Abstract
Background and Aims
Hemodialysis (HD) patients are educated and counseled during the HD procedure. There are few studies assessing whether cognitive performance varies with dialysis.
Methods
Using a randomized cross-over design, 40 patients were assigned to one of two sequences: testing 1 h before dialysis followed 1 month later by testing during the first hour of dialysis (n = 21) versus testing during the first hour of dialysis followed 1 month later by 1 h before dialysis (n = 19). Cognitive tests were administered at each testing period. Mixed regression models evaluated for a dialysis effect (difference between test performance before vs. during dialysis) while adjusting for potential learning (difference between first and second tests).
Results
In models accounting for period of testing, there was no difference in test performance between 1 h before versus during the first hour of HD for all administered cognitive tests (p > 0.05). A learning effect was detected between first and second test administration in two tests, specifically, the Word List Learning and the Digit Symbol Substitution Test.
Conclusions
We found no difference in cognitive performance depending on the time of testing, suggesting that cognitive tests performed during the first hour of dialysis are a valid assessment of cognitive performance.
Keywords: Cognition, Hemodialysis, Randomized cross-over trial
Introduction
Cognitive impairment is common in hemodialysis (HD) patients [1–3] and is associated with lower quality of life, decreased patient compliance and increased mortality [4–6] . Reflecting the complex medical issues associated with kidney failure, there are multiple factors contributing to cognitive impairment in this population [4] . In addition to factors such as cerebrovascular disease [7, 8] and an aging dialysis population [9] , both uremia and the HD procedure itself [10, 11] have been suggested as potential causes of poor cognitive function [12] .
Despite decades of experience with HD, it remains unknown how and whether the HD procedure affects the brain [13] . Critically, as the majority of teaching and shared decision-making between dialysis patients and providers occurs during the dialysis procedure, little research has explored the effect of HD on cognitive performance. Attempting to address this question, one prior study showed a decline in cognitive function during HD compared to other testing times but was limited by small size and incomplete follow-up testing [14] . Accordingly, using a randomized, cross-over design in 40 prevalent HD patients, we assessed whether there are differences in cognitive function that are temporally related to the HD procedure.
Methods
Study Design
A randomized cross-over design was utilized to allow for the assessment of differences in cognitive performance with participants acting as his/her own control, addressing potential imbalances between the two randomized groups. Participants were block randomized via sealed envelopes into one of two arms (A-B): testing in the hour before dialysis initiation (A) followed by testing 1 month later during the first hour of dialysis (B), or (B-A): testing during the first hour of dialysis followed 1 month later by testing in the hour before dialysis initiation. A 1-month washout period was chosen so as to attenuate the learning effect from repeated testing while avoiding the potential for cognitive decline due to medical reasons and participant dropout that would occur over a longer time period. The first hour of dialysis was chosen to allow for completion of the full battery of tests; preliminary results indicated that fatigue limited participants from completing the full battery when tested during later in the dialysis procedure.
Participant Selection
Patients receiving chronic in-center HD at five Dialysis Clinic Inc. (DCI) units and one hospital-based unit (St. Elizabeth’s Medical Center) in the greater Boston area were evaluated using previously described criteria for entry into the Cognition and Dialysis Study; these included age >18 years, no non-access-related hospitalizations within 1 month, receipt of HD therapy for longer than 1 month, and single-pool Kt/V >1.0 [7] . To allow for accurate assessment with the cognitive battery, other eligibility criteria included English fluency as well as sufficient visual and hearing acuity. To minimize cognitive testing floor effects and reflecting inability to provide consent, individuals with Mini-Mental State Examination [15] (MMSE) ≤ 10 and/or advanced dementia based on medical record review were not eligible. The Tufts Medical Center/Tufts University Institutional Review Board approved the study and all participants who underwent cognitive testing signed informed consent. The clinical and research activities being reported are consistent with the Declaration of Helsinki.
