Abstract
We report here for the first time the rapid parallel production of bioactive folded cyclotides by using Fmoc-based solid-phase peptide synthesis in combination with a tea-bag approach. Using this approach we efficiently synthesized 15 different analogs of the CXCR4 antagonist cyclotide MCo-CVX-5c. Cyclotides were cyclized using a single-pot cyclization/folding reaction in the presence of reduced glutathione. Natively folded cyclotides were quickly purified from the cyclization/folding crude by activated thiol sepharose-based chromatography. The different folded cyclotide analogs were finally tested for their ability to inhibit the CXCR4 receptor in a cell-based assay. These results indicate that this approach can be used for the efficient chemical synthesis of cyclotide-based libraries that can be easily interfaced with solution or cell-based assays for the rapid screening of novel cyclotides with improved biological properties.
Keywords: Protein design, protein engineering, protein-protein interactions, cyclotides, CXCR4
INTRODUCTION
Cyclotides are small globular microproteins (ranging from 28 to 37 residues) that posses a unique head-to-tail cyclized backbone, which is stabilized by three disulfide bonds forming a cystine-knot motif [1, 2] (Fig. 1). The cyclic cystine-knot (CCK) molecular scaffold provides a very rigid molecular platform [3–5] that confers an exceptional stability towards physical, chemical and biological degradation [1, 2]. Cyclotides can be considered natural combinatorial peptide libraries structurally constrained by the cystine-knot scaffold and head-to-tail cyclization, but in which hypermutation of essentially all residues is permitted with the exception of the strictly conserved cysteines that comprise the knot [6–8]. In addition, naturally-occurring cyclotides have shown to posses various pharmacologically-relevant activities [1, 9]. Cyclotides have been also engineered to target extracellular [10–12] and intracellular [13] molecular targets in animal models. Some of these novel cyclotides have been shown to be orally bioavailable [11] and able to efficiently cross cellular-membranes [14–16]. Altogether, these features make the cyclotide scaffold an excellent molecular framework for the design of novel peptide-based therapeutics [2, 17].
Figure 1.
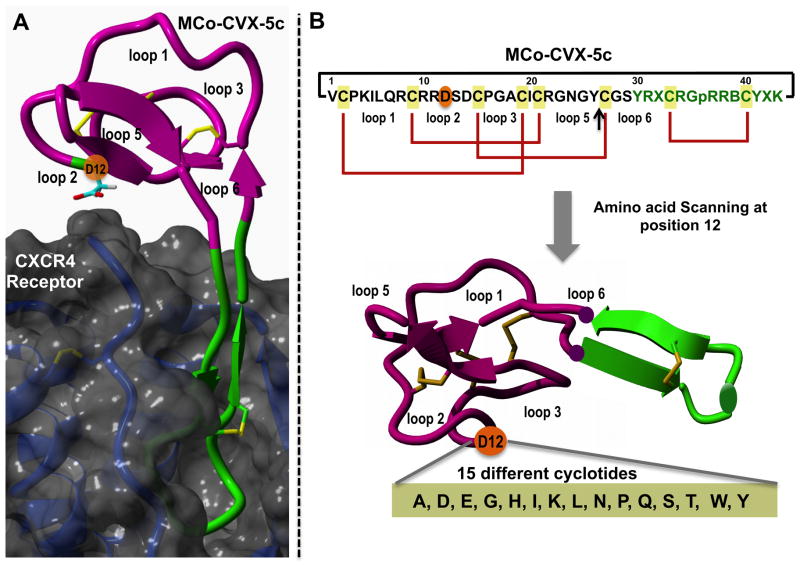
Design of the amino acid scanning library for the position 12 in loop 2 of cyclotide MCo-CVX-5c (WT). A. Structural model of the molecular complex between the cyclotide MCo-CVX-5c (WT) and the CXCR4 receptor. As shown in the model, the residue Asp12 located in loop 2 is in close proximity to the extracellular surface of the receptor CXCR4. B. Sequence and disulfide connectivities (red) of cyclotide MCo-CVX-5c and all their analogs. A black line is used to indicate the backbone cyclization between the a-NH2 and the a-carboxylate groups of residues 1 and 43, respectively. The cyclization site is indicated with an arrow. Single letter codes B, X, and p represent the amino acids 2-naphthylalanine, citruline, and D-proline, respectively. Molecular graphics were built with Yasara (www.yasara.org).
Naturally-occurring cyclotides are ribosomally produced in plants from precursors that comprise between one and three cyclotide domains [18–21]. However, the mechanism of excision of the cyclotide domains and ligation of the free N- and C-termini to produce the circular peptides has not yet been completely elucidated although it has been speculated that asparaginyl endopeptidases are involved in the cyclization process [22–24]. Cyclotides can be also produced recombinantly using standard microbial expression systems by making use of modified protein splicing units [25–28] allowing for the first time the production of biologically-generated libraries of these microproteins [26].
The relative small size of cyclotides makes it possible also to employ chemical tools for the generation of synthetic combinatorial libraries based on this scaffold for the screening and selection of optimized sequences for a particular biological activity. Chemical libraries present some advantages over biologically produced libraries, for example chemical libraries are not constrained to natural amino acids and can include both unnatural and D-amino acids, in addition to secondary structures not tolerated by the ribosome. As unnatural and D-amino acids are less susceptible to proteases and peptidases than natural L-amino acids, chemical libraries have the potential to rapidly identify stable and bioactive peptide sequences. In addition, chemical libraries also allow the incorporation of post-translational modifications, such as glycosylation and phosphorylation, which are not accessible in bacterial expression systems.
The chemical synthesis of several naturally-occurring and engineered cyclotides has been already accomplished by solid-phase peptide synthesis using either Boc- [29, 30] or Fmoc-based [10, 13, 14, 31–33] chemistry. All of them use an intramolecular native chemical ligation (NCL) [34, 35] to accomplish backbone cyclization followed by oxidative folding to produce the natively folded cyclotide. Our group has recently reported that the cyclization and oxidative folding reactions can be efficiently performed in a one-pot reaction when carried out in aqueous phosphate buffer at pH 7.2 in the presence of reduced glutathione (GSH) [10, 13, 14]. Under these conditions the production of folded cyclotides can be accomplished very efficiently in one step from the corresponding linear precursor with minimal purification [10, 13, 14]. This significantly minimizes the steps required for the production of cyclotides, which is key for the production of large libraries. This approach has been also recently used for the rapid and efficient production of other disulfide-rich backbone cyclized polypeptides such as the cyclic defensing RTD-1 [36].
