Abstract
Identifying neurocognitive processes associated with effective inhibitory control is particularly relevant for individuals at high risk for disruptive behaviors, such as maltreated children. Performance feedback processing during a flanker task was investigated in maltreated preschool-aged children (N = 67) via an event-related potential component, the feedback-related negativity (FRN). The functionality of the FRN in children with high impulsivity was of interest, as impulsivity was associated with an exaggerated FRN in previous research. Results showed that high impulsivity was associated with an exaggerated FRN and greater post-error slowing. For children with high impulsivity, there was a correlation between the FRN and accuracy, which was not found in children with low impulsivity. This suggests that an exaggerated FRN is particularly important for children with high impulsivity to maintain effective inhibitory control.
Keywords: feedback-related negativity, impulsivity, child maltreatment, flanker task
Effective inhibitory control is a key developmental skill associated with important outcomes, including school achievement, social-emotional competency, and reduced problem behaviors (Espy et al., 2004; Nigg et al., 2006; Raaijmakers et al., 2008; St Clair-Thompson & Gathercole, 2006). Understanding the development of this skill is particularly important for high-risk children such as those who have experienced maltreatment, as these children commonly experience difficulties with inhibitory control and high levels of associated disruptive and health-risk behaviors (Chamberlain & Sahakian, 2007; Pardini, Lochman, & Wells, 2004; Pears, Fisher, Bruce, Kim, & Yoerger, 2010; Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004; Riggs, Alario, & McHorney, 1990; Stirling & Amaya-Jackson, 2008). Event-related potential (ERP) techniques are a promising tool for understanding the neurocognitive processes associated with the development of inhibitory control in at-risk samples (van Noordt & Segalowitz, 2012). In the present study, we examined the association between caregiver-reported impulsivity and processing of performance feedback during an inhibitory control task, as indexed by peak-to-peak amplitude of the FRN, in a sample of maltreated preschool-aged children. We further examined the role of impulsivity as a moderator of the relation between the FRN and behavioral performance on an inhibitory control task.
The Feedback-Related Negativity (FRN)
The FRN, as conceptualized within the context of reinforcement learning theory, serves as a candidate ERP component relevant to the development of inhibitory control in children (Holroyd, Baker, Kerns, & Müller, 2008; Holroyd & Coles, 2002; McDermott, Pérez-Edgar, & Fox, 2007). In this model, negative events (i.e., negative feedback or outcomes worse than expected) elicit a large negative ERP deflection, termed the FRNn, approximately 300 ms after the event. A less pronounced negative deflection, termed the FRNp, is elicited by positive events and is theorized to reflect the reduced need to attend to expected feedback or outcomes (Holroyd & Coles, 2002; Montague, Hyman, & Cohen, 2004; van Meel, Oosterlaan, Heslenfeld, & Sergeant, 2005). The difference in magnitude between an individual’s electrophysiological responses to negative versus positive feedback comprises the FRN difference score (or FRNd). Source localization studies suggest that the FRN (a general term used to refer to the FRNn, FRNp, and FRNd) is generated in the anterior cingulate cortex and is involved in learning, punishment sensitivity, and response monitoring (Holroyd & Coles, 2002; Montague et al., 2004; van Meel et al., 2005).
Processing of negative feedback is believed to be critical to effective inhibitory control, because an individual must be attuned to what he or she is doing incorrectly to effectively update response strategies (Holroyd et al., 2008; Holroyd & Coles, 2002; McDermott et al., 2007; Potts, Martin, Kamp, & Donchin, 2011). Although internal response monitoring is associated with effective inhibitory control in older children, response monitoring following external feedback (e.g., positive reinforcement or privilege removal) is particularly important for adaptive preschool behavior (Hillman, Buck, Themanson, Pontifex, & Castelli, 2009; Santesso & Segalowitz, 2008; Simonsen, Fairbanks, Briesch, Myers, & Sugai, 2008). Children in this age group are not necessarily expected to have internalized rules, but are expected to change their behavior following feedback that their behavior is not appropriate (LoCasale-Crouch et al., 2007; Rimm-Kaufman, Pianta, & Cox, 2000; Smetana, 1981). Indeed, previous research has found that younger children exhibit stronger reactions to external feedback than older adolescents or adults, as measured by heart rate slowing and the FRNd (Crone, Somsen, Zanolie, & Van der Molen, 2006; Eppinger, Mock, & Kray, 2009).
Although FRN responses during inhibitory control tasks have been observed in children (Eppinger et al., 2009), certain individual characteristics seem to affect the development of the neural systems involved in feedback processing. Maltreatment experiences, for example, have been found to predict blunted FRNd responses during an inhibitory control task (Bruce, McDermott, Fisher, & Fox, 2009). This propensity has been theorized to relate to unpredictable feedback within inconsistent or dangerous caregiving environments (Bruce et al., 2009). Notably, early life stress has also been associated with atypical activation of the ACC and associated brain regions in humans and animals, further highlighting the relevance of understanding the FRN in at-risk samples (Segalowitz & Dywan, 2009).
Previous research investigating the FRN typically involves one of two types of feedback: feedback that includes an accuracy element (e.g., performance feedback) and feedback that solely conveys the receipt of loss or reward (e.g., utilitarian feedback; van der Helden, Boksem, & Blom, 2010; van Meel, Heslenfeld, Oosterlaan, Luman, & Sergeant, 2011). Research employing performance feedback (investigated in the current study) has primarily been conducted with adults, with the results suggesting that a larger FRNd is associated with higher accuracy or increased learning. This has been demonstrated using multiple ERP analysis methods, including the difference between the maximum negative amplitude of the FRNn and FRNp (Bellebaum & Daum, 2008; van der Helden et al., 2010) and the difference between peak-to-peak amplitude of the FRNn and FRNp (i.e., the maximum negative amplitude of the FRNn and FRNp and the maximum positive amplitude of the preceding peak; Frank, Woroch, & Curran, 2005; Hämmerer, Müller, & Lindenberger, 2011). Broadly, these studies suggest that a larger FRNd is adaptive: participants are more likely to achieve high levels of task performance through the increased saliency of negative feedback.
