Introduction
Pancreatic cancer (PCa) is the fourth most common cause of all cancer-related deaths in the USA. Currently, the overall median survival is less than 1 year and 5-year survival rate is about 5 % [1]. The poor outcome for PCa is largely due to the fact that the vast majority of patients (pts) are diagnosed at an advanced disease stage or with occult metastases that soon reveal themselves despite no clinical or radiographic clues. Therefore, early and accurate diagnosis is critical to appropriately risk stratify and intervene to improve the clinical outcomes of pts with PCa.
Diagnostic modalities in current use include cross-sectional imaging and the use of the non-specific serum biomarker carbohydrate antigen 19-9 (CA-19-9) and occasionally carcinoembryonic antigen (CEA). Radiographic imaging of PCa can be challenging due to the potential absence of a discrete pancreatic mass or presence of alternate symptom etiologies including pancreatic inflammation. Even in the setting of an appropriate clinical context with supportive imaging and serum biomarkers, assurance of histological or cytological confirmation of PCa is directly related to adequate tissue acquisition [2]. Methodologies for pancreatic tissue acquisition are invasive, of relative low diagnostic yield, and associated with potential complications such as pancreatitis, bleeding, duodenal perforations, and infections [3]. Therefore, novel non-invasive or minimally invasive methods of accurate PCa cell acquisition represent a clinical unmet need.
Progression of most solid cancers is associated with intravasation of cancer cells into the pt’s circulatory system with dissemination to metastatic sites. These circulating tumor cells (CTCs), measured by collection and detection of epithelial cells in the peripheral circulation, are known to be detectable in pts with solid tumors from early through advanced disease [4]. In addition to diagnostic sampling, CTCs have many potential clinical applications in the management of patients with solid tumors. These include, but are not limited to, risk stratification/prognostication, monitoring of response to therapy, characterizing the tumor’s molecular alterations, screening for early relapse, and other potential ways to personalize therapy [5–9]. In this brief report, we describe the successful isolation and molecular characterization of CTCs from a pt with PCa in whom traditional acquisition of tissue for diagnosis and management was unsuccessful despite multiple traditional attempts.
Clinical Case
Patient was a 65-year-old Caucasian male with a remote history of a Billroth-II partial gastrectomy for a bleeding ulcer 30 years ago. He was in his usual state of health until reporting to his primary care provider a 2–3-month history of near constant and progressive mid-back discomfort. He denied any trauma to his back, radiculopathy, or neuromuscular weakness. Clinical examination was unremarkable with MRI of his back being consistent with degenerative joint disease non-responsive to physical therapy with non-steroidal anti-inflammatories. Two weeks later, he developed jaundice (total bilirubin 11 mg/dL), darkening urine, and fevers, all clinically consistent with obstructive jaundice and ascending cholangitis. Liver ultrasound demonstrated dilated common and intrahepatic bile ducts. CT scan of the abdomen confirmed the presence of a 3 × 2.8 cm hypodense pancreatic mass in the uncinate process which encased the superior mesenteric artery and vein with occlusion of the superior mesenteric vein with associated pancreatic ductal obstruction and periportal and regional pancreatic lymphadenopathy (Fig. 1a). PET scan confirmed focal FDG-18-glucose uptake (SUV = 2.8) at the pancreatic head mass (Fig. 1a insert). Serum CA19-9 level was 93 U/mL. Diagnostic and therapeutic ERCP was undertaken for diagnosis and biliary stent placement. Although technically challenging given the prior Billroth-II anatomy, it confirmed an extrinsic compression resulting in intrapancreatic biliary stricture with successful deployment of a biliary stent. Cytology was determined to be adequate, but was without malignant cells identified. Discussion at multidisciplinary PCa case conference confirmed the clinical diagnosis of locally advanced pancreatic adenocarcinoma. Peripheral blood was voluntarily obtained (see “Materials and Methods”) for CTC assessment and institutional oncology biorepository after informed consent.
