost all eukaryotes. The Val column had almost exclusively prokaryotic or archaeal species (341 of 348 entries). In this column, the only eukaryotic species included Entamoeba histolytica and Giardia intestinalis, organisms that are known to have acquired Fe-S cluster assembly components from bacteria by gene transfer [44]. A single eukaryotic plant species, wheat or Aegilops tauschii, was found in this column. However, wheat also had another Isu gene using Met, and so it follows the theme of retaining more than one type of Isu, perhaps for adaptive advantages. Along the same lines, Bos mutus, a yak with a high altitude habitat, carried an Isu with Ile but also retained the more typical eukaryotic Isu with Met. The IscU proteins with leucine were found predominantly in prokaryotic species (181 of 199 entries). The outlier eukaryotic species on this list included selected apicomplexa (e.g. Plasmodium falciparum, P. vivax, P. yoelli), fungi (e.g. Cryptococcus and Aspergillus species) and mitosome containing microsporidia (e.g. Nematocida parisii).
The diversity of Isu1/IscU proteins is further increased by the expression of different splice forms in some species. Alternatively spliced forms of the scaffold protein genes were found in humans and armadillo. In humans, the X1 splice form inserts an Arg, and isoform X4 inserts a Lys at position X. The Lys containing isoform has been associated with destabilized protein and development of a human disease characterized by exercise intolerance and mitochondrial myopathy [45]. The substitutions of the Met 141 in yeast Isu1 with Lys and Arg were tested, and neither one was able to support scaffold activity or frataxin-bypass activity (Fig 1B). Nonetheless, it is still possible that these splice forms with Lys or Arg amino acid substitutions could serve a special function or afford an adaptive advantage under some special circumstances or in specific tissues.
In summary, Isu1/IscU amino acid sequences with Met at position X of the motif LPPVK LH CSX LA were predominantly found in eukaryotes but also occurred in several Rickettsia species. All contained frataxin in their genomes. Isu1/IscU proteins with the amino acids Cys, Ile, Val or Leu at position X were present primarily in prokaryotes, some with and some without frataxin in their genomes (Fig 6).
Discussion
Frataxin was initially identified by reverse genetics after the Friedreich’s ataxia disease gene was cloned in 1996 [2]. Soon afterwards the protein was linked to iron metabolism by characterization of the striking iron homeostatic phenotype of the yeast deletion strain [23]. However, a more detailed understanding of frataxin’s function has been elusive. Recently, frataxin has been convincingly implicated in mitochondrial Fe-S cluster assembly [10]. Frataxin interacts with components of the mitochondrial Fe-S cluster machinery, including Nfs1, Isd11 and Isu1 [5]. It is vital for formation of Fe-S cluster intermediates on the Isu1 scaffold, a key step in the Fe-S cluster assembly process [3]. A suppressor Isu1 carrying the amino acid substitution Met to Ile at position 141 can correct or bypass most of the Δyfh1 phenotypes that result from lack of frataxin [28]. The change introduces the amino acid used by E. coli in the highly homologous IscU protein. The Δyfh1 yeast deletion strain is severely compromised and slow growing, whereas the E. coli strain deleted for the homologous frataxin protein is mildly compromised and grows normally. Here we have delved into the phenomenon of bypass of frataxin deletion, performing a number of genetic and biochemical experiments.
The suppressor Isu1 acts as a genetic dominant and requires Nfs1 for its activity
The suppressor ISU1-Ile bypassed the requirement for YFH1 when it was introduced on a plasmid. Similarly, the corresponding substitution in a plasmid carrying the paralogous ISU2-Ile conferred bypass activity. The effects were observed with chromosomal ISU1 and ISU2 genes remaining intact, thereby reflecting genetic dominance and suggesting a gain of function. It is therefore interesting to consider more specifically the nature of the gained function. One explanation could be that Nfs1/Isd11 exhibits only basal cysteine desulfurase activity, and that frataxin is needed to act as a positive effector, thereby inducing the optimal activated level of cysteine desulfurase [13,29]. The wild-type Isu1 has no stimulatory activity on its own and may even be inhibitory [13,33], but the suppressor Isu1-Ile is able to substitute for frataxin by stimulating the Nfs1/Isd11 cysteine desulfurase [29,30]. If both wild-type Isu1 and suppressor Isu1-Ile are present in the cell simultaneously, the stimulatory effect of the suppressor is the dominant effect, providing bypass activity. An implied consequence of this scenario is that the suppressor Isu1-Ile will require an active form of Nfs1 for it to be effective. Thus it makes sense that the hypomorphic allele of NFS1, nfs1-14 [46], with decreased cysteine desulfurase activity, would not support bypass. The results provide genetic support for the hypothesis that frataxin and the bypass Isu1 work to produce their effects on Fe-S cluster assembly, at least in part, by boosting the activity of the cysteine desulfurase.
The suppressor Isu1 was shown to stimulate persulfide formation on Nfs1, similar to frataxin [29,30]. Subsequently, sulfur for Fe-S cluster synthesis must be transferred to the Isu1 scaffold and assembled with iron to form the Fe-S cluster intermediate. It is generally assumed that this process occurs in a protein complex with other Isu1 molecules present [35,36]. Therefore, we wondered if the Nfs1 stimulatory effect of the suppressor Isu1 could promote Fe-S cluster formation in trans on a normal copy of Isu1 or Isu2. However, at least in a series of genetic experiments, this was not the case. The survey of all the possible M141 amino acid substitutions identified a set of best scaffolds, moderate scaffolds and poor scaffolds, based on complementing activity in the GAL1-ISU1/Δisu2 strain (Fig 1B). The same set of plasmids, scored for frataxin-bypassing capability, identified activity for M141 with Cys, Ile, Leu or Val changes, and these all fell into the top category for scaffold activity. Furthermore, if a second site substitution abolishing scaffold activity (e.g. changing a critical Cys to Ala) was introduced into the M141I-Isu1 protein sequence, and the doubly substituted Isu1 was introduced into a Δyfh1 shuffle strain with wild-type ISU1 and ISU2 present, bypass activity was abrogated (Fig 1C). Thus most likely the suppressor Isu1 does not productively interact with the wild-type Isu1 to mediate bypass, but instead it replaces the wild-type Isu1 in providing both bypass and scaffold functions.