The production of combinatorial peptide-based libraries requires the parallel synthesis of multiple peptides simultaneously. The ‘tea-bag’ approach was one the first approaches developed for the generation of combinatorial peptide libraries [37]. This approach is well suited for the production of medium-sized libraries (≈3 × 102) to perform antigenic mapping [38], and to produce amino acid scanning [39, 40] and positional scanning libraries [41]. This approach, however, has been mostly used for the production of small linear peptides (10–15 residues) and it has never been explored for the production of libraries of large complex folded polypeptides such as cyclotides.
In this work we report for the first time the rapid parallel synthesis of a small library of folded bioactive cyclotides using a tea-bag approach in combination with optimized cyclization/folding protocols that require minimal purification steps. As model system we used the cyclotide MCo-CVX-5c (Fig. 1), a potent antagonist of the CXCR4 reporter [10]. Using this approach we were able to rapidly produce 15 cyclotides based on MCo-CVX-5c that were tested to rapidly evaluate structural activity relationships.
RESULTS AND DISCUSION
Library design
We used the cyclotide MCo-CVX-5c (Fig. 1) as a model to evaluate the parallel synthesis of cyclotides using a tea-bag approach. This cyclotide was engineered to inhibit the CXCR4 receptor by grafting a topologically modified CVX15-based peptide onto the loop 6 of cyclotide MCoTI-I (Fig. 1) [10]. This cyclotide has been shown to be a potent CXCR4 antagonist and an efficient HIV-1 cell-entry blocker [10]. In order to improve the activity of this cyclotide we decide to explore potential mutations located in the neighboring loops to the loop used for the grafting of the bioactive peptide CVX15 that could stabilize the interaction between the cyclotide and the receptor. To accomplish this task we first built a model of cyclotide MCo-CVX-5c bound to the CXCR4 receptor (Fig. 1A). This was accomplished as previously described [10] by using the solution structure of cyclotide MCoTI-II (PDB: 1IB9) [42] and the crystal structure of the peptide CVX15 bound to the CXCR4 receptor (PDB: 3OE0) [43] as molecular templates. According to this model, residue 12 in loop 2 is in close proximity to the CXCR4 extracellular surface receptor. Accordingly, to explore the effect of this residue on the biological activity of MCo-CVX-5c we decided to make a small library of cyclotides based on MCo-CVX-5c where the native Asp residue in position was mutated to different residues containing different chemical functionalities (Fig. 1B). The set of amino acids that were tested (Figs. 1B and C) include negative (Glu) and positive (Lys, His) charged, aliphatic (Ala, Ile, Leu) and aromatic (Tyr, Trp), hydrophilic (Asn, Gln, Ser, Thr), N-alkylated (Pro) and small (Gly) residues.
Library synthesis
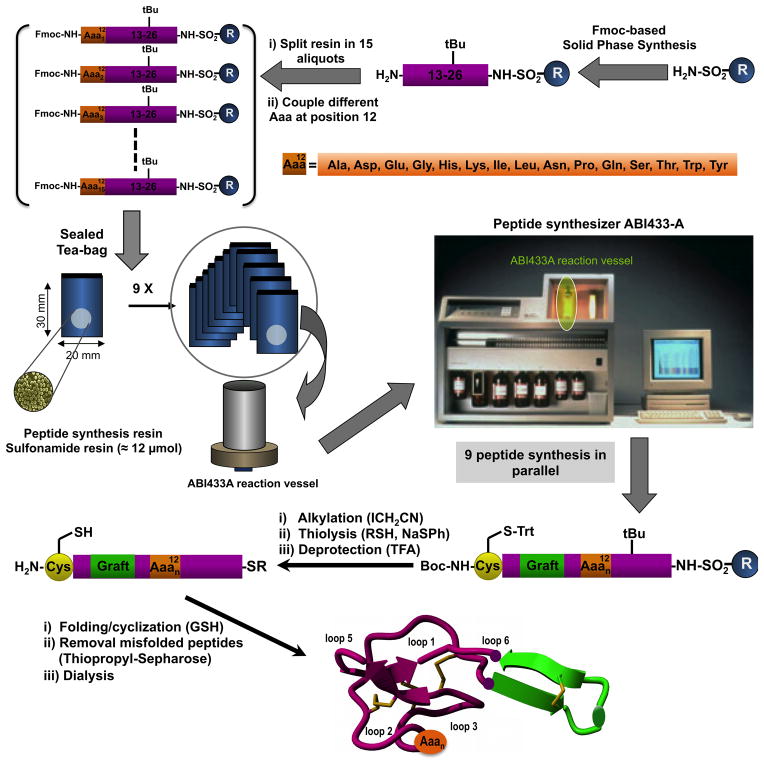
All 15 cyclotides (including the control cyclotide MCo-CVX-5c, called WT from here on) (Table 1) were chemically synthesized employing Fmoc-based solid-phase peptide synthesis on a sulfonamide resin [44–46] and a tea-bag approach. We used the peptide bond between residues Tyr26-Cys27 as the cyclization site. This cyclization site has shown to provide very good cyclization yields with different MCoTI-based cyclotide sequences [13, 14], including cyclotide WT [10]. The synthesis of the common C-terminal sequence for all cyclotides (peptide segment from residue Tyr26 to Ser13) was performed at 0.1 mmol scale on an automated peptide synthesizer ABI433A. At this point the resin was divided in 15 equimolar aliquots containing around 4–6 μmol each (≈ 25 mg) (Fig. 2). Each resin was manually coupled in parallel with the corresponding Fmoc-activated amino acid derivative. Once the couplings were complete, each resin was placed in an individual sealed poly-propylene ‘tea-bag’ (measuring around 30 × 20 mm), properly tagged for identification and the synthesis was continued on the synthesizer using the ABI433A 0.25 mmol scale reaction vessel, which has a capacity of 42 mL (Fig. 2). This reaction vessel was able to accommodate up to 9 ‘tea bags’ with the dimensions described above. The number of ‘tea-bags’, however, could likely be easily increased by using smaller ‘tea-bags’. Accordingly, the synthesis of the common N-terminal segment for the 15 different cyclotides was performed in two steps, each with 9 and 6 ‘tea bags’, respectively. Once the synthesis of all the cyclotide analogs was complete, activation of the corresponding peptide sulfonamide-resins was performed in parallel with iodoacetonitrile, followed by cleavage with ethyl mercaptoacetate and acidolytic deprotection provided the fully deprotected linear peptide α-thioesters precursors (Fig. 2). The peptide crudes were characterized by HPLC and ES-MS and in all the cases the major product was the corresponding linear precursor thioester (Figs. S1 and S2).