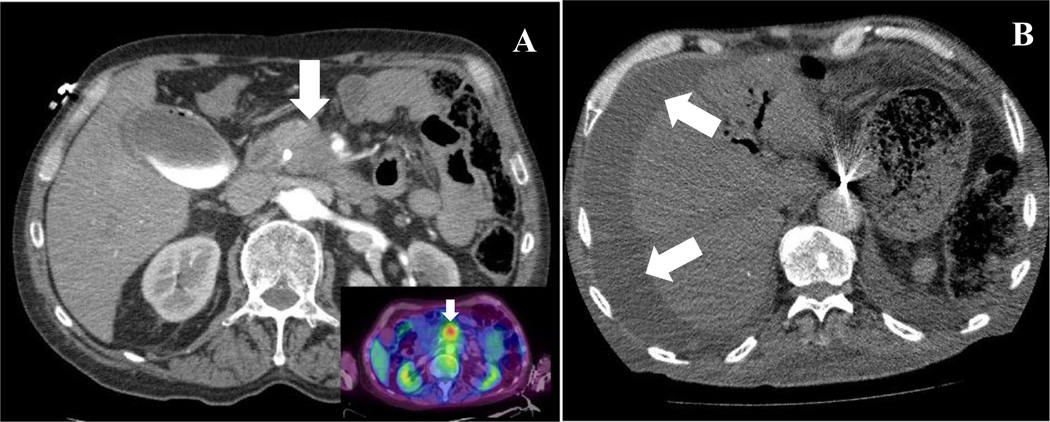
Fig. 1.
a CT scan demonstrating pancreatic head mass (arrow) with involvement of SMV and SMA; Inset: FDG-18-glucose uptake in area of pancreatic mass. b CT scan demonstrating ascites (arrows) and peritoneal implants (not shown). SMV superior mesenteric vein, SMA superior mesenteric artery
Palliative chemotherapy was initiated. After several cycles, the pt had a biochemical response with CA19-9 reduction to a nadir of 26 U/mL, without overt progression of metastases, but continued clinical symptoms. Palliative radiotherapy was provided after endoscopic biliary stent revision. Repeat endoscopic brushings and biopsies at that time were again non-diagnostic for malignancy. After completion of palliative radiotherapy, his serum CA19-9 level began to rise to 322 U/mL and he developed increasing abdominal girth and anorexia. Repeat imaging confirmed new ascites (Fig. 1b), mesenteric stranding, and caking c/w carcinomatosis and bilobar subcentimeter pulmonary nodules. Diagnostic and therapeutic large volume paracentesis was undertaken with results consistent with malignant ascites, but again, cytology was negative for tumor cells. The pulmonary lesions were deemed too small for accurate sampling. Given his clinical decline in performance status, supportive care was provided over the ensuing few weeks. He passed away 7 months after his symptomatic presentation.
Materials and Methods
A total of 6 mL of the pt’s whole blood was obtained through peripheral phlebotomy shortly after symptomatic presentation and analyzed using our recently described novel microfluidic device (Fig. 2) [10]. Quantification and characterization is detailed in the Supplemental Material (Materials and Methods section). A total of nine (9) confirmed CTCs were counted from the 6 mL of whole blood collected at the initial time point.
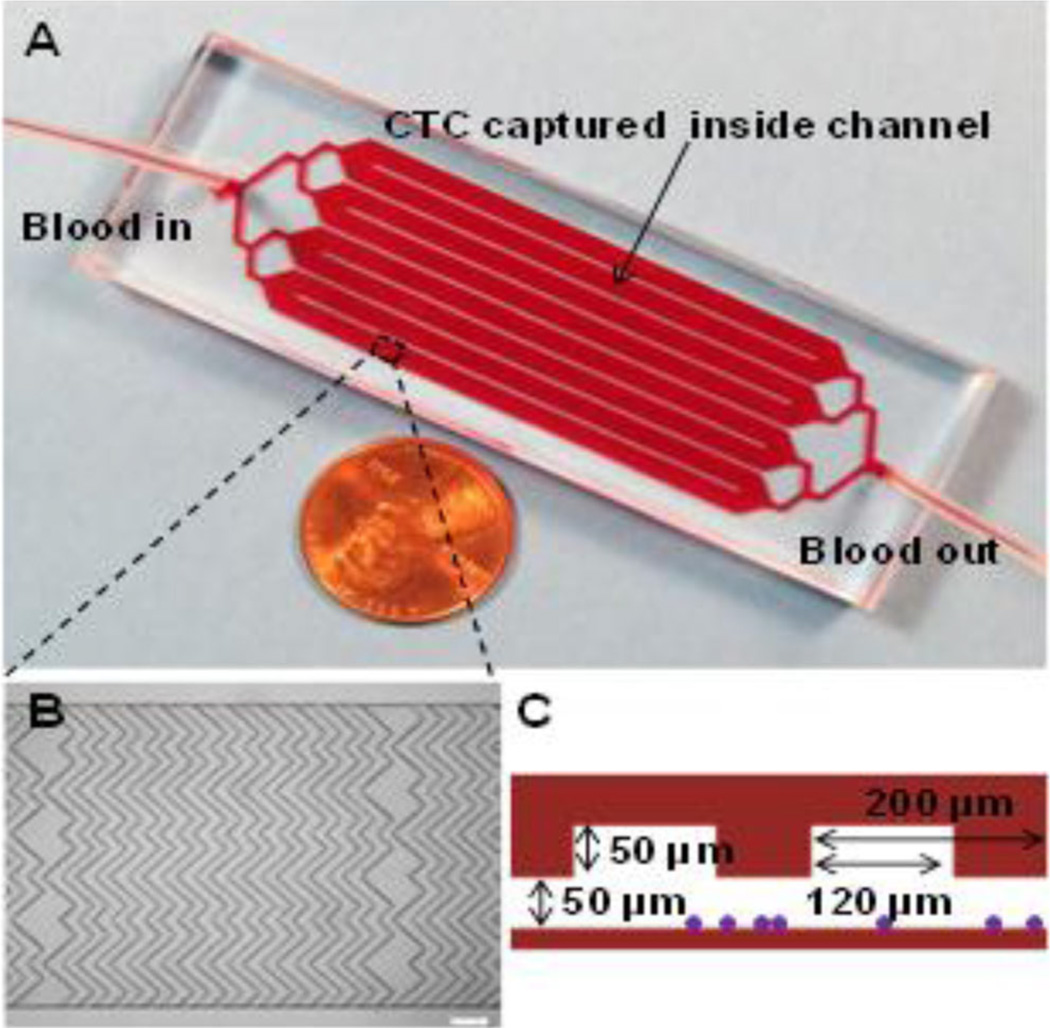
Fig. 2.
Microfluidic device used for this study. a Picture of microfluidic device consisting of eight parallel channels with single inlet and outlet. b Micrograph (×4 bright field) of the staggered herringbone grooves inside a channel that enhance mixing and promote the interactions between cells and antibodies on the channel surfaces, scale bar = 200 µm. c Cross-sectional illustration of a representative channel with groove and channel depth of both 50 µm with a groove width of 120 µm and pitch of 200 µm; purple dots illustrate cells captured inside a channel
Discussion