The second type of task (not investigated in the current study) involves utilitarian feedback during gambling/guessing tasks. In these studies, a larger FRNd (difference between the maximum negative amplitudes and peak-to-peak amplitudes) is associated with high punishment sensitivity or high reward saliency (Moser & Simons, 2009; Santesso et al., 2012). However, in these studies, it is less clear what represents an ‘adaptive’ response, as an exaggerated FRN may be helpful (high-task engagement) or pathological (exaggerated punishment sensitivity; Santesso et al., 2012; van Noordt & Segalowitz, 2012).
The Role of Impulsivity
Individual differences in impulsivity are also important for understanding inhibitory control performance and feedback processing. For example, children with high impulsivity typically exhibit poor performance on tasks assessing inhibitory control (Casey et al., 1997). From these results, it might be expected that children with high impulsivity would display a smaller FRNd. However, children with ADHD have actually been found to have an exaggerated FRNd (difference between the maximum negative amplitudes) compared to their peers during tasks using utilitarian feedback (although this finding has not been consistently replicated; Rosch & Hawk, 2013; van Meel et al., 2005). Critically, previous research has not investigated whether children with high impulsivity also exhibit an exaggerated FRNd during tasks using performance feedback. Thus, it is unclear if the exaggerated FRNd in response to utilitarian feedback among these children reflects a less adaptive response (e.g., exaggerated reactivity to feedback) or a more adaptive response (e.g., the need for a ‘louder’ feedback processing signal to internalize rules and facilitate goal-directed behavior).
Present Study
In the present study, we examined the associations between the peak-to-peak amplitude of the FRN (FRNd, FRNn, and FRNp), caregiver-reported impulsivity, and behavioral performance on an inhibitory control task, the flanker task, in maltreated preschool-aged children in foster care. Examining the function of individual differences in impulsivity in predicting the FRN and inhibitory control task accuracy and reaction time is particularly important for these children, as they are at risk for externalizing behavior problems. Furthermore, the findings may help to explain the adaptive (or maladaptive role) in atypical FRNd responses previously observed in clinically impulsive populations.
Consistent with previous literature using performance feedback, we hypothesized that there would be a negative relation between the peak-to-peak amplitude of the FRNd and accuracy on the flanker task (e.g., a more pronounced FRNd would be associated with higher accuracy). We also expected that impulsivity would be negatively related to accuracy. Finally, it was hypothesized that the FRNd during this performance-feedback task would negatively relate to impulsivity (an exaggerated FRNd in children with high impulsivity), as this association has been found in tasks involving utilitarian feedback. However, it was uncertain if an exaggerated FRNd would be adaptive (i.e., a more pronounced FRNd would allow children with high impulsivity to maintain effective inhibitory control performance) or maladaptive (i.e., an exaggerated FRNd would negatively affect the inhibitory control performance of children with high impulsivity). For this reason, the association between the FRN (FRNd, FRNn, and FRNp) and behavioral performance (specifically incongruent trial accuracy and reaction time) was examined separately for children with high and low levels of caregiver-reported impulsivity. The additional behavioral index of response monitoring, post-error slowing, was of interest, but it was unknown if this would relate to accuracy given inconsistent findings of post-error slowing in previous samples of young children (Brooker & Buss, 2014). Examining the functional utility of the FRN during an inhibitory control task in a sample at risk for disruptive behaviors may allow us to identify the neurocognitive processes associated with effective inhibitory control and to design targeted interventions for these vulnerable children.
Method
Participants
The present sample included 67 preschool-aged children recruited from a larger efficacy trial of a school-readiness intervention for maltreated children in foster care (n = 192; Pears et al., 2013). Eligibility requirements for the larger efficacy trial included: current placement in kinship or non-kinship foster care, plans to enter kindergarten in the fall, non-involvement in treatment protocols closely related to that being tested, and being a monolingual or bilingual English speaker. Because a number of participants from the larger efficacy trial were unable to travel to the electroencephalogram (EEG) laboratory, EEG data were collected from only a subsample of the participants. In total, EEG data were collected from 84 participants. Of these children, 17 children were subsequently excluded from the analyses presented here: 13 children because of excessive artifact in the EEG data or an inadequate number of artifact-free ERP trials (described below) and 4 children because of poor behavioral performance (failing to respond to trials, i.e., > 50% errors of omission, or pressing the same button for every trial, i.e., <10% of correct trials on one color).
Of the children in the present study, 44.8% were male. Further, 59.7% were European American, 22.4% were Hispanic, and the remaining 17.9% were African American, Native American, or multiple races/ethnicities. The mean age of participants was 5.19 years (SD = .30). Only baseline data collected prior to the start of the intervention were utilized for the present study, thus details about the intervention are described elsewhere (Pears et al., 2013). The demographic variables including age and race/ethnicity were not significantly related to any of the study variables, and, accordingly, are not included in the following analyses. One exception was gender which was related to reaction time; however, since this was not a primary variable of interest and since preliminary analyses controlling for gender did not significantly alter the pattern of results presented in the models below, we did not include gender in the subsequent analyses.
Children’s Behavior Questionnaire (CBQ)
The CBQ is a caregiver-report measure that was validated for children aged 3 to 7 years and yields 15 theoretically-derived scales relating to arousability, self-regulation, and emotional reactivity (Rothbart, Ahadi, Hershey, & Fisher, 2001). Caregivers rate how true 195 statements are for their child on a Likert scale from 1 (extremely untrue) to 7 (extremely true). From this measure, we were interested in examining the impulsivity scale given the aforementioned associations with the FRN and inhibitory control task performance.