Demographic, clinical and laboratory factors were ascertained at the time of first cognitive testing. Education (<12th grade, high school graduate, and ≥ 2 years of college) was obtained via patient questionnaire. Demographic data and medical history were determined by patient history or documentation in the medical chart. Cause of ESRD and dialysis vintage were obtained from the DCI or St. Elizabeth’s electronic record as were the mean monthly systolic and diastolic blood pressures, body mass index (BMI), predialysis blood tests including hematocrit, phosphorus, intact parathyroid hormone, albumin, and single-pool Kt/V. All DCI laboratory tests were measured in a central laboratory in Nashville, Tenn.
Outcomes
Participants were administered a battery of cognitive tests by research assistants following training and direct observation by the study neuropsychologist (T.M.S.). All tests were administered in the same location within each HD unit with effort made to limit noise and interruptions, with individual tests administered in the same order, regardless of testing period (before vs. during). The neuropsychological battery included well-validated commonly used cognitive tests that possess high inter- and intra-rater reliability, with many having established age, sex, and/or educationmatched normative scores. Tests included the MMSE [15] , the North American Adult Reading Test (NAART) [16] , the Wechsler Memory Scale-III (WMS-III) Word List Learning Subtest [17] , the Wechsler Adult Intelligence Scale-III (WAIS-III) Block Design and Digit Symbol-Coding Subtests [17] , and Trail Making Tests A and B [18] , Mental Alternations [19] and the Controlled Oral Word Association Test (COWAT) [20] . The overall battery assesses a broad range of functioning including global ability, supraspan learning, auditory retention, visual retention, attention/mental processing speed, visual construction/fluid reasoning, and motor speed.
Statistical Analysis
Primary analyses were done using a mixed model for each cognitive score of the form:
where SEQi is the sequence or the randomized group assigned (A-B or B-A), PERIODk is the kth testing period (first or second) and TIME is whether the testing was done prior to (A) or during (B) dialysis. Sequence, period and time are fixed effects, while subject is a random effect which accounts for correlation in test scores for each individual participant. The primary study question of a dialysis effect, representing the difference in cognitive scores between the two testing times (A and B), was tested with the TIME term, while the PERIOD effect captures potential learning (if performances on second tests are different than first tests). Demographic and clinical characteristics for study participants were reported as means with standard deviations (SDs) for continuous variables or percentages for categorical variables and compared using t tests, Wilcoxon signed rank tests and χ2 tests as appropriate between the two randomized groups. Pre-study power calculations were based on a sample size of 40 patients. We conservatively assumed a correlation of 0.5 between results of cognitive testing performed prior to HD and during HD, which allows an 80% power to detect a difference of 0.45 SDs between testing periods. For a SD of 2.9 for the MMSE, as observed in our baseline data, there is 80% power to detect a difference of 1.3. Similarly, using a SD of 85 for the Trails B, we calculated 80% power to detect a difference of 38. These power calculations are identical for testing the hypothesis regarding ‘learning’ between the first and second time periods. All analyses were performed using SAS, version 9.3 (SAS Institute, Cary, N.C., USA). Differences were considered statistically significant at a p value <0.05.