Table 1.
Nomenclature used for all the MCo-CVX-based cyclotide used in this work. All the cyclotides sequence are based on cyclotide MCo-CVX-5c
| Aaa12a | Cyclotide name | Molecular weight/Dab |
|---|---|---|
| Asp | WT | 5056.7 ± 0.8 Da (5056.9 Da) |
| Ala | D12A | 5012.8 ± 0.3 Da (5012.9 Da) |
| Glu | D12E | 5070.8 ± 0.8 Da (5070.9 Da) |
| Gly | D12G | 4998.6 ± 0.6 Da (4998.9 Da) |
| His | D12H | 5078.9 ± 0.8 Da (5078.9 Da) |
| Lys | D12K | 5069.8 ± 0.6 Da (5070.0 Da) |
| Ile | D12I | 5054.8 ± 0.6 Da (5054.9 Da) |
| Leu | D12L | 5054.7 ± 0.5 Da (5054.9 Da) |
| Asn | D12N | 5055.9 ± 0.5 Da (5055.9 Da) |
| Pro | D12P | 5039.5 ± 0.6 Da (5038.9 Da) |
| Gln | D12Q | 5069.9 ± 0.1 Da (5069.9 Da) |
| Ser | D12S | 5028.7 ± 0.4 Da (5028.9 Da) |
| Thr | D12T | 5043.1 ± 1.0 Da (5042.9 Da) |
| Trp | D12W | 5127.9 ± 1.0 Da (5128.0 Da) |
| Tyr | D12Y | 5104.5 ± 1.0 Da (5105.0 Da) |
Amino acid at position 12 in loop 2 of cyclotide MCo-CVX-5c (Fig. 1)
Found molecular weight. Expected average isotopic molecular weight is given in parenthesis
Figure 2.
Synthetic scheme used for the parallel production of the amino acid scanning library at position 12 of cyclotide MCo-CVX-5c (WT) using a ‘tea-bag’ approach.
Library cyclization/folding and purification
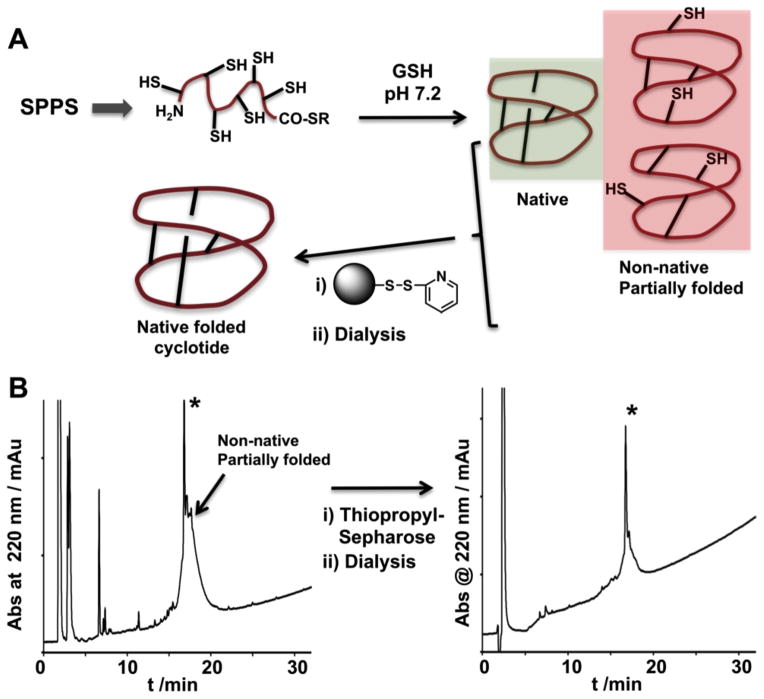
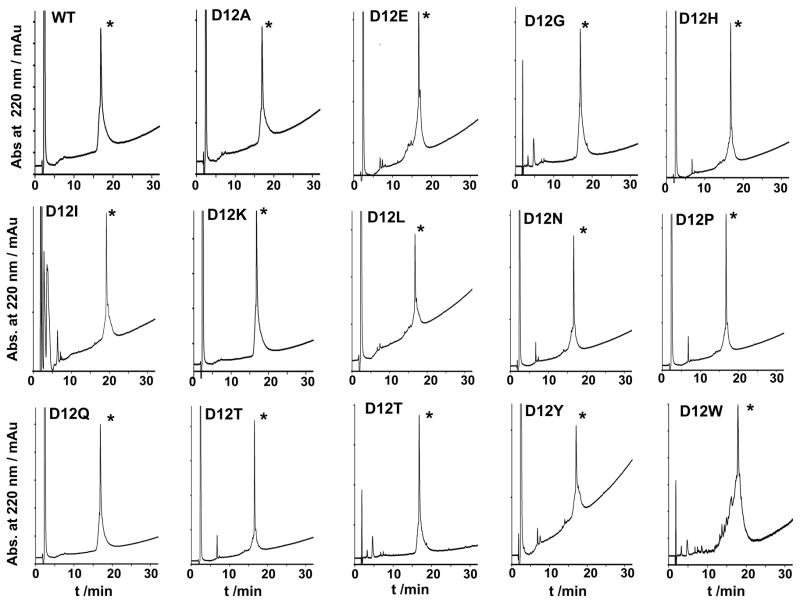
The corresponding peptide thioester precursors were directly cyclized and folded in parallel in a one-pot reaction using sodium phosphate buffer at pH 7.2 in the presence of 1 mM GSH. Under these conditions cyclotide WT has been shown to cyclize and adopt a native cyclotide as determined by NMR [10]. The cyclization/folding reactions were followed by HPLC and shown to be complete in 24–96 h. Most of the cyclotides were able to cyclize and fold with yields similar to that of WT (≈80%), only cyclotides D12I, D12L, D12Y and D12W showed lower yields with several late-eluting by-products that were attributed to partially folded and/or GSH-adducts as determined by ES-MS analysis (Figs. 3 and S3). The formation of alternative structures with shuffled S-S bonds was not observed with any of the cyclotides studied in this work. The conditions used in this work for the oxidative folding, which involve thermodynamic control under slightly reductive conditions, favor the accumulation of products containing the more stable cyclotide scaffold (Fig. 3) [10]. Removal of partially folded and/or GSH-peptide adducts was readily accomplished by using activated thiopropyl-sepharose chromatography. Activated thiopropyl-sepharose beads contain a reactive disulfide that reacts covalently with molecules containing free thiol groups therefore removing any partially folded product from the crude reaction (Fig. 3A). Accordingly, fully folded cyclotides are eluted from the column along 2-mercaptopydone, which can be easily removed by dialysis. As shown in Fig. 4, this approach was successfully used to produce 14 of the 15 cyclotides in high purity (≈80–90%, as determined by HPLC and ES-MS). For most cell-based assays this level of purity is usually satisfactory [10]. In addition, we tested the activity of some of the cyclotides purified by HPLC and compared them with the activities obtained with the same cyclotides purified just using the activated thiopropyl-sepharose, and the values were practically similar [10].
Figure 3.
Purification of folded cyclotides from the one-pot cyclization-folding reaction using activated thiopropyl-sepharose beads. A. Principle for the removal of unfolded and/or partially folded cyclotides from the cyclization-folding reaction using activated thiopropyl sepharose beads. Unfolded and/or partially folded peptides have free thiol groups that are captured and immobilized onto the sepharose beads. Fully folded cyclotides do not contain any free thiol available to react with the sepharose beads and therefore are not captured. The capture reaction also produces 2-mercaptopyridone that can be easily removed in a separate dialysis step. B. Analytical HPLC