Previous research with a normative sample (N = 517) reported an average impulsivity score of 4.53 (SD = .73) on the CBQ for children ages 4–5 (Rothbart et al., 2001), suggesting the mean score for the present sample (M = 4.82, SD = 1.14) was about average. We used a median score of 4.83 on the impulsivity scale to identify children with high (n = 35) or low (n = 32) impulsivity and included three children with the median impulsivity (4.83) in the high group. Children in the high impulsivity group (M = 5.69, SD = .63) had significantly higher levels of impulsivity than did children in the low impulsivity group (M =3.86, SD = .73), t(65) = −11.06, p < .001. The average score of the high impulsivity group is similar to average impulsivity scores found in children with ADHD (M = 5.06, SD = .94) and higher than average scores found in normative (M = 4.53, SD = .73) and non-ADHD (M = 3.59, SD = .98) samples (Foley, McClowry, & Castellanos, 2008; Rothbart et al., 2001). This suggests that children in the high impulsivity group in the present study had elevated levels of impulsivity similar to those in a clinical population. The demographic variables including age and race/ethnicity were not significantly related to any of the study variables, and, accordingly, are not included in the following analyses. One exception was gender, which was related to reaction time; however, since this was not a primary variable of interest and since preliminary analyses controlling for gender did not significantly alter the pattern of results presented in the models below, we did not include gender in the subsequent analyses.
Flanker Task
The flanker task employed in this study was adapted for young children (McDermott et al., 2007) and was presented using the STIM stimulus presentation system (James Long Company, Caroga Lake, NY). Throughout the task, a small fixation point is displayed in the center of the computer screen. Each trial begins with a 300 ms warning cue indicated by a small asterisk, followed by a 500 ms fixation point. A horizontal row of five 1-inch circles, with the central circle directly above the fixation point, is then presented for 700 ms. The task includes congruent trials, which consist of five red circles or five green circles, and incongruent trials, which consist of a central red circle flanked by green circles or a central green circle flanked by red circles. The children held a button box with a green pushbutton and a red pushbutton. Instructions were to press the button that corresponded to the color of the central circle regardless of the color of the flanking circles as quickly and correctly as possible. The children were given up to 1300 ms to respond. The 800 ms feedback display is presented 450 ms after the response. The feedback display consists of a 1-inch yellow face, which is smiling to indicate a correct response or frowning to indicate an incorrect response. The inter-trial interval was between 0 and 500 ms. The task took the children about 20 min to complete and consisted of 3 blocks of 60 trials each. To ensure that the children understood the task instructions, the children’s color vision, color familiarity, and comprehension were assessed and the children completed eight practice trials prior to beginning the task.
Electrophysiological Recording and Analysis
A calibration file was collected by running a 50-μV, 10-Hz calibration signal through all EEG channels prior to collecting the EEG data. A Lycra cap fitted with tin electrodes in accordance with the International 10–20 System (Jasper, 1958) was used to record the EEG data. With the Cz serving as the reference site and Afz serving as the ground site, recordings were obtained from the mastoids (M1 and M2) and 26 scalp sites. Two channels of electrooculogram (EOG) were recorded with a mini-electrode placed above and below the left eye for vertical EOG and a mini-electrode placed at the outer canthus of each eye for horizontal EOG. Electrode impedances were tested to ensure that all of the sites had an impedance reading of 10 KS or less before and after EEG data collection.
The EEG signals were amplified by a 32-channel Mode lBA bioamplifier using filter settings of 0.1 Hz and 100 Hz. The data was digitized using a sampling rate of 512 Hz with an IOtech DaqBook A/D converter and re-referenced offline using an average mastoid configuration. The ERP Analysis system (James Long Company, Caroga Lake, NY) was used for artifact scoring with artifact due to vertical eye movements regressed out and epochs with signals +/− 200 μV excluded from analyses. Trials with reaction times of less than 300 ms and errors of omission were excluded from EEG analyses. EEG data was then time-locked to the onset of feedback display, corrected using a baseline window of −150 to −50 ms relative to feedback display, and quantified separately for negative versus positive feedback trials. A 15 Hz low-pass filter was used for digital re-filtering. The FRNn and FRNp were quantified as the peak-to-peak amplitude (or the difference between the maximum positive peak between 160 – 260 ms and the maximum negative peak between 300 – 600 ms) for negative and positive feedback, respectively. Electrode sites Fz and FCz were examined, as these sites have been used in previous FRN research (Santesso et al., 2008). To be included in analyses, a child had to have at least 10 artifact-free negative feedback trials and 10 artifact-free positive feedback trials. Although this criterion is less stringent than is typically used in adult samples, it is consistent with previous research with at-risk developmental samples (Brooker & Buss, 2014; Bruce et al., 2009; Suárez-Pellicioni, Núñez-Peña, & Colomé, 2013). The average number of total trials per participant was 120.33 (SD = 35.05, range 28–167), with an average of 88.33 trials (SD = 32.75, range 16–125) for positive feedback and 32.00 trials (SD = 13.65, range (10–64)) for negative feedback. There were no significant differences in these trial counts between children with high and low impulsivity (p >.05).
The Institutional Review Board (IRB) of the research center as well as the Public Health IRB for the state approved all study procedures.
Statistical Analyses
All reaction time variables were inverse-transformed following standard reaction time analysis recommendations (Whelan, 2010). Post-error slowing on incongruent trials was calculated by subtracting each participant’s transformed average reaction time on incongruent trials following errors of commission from each participant’s transformed average reaction time on incongruent trials following correct trials. Bivariate Pearson correlations were examined to determine associations between the amplitude of the FRN (FRNd, FRNn, and FRNp), accuracy on the incongruent trials, reaction time on correct incongruent trials, post-error slowing on incongruent trials, and impulsivity. Independent sample t-tests were conducted to determine if there were significant differences on variables of interest based on impulsivity group. The correlations between the amplitude of the FRN, accuracy on the incongruent trials, reaction time on correct incongruent trials, post-error slowing on incongruent trials, was then examined for children with high and low impulsivity separately. These Pearson correlation coefficients were statistically transformed using Fisher’s r to z transformation online calculator and then were compared using a two-tailed Z-test (Preacher, 2002). All other analyses were conducted in SPSS v.19 (IBM Corp., 2010).
Results
Behavioral Measures
Flanker task behavioral performance analyses indicated a mean correct response rate of 57.6% (SD = 14.2), which included a high percentage of errors of omission (M = 18.8%, SD = 12.0). Paired sample t-tests indicated the presence of a flanker interference effect: significantly more correct trials on congruent vs. incongruent trials, t(66) = −6.36, p < 0.001. Specifically, there were fewer errors of commission on congruent vs. incongruent trials, t(66) = 6.40, p < 0.001, but no significant differences on errors of omission on congruent vs. incongruent trials, t(66) = .65, ns.