Results
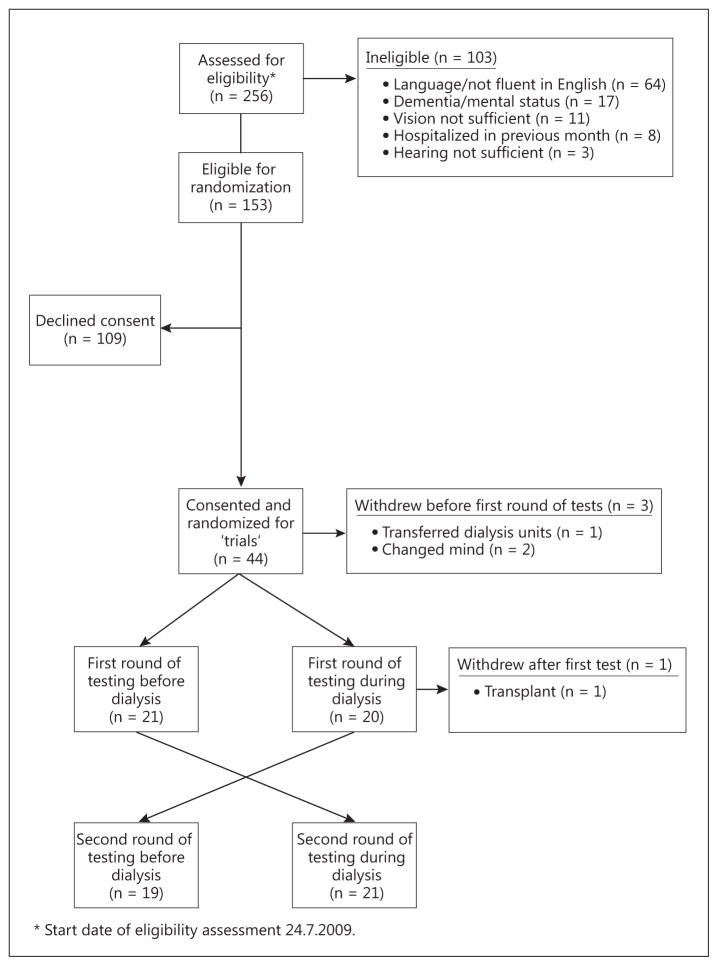
We assessed 256 patients for eligibility; 103 were deemed ineligible for study enrollment, with lack of English fluency (n = 64), dementia (n = 17), and insufficient vision (n = 11) the most common criteria (fig. 1). Of those eligible for randomization (n = 153), 109 declined consent for entry into the study. Compared to those who consented, the patients who declined consent were significantly older (67 vs. 59 years) and were more likely to have diabetes as a cause of ESRD (23 vs. 10%), but were otherwise similar with regard to sex, race, and dialysis vintage.
Fig. 1.
Participant flow diagram.
Of the remaining 44 patients, 3 withdrew prior to start of testing, leaving 41 study participants that were randomized, with 21 assigned to arm A-B (before followed by during dialysis) and 20 to arm B-A (during followed by before dialysis) (fig. 1). One subject underwent kidney transplantation after the first set of testing and did not complete a second round of testing. Of the 40 patients with two full sets of tests, the mean age was 59 years; 40% were women, 23% African-American, and 56% had some college education ( table 1 ). Arm A-B had fewer patients with a history of diabetes, higher education status, and lower BMI, but was otherwise similar to arm B-A for other patient characteristics. Day of the week of testing (Monday/Tuesday vs. other) was randomly distributed between the two arms for each testing period (p > 0.2 for both).
Table 1.
Study participant demographics and clinical characteristics
| Total (n = 40) | Before→during dialysis (n = 21) | During→before dialysis (n = 19) | p value | |
|---|---|---|---|---|
| Age, years | 58.9 ± 17.1 | 58.1 ± 18.0 | 59.8 ± 16.4 | 0.8 |
| Female | 40 | 29 | 53 | 0.1 |
| Black | 23 | 24 | 21 | 0.8 |
| Education | ||||
| <12th grade | 10 | 5 | 16 | 0.06 |
| High school graduate | 33 | 20 | 47 | |
| >2 years at college | 56 | 75 | 37 | |
| Stroke | 5 | 5 | 5 | 0.9 |
| Peripheral vascular disease | 8 | 10 | 5 | 0.6 |
| Hypertension | 93 | 91 | 95 | 0.6 |
| Diabetes | 38 | 19 | 58 | 0.01 |
| Heart failure | 28 | 19 | 37 | 0.2 |
| Coronary artery disease | 28 | 29 | 26 | 0.9 |
| Primary cause of ESRD | ||||
| Diabetes | 10 | 5 | 16 | 0.7 |
| Hypertension | 10 | 10 | 11 | |
| Other | 10 | 14 | 5 | |
| Unknown | 45 | 43 | 47 | |
| Glomerulonephritis | 25 | 29 | 21 | |
| Smoking history | ||||
| Never | 40 | 30 | 50 | 0.4 |
| Past | 55 | 65 | 44 | |
| Current | 5 | 5 | 6 | |
| Kt/V | 1.51 ± 0.23 | 1.55 ± 0.24 | 1.47 ± 0.22 | 0.3 |
| Hematocrit, % | 35.2 ± 4.7 | 35.7 ± 3.1 | 34.6 ± 6.1 | 0.5 |
| Phosphorus, mg/dl | 5.8 ± 1.6 | 5.9 ± 1.5 | 5.8 ± 1.7 | 0.9 |
| Parathyroid hormone, pg/ml | 295 (214, 487) | 355 (252, 602) | 260 (212, 372) | 0.2 |
| Albumin, g/dl | 3.8 ± 0.3 | 3.8 ± 0.3 | 3.8 ± 0.3 | 0.9 |
| Dialysis vintage, months | 18 (5, 36) | 17 (5, 34) | 18 (7, 52) | 0.7 |
| Body mass index | 27.8 ± 6.4 | 25.6 ± 4.1 | 30.2 ± 7.7 | 0.02 |
| First test on Monday/Tuesday | 43 | 48 | 37 | 0.2 |
Summary statistic presented as mean (SD), median (25th, 75th) or percentage of total.