analysis of the purification of cyclotide D12L using activated thiopropyl-sepharose beads followed by dialysis. The cyclization-folding reaction in this cyclotide analog produced some late eluting by products that were attributed to partially folded cyclotides and GSH-adducts. All these by-products contain free-thiol groups that were efficiently captured by the activated sepharose beads yielding folded cyclotide with a high level of purity as determined by HPLC and ES-MS (Fig. S4). HPLC analysis was performed using a linear gradient of 0–70% solvent B over 30 minutes. An asterisk marks the product corresponding to the folded cyclotide as determined by ES-MS analysis.
Figure 4.
Analytical HPLC analysis of all the cyclotides used in this work after being purified by activated thiopropyl-seharose beads followed by dialysis. HPLC analysis was performed using a linear gradient of 0–70% solvent B over 30 minutes. An asterisk marks the product corresponding to the folded cyclotide as determined by ES-MS analysis.
Only cyclotide, D12W, was obtained with a lower degree of purity (≥70%, as determined by HPLC and ES-MS). This however was attributed to the higher reactivity of Trp-containing peptides of the linear peptide precursor. It is also important to note that this purification approach can be easily used in parallel for the purification of a single cyclotide compound but also and more importantly for the purification of mixtures of cyclotides as it relies only on the ability of the polypeptide/s to adopt a cyclotide fold with no free thiol groups. Consequently, this approach to remove unfolded and partially folded compounds is potentially compatible with the production of positional scanning libraries using the cyclotide scaffold.
Biological activity against the CXCR4 receptor
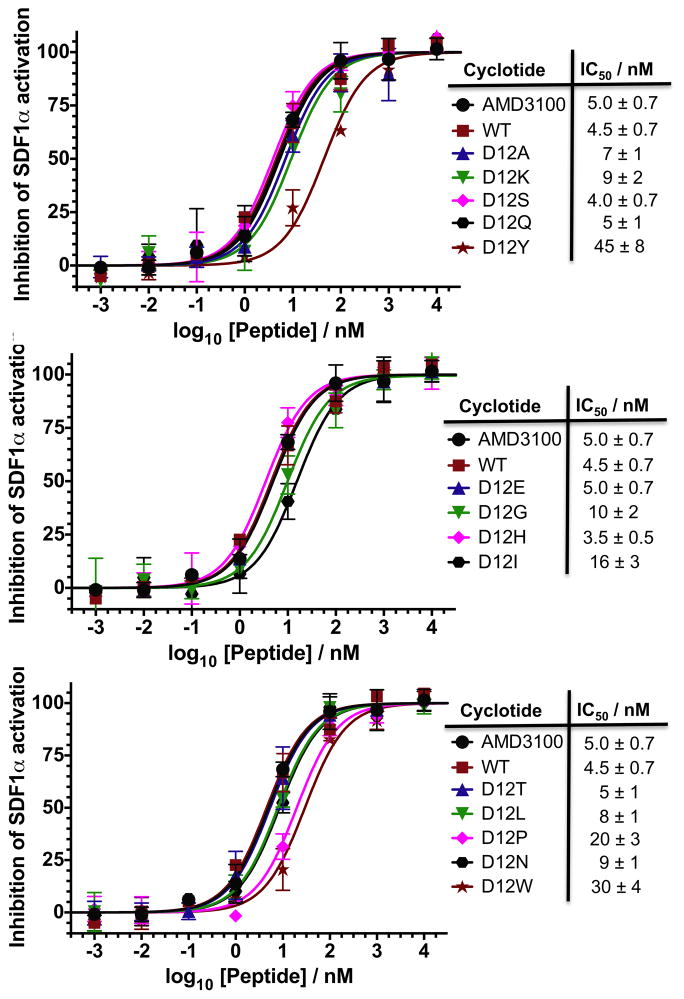
To evaluate the effect of the different mutations on the biological activity of the corresponding cyclotide analogs, we tested the ability of the CVX15-grafted cyclotides to inhibit SDF1α-mediated CXCR4 activation using a CXCR4-β-lactamase U2OS cell-based fluorescence assay (Fig. 5). All the cyclotides tested were able to inhibit SDF1α-mediated CXCR4 activation in a dose dependent manner with IC50 values ranging from 3.5 ± 0.1 nM (D12H) to 45 ± 8 nM (D12Y). The empty scaffold cyclotide MCoTI-I has already been shown to have no inhibitory activity in this assay [10] and was not included in this study. The small molecule AMD3100 [47] was also included as positive control. As previously described the activity of the WT cyclotide was similar to that of AMD3100 in this type of assay, indicating the relatively high potency of this cyclotide. As expected, replacement of Asp (WT) by Glu (D12E) did not change the activity of the resulting cyclotide. Most of the remaining mutations tested in this work did not significantly change the activity of the resulting cyclotides. Intriguingly replacing the original Asp amino acid by a positive charged residue had no or very little effect on the biological activity of the resulting cyclotides. For example cyclotide D12K (IC50 = 9 ± 2 nM) was only about two times less potent than the WT cyclotide, while cyclotide D12H (IC50 = 3.5 ± 0.5 nM) had practically the same activity of the WT cyclotide. Cyclotide D20P (IC50 = 20 ± 3 nM) also showed a slightly decrease in activity, about 4 times less active than WT), which could be attributed to the conformational restrictions imposed by the Pro residue. Among all the analogues tested in this study, only cyclotides D12Y (IC50 = 45 ± 8 nM) and D12W (IC50 = 30 ± 4 nM) had a significant decrease in biological activity, being both almost 10 times less active than the WT cyclotide. Altogether these results indicate that position 12 is quite tolerant to mutations, and only the introduction of aromatic residues (Trp and Tyr) seems to have a significant detrimental effect on the biological activity of the resulting cyclotides.
Figure 5.