In this report, we describe the successful isolation, enumeration, and molecular characterization of CTCs from a pt with PCa in whom acquisition of diagnostic tissue for histopathologic or cytological confirmation was unsuccessful despite multiple and diverse attempts. Isolation and enumeration of CTCs in pts with a variety of solid tumors is well established with several studies indicating that CTCs may add prognostic value beyond traditional staging [3–9]. The use of “liquid biopsies” through simple phlebotomy for the diagnosis and monitoring of hematologic malignancies (e.g., chronic and acute leukemias) is well characterized and serves as a well-accepted clinical tool for non-invasive diagnosis, molecular characterization, and disease response monitoring. A similar potential tool in solid tumors utilizes CTCs. However, many studies suffer from relatively small sample sizes and a diversity of CTC assay types, accounting for some of the inconsistencies that have been noted.
Despite years of work, only the CellSearch® (Veridex; Janssen Diagnostics, LLC) is FDA-approved for use in pts with metastatic breast, prostate, or colorectal cancers [11, 12]. Successfully identifying CTCs in patients with PCa has been challenging in part owing to the absolute low number of CTCs circulating in the majority of pts, despite most having advanced and metastatic disease [8, 15]. For example, the FDA-approved CellSearch system was able to isolate and enumerate CTCs from patients with widely metastatic PCa only at a rate of 2±6 per 7.5 mL of blood compared 1–23,618 per 7.5 mL of blood in women with metastatic breast cancer [5–8]. In another study, the CellSearch system was able to a make positive CTC identification (≥1 per 7.5 ml of blood) in only 11 of 26 pts investigated even though 24 of them had incurable PCa with 12 having clinical evidence of metastases [13]. It is also notable that four of these pts with clinically metastatic PCa had no identifiable CTCs using the CellSearch system. A third study reported a median CTC count of 0 in 54 patients with PCa also using the CellSearch system [14]. It is noteworthy that the pt in our report did not have overt metastatic disease at the time of presentation. The lower absolute total tumor burden might contribute to even fewer CTCs in pts with non-metastatic disease. The CTC device and assay used in this report has a demonstrated higher CTC capture efficiency due to novel bioengineering augmentation and optimization of the flow rate and device geometry which may help overcome some of these previously identified limitations [10]. Together, these data demonstrate some of the unique challenges in advancing translational scientific and clinical progress in PCa.