In unadjusted analyses assessing whether the timing of testing affected performance (a dialysis effect), there was no difference in test scores between the testing before HD group and the testing during HD group ( table 2 ; all p values >0.05). A learning effect was seen in unadjusted analyses from the first period to the second period for the WAIS-III Word List Learning Test, specifically the Total Recall and Delayed Recall subscores [first period mean score (SD) vs. second period mean score (SD) of 24.6 (6.8) vs. 27.5 (7.7) and 4.5 (2.5) vs. 5.4 (3.1), respectively] ( table 2 ). A learning effect was also seen for the Digit Symbol Substitution Test, with first versus second period scores of 45.2 (17.4) versus 48.4 (19.8) ( table 2 ).
Table 2.
Unadjusted participant performance on neurocognitive tests
| Test | First test (n = 40) | Second test (n = 40) | p value | Before HD (n = 40) | During HD (n = 40) | p value |
|---|---|---|---|---|---|---|
| MMSE | 26.7 ± 2.6 | 27.0 ± 2.6 | 0.4 | 26.9 ± 2.6 | 26.8 ± 2.6 | 0.8 |
| Verbal IQ (NAART) | 102.8 ± 14.2 | 102.9 ± 13.6 | 0.8 | 102.5 ± 14.3 | 103.2 ± 13.4 | 0.2 |
| Word List Learning | ||||||
| Delayed Recall | 4.5 ± 2.5 | 5.4 ± 3.1 | 0.003 | 5.0 ± 2.9 | 4.9 ± 2.8 | 0.8 |
| Total Recall | 24.6 ± 6.8 | 27.5 ± 7.7 | <0.001 | 25.9 ± 7.3 | 26.2 ± 7.5 | 0.7 |
| Recognition | 20.5 ± 2.8 | 20.6 ± 3.0 | 0.8 | 20.8 ± 2.9 | 20.4 ± 2.8 | 0.4 |
| Block Design | 26.7 ± 12.0 | 28.0 ± 10.5 | 0.07 | 27.2 ± 11.8 | 27.6 ± 10.7 | 0.9 |
| Digit Span | ||||||
| Digits Forward | 10.5 ± 2.2 | 10.2 ± 2.3 | 0.3 | 10.2 ± 2.2 | 10.4 ± 2.4 | 0.4 |
| Digits Backward | 5.7 ± 2.1 | 5.7 ± 2.0 | 0.9 | 5.8 ± 1.9 | 5.6 ± 2.2 | 0.7 |
| Digit Symbol Substitution | 45.2 ± 17.4 | 48.4 ± 19.8 | 0.02 | 47.6 ± 17.4 | 45.9 ± 19.9 | 0.2 |
| Trail Making Tests | ||||||
| Trails A, s | 58.9 ± 40.8 | 56.6 ± 47.9 | 0.6 | 57.2 ± 40.8 | 58.3 ± 48.0 | 0.8 |
| Trails B, s | 138.1 ± 82.9 | 136.8 ± 83.7 | 0.9 | 132.6 ± 83.8 | 142.3 ± 82.5 | 0.3 |
| Mental Alternation | 22.2 ± 9.3 | 22.5 ± 8.8 | 0.6 | 22.7 ± 8.0 | 22.0 ± 9.9 | 0.4 |
| COWAT | ||||||
| Animals | 16.9 ± 5.4 | 17.8 ± 6.4 | 0.3 | 17.5 ± 5.3 | 17.1 ± 6.6 | 0.6 |
| Supermarket Items | 22.9 ± 6.6 | 22.2 ± 7.9 | 0.6 | 23.0 ± 6.5 | 22.1 ± 7.9 | 0.4 |
All scores are the number correct, with higher values indicating better performance, except for the Trail Making Tests, where higher time to complete identifies worse performance. Scores are for the specified groups (each participant appears twice) and are not adjusted for the timing or period of testing.