Paired sample t-tests with inverse-transformed reaction time variables did not support a significant flanker interference effect. In fact, participants responded significantly faster on correct incongruent vs. correct congruent trials, t(66) = 2.76, p < .01. Paired sample t-tests also did not suggest that participants exhibited post-error slowing overall, with no significant differences in reaction time on incongruent trials following correct vs. error of commission trials, t(66) = −.45, ns. We did, however, compute each participant’s post-error slowing to examine if individual differences in post-error slowing were significantly related to other variables of interest. See Table 1 for additional descriptive information.
Table 1.
Behavioral Measures from the Flanker Task
| Congruent | Incongruent | Total | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| M (SD) | Range | M (SD) | Range | M (SD) | Range | |
| Percent correct | 61.95 (15.34) | 25.9 – 91.7 | 55.77 (14.41) | 23.81 – 85.17 | 57.63 (14.24) | 24.44 – 87.50 |
| Percent errors of omissions | 18.47 (12.84) | 0.0 – 53.7 | 18.95 (12.12) | 1.19 – 46.03 | 18.81 (12.03) | .83 – 47.78 |
| Percent errors of commission | 19.57 (8.42) | 5.6 – 42.6 | 25.3 (9.4) | 5.56 – 50.0 | 23.57 (8.49) | 6.11 – 42.22 |
| Correct incongruent trial reaction time (ms) | 745.50 (113.97) | 445.85 – 930.16 | 730.08 (135.07) | 378.12 – 956.56 | 735.92 (124.54) | 400.90 – 944.26 |
Note: Raw reaction time data is reported here for ease of interpretation, but inverse-transformed data was used in all analyses.
Electrophysiological Measures
Amplitude of the FRN to positive (FRNp) and negative (FRNn) feedback was analyzed using a repeated measures ANOVA with feedback type (positive and negative) and electrode site (Fz and FCz) as within-subject factors. These analyses indicated a main effect of feedback type, F(1,65) = 14.46, p < 0.001, with positive feedback trials eliciting a smaller peak-to-peak amplitude (M = −7.32 μV, SD = .67) compared to negative feedback trials (M = −10.02 μV, SD = .89). Additionally, there was a main effect of electrode site, F(1,65) = 9.06, p > 0.01, with a more negative peak-to-peak amplitude at Fz (M = −8.95 μV, SD = .70) than at FCz (M = −8.39 μV, SD = .72). There was not a significant feedback type x electrode site interaction.
At electrode sites Fz and FCz, the peak-to-peak amplitude of both the FRNn and FRNp were significantly correlated, r = .96, p < 0.001 and r = .96, p < 0.001, respectively, and thus each component was averaged across sites to create a more reliable index of the FRNn and FRNp for the remaining analyses (presented in Table 2). The amplitude of the difference wave FRN (FRNd) was then calculated by subtracting the peak-to-peak amplitude of the FRNp from the peak-to-peak amplitude of the FRNn. For the FRNd, a larger negative number indicates a greater difference between the FRNp and the FRNn.
Table 2.
Bivariate Correlations
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Incongruent accuracy | 1.00 | ||||||
| 2. Impulsivity | −.16 | 1.00 | |||||
| 3. FRNd | −.11 | −.27* | 1.00 | ||||
| 4. FRNn | −.21† | −.21† | .67** | 1.00 | |||
| 5. FRNp | −.16 | .01 | −.18 | .61** | 1.00 | ||
| 6. Correct incongruent trial reaction time (ms) | −.49** | .26* | .11 | .05 | −.04 | 1.00 | −.13 |
| 7. Post-error slowing (ms) | −.20 | −.28* | −.08 | −.06 | −.01 | −.48** | 1.00 |
p < 0.01,
p < 0.05,
p < 0.10
Note: The reaction time and post-error slowing variables are inverse transformed. Therefore, correlations with these variables should be interpreted in the opposite direction (e.g., a negative correlation between impulsivity and post-error slowing suggests that higher impulsivity is associated with higher post-error slowing).
Correlational Analyses
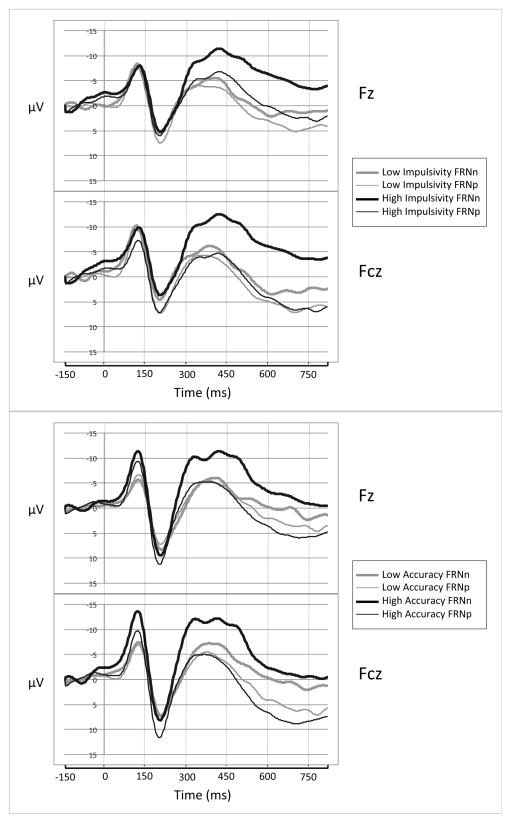
Correlational analyses with the whole sample are presented in Table 2. Incongruent trial accuracy was significantly correlated with correct incongruent trial reaction time, with longer reaction times associated with higher accuracy. Incongruent trial accuracy was not significantly associated with the FRNd or FRNp. Incongruent trial accuracy was, however, marginally negatively correlated with amplitude of the FRNn, with a more pronounced FRNn associated with higher accuracy. (See Figure 1 for grand average ERP waveforms to positive and negative feedback trials using an extreme tertile split on incongruent trial accuracy and impulsivity.) Impulsivity was not associated with incongruent trial accuracy, but was significantly correlated with correct incongruent trial reaction time (i.e., higher impulsivity, shorter reaction time) and post-error slowing (i.e., higher impulsivity, more post-error slowing). Impulsivity was also significantly negatively correlated with the FRNd, and marginally negatively correlated with the FRNn (i.e., higher impulsivity, a more pronounced FRNd/FRNn).