Prokaryotic versus eukaryotic cysteine desulfurases
Eukaryotic and prokaryotic Fe-S cluster machineries are highly conserved. Both yeast and E. coli utilize cysteine desulfurases, scaffold proteins and frataxin homologs. However major differences in the frataxin deletion phenotypes have been reported, with essentiality or severely deleterious phenotypes in the eukaryotic case [4], and normal growth and relatively mild phenotypes in E. coli [27]. Why the difference? One possibility is that E. coli possesses a redundant Fe-S cluster assembly system, the SUF system, which may compensate for lack of frataxin [47]. However this does not entirely account for the phenotypic differences, because the SUF system is not generally deployed under standard growth conditions. Significantly, the cysteine desulfurases show key differences. Most prokaryotic cysteine desulfurases such as IscS are constitutively active, although some regulatory changes in activity have been described [15]. The eukaryotic cysteine desulfurases, on the other hand, seem to be largely inactive in their basal state. Activation is required, and this activation involves frataxin. Purified Nfs1 is able to bind the substrate cysteine in its PLP containing substrate-binding site. However, binding is inefficient, and frataxin interaction increases exposure and utilization of substrate-binding sites of the enzyme [29]. The suppressor Isu1-Ile protein is able to generate a similar alteration of Nfs1, mimicking the effect of frataxin on Nfs1, and providing a plausible explanation for its frataxin-bypass activity. Nfs1 enzyme with the substrate bound must still undergo another activation step, mediated by Isd11. Isd11 triggers a conformational change and persulfide formation, thereby generating the intermediate for Fe-S cluster assembly [29]. Here we have seen that not only the Ile but also the Cys, Val, Leu substituted forms of Isu1 are able to stimulate persulfide formation on Nfs1 in the absence of frataxin. Thus these bypassing alleles of Isu1 activate Nfs1 independently of frataxin, rendering the mitochondria more prokaryote-like.
Frataxin-bypassing amino acid substitutions in Isu1 and how they may work
More than 17,000 amino acid changes of ISU1 were surveyed, but only a small subset of those conferred bypass activity. In all cases, the active changes altered the amino acid at position 141 of Isu1, introducing the amino acids Cys, Ile, Leu, or Val. The mutagenesis was not exhaustive, but nonetheless, the data support the very restricted nature of the changes that confer this activity. The biochemical features of these newly discovered bypass mutants (Isu1 with Cys, Leu, or Val substituted at position 141) were similar to the original one (Isu1 with Met replaced by Ile). The various mutant forms of Isu1 conferred improved growth in the absence of frataxin and more efficient Fe-S cluster assembly in mitochondria. The persulfide-forming activity was increased in mitochondria lacking frataxin, indicating enhanced cysteine desulfurase activity.
How does the Isu1 suppressor work? Isu1 is a central component of the Fe-S cluster assembly complex consisting of Nfs1/Isd11/Isu1/Yfh1. The components are highly conserved with their bacterial homologs, with the exception of Isd11. Structural information has been obtained only for the bacterial components [11,36]. For Isu1/IscU the structure includes alpha helices framing a platform of beta sheets, with three conserved cysteines oriented towards a binding pocket in the core and able to coordinate the Fe-S cluster intermediate (Fig 7). The amino acid motif LPPVK is found towards the beginning of a long C-terminal alpha helix [11,39]. Interestingly, the PVK motif (Fig 7, green in Fe-S scaffold) was shown to include the frataxin-binding site [33]. The suppressor residue Ile (Fig 7, red ball-and-stick) is predicted to lie on an exposed surface of this helix, opposite the Fe-S liganding Cys, which is found on the opposite interior face of the helix (Fig 7, blue ball-and-stick). Thus the Met to Ile amino acid change near to this frataxin-binding site on Isu1 might mimic the effects of frataxin binding. Substitutions of Cys, Val, or Leu would be predicted to produce similar changes.
Fig 7. The Fe-S cluster assembly complex of E. coli.
The Protein Data Bank (PDB) file of the bacterial Fe-S cluster assembly complex [36] was kindly provided by Annalisa Pastore (King’s College London). The image was drawn using the Stanford Chimera program. The cysteine desulfurase is IscS for E. coli or Nfs1 for yeast; frataxin is CyaY for E. coli or Yfh1 for yeast; the Fe-S scaffold is IscU for E. coli or Isu1/Isu2 for yeast. Highlighted features of IscU/Isu1 are the suppressor amino acid Ile (red), the Fe-S cluster cysteine ligands (blue), and the PVK frataxin-binding site (green). The reciprocal interaction site on frataxin includes a tryptophan (green). The PLP cofactor of IscS/Nfs1 is shown as yellow spheres, and a dotted purple line is used to indicate the approximate position of the active site mobile loop, which is not seen in the structures.