Mixed models assessing for a dialysis effect (primary study question, before vs. during), and adjusting for period (learning, first vs. second test regardless of before or during dialysis) and sequence (A-B vs. B-A) demonstrated no difference in tests conducted before versus during dialysis sessions ( table 3 ). Using the same mixed models, a learning effect was again seen, with a difference (95% CI) of 2.9 (1.5, 4.3) and 0.9 (0.3, 1.5) for the Total Recall and Delayed Recall subtests, as well as a difference of 3.3 (0.7, 5.8) for the Digit Symbol Substitution Test ( table 3 ).
Table 3.
Results of mixed regression models assessing timing (difference between test performance before vs. during dialysis) and learning (difference between 1st and 2nd tests)
| Test | Timing β (95% CI) (dialysis effect) | p value | Period β (95% CI) (learning effect) | p value |
|---|---|---|---|---|
| MMSE | 0.09 (−0.62, 0.80) | 0.8 | −0.28 (−0.99, 0.43) | 0.4 |
| Verbal IQ (NAART) | −0.66 (−1.75, 0.43) | 0.2 | −0.15 (−1.23, 0.94) | 0.8 |
| Word List Learning | ||||
| Delayed Recall | 0.15 (−0.43, 0.72) | 0.6 | −0.91 (−1.48, −0.34) | 0.003 |
| Total Recall | −0.23 (−1.60, 1.14) | 0.7 | −2.91 (−4.29, −1.54) | <0.001 |
| Recognition | 0.41 (−0.46, 1.27) | 0.4 | −0.12 (−0.98, 0.74) | 0.8 |
| Blocks Design | 0.03 (−1.75, 1.80) | 0.9 | −1.60 (−3.38, 0.18) | 0.08 |
| Digit Span | ||||
| Digits Forward | −0.24 (−0.77, 0.29) | 0.4 | 0.29 (−0.24, 0.81) | 0.3 |
| Digits Backward | 0.11 (−0.48, 0.70) | 0.7 | 0.03 (−0.56, 0.62) | 0.9 |
| Digit Symbol Substitution | 1.89 (−0.67, 4.45) | 0.14 | −3.27 (−5.83, −0.71) | 0.01 |
| Trail Making Tests | ||||
| Trails A | −1.19 (−8.89, 6.50) | 0.8 | 2.38 (−5.31, 10.08) | 0.5 |
| Trails B | −9.77 (−27.91, 8.38) | 0.3 | 1.81 (−16.33, 19.96) | 0.8 |
| Mental Alternation | 0.80 (−0.96, 2.55) | 0.4 | −0.46 (−2.22, 1.29) | 0.6 |
| COWAT | ||||
| Animals | 0.47 (−1.26, 2.20) | 0.6 | −0.90 (−2.63, 0.83) | 0.3 |
| Supermarket Items | 0.92 (−1.38, 3.22) | 0.4 | 0.65 (−1.64, 2.95) | 0.6 |
Period (learning effect) = difference in scores for first time taking test and second time taking test; dialysis effect = difference in scores for testing before and during first hour of dialysis. For all tests, except the Trail Making Tests, a negative β coefficient is consistent with learning, while for the Trail Making Tests a positive β coefficient indicates learning. Similarly, for all tests except the Trail Making Tests, a positive timing β coefficient suggests before dialysis performance is superior to during dialysis, while for the Trail Making Tests, a negative timing β coefficient suggests before dialysis performance is superior to during dialysis.