Figure 1.
Grand Average ERP Waveforms to Positive and Negative Feedback Trials for Children with High and Low Incongruent Trial Accuracy and Impulsivity
Note: The top and bottom third of children are contrasted in the ERP waveforms for display purposes only. All of the children are included in the analyses.
Different Pattern of Relations based on Impulsivity
As described above, we identified children with low impulsivity (N = 32) and high impulsivity (n = 35). A series of independent sample t-tests indicated that there were no significant differences in flanker task accuracy, correct incongruent trial reaction times, or post-error slowing between children with high and low impulsivity. There was a significantly more pronounced FRNd in children with high impulsivity compared to children with low impulsivity, t(65) = 2.82, p < .01, which was driven by a significantly different FRNn, t(65) = 2.16, p < .05, but minimal differences on the FRNp, t(65) = −.07, ns. See Table 3 for descriptive information on all variables between impulsivity groups.
Table 3.
Task Descriptives Split by High/Low Impulsivity
| Overall Group (n = 71) | Low Impulsivity (n = 32) | High Impulsivity (n = 35) | Difference between groups | |
|---|---|---|---|---|
| Incongruent accuracy | 55.77 (14.41) | 56.60 (15.08) | 55.02 (13.96) | ns |
| Impulsivity | 4.82 (1.13) | 3.86 (.73) | 5.69 (.63) | p < .001 |
| FRNd | −2.62 (5.76) | −.65 (5.79) | −4.43 (5.18) | p < .01 |
| FRNn | −9.95 (7.19) | −8.02 (7.04) | −11.71 (6.96) | p <.05 |
| FRNp | −7.32 (5.43) | −7.34 (5.39) | −7.27 (5.53) | ns |
| Correct incongruent trial reaction time (ms) | 730.08 (135.06) | 753.67 (115.20) | 708.51 (149.36) | ns |
| Post-error slowing (ms) | 7.05 (118.66) | −10.64 (111.30) | 23.23 (124.38) | ns |
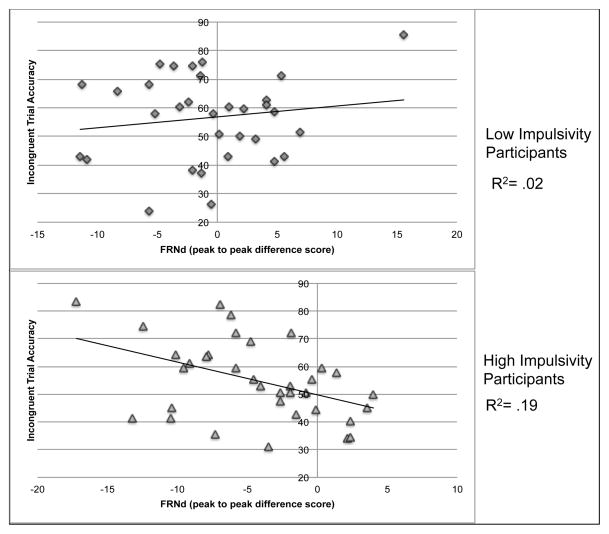
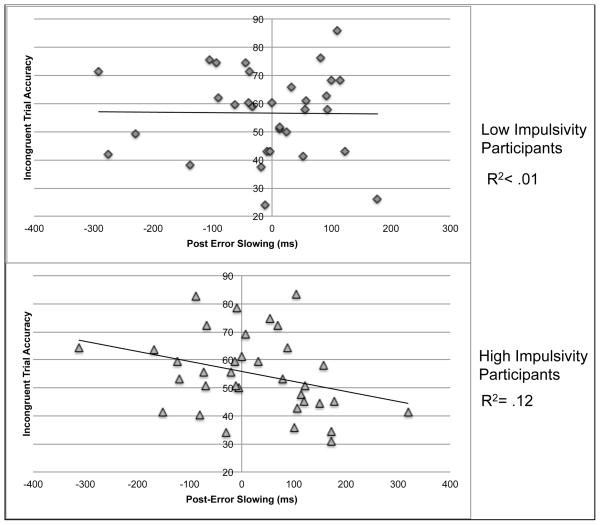
For children with low impulsivity, there was no significant association between incongruent trial accuracy and amplitude of the FRNd, r = .15, ns. There was, however, a significant association between the amplitude of the FRNd and incongruent trial accuracy in the children with high impulsivity, r = −.44, p < 0.01. These correlations were determined to be significantly different based on Fisher’s r to z transformation and associated Z-test, Z = 2.43, p < .05 (Figure 2.). There was also a significant association between incongruent trial accuracy and post-error slowing in the children with high impulsivity, r = .34, p < .05, which was not present in the children with low impulsivity, r = .02, ns (Figure 3.). However, these correlation coefficients were not significantly different, Z = −1.30, p > .05. In both groups, there were significant associations with incongruent trial accuracy and correct incongruent trial reaction time with faster reaction time associated with lower accuracy. Scatterplots of the association between the amplitude of the FRNd and incongruent trial accuracy and between post-error slowing and incongruent trial accuracy for children with high and low impulsivity are displayed in Figure 2.
Figure 2.
The Relationship between FRNd and Accuracy Split by High/Low Impulsivity
Figure 3.
The Relationship between Post-Error Slowing and Accuracy Split by High/Low Impulsivity
Note: The raw post-error slowing reaction time data is presented in the figure for display purposes, but R2 values are from the inverse-transformed data, on which all statistics were performed.