Conformational changes of the Fe-S cluster assembly complex facilitating Fe-S cluster intermediate formation might ensue. Nfs1 might be altered in a way that exposes substrate-binding sites for interacting with cysteine [29]. (Fig 7, yellow balls for PLP in the binding site). The sulfur intermediate, converted to a persulfide and bound to the flexible loop of Nfs1 (Fig 7, magenta dotted line and adjacent residues), might be more readily transferred to the modified Isu1. The Cys 139, an Fe-S cluster ligand on Isu1, that is one helix turn away from the Ile suppressor residue, might be rendered more accessible for persulfide transfer (Fig 7). Iron delivery remains the least characterized step in Fe-S cluster formation. Frataxin might facilitate iron entry into the Fe-S cluster assembly complex by initiating a conformational change that promotes transfer of mitochondrial iron from a physiological ligand (still undefined) to Isu1. Alternatively, frataxin might play a more direct role in binding iron and delivering it to Isu1 as has been shown for Yfh1 [19]. The Isu1-Ile protein (or bacterial IscU) might accomplish a similar function by exposing iron-binding sites on Isu1 [19]. The iron delivery step in Fe-S cluster assembly will need to be better understood to support or to refute these possibilities.
Iron homeostasis and heme
Iron homeostasis was improved, correlating with the improvements in cytochrome c, a mitochondrial heme protein. In previously published work, cytochromes were virtually absent in Δyfh1 and restored by the ISU1-Ile allele as assessed by low temperature spectra of whole cells [28]. Other heme proteins such as cytochrome c peroxidase, Ccp1, were similarly affected [30]. Heme synthesis, measured by 55Fe incorporation into porphyrin in isolated mitochondria, was very low in Δyfh1 and recovered in the presence of the substituted ISU1 [28]. What is the mechanistic connection between a change in the Fe-S cluster assembly scaffold and these effects on heme synthesis? Heme synthesis occurs inside mitochondria and involves the insertion of iron into porphyrin by the enzyme ferrochelatase to make protoheme. Porphyrin and ferrochelatase activity are not lacking, and in fact, zinc protoporphyrin accumulates in the Δyfh1 mutant [25]. Instead iron is the likely source of the trouble. Iron accumulating in Δyfh1 mitochondria (and in other Fe-S cluster deficient cells) exhibits changes in its physical properties and solubility that could lead to loss of bioavailability [25]. The data suggest that Fe-S cluster synthesis acts upstream of heme synthesis in promoting iron bioavailability for heme synthesis, although many mechanistic details are still lacking.
Taxonomy of Isu1/IscU sequences with or without frataxin
Isu1/IscU amino acid sequences sorted according to the amino acid appearing in position 141 fall into clearly defined grouping, underscoring the importance of this residue for Isu1/IscU function. The amino acid Met appeared almost exclusively in eukaryotic organisms. The only significant exceptions were several prokaryotic Rickettsia species, which were classified as proteobacteria. In all of these organisms with IscU-Met, frataxin was also present in their genomes. We can imagine a scenario in which IscU-Met and frataxin originated together in proteobacterial ancestors of mitochondria such as Rickettsia, and from there gave rise to modern mitochondria via horizontal gene transfer during the endosymbiotic event (Fig 6). A key point is that the Met amino acid was found only in organisms with frataxin, as if Met serves to “lock in” frataxin by ensuring frataxin dependence of Fe-S cluster assembly. The co-dependence of these two elements, the IscU-Met and frataxin, appears to be quite profound, as they have been retained together throughout all the eukaryotic branches of the tree of life. In the prokaryotic world, on the other hand, various IscU variants were found, and various amino acids were found at position X adjacent to the PVK frataxin-binding motif of the IscU homologs. The amino acids Cys, Ile, Leu, or Val which conferred frataxin-bypass in biochemical experiments in yeast, were found in IscU proteins of species with or without frataxin homologs (Fig 6). Thus the evolutionary record suggests that these amino acids may be associated with relative frataxin independence of Fe-S cluster assembly. The advantages of retaining IscU-Met and frataxin versus IscU-Ile and no frataxin remain to be ascertained, as both arrangements are able to support efficient and regulated Fe-S cluster assembly.
Implications for Friedreich's ataxia
Friedreich’s ataxia is a progressive degenerative disease affecting neurons such as dorsal root ganglia and cardiomyocytes, and certain other tissues. The disease is caused by frataxin deficiency in affected tissues and is associated with defective Fe-S cluster assembly [24,48]. The efficacy of frataxin-bypass in yeast can be viewed as a reprogramming of mitochondrial Fe-S cluster assembly to a more prokaryotic type, such that the cysteine desulfurase and other features of the assembly process become more frataxin-independent. The discovery of several substitutions, changes to Cys, Ile, Leu, or Val, that are able to confer frataxin-bypass suggests that there may be common structural features of these Isu proteins that could be mimicked by small molecules [49]. The genetic dominance of the suppressor activity in the Δyfh1 yeast also suggests that such an approach could be effective in a therapeutic setting in human mitochondria where frataxin is deficient and normal Isu1 is still present.
Materials and Methods
Construction of Isu1 mutant plasmids and yeast strains
A set of plasmids was generated containing modified versions of the Isu1 coding sequence (between NdeI and XhoI), carried between the native Isu1 promoter (700 bp between EagI and NdeI) and terminator (200 bp between BamHI and SacI) on a centromere based plasmid, YCplac22. Residue 141 of the full length Isu1 precursor protein was changed from M to each of the other 19 amino acids, using QuikChange mutagenesis (Agilent Technologies Inc.). In addition, N123D and N123A mutants were generated. Cysteine mutants of the Isu1 coding sequence were constructed in which C69, C96 and C139 were each changed to A. The M141I change was then introduced into each of these cysteine mutants, creating plasmids C69A-M141I, C96A-M141I, and C139A-M141I. A cysteine swap mutant was created in which C139A was combined with M141C in the full length Isu1. The various mutant forms of Isu1 were tested for Yfh1-bypassing function and scaffold function by transforming into strains YFH1 shuffle and GAL1-ISU1/Δisu2, respectively (Table 1). For testing genetic dominance of the ISU1 bypass suppressor, the suppressor allele (YCplac22-ISU1-M141I) was introduced into strain 70–31 containing genomic copies of ISU1 and ISU2 (Table 1), and the covering YFH1 plasmid was removed by fluoroorotic acid (FOA) treatment. For testing ISU2 function, the native ISU2 sequence was amplified from plasmid pGP564-ISU2 including 700 bp 5’ and 200 bp 3’ of the coding sequence, and cloned between restriction sites HindIII and BamHI in YCplac22, making plasmid YCplac22-ISU2. The M133 was changed to Ile by site directed mutagenesis, creating YCplac22-ISU2-M133I. The ISU2 coding sequence was inserted into NdeI-XhoI sites in place of the ISU1 coding creating YCplac22-ISU2coding. The M133 was changed to Ile by site directed mutagenesis creating plasmid YCplac22-ISU2coding-M133I.