Discussion
In this study, we found no evidence of lower cognitive performance associated with testing during the first hour of dialysis compared to during the hour prior to dialysis initiation. This result was consistent across a broad array of cognitive tests that test multiple cognitive domains. Although a learning effect was observed in two of the eight tests, including one test focused on memory and a second that incorporates working memory, this effect was adjusted for in the primary analyses evaluating timing of testing. These results suggest that testing early in the course of a HD treatment may be an accurate proxy for testing while not in the HD unit. This finding has multiple important implications. First, it provides evidence that it is reasonable to provide patient education and to initiate shared decision-making during this period, assuming a particular patient’s baseline cognitive function allows for successful education. Second, for research purposes, it confirms the validity of performing cognitive testing during a dialysis procedure. This is relevant as prior studies detailing cognitive function in dialysis patients were conducted during the dialysis procedure [7, 14] .
Two prior studies have addressed similar questions regarding the timing of cognitive testing. Murray et al. [14] performed cognitive testing in 33 HD patients at four different time periods: 1 h before, during (45–90 min from start), 1 h after, and 24–30 h after. Contrary to our findings, they noted a modest decrease in cognitive performance during dialysis sessions compared to 1 h before, 1 h after, and 24 h after. However, many subjects (15 of the total 33 who were recruited) did not complete all testing at all four time periods, potentially affecting the study findings. Additionally, testing during the dialysis procedure was done 45–90 min from the start of HD, while our study conducted testing shortly after dialysis initiation. Williams et al. [21] also explored temporal variation in cognitive performance among 20 HD patients. In contrast to our study, this study did not randomly vary the timing of testing and did not test cognitive performance during HD, instead administering tests 1 h after dialysis, as well as 24 and 67 h after dialysis. They found that cognitive function was worst at 67 h after dialysis, compared to 1 and 24 h after dialysis. Notably, testing was limited to three tests addressing attention, memory, and intelligence, and findings were not consistent across all three of these tests.
There are several possible explanations for our results. Although it is well known that HD patients have high rates of cognitive impairment, HD-induced cognitive impairment may be mediated only through chronic and repeated dialysis sessions. The effect may occur through repeated episodes of hypotension [12] , microbleeds in the brain associated with anticoagulant use [22] , or chronic dialysis disequilibrium [23] . Moreover, it has become increasingly evident that cognitive impairment in HD patients is partly and perhaps predominantly due to cerebrovascular disease, a process which, although at times acute and catastrophic, is also chronic and subtle [24] , and therefore less likely to fluctuate in the setting of a single HD session.
We acknowledge that our study findings may be limited by our modest sample size, which may hinder the ability to detect small differences in cognitive performance between testing times. Attempting to partially address this limitation, our choice of a cross-over study design was in part made to increase the statistical power. Another potential limitation of our study is the ‘washout’ period used between testing. It is possible that this 1- month interval was either too short, allowing for excess learning, or too long, allowing for cognitive decline. Given the poor health status of many dialysis patients, a longer interval between testing days also increases the possibility of hospitalization or major illness, both of which could affect cognitive performance. In this context, we felt a 1-month interval was most appropriate, despite a higher likelihood for learning to occur. However, a significant learning effect was detected in only two tests, and the mixed model statistical analysis was designed to account and adjust for the possibility of learning between testing periods. A further limitation is that our patient sample was younger, had less comorbidity, and was, on average, more educated than the average US HD patient, which may limit the generalizability of our results. Each of these differences would predispose towards better baseline cognitive function and thus could potentially make our participants less susceptible to cognitive decline associated with the HD procedure. In addition, this potential bias could imply that we did not have enough power to detect a difference in testing times. However, this difference would most likely be modest, making it less clinically meaningful. It is also important to note that even this younger population has a high prevalence of cognitive impairment when compared with normative data [25] . Similarly, as shown by Murray et al. [3] among participants age 55–64 years, only 26% had either normal cognitive function or only mild cognitive impairment, while 45% had moderate cognitive impairment and 29% had severe cognitive impairment. Given that our mean age falls within this group and that the data from Murray et al. suggest an impressive burden of cognitive impairment in even this younger group, we do not view the inclusion of younger patients overall as a major limitation. Finally, our study does not include testing on a non-HD day, which with potentially fewer uremic symptoms and a lack of post-dialysis fatigue could be argued to provide a better comparison to testing during HD. Our choice of testing times was based on the practical aspect of testing during times in which a patient is likely to have interaction with a medical provider.