Biological characterization of all the MCo-CVX-based cyclotides produced in this work. Competitive inhibition of SDF1α-mediated CXCR4 activation by the different cyclotide analogs. The small molecule CXCR4 antagonist AMD3100 was used as control. The assay was performed using CXCR4-bla U2OS cells as described in the Materials and Methods section.
CONCLUSIONS
In summary, we have shown for the first time that bioactive folded MCoTI-based cyclotides can be efficiently produced in parallel using a ‘tea-bag’ approach in combination with high efficient cyclization-folding protocols. The approach described in this work also includes an efficient purification procedure to rapidly remove non-folded or partially folded cyclotides from the cyclization-folding crude. This procedure can be easily used in parallel for the purification of individual compounds, but also and more importantly for the purification of cyclotide mixtures. Therefore making it potentially compatible with the synthesis of positional scanning libraries in order to perform efficient screening of large libraries.
These protocols were successfully used for the production of small library of bioactive cyclotides based on the cyclotide MCo-CVX-5c (WT), which is a potent CXCR4 antagonist recently developed in our group [10]. Biological evaluation of this library allowed rapidly assessment of the effects of single mutations on the activity of the corresponding cyclotide analogues. The results performed in the position 12 of loop 2 indicated that none of mutations improved significantly the activity of the WT cyclotide, and that this position was quite tolerant to most of the mutations tested in this work, except for residues containing aromatic side-chains. These results open the possibility to study other positions and/or loops within this bioactive cyclotide through the use of amino acid or positional scanning libraries. In summary, our results demonstrate for the first time the efficient parallel synthesis of bioactive folded cyclotides thereby providing a powerful chemical tool to perform structure activity relationships in large complex polypeptides such as cyclotides in a rapid and efficient fashion.
MATERIALS AND METHODS
Analytical HPLC was performed on a HP1100 series instrument with 220 nm and 280 nm detection using a Vydac C18 column (5 mm, 4.6 × 150 mm) at a flow rate of 1 mL/min. All runs used linear gradients of 0.1% aqueous trifluoroacetic acid (TFA, solvent A) vs. 0.1% TFA, 90% acetonitrile in H2O (solvent B). UV-vis spectroscopy was carried out on an Agilent 8453 diode array spectrophotometer, and fluorescence analysis on a Jobin Yvon Flurolog-3 spectroflurometer. Electrospray mass spectrometry (ES-MS) analysis was routinely applied to all cyclized peptides. ES-MS experiments were performed on a Applied Biosystems API 3000 triple quadrupole mass spectrometer.
Preparation of Fmoc-Tyr(tBu)-F
Fmoc-Tyr(tBu)-F was prepared using diethylaminosulfur trifluoride DAST as previously described [14] and quickly used afterwards. Briefly, DAST (160 μL, 1.2 mmol) was added drop wise at 25° C under nitrogen current to a stirred solution of Fmoc-Tyr(tBu)-OH (459.6 mg, 1 mmol) in 10 mL of dry dichloromethane (DCM), containing dry pyridine (81 μL, 1 mmol). After 20 minutes, the mixture was washed with ice-cold water (3 × 20 mL). The organic layer was separated and dried over anhydrous MgSO4. The solvent was removed under reduced pressure to give the corresponding Fmoc-amino acyl fluoride as white solid that was immediately used.
Loading of 4-sulfamylbutyryl AM resin with Fmoc-Tyr(tBu)-F
Loading of the first residue was accomplished using Fmoc-Tyr(tBu)-F as previously described [10]. Briefly, 4-sulfamylbutyryl AM resin (420 mg, 0.33 mmol) (Novabiochem) was swollen for 20 minutes with dry DCM and then drained. A solution of Fmoc-Tyr(tBu)-F (≈461 mg, 1 mmol) in dry DCM (2 mL) and di-isopropylethylamine (DIEA) (180 μL, 1 mmol) was added to the drained resin and reacted at 25° C for 1 h. The resin was washed with dry DCM (5 × 5 mL), dried and kept at −20° C until use.