Mutant strains expressing alleles of Isu1 (Cys, Ile, Leu, or Val) in single copy
Strain GAL1-ISU1/Δisu2 was transformed with plasmid YCplac22-ISU1 in which M141 was changed to C, I, L or V and the chromosomal GAL1 promoter was turned off by shifting cells from galactose to glucose as the carbon source. The YFH1 gene was deleted by transforming with plasmid pRS405-gamma-yfh1 linearized with BamHI, and selecting for transformants on defined leucine drop-out medium in an argon-filled chamber. These low oxygen conditions were previously shown to mitigate the mutant phenotype and allow for stable propagation of the knockouts [30]. The knockouts were confirmed by PCR of the YFH1 locus. The strains were denoted Δyfh1 [ISU1] (115–26), Δyfh1 [ISU1-Cys] (116–54), Δyfh1 [ISU1-Ile] (115–28), Δyfh1 [ISU1-Leu] (116–53), and Δyfh1 [ISU1-Val] (116–51). Congenic wild-type strain YFH1 [ISU1] was included as a control.
The strains were thawed from -80°C vials and inoculated to CSM-Trp/2% raffinose defined medium agar plates kept in an argon-filled jar. Cells were inoculated from the plates into liquid medium of the same composition. In general, small cultures of 50 ml were grown in argon-filled bottles for two days without shaking, expanded to 100 ml cultures while shaking in air, and then diluted again into 1 L cultures supplemented with 10 μM ferrous ascorbate. The 1 L cultures were grown at 30°C for 16 h, and the total time exposed to air was approximately 24 h for the slow-growing strains.
Iron measurements and enzyme assays
Cellular and subcellular iron levels were determined by growing cells for at least four doublings in standard defined medium supplemented with 58 nM 55FeCl3, 10 μM unlabled ferric chloride and 100 μM ascorbic acid. Cells were washed free of unincorporated iron, and then they were ruptured by vortexing with glass beads in the presence of 50 mM Hepes/KOH, pH 7.5, 150 mM NaCl, 0.6 M sorbitol. Differential centrifugation was used to remove unbroken cells and to separate the remainder into mitochondrial and post-mitochondrial fractions [50]. The post-mitochondrial fraction included both cytoplasm and vacuoles, as no effort was made to separate these two cellular components. Mitochondria were shown to be mostly intact by evaluation of mitochondrial marker proteins, which remained with the mitochondrial fraction. Mitochondria were lysed for 10 min at room temperature in the presence of 0.1% Triton X-100 in hypotonic buffer (50 mM Hepes/KOH, pH 7.5, 150 mM NaCl). The supernatant (soluble) and pellet (insoluble) portions were separated by centrifugation at 20,000 x g for 30 min. Iron content was determined by scintillation counting for 55Fe, and protein content was measured by bicinchoninic acid assay (BCA, Pierce) [28]. For whole cells, iron content was reported as pmol iron per million cells, whereas for the cellular fractions iron content was reported as pmol iron per microgram protein. An in-gel activity assay for aconitase was performed. Briefly, mitochondria were lysed in buffer consisting of 50 mM Tris-HCl pH 8, 50 mM NaCl, 1% TX-100, 10% v/v glycerol, 2 mM Na-citrate and 15 U catalase. Samples were loaded on a native acrylamide gel containing 132 mM Tris base, 132 mM boric acid, 3.6 mM sodium citrate. The signal was developed in the gel by incubating in developing buffer containing 100 mM Tris-HCl, pH 8, 1 mM NADP, 2.5 mM cis-aconitic acid, 5 mM MgCl2, 1.5 mM methylthiazolyldiphenyl-tetrazolium bromide (MTT), 0.3 mM phenazine methosulfate, and 5 U/ml isocitrate dehydrogenase [51,52].
Organelle assays for persulfide and Fe-S cluster synthesis
Persulfide formation on Nfs1 present in intact mitochondria was measured as described [16]. Isolated mitochondria were depleted for endogenous nucleotides and NADH by incubation for 10 min at 30°C in buffer (20 mM Hepes/KOH, pH 7.5, 0.6 M sorbitol). These mitochondria were incubated with 35S-cysteine (10 μCi) for 15 min at 30°C. Mitochondria were recovered, proteins were separated by non-reducing SDS gel, and the persulfide was viewed by radioautography. Fe-S cluster formation was measured as described [12]. Mitochondria were incubated with 35S-cysteine for 30 min in the presence of added ATP (4 mM), GTP (1 mM), NADH (5 mM) and iron (10 μM ferrous ascorbate). Mitochondria were recovered and the soluble proteins were released by freeze-thaw and sonication and separated on a native gel. The signal associated with newly formed [Fe-35S] aconitase was visualized by radioautography.