Our study has several strengths. First, the cognitive battery used in this study is comprehensive, incorporating multiple previously validated cognitive tests. Thus the null findings across all cognitive tests provide additional validity to the results. Second, our study uses a randomized cross-over design, allowing each subject to serve as his/her own control which may reduce the effect of confounding created by imbalances between randomized groups. Finally, despite a modest sample size, our study is the largest and most complete to date assessing whether there is difference in cognitive performance depending on timing of testing.
In conclusion, we did not detect a difference in cognitive performance if patients were tested before or during the first hour of dialysis. We recognize these findings are not definitive given the modest size, the select sample, and variability in the day of testing, but these results are relatively reassuring as a significant percentage of provider- patient interaction occurs during the dialysis treatment. It is important to note that as we did not perform testing later in dialysis sessions, we are unable to comment as to whether these findings apply to the entire dialysis session. Future studies should explore the risk factors for cognitive impairment and attempt to identify interventions which may improve cognitive function in dialysis patients.
Acknowledgments
The study was funded through grants R21 DK068310 (M.J.S.), K23 DK71636 (D.E.W.), a Carl Gottschalk Career Development Grant from the American Society of Nephrology (D.E.W.) and R01 DK078204 (M.J.S.). We would like to acknowledge the tremendous assistance of Dialysis Clinic, Inc. and, in particular, the staff and patients at the five DCI units in the Boston area and St. Elizabeth’s Dialysis unit, whose generous cooperation made this study possible.
Footnotes
Disclosure Statement
The authors have conflicts of interest to disclose.
The results of the present study were presented in abstract form at the American Society of Nephrology Annual Meeting in San Diego, Calif., 2012.
References
- 1.Buchman AS, Tanne D, Boyle PA, Shah RC, Leurgans SE, Bennett DA. Kidney function is associated with the rate of cognitive decline in the elderly. Neurology. 2009;73:920–927. doi: 10.1212/WNL.0b013e3181b72629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elias MF, Elias PK, Seliger SL, Narsipur SS, Dore GA, Robbins MA. Chronic kidney disease, creatinine and cognitive functioning. Nephrol Dial Transplant. 2009;24:2446–2452. doi: 10.1093/ndt/gfp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, Smith GE, Hochhalter AK, Collins AJ, Kane RL. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67:216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 4.Kurella M, Mapes DL, Port FK, Chertow GM. Correlates and outcomes of dementia among dialysis patients: The Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2006;21:2543–2548. doi: 10.1093/ndt/gfl275. [DOI] [PubMed] [Google Scholar]
- 5.Gokal R. Quality of life in patients undergoing renal replacement therapy. Kidney Int Suppl. 1993;40:S23–S27. [PubMed] [Google Scholar]
- 6.Griva K, Stygall J, Hankins M, Davenport A, Harrison M, Newman SP. Cognitive impairment and 7-year mortality in dialysis patients. Am J Kidney Dis. 2010;56:693–703. doi: 10.1053/j.ajkd.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Weiner DE, Scott TM, Giang LM, Agganis BT, Sorensen EP, Tighiouart H, Sarnak MJ. Cardiovascular disease and cognitive function in maintenance hemodialysis patients. Am J Kidney Dis. 2011;58:773–781. doi: 10.1053/j.ajkd.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vupputuri S, Shoham DA, Hogan SL, Kshirsagar AV. Microalbuminuria, peripheral artery disease, and cognitive function. Kidney Int. 2007;73:341–346. doi: 10.1038/sj.ki.5002672. [DOI] [PubMed] [Google Scholar]