Chemical synthesis of the cyclotide-based library
All peptides were synthesized as described in Fig. 2 and was carried out by solid-phase synthesis on an automatic peptide synthesizer ABI433A (Applied Biosystems) using the Fast-Fmoc chemistry with 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU)/diisopropylethylamine (DIEA) activation protocol at 0.1 mmole scale on a Fmoc-Tyr(tBu)-sulfamylbutyryl AM resin. Side-chain protection compatible with Fmoc-chemistry was employed as previously described for the synthesis of peptide α-thioesters by the Fmoc-protocol, except for the N-terminal Cys residue, which was introduced as Boc-Cys(Trt)-OH. Before coupling of residue 12, the peptide-resin was manually resuspended in DCM:dimethylformamide (DMF)(1:1) and split into 15 equal aliquots. Each peptide-resin aliquot was placed in a separate 1 mL polypropylene column (Qiagen). The resins were manually deprotected with 20% 4-methylpiperidine in DMF (3 × 5 min) and then individually coupled with ach of the different 15 Fmoc-Aaa-OH (Figs. 1 and 2). Couplings were performed using HBTU and DIEA for 45 min. The resins were then washed, dried, placed in individual tagged ‘tea-bags’ (30 × 20 mm) and sealed. The synthesis was continued using the peptide synthesizer 0.25 mmol reaction vessel, which can hold up to 9 different tea-bags of the dimensions reported above. This required 2 synthetic steps were 9 and 6 ‘tea-bags’ were used in the reaction vessel, respectively. Following chain assembly, the alkylation, thiolytic cleavage and side chain deprotection were performed for individual peptides in 1 mL polypropylene columns as previously described [14]. Briefly, ≈10 mg of protected peptide-resin were first alkylated two times with ICH2CN (17.4 μL, 0.24 mmol; previously filtered through basic alumina) and DIEA (8.2 μL, 0.046 mmol) in N-methylpyrrolidone (NMP) (0.22 mL) for 24 h. The resin was then washed with NMP (3 × 5 mL) and DCM (3 × 5 mL). The alkylated peptide resin was cleaved from the resin with HSCH2CH2CO2Et (20 μL, 0.18 mmol) in the presence of a catalytic amount of sodium thiophenolate (NaSPh, 0.3 mg, 2.2 μmol) in DMF:DCM (1:2 v/v, 0.12 mL) for 24 h. The resin was then dried at reduced pressure. The side-chain protecting groups were removed by treating the dried resin with trifluoroacetic acid (TFA):H2O:tri-isopropylsilane (TIS) (95:3:2 v/v, 0.5 mL) for 3–4 h at room temperature. The resin was filtered and the linear peptide thioester was precipitated in cold Et2O. The crude material was dissolved in the minimal amount of H2O:MeCN (4:1) containing 0.1% TFA and characterized by HPLC and ES-MS as the desired grafted MCoTI-I linear precursor α-thioester (Fig. S1 and S2). Cyclization and folding was accomplished by flash dilution of the linear α-thioester TFA crude to a final concentration of ≈20–50 μM into freshly degassed 2mM reduced glutathione (GSH), 0.1 M sodium phosphate buffer at pH 7.2 for 96 h.
Purification of folded cylotides using activated thio-propyl sepharose beads
Following oxidative folding, the pH of the cyclization-folding crude was raised to pH 8.0 by slowly adding a solution of 0.2 M Na3PO4 in water. After 4 h, the resulting crudes were treated with pre-swollen activated thio-propyl sepharose beads (20 μl of swollen beads/mL of cyclization-folding crude) (Pharmacia) for 1 h. The beads were then drain and washed with 10% HOAc in water (2 × 200 μL). The combined liquid fractions were pooled and dialyzed using a slide-A-lyzer mini dialysis (Thermoscientific) with a cut-off of 3.5 kDa against 0.1 M HOAc (2 × 14 mL). The dialyzed samples were then analyzed by HPLC and ES-MS for purity (Figs. 4 and S4), lyophilized and stored at -20° C. Purified cyclotides were quantified by UV-vis spectroscopy at 280 nm using a molar absortivity coefficient of 10,470 M−1 cm−1 for all the cyclotides except for D12Y (11,810 M−1 cm−1) and D12W (16,020 M−1 cm−1).
Cell-based CXCR4 competitive binding assays
Briefly, Tango™ CXCR4-bla U2OS cells (Life Technologies) were seeded at 11,000 cells/well in 384-well tissue culture plate for 24 h in DMEM supplemented with 1% FBS. Cells were pre-treated with various concentrations of inhibitors for 30 minutes prior to the addition of 30 nM of SDF-1α and incubated for 5 h at 37°C. β-Lactamase substrate LiveBLAzer™-FRET B/G Substrate (Invitrogen) was then incubated with treated cells for 2 h at room temperature and fluorescence signal was measured by Synergy H1 plate reader (Bio Tek) at 409/460 nm (substrate cleaved) and 409/530nm (substrate uncleaved).
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health Research Grant R01GM090323 (JAC) and the Southern California Clinical and Translational Science Institute (JAC and NN).