Regardless, the future of clinical oncology care will most certainly include more readily available and accurate tools for cancer screening, prognosis, molecular profiling, therapeutic response, and monitoring of remission. CTCs and other readily available circulating cancer biomarkers (contained within a “liquid biopsy”) have the potential to fulfill that critical role. This approach has the potential to be a clinical and research “game changer” as individualized and personalized pt treatments increasingly require accurate determination of their cancer’s molecular profile, both at baseline as well as during the course of treatment. Importantly, this approach may also offer the development of a screening method for pts at risk for PCa as there is no currently recommended screening program or early diagnostic tool [15]. Earlier PCa diagnosis, well before most develop symptoms, offers a greater chance for cure.
In summary, this brief report illustrates some of the challenges and potential value inherent to solid tumor CTC detection, enumeration, and molecular characterization. Although descriptive in a pt with PCa, the principles are generalizable to all solid tumors. Further efforts will undoubtedly lead to successful incorporation of CTC use in the clinical management of pts with PCa and a better fundamental understanding of the mechanism of cancer metastases and therapeutic resilience.
Supplementary Material
Acknowledgments
The authors acknowledge the Intramural University of Florida faculty funds
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12029-014-9638-3) contains supplementary material, which is available to authorized users.
Conflict of Interest The authors have no financial conflicts of interest.
Contributor Information
Thomas J. George, Jr., Email: thom.george@medicine.ufl.edu, Department of Medicine, University of Florida, Gainesville, FL 32610, USA; Division of Hematology/Oncology, Department of Medicine, University of Florida, Gainesville, FL 32610, USA.
Olorunseun O. Ogunwobi, Email: ogunwobi@genectr.hunter.cuny.edu, Department of Biological Sciences, Hunter College of the City University of New York, 695 Park Avenue, Room 932HN, New York, NY 10065, USA.
Weian Sheng, Department of Mechanical and Aerospace Engineering, University of Florida, Gainesville, FL 32610, USA.
Z. Hugh Fan, Department of Mechanical and Aerospace Engineering, University of Florida, Gainesville, FL 32610, USA.
Chen Liu, Department of Pathology, Immunology, and Laboratory Medicine, University of Florida, Gainesville, FL 32610, USA.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Kim TH, Choi KH, Song HS, Kim JW, Jeon BJ. Histology combined with cytology by endoscopic ultrasound-guided fine needle aspiration for the diagnosis of solid pancreatic mass and intra-abdominal lymphadenopathy. Gut Liver. 2013;7:605–610. doi: 10.5009/gnl.2013.7.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Toole D, Palazzo L, Arotcarena R, et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470–474. doi: 10.1067/mge.2001.112839. [DOI] [PubMed] [Google Scholar]
- 4.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 5.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Wang K, Xu J, Huang J, Zhang T. Prognostic significance of circulating tumor cells in non-small-cell lung cancer patients: a meta-analysis. PLoS ONE. 2013;8(11):e78070. doi: 10.1371/journal.pone.0078070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 8.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 9.Hughes AD, Marshall JR, Keller E, Powderly JD, Greene BT, King MR. Differential drug responses of circulating tumor cells within patient blood. Cancer Lett. 2013 doi: 10.1016/j.canlet.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheng W, Ogunwobi OO, Chen T, et al. Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab Chip. 2014;14:89–98. doi: 10.1039/c3lc51017d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller V, Riethdorf S, Rack B, Janni W, Fasching PA, Solomayer E, et al. Prognostic impact of circulating tumor cells assessed with the Cell Search System™ and AdnaTest Breast™ in metastatic breast cancer patients: the detect study. Breast Cancer Res. 2012;14(4):R118. doi: 10.1186/bcr3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller MC, Doyle GV, Terstappen LWMM. Significance of circulating tumor cells detected by the cell search system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010;2010:617421. doi: 10.1155/2010/617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurihara T, Itoi T, Sofuni A, et al. Detection of circulating tumor cells in patients with pancreatic cancer: a preliminary result. J Hepato-Biliary-Pancreat Surg. 2008;15:189–195. doi: 10.1007/s00534-007-1250-5. [DOI] [PubMed] [Google Scholar]
- 14.Khoja L, Backen A, Sloane R, et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106:508–516. doi: 10.1038/bjc.2011.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tjensvoll K, Nordgard O, Smaaland R. Circulating tumor cells in pancreatic cancer patients: methods of detection and clinical implications. Int J Cancer. 2014;134:1–8. doi: 10.1002/ijc.28134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.