- 9.Murray AM. Cognitive impairment in the aging dialysis and chronic kidney disease populations: an occult burden. Adv Chronic Kidney Dis. 2008;15:123–132. doi: 10.1053/j.ackd.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishida I, Hirakata H, Sugimori H, Omae T, Hirakata E, Ibayashi S, Kubo M, Fujishima M. Hemodialysis causes severe orthostatic reduction in cerebral blood flow velocity in diabetic patients. Am J Kidney Dis. 1999;34:1096–1104. doi: 10.1016/s0272-6386(99)70016-8. [DOI] [PubMed] [Google Scholar]
- 11.Stefanidis I, Bach R, Mertens PR, Liakopoulos V, Liapi G, Mann H, Heintz B. Influence of hemodialysis on the mean blood flow velocity in the middle cerebral artery. Clin Nephrol. 2005;64:129–137. doi: 10.5414/cnp64129. [DOI] [PubMed] [Google Scholar]
- 12.Mizumasa T, Hirakata H, Yoshimitsu T, Hirakata E, Kubo M, Kashiwagi M, Tanaka H, Kanai H, Fujimi S, Iida M. Dialysis-related hypotension as a cause of progressive frontal lobe atrophy in chronic hemodialysis patients: a 3-year prospective study. Nephron Clin Pract. 2004;97:c23–c30. doi: 10.1159/000077592. [DOI] [PubMed] [Google Scholar]
- 13.Madero M, Sarnak MJ. Does hemodialysis hurt the brain? Semin Dial. 2011;24:266–268. doi: 10.1111/j.1525-139X.2011.00857.x. [DOI] [PubMed] [Google Scholar]
- 14.Murray AM, Pederson SL, Tupper DE, Hochhalter AK, Miller WA, Li Q, Zaun D, Collins AJ, Kane R, Foley RN. Acute variation in cognitive function in hemodialysis patients: a cohort study with repeated measures. Am J Kidney Dis. 2007;50:270–278. doi: 10.1053/j.ajkd.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. ‘Minimental state’ A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Uttl B. North American Adult Reading Test: age norms, reliability, and validity. J Clin Exp Neuropsychol. 2002;24:1123–1137. doi: 10.1076/jcen.24.8.1123.8375. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. (WAIS-III) and Wechsler Memory Scale (WMS-III): Technical Manual. 3. San Antonio: Psychological Corporation; 2002. Wechsler Adult Intelligence Scale. [Google Scholar]
- 18.Heaton RK, Grant I, Matthews CG. Comprehensive Norms for an Expanded Halstead- Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Odessa/FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 19.Teng E. The Mental Alternations Test (MAT) Clin Neuropsychol. 1995;9 [Google Scholar]
- 20.Benton AL, Hamsher KS. Multilingual Aphasia Examination: Manual of Instructions. Iowa City: Department of Neurology and Psychology, University of Iowa; 1983. [Google Scholar]
- 21.Williams MA, Sklar AH, Burright RG, Donovick PJ. Temporal effects of dialysis on cognitive functioning in patients with ESRD. Am J Kidney Dis. 2004;43:705–711. doi: 10.1053/j.ajkd.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe A. Cerebral microbleeds and intracerebral hemorrhages in patients on maintenance hemodialysis. J Stroke Cerebrovasc Dis. 2007;16:30–33. doi: 10.1016/j.jstrokecerebrovasdis.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Arieff AI. Dialysis disequilibrium syndrome: current concepts on pathogenesis and prevention. Kidney Int. 1994;45:629–635. doi: 10.1038/ki.1994.84. [DOI] [PubMed] [Google Scholar]
- 24.Drew DA, Bhadelia R, Tighiouart H, et al. Anatomic brain disease in hemodialysis patients: a cross-sectional study. Am J Kidney Dis. 2013;61:271–278. doi: 10.1053/j.ajkd.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarnak MJ, Tighiouart H, Scott TM, et al. Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology. 2013;80:471–480. doi: 10.1212/WNL.0b013e31827f0f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]