Genetic screen for Isu1 alleles with bypass activity
Pools of mutagenized linear fragments of 1443 bp including the coding region of the ISU1 gene were generated by mutagenic PCR in the presence of 1 mM MnCl2 and altered nucleotide concentrations (2 mM dATP, 2 mM dGTP, 10 mM dTTP, 10 mM dCTP). The primers used were: A (5’ TTTTTTCGGCCGTTCTTTTCTTTTTCTTGCACTACC 3’) and B (5’ TGATTTGAGCTCagcacgtccgtcccgctttcaccctgg 3’), and the templates were different YCplac22-ISU1 plasmids with M, Y, H or F at position 141 of the coding region. Each mutagenized pool was co-transformed with a gapped plasmid (pRS416-ISU1 digested with MscI and BamHI) into the YFH1 shuffle strain 109–9 (Δyfh1::TRP1 Δisu1::HIS3MX6 ISU2 cyh2 [pRS318-CYH2-LEU2-YFH1], Table 1). After selecting for transformants on uracil drop-out medium with glucose as the carbon source, the colonies were replicated to uracil drop-out, cycloheximide medium with glucose or raffinose as the carbon source. The large colonies appearing after several days on the raffinose plates were analyzed further. Colony PCR was used to check for absence of YFH1. Transformants still harboring YFH1 after counterselection were discarded. The remaining clones were expanded, and the plasmid-borne ISU1 alleles were rescued in E. coli and the DNA was sequenced.
E. coli IscU constructs and yeast strains for mitochondrial targeting
The coding region of E. coli IscU was amplified from genomic E. coli DNA from strain DH5 alpha and inserted into the XbaI and XhoI sites of a YCplac22 derived plasmid. In this plasmid, the ISU1 promoter consisting of 700 bp, is followed by the first 22 amino acids of the cytochrome c oxidase subunit IV (CoxIV) mitochondrial signal sequence [53], and 200 bp of ISU1 terminator. The resulting plasmid was checked by DNA sequencing and confirmed to code for a CoxIV-IscU fusion protein with amino terminus as follows: MLSLRQSIRFFKPATRT^LCSSRHMAYSEKVID. The caret indicates the predicted signal sequence cleavage site by the mitochondrial processing peptidase, and the bolded letters indicate amino acids of E. coli IscU. The rest of the IscU sequence was confirmed to be the same as listed in Genbank accession No. AAJU02000016.1. The amino acid I108 of IscU (equivalent to M107 in signal-cleaved mature Isu1 or M141 in the full length precursor form of Isu1) was changed from I to M by QuikChange mutagenesis, generating a plasmid for expressing IscU-Met in mitochondria. The mitochondrial-targeted iscU plasmids were introduced into strain GAL1-ISU1/Δisu2, generating strains YFH1 [iscU] and YFH1 [iscU-Met]. YFH1 was deleted in these strains by transforming with pRS405-gamma-yfh1, linearized at BamHI, followed by selection on leucine drop-out medium in an argon-filled anaerobic jar. This created strains Δyfh1 [iscU] and Δyfh1 [iscU-Met] (Table 1). Deletion of YFH1 was verified by PCR. Strain GAL1-ISU1/Δisu2 transformed with YCplac22-ISU1 served as the control strain YFH1 [ISU1]. All strains were grown in an argon-filled chamber or in argon-bubbled medium, and cells were then exposed to air in defined raffinose medium for iron labeling and mitochondrial isolation.
Bioinformatic analysis of Isu1/IscU protein sequences
Isu1/IscU related sequences were collected by using BLAST similarity to the Isu1 of Saccharomyces cerevisiae S288c from the RefSeq non-redundant database. All related entries in RefSeq (6064) were aligned using the Muscle program [43], according to the amino acid at position X (equivalent to Isu1 residue 141) of the amino acid motif LPPVK LH CSX LA. The lists were manually edited, and duplicates were removed, retaining one entry for each species. For each entry, the sequence was scored + if it contained 8 or more amino acids identical to the query, and 0 if contained 7 or less. The sequences were scored * for exceptions if they deviated from the rule that eukaryotic Isu proteins use only methionine at that amino acid position and prokaryotic Isu proteins do not use methionine at that position.
Supporting Information
The Δyfh1 shuffle strain 70–31 strain was transformed with ISU2-containing plasmids and control plasmids, and the covering YFH1 plasmid was removed by FOA treatment. The plasmids are: 1) YCplac22-ISU2coding-Met, 2) YCplac22-ISU2coding-Ile 3) YCplac22-ISU2-Met, 4) YCplac22-ISU2-Ile, 5) YCplac22, 6), YCplac22-ISU1-Met, 7) YCplac22-ISU1-Ile, 8) YCplac22-YFH1. Frataxin-bypass was observed for ISU2-Ile and ISU2coding-Ile in which the mutated coding sequence of ISU2 was expressed from the ISU1 promoter.
(TIF)
The ISU1 coding sequence was amplified by error-prone PCR from the native sequence (M141) or from mutated sequences plasmids (Y141, H141 or F141) and co-transformed with a gapped ISU1 plasmid into the YFH1 shuffle strain. Following removal of the YFH1 plasmid, clones with improved growth were identified, and the plasmid borne ISU1 sequence was determined. The table lists amino acid changes in the ISU1 coding of the “hits” with improved growth and the corresponding nucleotide sequence of the codon at position 141 of ISU1. Among the hits, M141I was selected 6 times, M141V was selected 24 times, M141L was selected 4 times, and M141C was selected 17 times. No selected clones had the wild-type sequence, M141. As a control, 42 unselected clones were evaluated from the randomized pool starting with M141, and these all retained the M141 codon.
(XLSX)
Isu1/IscU proteins from the RefSeq non-redundant database (total number of 6064) were aligned according to the amino acid at position X of the 12 amino acid motif LPPVK LH CSX LA. In all, 21 lists were collected, one for each amino acid at position X and a no-alignment list. The lists were manually edited, retaining one entry for each species. For each entry, columns were annotated with the species name, accession number, taxonomic category, and the sequence of the 12 amino acid motif. The sequences were scored + if they contained 8 or more amino acids identical with the motif present in yeast Isu1 and 0 if they contained 7 or less identical amino acids. The sequences were scored * for exceptions if they deviated from the rule that Isu1/IscU proteins use methionine at position X and prokaryotic proteins do not use methionine at that position.