Discussion
We examined the associations between child impulsivity, an ERP index of performance feedback processing, and accuracy and reaction time on a task assessing inhibitory control in a sample of maltreated preschool-aged children in foster care. This population is at risk for disruptive behaviors and the associated psychopathology (e.g., ADHD and oppositional defiant disorder) and, as such, is an important group in which to understand the underlying neural bases of these behaviors. Individual differences in impulsivity significantly predicted the amplitude of the FRNd (difference between the peak-to-peak amplitude of the FRN to negative vs. positive feedback). A more pronounced FRNn marginally predicted higher accuracy in the entire sample, although the FRNd did not. Notably, however, results suggest that an exaggerated FRNd may be particularly important to effective inhibitory control performance in children with high impulsivity. As elaborated below, these results have important implications for understanding children’s emerging inhibitory control skills.
Our results extend research seeking to understand the association between a more pronounced FRNd and task accuracy and learning in adults and adolescents (Bellebaum & Daum, 2008; Cohen & Ranganath, 2007). In the current study, the FRNd was not significantly associated with flanker task accuracy in the whole sample; however, in the subsample of children with high impulsivity, the FRNd was highly associated with accuracy. We suggest, that the FRNd may not reliably predict improved inhibitory control accuracy in young children (although the FRNn, specifically, was marginally predictive of accuracy in the whole sample), but may be predictive for specific subgroups of children. Prior developmental studies have examined the role of the FRNd in tasks using performance feedback, but not in populations under the age of 9 (Eppinger et al., 2009; Hämmerer et al., 2011; Santesso, Dzyundzyak, & Segalowitz, 2011; Zottoli & Grose-Fifer, 2012). Our findings suggest that the FRNd may have a functionally similar role in processing performance feedback for some, but not all, maltreated preschool-aged children.
It is also noteworthy that impulsivity predicts an exaggerated FRNd. This finding is consistent with the developmental and adult literature examining the FRNd in populations with high impulsivity. Specifically, developmental studies using utilitarian feedback report exaggerated FRNd to negative feedback in children with ADHD (Holroyd et al., 2008; van Meel et al., 2005), and recent findings with adults link extraversion to an exaggerated FRNd during a gambling task (Smillie, Cooper, & Pickering, 2011). However, to our knowledge, the FRNd has not been examined in individuals with high impulsivity during tasks with performance feedback. Unlike previous studies that could not establish the functional utility or impairment conferred by an exaggerated FRNd in children with high impulsivity, our findings suggest that this electrophysiological response may not be maladaptive. Instead, these results suggest that it is precisely the children with high impulsivity who need a more pronounced FRNd to effectively update performance strategies. Findings regarding a significant relationship between accuracy and post-error slowing in the children with high impulsivity, but not low impulsivity, also suggest that response-monitoring behaviors in children with high impulsivity may be particularly important to achieving accurate performance on an inhibitory control task.
Given the association between a more pronounced FRNd and better accuracy in children with high impulsivity, it will be important to determine whether feedback processing may serve as a stepping stone for scaffolding gains in inhibitory control over time. Since children who experience early life adversity such as low socioeconomic status, maltreatment, and family chaos are more likely to show high impulsivity, an exaggerated FRNd may be particularly important among individuals with these backgrounds for engaging effective inhibitory control behavior (Braquehais, Oquendo, Baca-Garcia, & Sher, 2010; Ouyang, Fang, Mercy, Perou, & Grosse, 2008).
A possible explanation for the lack of an association between the FRNd and flanker task accuracy in children with low impulsivity might be that children with this characteristic do not require as pronounced a response to external feedback to monitor their behavior. Prior developmental ERP and functional magnetic resonance imaging (fMRI) studies have reported increased brain activity in inhibitory control tasks in children when compared to adults, which is theorized to relate to the increased effort required to perform tasks effectively (Luna, Padmanabhan, & O’Hearn, 2010). In line with this conceptualization, children with low impulsivity may have a more efficient feedback processing network and thus requires less neural activity to effectively update performance strategies. Of additional note is the lack of a significant relationship between impulsivity and flanker task accuracy. Although we expected that children with high impulsivity would exhibit poorer accuracy on the flanker task, we note that the relatively small sample size and high standard deviation in accuracy may obscure relevant differences. It is also possible that the children with high impulsivity were able to maintain higher accuracy through increased effort and feedback processing, as reflected by the significantly larger FRNd. Although higher impulsivity was associated with faster reaction time, as expected, our results suggest that children with high impulsivity were able to exhibit adaptive response monitoring following incorrect trials, as reflected by the association between impulsivity and post-error slowing.
This study had a number of limitations. One methodological issue was the relatively high rate of errors of omission. This suggests that some children had difficulties with task performance, task motivation, or sustained attention. We were also limited by a moderate sample size and an ERP trial inclusion criterion that was lower than that typically used in the adult literature. However, we note that, compared to previous ERP research with young children, the sample size was comparable (or larger) and the inclusion criterion was similar (or more stringent), particularly considering the high-risk nature of this sample (Brooker & Buss, 2014; Bruce et al., 2009; Mai et al., 2011). Additionally, the children had different numbers of artifact-free trials with positive vs. negative feedback based on their performance. Finally, given the cross sectional nature of the study, the directionality of associations needs to be interpreted with caution.
In the present study, we focused on the regulatory concept of impulsivity given the small but existent literature on the FRNd and impulsivity in other samples, such as children with ADHD and adults with high and low impulsivity (Martin & Potts, 2009; van Meel et al., 2005). However, future research investigating other self-regulatory processes such as self-regulation and behavioral inhibition is warranted. Additionally, research directly comparing individual differences in the FRNd to utilitarian and performance feedback would be of interest. It is important to understand the extent to which atypical exaggeration (or blunting) of the electrophysiological response is related to clinical symptoms and reflects either inefficient cognitive processing or an effective compensatory strategy.
Our results suggest that an exaggerated FRNd may be particularly adaptive for achieving higher inhibitory control accuracy in children with high impulsivity. This may be particularly relevant for young children, as children in these populations may have less developed inhibitory control abilities. Future research should aim to investigate how differences in ERP components believed to reflect feedback processing at young ages predict differential susceptibility to negative outcomes, including disruptive behaviors. Such knowledge may help us to better target interventions to improve feedback processing skills and moderate the long-term risk associated with early adversities, such as child maltreatment.