References
- 1.Daly NL, Rosengren KJ, Craik DJ. Discovery, structure and biological activities of cyclotides. Adv Drug Deliv Rev. 2009;61:918–930. doi: 10.1016/j.addr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Gould A, Ji Y, Aboye TL, Camarero JA. Cyclotides, a novel ultrastable polypeptide scaffold for drug discovery. Curr Pharm Des. 2011;17:4294–4307. doi: 10.2174/138161211798999438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puttamadappa SS, Jagadish K, Shekhtman A, Camarero JA. Backbone Dynamics of Cyclotide MCoTI-I Free and Complexed with Trypsin. Angew Chem Int Ed Engl. 2010;49:7030–7034. doi: 10.1002/anie.201002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puttamadappa SS, Jagadish K, Shekhtman A, Camarero JA. Erratum in: Backbone Dynamics of Cyclotide MCoTI-I Free and Complexed with Trypsin. Angew Chem Int Ed Engl. 2011;50:6948–6949. doi: 10.1002/anie.201002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daly NL, Thorstholm L, Greenwood KP, King GJ, Rosengren KJ, Heras B, Martin JL, Craik DJ. Structural insights into the role of the cyclic backbone in a squash trypsin inhibitor. J Biol Chem. 2013;288:36141–36148. doi: 10.1074/jbc.M113.528240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin J, Kimura RH, Woo YH, Camarero JA. In vivo biosynthesis of an Ala-scan library based on the cyclic peptide SFTI-1. Amino Acids. 2010;38:1313–1322. doi: 10.1007/s00726-009-0338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonsen SM, Sando L, Rosengren KJ, Wang CK, Colgrave ML, Daly NL, Craik DJ. Alanine scanning mutagenesis of the prototypic cyclotide reveals a cluster of residues essential for bioactivity. J Biol Chem. 2008;283:9805–9813. doi: 10.1074/jbc.M709303200. [DOI] [PubMed] [Google Scholar]
- 8.Huang YH, Colgrave ML, Clark RJ, Kotze AC, Craik DJ. Lysine-scanning mutagenesis reveals an amendable face of the cyclotide kalata B1 for the optimization of nematocidal activity. J Biol Chem. 2010;285:10797–10805. doi: 10.1074/jbc.M109.089854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia AE, Camarero JA. Biological activities of natural and engineered cyclotides, a novel molecular scaffold for peptide-based therapeutics. Curr Mol Pharmacol. 2010;3:153–163. doi: 10.2174/1874467211003030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aboye TL, Ha H, Majumder S, Christ F, Debyser Z, Shekhtman A, Neamati N, Camarero JA. Design of a novel cyclotide-based CXCR4 antagonist with anti-human immunodeficiency virus (HIV)-1 activity. J Med Chem. 2012;55:10729–10734. doi: 10.1021/jm301468k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong CT, Rowlands DK, Wong CH, Lo TW, Nguyen GK, Li HY, Tam JP. Orally active peptidic bradykinin B1 receptor antagonists engineered from a cyclotide scaffold for inflammatory pain treatment. Angew Chem Int Ed Engl. 2012;51:5620–5624. doi: 10.1002/anie.201200984. [DOI] [PubMed] [Google Scholar]
- 12.Chan LY, Gunasekera S, Henriques ST, Worth NF, Le SJ, Clark RJ, Campbell JH, Craik DJ, Daly NL. Engineering pro-angiogenic peptides using stable, disulfide-rich cyclic scaffolds. Blood. 2011;118:6709–6717. doi: 10.1182/blood-2011-06-359141. [DOI] [PubMed] [Google Scholar]
- 13.Ji Y, Majumder S, Millard M, Borra R, Bi T, Elnagar AY, Neamati N, Shekhtman A, Camarero JA. In Vivo Activation of the p53 Tumor Suppressor Pathway by an Engineered Cyclotide. J Am Chem Soc. 2013;135:11623–11633. doi: 10.1021/ja405108p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contreras J, Elnagar AY, Hamm-Alvarez SF, Camarero JA. Cellular uptake of cyclotide MCoTI-I follows multiple endocytic pathways. J Control Release. 2011;155:134–143. doi: 10.1016/j.jconrel.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cascales L, Henriques ST, Kerr MC, Huang YH, Sweet MJ, Daly NL, Craik DJ. Identification and characterization of a new family of cell-penetrating peptides: cyclic cell-penetrating peptides. J Biol Chem. 2011;286:36932–36943. doi: 10.1074/jbc.M111.264424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Souza C, Henriques ST, Wang CK, Craik DJ. Structural parameters modulating the cellular uptake of disulfide-rich cyclic cell-penetrating peptides: MCoTI-II and SFTI-1. European journal of medicinal chemistry. 2014 doi: 10.1016/j.ejmech.2014.06.047. [DOI] [PubMed] [Google Scholar]
- 17.Henriques ST, Craik DJ. Cyclotides as templates in drug design. Drug Discov Today. 2010;15:57–64. doi: 10.1016/j.drudis.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Mylne JS, Chan LY, Chanson AH, Daly NL, Schaefer H, Bailey TL, Nguyencong P, Cascales L, Craik DJ. Cyclic peptides arising by evolutionary parallelism via asparaginyl-endopeptidase-mediated biosynthesis. Plant Cell. 2012;24:2765–2778. doi: 10.1105/tpc.112.099085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poth AG, Mylne JS, Grassl J, Lyons RE, Millar AH, Colgrave ML, Craik DJ. Cyclotides associate with leaf vasculature and are the products of a novel precursor in petunia (Solanaceae) J Biol Chem. 2012;287:27033–27046. doi: 10.1074/jbc.M112.370841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poth AG, Colgrave ML, Lyons RE, Daly NL, Craik DJ. Discovery of an unusual biosynthetic origin for circular proteins in legumes. Proc Natl Acad Sci U S A. 2011;108:1027–1032. doi: 10.1073/pnas.1103660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jennings C, West J, Waine C, Craik D, Anderson M. Biosynthesis and insecticidal properties of plant cyclotides: the cyclic knotted proteins from Oldenlandia affinis. Proc Natl Acad Sci U S A. 2001;98:10614–10619. doi: 10.1073/pnas.191366898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillon AD, Saska I, Jennings CV, Guarino RF, Craik DJ, Anderson MA. Biosynthesis of circular proteins in plants. Plant J. 2008;53:505–515. doi: 10.1111/j.1365-313X.2007.03357.x. [DOI] [PubMed] [Google Scholar]