(XLSX)
Acknowledgments
We thank Annalisa Pastore (King's College London) for providing PDB files of the bacteria Fe-S cluster assembly complex. We thank Agostinho Rocha (University of Pennsylvania, Philadelphia), and we thank Timothy Stemmler (Wayne State University, Detroit) and April Kosowski (Wayne State University, Detroit) for help with making Fig 7.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Institutes of Health Grant R37DK53953 to AD, National Institutes of Health Grant R01GM107542 to DP, and by a grant from the Friedreich's Ataxia Research Alliance (http://www.curefa.org/) to DP and AD. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gibson TJ, Koonin EV, Musco G, Pastore A, Bork P (1996) Friedreich's ataxia protein: phylogenetic evidence for mitochondrial dysfunction. Trends Neurosci 19: 465–468. [DOI] [PubMed] [Google Scholar]
- 2. Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, et al. (1996) Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271: 1423–1427. [DOI] [PubMed] [Google Scholar]
- 3. Gerber J, Muhlenhoff U, Lill R (2003) An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1. EMBO Rep 4: 906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmucker S, Martelli A, Colin F, Page A, Wattenhofer-Donze M, Reutenauer L, et al. (2011) Mammalian frataxin: an essential function for cellular viability through an interaction with a preformed ISCU/NFS1/ISD11 iron-sulfur assembly complex. PLoS One 6: e16199 10.1371/journal.pone.0016199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang T, Craig EA (2008) Binding of yeast frataxin to the scaffold for Fe-S cluster biogenesis, Isu. J Biol Chem 283: 12674–12679. 10.1074/jbc.M800399200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lill R, Hoffmann B, Molik S, Pierik AJ, Rietzschel N, Stehling O, et al. (2012) The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim Biophys Acta 1823: 1491–1508. 10.1016/j.bbamcr.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 7. Roche B, Aussel L, Ezraty B, Mandin P, Py B, Barras F (2013) Iron/sulfur proteins biogenesis in prokaryotes: formation, regulation and diversity. Biochim Biophys Acta 1827: 455–469. 10.1016/j.bbabio.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 8. Johnson DC, Dean DR, Smith AD, Johnson MK (2005) Structure, function, and formation of biological iron-sulfur clusters. Annu Rev of Biochem 74: 247–281. [DOI] [PubMed] [Google Scholar]
- 9. Colin F, Martelli A, Clemancey M, Latour JM, Gambarelli S, Zeppieri L, et al. (2013) Mammalian frataxin controls sulfur production and iron entry during de novo Fe4S4 cluster assembly. J Am Chem Soc 135: 733–740. 10.1021/ja308736e [DOI] [PubMed] [Google Scholar]
- 10. Pastore A, Puccio H (2013) Frataxin: a protein in search for a function. J Neurochem 126 Suppl 1: 43–52. 10.1111/jnc.12220 [DOI] [PubMed] [Google Scholar]
- 11. Shi R, Proteau A, Villarroya M, Moukadiri I, Zhang L, Trempe JF, et al. (2010) Structural basis for Fe-S cluster assembly and tRNA thiolation mediated by IscS protein-protein interactions. PLoS Biol 8: e1000354 10.1371/journal.pbio.1000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pandey A, Golla R, Yoon H, Dancis A, Pain D (2012) Persulfide formation on mitochondrial cysteine desulfurase: enzyme activation by a eukaryote-specific interacting protein and Fe-S cluster synthesis. Biochem J 448: 171–187. 10.1042/BJ20120951 [DOI] [PubMed] [Google Scholar]
- 13. Tsai CL, Barondeau DP (2010) Human frataxin is an allosteric switch that activates the Fe-S cluster biosynthetic complex. Biochemistry 49: 9132–9139. 10.1021/bi1013062 [DOI] [PubMed] [Google Scholar]
- 14. Iannuzzi C, Adinolfi S, Howes BD, Garcia-Serres R, Clemancey M, Latour JM, et al. (2011) The role of CyaY in iron sulfur cluster assembly on the E. coli IscU scaffold protein. PLoS One 6: e21992 10.1371/journal.pone.0021992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bridwell-Rabb J, Iannuzzi C, Pastore A, Barondeau DP (2012) Effector role reversal during evolution: the case of frataxin in Fe-S cluster biosynthesis. Biochemistry 51: 2506–2514. 10.1021/bi201628j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pandey A, Yoon H, Lyver ER, Dancis A, Pain D (2012) Identification of a Nfs1p-bound persulfide intermediate in Fe-S cluster synthesis by intact mitochondria. Mitochondrion 12: 539–549. 10.1016/j.mito.2012.07.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Richards TA, van der Giezen M (2006) Evolution of the Isd11-IscS complex reveals a single alpha-proteobacterial endosymbiosis for all eukaryotes. Mol Biol Evol 23: 1341–1344. [DOI] [PubMed] [Google Scholar]
- 18. Bandyopadhyay S, Chandramouli K, Johnson MK (2008) Iron-sulfur cluster biosynthesis. Biochem Soc Trans 36: 1112–1119. 10.1042/BST0361112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cook JD, Kondapalli KC, Rawat S, Childs WC, Murugesan Y, Dancis A, et al. (2010) Molecular details of the yeast frataxin-Isu1 interaction during mitochondrial Fe-S cluster assembly. Biochemistry 49: 8756–8765. 10.1021/bi1008613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pastore C, Franzese M, Sica F, Temussi P, Pastore A (2007) Understanding the binding properties of an unusual metal-binding protein—a study of bacterial frataxin. FEBS J 274: 4199–4210. [DOI] [PubMed] [Google Scholar]