Acknowledgments
Support for this article was provided by the following grants: R01 DA021424, P30 DA023920, and P50 DA035763 NIDA, U.S. PHS; and P50 MH078105 NIMH, U.S. PHS. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the funding organization. The authors thank Deena Scheidt and Angie Relling for project management, Diana Strand for editorial assistance, and the staff and families of the Kids In Transition to School project for their ongoing dedication and participation.
Contributor Information
Leslie E. Roos, Department of Psychology, University of Oregon
Katherine Pears, Oregon Social Learning Center.
Jacqueline Bruce, Oregon Social Learning Center.
Hyoun K. Kim, Oregon Social Learning Center
Philip A. Fisher, Department of Psychology, University of Oregon
References
- Bellebaum C, Daum I. Learning-related changes in reward expectancy are reflected in the feedback-related negativity. European Journal of Neuroscience. 2008;27:1823–1835. doi: 10.1111/j.1460-9568.2008.06138.x. [DOI] [PubMed] [Google Scholar]
- Braquehais MD, Oquendo MA, Baca-García E, Sher L. Is impulsivity a link between childhood abuse and suicide? Comprehensive Psychiatry. 2010;51:121–129. doi: 10.1016/j.comppsych.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA. Toddler fearfulness is linked to individual differences in error-related negativity during preschool. Developmental Neuropsychology. 2014;39:1–8. doi: 10.1080/87565641.2013.826661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J, McDermott JM, Fisher PA, Fox NA. Using behavioral and electrophysiological measures to assess the effects of a preventive intervention: A preliminary study with preschool-aged foster children. Prevention Science. 2009;10:129–140. doi: 10.1007/s11121-008-0115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Rapoport JL. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Current Opinion in Psychiatry. 2007;20:255–261. doi: 10.1097/YCO.0b013e3280ba4989. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Ranganath C. Reinforcement learning signals predict future decisions. The Journal of Neuroscience. 2007;27:371–378. doi: 10.1523/JNEUROSCI.4421-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Somsen RJ, Zanolie K, Van der Molen MW. A heart rate analysis of developmental change in feedback processing and rule shifting from childhood to early adulthood. Journal of Experimental Child Psychology. 2006;95:99–116. doi: 10.1016/j.jecp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Mock B, Kray J. Developmental differences in learning and error processing: Evidence from ERPs. Psychophysiology. 2009;46:1043–1053. doi: 10.1111/j.1469-8986.2009.00838.x. [DOI] [PubMed] [Google Scholar]
- Espy KA, McDiarmid MM, Cwik MF, Stalets MM, Hamby A, Senn TE. The contribution of executive functions to emergent mathematic skills in preschool children. Developmental Neuropsychology. 2004;26:465–486. doi: 10.1207/s15326942dn2601_6. [DOI] [PubMed] [Google Scholar]
- Foley M, McClowry SG, Castellanos FX. The relationship between attention deficit hyperactivity disorder and child temperament. Journal of Applied Developmental Psychology. 2008;29:157–169. doi: 10.1016/j.appdev.2007.12.005. [DOI] [Google Scholar]
- Frank MJ, Woroch BS, Curran T. Error-related negativity predicts reinforcement learning and conflict biases. Neuron. 2005;47:495–501. doi: 10.1016/j.neuron.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Hämmerer D, Li SC, Müller V, Lindenberger U. Life span differences in electrophysiological correlates of monitoring gains and losses during probabilistic reinforcement learning. Journal of Cognitive Neuroscience. 2011;23:579–592. doi: 10.1162/jocn.2010.21475. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Buck SM, Themanson JR, Pontifex MB, Castelli DM. Aerobic fitness and cognitive development: Event-related brain potential and task performance indices of executive control in preadolescent children. Developmental Psychology. 2009;45:114–129. doi: 10.1037/a0014437. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Baker TE, Kerns KA, Müller U. Electrophysiological evidence of atypical motivation and reward processing in children with attention-deficit hyperactivity disorder. Neuropsychologia. 2008;46:2234–2242. doi: 10.1016/j.neuropsychologia.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037//0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS statistics for windows, Version 19.0. Armonk, NY: Author; 2010. [Google Scholar]
- Jasper HH. The ten twenty electrode system of the international federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- LoCasale-Crouch J, Konold T, Pianta R, Howes C, Burchinal M, Bryant D, Barbarin O. Observed classroom quality profiles in state-funded pre-kindergarten programs and associations with teacher, program, and classroom characteristics. Early Childhood Research Quarterly. 2007;22:3–17. doi: 10.1016/j.ecresq.2006.05.001. [DOI] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai X, Tardif T, Doan SN, Liu C, Gehring WJ, Luo YJ. Brain activity elicited by positive and negative feedback in preschool-aged children. PloS one. 2011;6:e18774. doi: 10.1371/journal.pone.0018774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LE, Potts GF. Impulsivity in decision-making: An event-related potential investigation. Personality and Individual Differences. 2009;46:303–308. doi: 10.1016/j.paid.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JM, Pérez-Edgar K, Fox NA. Variations of the flanker paradigm: Assessing selective attention in young children. Behavior Research Methods. 2007;39:62–70. doi: 10.3758/BF03192844. [DOI] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Moser JS, Simons RF. The neural consequences of flip-flopping: The feedback-related negativity and salience of reward prediction. Psychophysiology. 2009;46:313–320. doi: 10.1111/j.1469-8986.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Ouyang L, Fang X, Mercy J, Perou R, Grosse SD. Attention-deficit/hyperactivity disorder symptoms and child maltreatment: A population-based study. The Journal of Pediatrics. 2008;153:851–856. doi: 10.1016/j.jpeds.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Pardini D, Lochman J, Wells K. Negative emotions and alcohol use initiation in high-risk boys: The moderating effect of good inhibitory control. Journal of Abnormal Child Psychology. 2004;32:505–518. doi: 10.1023/B:JACP.0000037780.22849.23. [DOI] [PubMed] [Google Scholar]
- Pears KC, Fisher PA, Bruce J, Kim HK, Yoerger K. Early elementary school adjustment of maltreated children in foster care: The roles of inhibitory control and caregiver involvement. Child Development. 2010;81:1550–1564. doi: 10.1111/j.1467-8624.2010.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears KC, Fisher PA, Kim HK, Bruce J, Healey CV, Yoerger K. Immediate effects of a school readiness intervention for children in foster care. Early Education and Development. 2013;24:771–791. doi: 10.1080/10409289.2013.736037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts GF, Martin LE, Kamp SM, Donchin E. Neural response to action and reward prediction errors: Comparing the error-related negativity to behavioral errors and the feedback-related negativity to reward prediction violations. Psychophysiology. 2011;48:218–228. doi: 10.1111/j.1469-8986.2010.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ. Calculation for the test of the difference between two independent correlation coefficients [Computer software] 2002 May; Available from http://quantpsy.org.