- 23.Saska I, Gillon AD, Hatsugai N, Dietzgen RG, Hara-Nishimura I, Anderson MA, Craik DJ. An asparaginyl endopeptidase mediates in vivo protein backbone cyclization. J Biol Chem. 2007;282:29721–29728. doi: 10.1074/jbc.M705185200. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen GK, Wang S, Qiu Y, Hemu X, Lian Y, Tam JP. Butelase 1 is an Asx-specific ligase enabling peptide macrocyclization and synthesis. Nat Chem Biol. 2014;10:732–738. doi: 10.1038/nchembio.1586. [DOI] [PubMed] [Google Scholar]
- 25.Jagadish K, Borra R, Lacey V, Majumder S, Shekhtman A, Wang L, Camarero JA. Expression of fluorescent cyclotides using protein trans-splicing for easy monitoring of cyclotide-protein interactions. Angew Chem Int Ed Engl. 2013;52:3126–3131. doi: 10.1002/anie.201209219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin J, Wang W, Puttamadappa S, Shekhtman A, Camarero JA. Biosynthesis and biological screening of a genetically encoded library based on the cyclotide MCoTI-I. Chembiochem. 2009;10:2663–2670. doi: 10.1002/cbic.200900534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camarero JA, Kimura RH, Woo YH, Shekhtman A, Cantor J. Biosynthesis of a fully functional cyclotide inside living bacterial cells. Chembiochem. 2007;8:1363–1366. doi: 10.1002/cbic.200700183. [DOI] [PubMed] [Google Scholar]
- 28.Kimura RH, Tran AT, Camarero JA. Biosynthesis of the cyclotide kalata B1 by using protein splicing. Angew Chem Int Ed. 2006;45:973–976. doi: 10.1002/anie.200503882. [DOI] [PubMed] [Google Scholar]
- 29.Tam JP, Lu YA, Yang JL, Chiu KW. An unusual structural motif of antimicrobial peptides containing end-to-end macrocycle and cystine-knot disulfides. Proc Natl Acad Sci U S A. 1999;96:8913–8918. doi: 10.1073/pnas.96.16.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daly NL, Love S, Alewood PF, Craik DJ. Chemical synthesis and folding pathways of large cyclic polypeptides: studies of the cystine knot polypeptide kalata B1. Biochemistry. 1999;38:10606–10614. doi: 10.1021/bi990605b. [DOI] [PubMed] [Google Scholar]
- 31.Thongyoo P, Roque-Rosell N, Leatherbarrow RJ, Tate EW. Chemical and biomimetic total syntheses of natural and engineered MCoTI cyclotides. Org Biomol Chem. 2008;6:1462–1470. doi: 10.1039/b801667d. [DOI] [PubMed] [Google Scholar]
- 32.Thongyoo P, Tate EW, Leatherbarrow RJ. Total synthesis of the macrocyclic cysteine knot microprotein MCoTI-II. Chem Commun (Camb) 2006:2848–2850. doi: 10.1039/b607324g. [DOI] [PubMed] [Google Scholar]
- 33.Aboye TL, Clark RJ, Craik DJ, Göransson U. Ultra-stable peptide scaffolds for protein engineering-synthesis and folding of the circular cystine knotted cyclotide cycloviolacin O2. Chembiochem. 2008;9:103–113. doi: 10.1002/cbic.200700357. [DOI] [PubMed] [Google Scholar]
- 34.Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Synthesis of Proteins by Native Chemical Ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 35.Camarero JA, Muir TW. Chemoselective Backbone Cyclization of Unprotected Peptides. Chem Comm. 1997;1997:202–219. [Google Scholar]
- 36.Aboye TL, Li Y, Majumder S, Hao J, Shekhtman A, Camarero JA. Efficient one-pot cyclization/folding of Rhesus theta-defensin-1 (RTD-1) Bioorg Med Chem Lett. 2012;22:2823–2826. doi: 10.1016/j.bmcl.2012.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houghten RA. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985;82:5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sallberg M, Ruden U, Magnius LO, Norrby E, Wahren B. Rapid “tea-bag” peptide synthesis using 9-fluorenylmethoxycarbonyl (Fmoc) protected amino acids applied for antigenic mapping of viral proteins. Immunology letters. 1991;30:59–68. doi: 10.1016/0165-2478(91)90090-w. [DOI] [PubMed] [Google Scholar]
- 39.Baeza CR, Unden A. Studies on the structural requirement for ligand binding to the neuropeptide Y (NPY) receptor from rat cerebral cortex. FEBS Lett. 1990;277:23–25. doi: 10.1016/0014-5793(90)80800-x. [DOI] [PubMed] [Google Scholar]
- 40.Gilmore BF, Harriott P, Walker B. The inactivation of bovine cathepsin B by novel N-chloro-acetyl-dipeptides: application of the Houghten ‘tea bag’ methodology to inhibitor synthesis. Biochem Biophys Res Commun. 2005;333:1284–1288. doi: 10.1016/j.bbrc.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 41.Pinilla C, Appel JR, Houghten RA. Tea bag synthesis of positional scanning synthetic combinatorial libraries and their use for mapping antigenic determinants. Methods Mol Biol. 1996;66:171–179. doi: 10.1385/0-89603-375-9:171. [DOI] [PubMed] [Google Scholar]
- 42.Felizmenio-Quimio ME, Daly NL, Craik DJ. Circular proteins in plants: solution structure of a novel macrocyclic trypsin inhibitor from Momordica cochinchinensis. J Biol Chem. 2001;276:22875–22882. doi: 10.1074/jbc.M101666200. [DOI] [PubMed] [Google Scholar]
- 43.Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, Hamel DJ, Kuhn P, Handel TM, Cherezov V, Stevens RC. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Backes BJ, Ellman JA. An alkanesulfonamide “safety-catch” linker for solid-phase synthesis. J Org Chem. 1999;64:2322–2330. [Google Scholar]
- 45.Shin Y, Winans KA, Backes BJ, Kent SBH, Ellman JA, Bertozzi CR. Fmoc-Based Synthesis of Peptide-αThioesters: Application to the Total Chemical Synthesis of a Glycoprotein by Native Chemical Ligation. J Am Chem Soc. 1999;121:11684–11689. [Google Scholar]
- 46.Ingenito R, Bianchi E, Fattori D, Pessi A. Solid phase synthesis of peptide C-terminal thioesters by Fmoc/t-Bu chemistry. J Am Chem Soc. 1999;121:11369–11374. [Google Scholar]
- 47.De Clercq E. The AMD3100 story: the path to the discovery of a stem cell mobilizer (Mozobil) Biochem Pharmacol. 2009;77:1655–1664. doi: 10.1016/j.bcp.2008.12.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.