- 21. Kim JH, Frederick RO, Reinen NM, Troupis AT, Markley JL (2013) [2Fe-2S]-ferredoxin binds directly to cysteine desulfurase and supplies an electron for iron-sulfur cluster assembly but is displaced by the scaffold protein or bacterial frataxin. J Am Chem Soc 135: 8117–8120. 10.1021/ja401950a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shakamuri P, Zhang B, Johnson MK (2012) Monothiol glutaredoxins function in storing and transporting [Fe2S2] clusters assembled on IscU scaffold proteins. J Am Chem Soc 134: 15213–15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Babcock M, de Silva D, Oaks R, Davis-Kaplan S, Jiralerspong S, Montermini L, et al. (1997) Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science 276: 1709–1712. [DOI] [PubMed] [Google Scholar]
- 24. Santos R, Lefevre S, Sliwa D, Seguin A, Camadro JM, Lesuisse E (2010) Friedreich ataxia: molecular mechanisms, redox considerations, and therapeutic opportunities. Antioxid Redox Signal 13: 651–690. 10.1089/ars.2009.3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lesuisse E, Santos R, Matzanke BF, Knight SA, Camadro JM, Dancis A (2003) Iron use for haeme synthesis is under control of the yeast frataxin homologue (Yfh1). Hum Mol Genet 12: 879–889. [DOI] [PubMed] [Google Scholar]
- 26. Miao R, Martinho M, Morales JG, Kim H, Ellis EA, Lill R, et al. (2008) EPR and Mossbauer spectroscopy of intact mitochondria isolated from Yah1p-depleted Saccharomyces cerevisiae. Biochemistry 47: 9888–9899. 10.1021/bi801047q [DOI] [PubMed] [Google Scholar]
- 27. Li DS, Ohshima K, Jiralerspong S, Bojanowski MW, Pandolfo M (1999) Knock-out of the cyaY gene in Escherichia coli does not affect cellular iron content and sensitivity to oxidants. FEBS Lett 456: 13–16. [DOI] [PubMed] [Google Scholar]
- 28. Yoon H, Golla R, Lesuisse E, Pain J, Donald JE, Lyver ER, et al. (2012) Mutation in the Fe-S scaffold protein Isu bypasses frataxin deletion. Biochem J 441: 473–480. 10.1042/BJ20111637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pandey A, Gordon DM, Pain J, Stemmler TL, Dancis A, Pain D (2013) Frataxin directly stimulates mitochondrial cysteine desulfurase by exposing substrate-binding sites and a mutant Fe-S cluster scaffold protein with frataxin-bypassing ability acts similarly. J Biol Chem 288: 36773–36786. 10.1074/jbc.M113.525857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoon H, Knight SA, Pandey A, Pain J, Zhang Y, Pain D, et al. (2014) Frataxin-bypassing Isu1: characterization of the bypass activity in cells and mitochondria. Biochem J 459: 71–81. 10.1042/BJ20131273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garland SA, Hoff K, Vickery LE, Culotta VC (1999) Saccharomyces cerevisiae ISU1 and ISU2: members of a well-conserved gene family for iron-sulfur cluster assembly. J Mol Biol 294: 897–907. [DOI] [PubMed] [Google Scholar]
- 32. Andrew AJ, Song JY, Schilke B, Craig EA (2008) Posttranslational regulation of the scaffold for Fe-S cluster biogenesis, Isu. Mol Biol Cell 19: 5259–5266. 10.1091/mbc.E08-06-0622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manicki M, Majewska J, Ciesielski S, Schilke B, Blenska A, Kominek J, et al. (2014) Overlapping Binding Sites of the Frataxin Homologue Assembly Factor and the Heat Shock Protein 70 Transfer Factor on the Isu Iron-sulfur Cluster Scaffold Protein. J Biol Chem 289: 30268–30278. 10.1074/jbc.M114.596726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim JH, Tonelli M, Markley JL (2012) Disordered form of the scaffold protein IscU is the substrate for iron-sulfur cluster assembly on cysteine desulfurase. Proc Natl Acad Sci U S A 109: 454–459. 10.1073/pnas.1114372109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonomi F, Iametti S, Morleo A, Ta D, Vickery LE (2011) Facilitated transfer of IscU-[2Fe2S] clusters by chaperone-mediated ligand exchange. Biochemistry 50: 9641–9650. 10.1021/bi201123z [DOI] [PubMed] [Google Scholar]
- 36. Prischi F, Konarev PV, Iannuzzi C, Pastore C, Adinolfi S, Martin SR, et al. (2010) Structural bases for the interaction of frataxin with the central components of iron-sulphur cluster assembly. Nat Commun 1: 95 10.1038/ncomms1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bulteau AL, Dancis A, Gareil M, Montagne JJ, Camadro JM, Lesuisse E (2007) Oxidative stress and protease dysfunction in the yeast model of Friedreich ataxia. Free Radical Biol Med 42: 1561–1570. [DOI] [PubMed] [Google Scholar]
- 38. Sherman F (2005) The importance of mutation, then and now: studies with yeast cytochrome c. Mutat Res 589: 1–16. [DOI] [PubMed] [Google Scholar]
- 39. Markley JL, Kim JH, Dai Z, Bothe JR, Cai K, Frederick RO, et al. (2013) Metamorphic protein IscU alternates conformations in the course of its role as the scaffold protein for iron-sulfur cluster biosynthesis and delivery. FEBS Lett 587: 1172–1179. 10.1016/j.febslet.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shirai A, Matsuyama A, Yashiroda Y, Hashimoto A, Kawamura Y, Arai R, et al. (2008) Global analysis of gel mobility of proteins and its use in target identification. J Biol Chem 283: 10745–10752. 10.1074/jbc.M709211200 [DOI] [PubMed] [Google Scholar]