- Raaijmakers MA, Smidts DP, Sergeant JA, Maassen GH, Posthumus JA, Van Engeland H, Matthys W. Executive functions in preschool children with aggressive behavior: Impairments in inhibitory control. Journal of Abnormal Child Psychology. 2008;36:1097–1107. doi: 10.1007/s10802-008-9235-7. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Riggs S, Alario AJ, McHorney C. Health risk behaviors and attempted suicide in adolescents who report prior maltreatment. The Journal of Pediatrics. 1990;116:815–821. doi: 10.1016/S0022-3476(05)82679-4. [DOI] [PubMed] [Google Scholar]
- Rimm-Kaufman SE, Pianta RC, Cox MJ. Teachers’ judgments of problems in the transition to kindergarten. Early Childhood Research Quarterly. 2000;15:147–166. doi: 10.1016/S0885-2006(00)00049-1. [DOI] [Google Scholar]
- Rosch KS, Hawk LW. The effects of performance-based rewards on neurophysiological correlates of stimulus, error, and feedback processing in children with ADHD. Psychophysiology. 2013;50:1157–1173. doi: 10.1111/psyp.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, Fisher P. Investigations of temperament at three to seven years: The Children’s Behavior Questionnaire. Child Development. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Bogdan R, Birk JL, Goetz EL, Holmes AJ, Pizzagalli DA. Neural responses to negative feedback are related to negative emotionality in healthy adults. Social Cognitive and Affective Neuroscience. 2012;7:794–803. doi: 10.1093/scan/nsr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Dillon DG, Birk JL, Holmes AJ, Goetz E, Bogdan R, Pizzagalli DA. Individual differences in reinforcement learning: Behavioral, electrophysiological, and neuroimaging correlates. Neuroimage. 2008;42:807–816. doi: 10.1016/j.neuroimage.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Dzyundzyak A, Segalowitz SJ. Age, sex, and individual differences in punishment sensitivity: Factors influencing the feedback-related negativity. Psychophysiology. 2011;48:1481–1489. doi: 10.1111/j.1469-8986.2011.01229.x. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ. Developmental differences in error-related ERPs in middle-to late-adolescent males. Developmental Psychology. 2008;44:205. doi: 10.1037/0012-1649.44.1.205. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Dywan J. Individual differences and developmental change in the ERN response: Implications for models of ACC function. Psychological Research. 2009;73:857–870. doi: 10.1007/s00426-008-0193-z. [DOI] [PubMed] [Google Scholar]
- Simonsen B, Fairbanks S, Briesch A, Myers D, Sugai G. Evidence-based practices in classroom management: Considerations for research to practice. Education and Treatment of Children. 2008;31:351–380. doi: 10.1353/etc.0.0007. [DOI] [Google Scholar]
- Smetana JG. Preschool children’s conceptions of moral and social rules. Child Development. 1981;52:1333–1336. doi: 10.2307/1129527. [DOI] [Google Scholar]
- Smillie LD, Cooper AJ, Pickering AD. Individual differences in reward–prediction–error: Extraversion and feedback-related negativity. Social Cognitive and Affective Neuroscience. 2011;6:646–652. doi: 10.1093/scan/nsq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair-Thompson HL, Gathercole SE. Executive functions and achievements in school: Shifting, updating, inhibition, and working memory. The Quarterly Journal of Experimental Psychology. 2006;59:745–759. doi: 10.1080/17470210500162854. [DOI] [PubMed] [Google Scholar]
- Stirling J, Amaya-Jackson L. Understanding the behavioral and emotional consequences of child abuse. Pediatrics. 2008;122:667–673. doi: 10.1542/peds.2008-1885. [DOI] [PubMed] [Google Scholar]
- Suárez-Pellicioni M, Núñez-Peña MI, Colomé À. Abnormal error monitoring in math-anxious individuals: Evidence from error-related brain potentials. PloS one. 2013;8:e81143. doi: 10.1371/journal.pone.0081143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helden J, Boksem MA, Blom JH. The importance of failure: Feedback-related negativity predicts motor learning efficiency. Cerebral Cortex. 2010;20:1596–1603. doi: 10.1093/cercor/bhp224. [DOI] [PubMed] [Google Scholar]
- van Meel CS, Heslenfeld DJ, Oosterlaan J, Luman M, Sergeant JA. ERPs associated with monitoring and evaluation of monetary reward and punishment in children with ADHD. Journal of Child Psychology and Psychiatry. 2011;52:942–953. doi: 10.1111/j.1469-7610.2010.02352.x. [DOI] [PubMed] [Google Scholar]
- van Meel CS, Oosterlaan J, Heslenfeld DJ, Sergeant JA. Telling good from bad news: ADHD differentially affects processing of positive and negative feedback during guessing. Neuropsychologia. 2005;43:1946–1954. doi: 10.1016/j.neuropsychologia.2005.03.018. [DOI] [PubMed] [Google Scholar]
- van Noordt SJ, Segalowitz SJ. Performance monitoring and the medial prefrontal cortex: A review of individual differences and context effects as a window on self-regulation. Frontiers in Human Neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R. Effective analysis of reaction time data. The Psychological Record. 2010;58(3):9. [Google Scholar]
- Zottoli TM, Grose-Fifer J. The feedback-related negativity (FRN) in adolescents. Psychophysiology. 2012;49:413–420. doi: 10.1111/j.1469-8986.2011.01312.x. [DOI] [PubMed] [Google Scholar]