- 41. Gordon DM, Kogan M, Knight SA, Dancis A, Pain D (2001) Distinct roles for two N-terminal cleaved domains in mitochondrial import of the yeast frataxin homolog, Yfh1p. Hum Mol Genet 10: 259–269. [DOI] [PubMed] [Google Scholar]
- 42. Poor CB, Wegner SV, Li H, Dlouhy AC, Schuermann JP, Sanishvili R, et al. (2014) Molecular mechanism and structure of the Saccharomyces cerevisiae iron regulator Aft2. Proc Natl Acad Sci U S A 111: 4043–4048. 10.1073/pnas.1318869111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pais FS, Ruy Pde C, Oliveira G, Coimbra RS (2014) Assessing the efficiency of multiple sequence alignment programs. Algorithms Mol Biol 9: 4 10.1186/1748-7188-9-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van der Giezen M, Cox S, Tovar J (2004) The iron-sulfur cluster assembly genes iscS and iscU of Entamoeba histolytica were acquired by horizontal gene transfer. BMC Evol Biol 4: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Crooks DR, Jeong SY, Tong WH, Ghosh MC, Olivierre H, Haller RG, et al. (2012) Tissue specificity of a human mitochondrial disease: differentiation-enhanced mis-splicing of the Fe-S scaffold gene ISCU renders patient cells more sensitive to oxidative stress in ISCU myopathy. J Biol Chem 287: 40119–40130. 10.1074/jbc.M112.418889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li J, Kogan M, Knight SA, Pain D, Dancis A (1999) Yeast mitochondrial protein, Nfs1p, coordinately regulates iron-sulfur cluster proteins, cellular iron uptake, and iron distribution. J Biol Chem 274: 33025–33034. [DOI] [PubMed] [Google Scholar]
- 47. Dai Y, Outten FW (2012) The E. coli SufS-SufE sulfur transfer system is more resistant to oxidative stress than IscS-IscU. FEBS Lett 586: 4016–4022. 10.1016/j.febslet.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Puccio H, Anheim M, Tranchant C (2014) Pathophysiogical and therapeutic progress in Friedreich ataxia. Rev Neurol (Paris) 170: 355–365. 10.1016/j.neurol.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 49. Cotticelli MG, Rasmussen L, Kushner NL, McKellip S, Sosa MI, Manouvakhova A, et al. (2012) Primary and secondary drug screening assays for Friedreich ataxia. J Biomol Screen 17: 303–313. 10.1177/1087057111427949 [DOI] [PubMed] [Google Scholar]
- 50. Diekert K, de Kroon AI, Kispal G, Lill R (2001) Isolation and subfractionation of mitochondria from the yeast Saccharomyces cerevisiae. Methods Cell Biol 65: 37–51. [DOI] [PubMed] [Google Scholar]
- 51. Amutha B, Gordon DM, Gu Y, Lyver ER, Dancis A, Pain D (2008) GTP is required for iron-sulfur cluster biogenesis in mitochondria. J Biol Chem 283: 1362–1371. [DOI] [PubMed] [Google Scholar]
- 52. Tong WH, Rouault TA (2006) Functions of mitochondrial ISCU and cytosolic ISCU in mammalian iron-sulfur cluster biogenesis and iron homeostasis. Cell Metab 3: 199–210. [DOI] [PubMed] [Google Scholar]
- 53. Isaya G, Miklos D, Rollins RA (1994) MIP1, a new yeast gene homologous to the rat mitochondrial intermediate peptidase gene, is required for oxidative metabolism in Saccharomyces cerevisiae. Mol Cell Biol 14: 5603–5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Δyfh1 shuffle strain 70–31 strain was transformed with ISU2-containing plasmids and control plasmids, and the covering YFH1 plasmid was removed by FOA treatment. The plasmids are: 1) YCplac22-ISU2coding-Met, 2) YCplac22-ISU2coding-Ile 3) YCplac22-ISU2-Met, 4) YCplac22-ISU2-Ile, 5) YCplac22, 6), YCplac22-ISU1-Met, 7) YCplac22-ISU1-Ile, 8) YCplac22-YFH1. Frataxin-bypass was observed for ISU2-Ile and ISU2coding-Ile in which the mutated coding sequence of ISU2 was expressed from the ISU1 promoter.
(TIF)
The ISU1 coding sequence was amplified by error-prone PCR from the native sequence (M141) or from mutated sequences plasmids (Y141, H141 or F141) and co-transformed with a gapped ISU1 plasmid into the YFH1 shuffle strain. Following removal of the YFH1 plasmid, clones with improved growth were identified, and the plasmid borne ISU1 sequence was determined. The table lists amino acid changes in the ISU1 coding of the “hits” with improved growth and the corresponding nucleotide sequence of the codon at position 141 of ISU1. Among the hits, M141I was selected 6 times, M141V was selected 24 times, M141L was selected 4 times, and M141C was selected 17 times. No selected clones had the wild-type sequence, M141. As a control, 42 unselected clones were evaluated from the randomized pool starting with M141, and these all retained the M141 codon.
(XLSX)
Isu1/IscU proteins from the RefSeq non-redundant database (total number of 6064) were aligned according to the amino acid at position X of the 12 amino acid motif LPPVK LH CSX LA. In all, 21 lists were collected, one for each amino acid at position X and a no-alignment list. The lists were manually edited, retaining one entry for each species. For each entry, columns were annotated with the species name, accession number, taxonomic category, and the sequence of the 12 amino acid motif. The sequences were scored + if they contained 8 or more amino acids identical with the motif present in yeast Isu1 and 0 if they contained 7 or less identical amino acids. The sequences were scored * for exceptions if they deviated from the rule that Isu1/IscU proteins use methionine at position X and prokaryotic proteins do not use methionine at that position.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.