Abstract
Purpose
The purpose of this study was to estimate the effect of the Cognitive Orientation to daily Occupational Performance (CO-OP) approach compared to usual outpatient rehabilitation on activity and participation in people less than 3 months post stroke.
Methods
An exploratory, single blind, randomized controlled trial with a usual care control arm was conducted. Participants referred to 2 stroke rehabilitation outpatient programs were randomized to receive either Usual Care or CO-OP. The primary outcome was actual performance of trained and untrained self-selected activities, measured using the Performance Quality Rating Scale (PQRS). Additional outcomes included the Canadian Occupational Performance Measure (COPM), the Stroke Impact Scale Participation Domain, the Community Participation Index, and the Self Efficacy Gauge.
Results
Thirty-five (35) eligible participants were randomized; 26 completed the intervention. Post-intervention, PQRS change scores demonstrated CO-OP had a medium effect over Usual Care on trained self-selected activities (d=0.5) and a large effect on untrained (d=1.2). At a 3 month follow-up, PQRS change scores indicated a large effect of CO-OP on both trained (d=1.6) and untrained activities (d=1.1). CO-OP had a small effect on COPM and a medium effect on the Community Participation Index perceived control and the Self-Efficacy Gauge.
Conclusion
CO-OP was associated with a large treatment effect on follow up performances of self-selected activities, and demonstrated transfer to untrained activities. A larger trial is warranted.
Keywords: stroke, rehabilitation, Cognitive Orientation to daily Occupational Performance, cognition, participation, self-efficacy
Introduction
Individuals living with the effects of a stroke continue to experience significant challenges with their functional health, despite advances in rehabilitation. Approximately half of those living in the community after their stroke are dependent in activities of daily living, 1 and the majority have not achieved their individual functional goals.2 They experience participation restrictions,3–5 have limitations in performing meaningful, activities,6 and are significantly less active than age-matched controls.7 Evidence suggests treatments that incorporate repetitive task-specific training are the most effective of contemporary rehabilitation approaches to improve gait speed and upper extremity activities, and are recommended over traditional neurodevelopmental approaches.8,9 However, the effect seems to be limited to the specific tasks trained,9 and retention of learning has not been consistently demonstrated.10 In contrast, the Cognitive Orientation to daily Occupational Performance (CO-OP) approach, which superimposes cognitive and metacognitive elements on task-specific training, is associated with improvements in both trained and untrained activities, and the newly acquired skills are retained.11,12
CO-OP is a complex treatment approach that differs from other contemporary stroke rehabilitation approaches in that it combines theory and evidence from both motor and cognitive sciences, and situates them in an educational, client-centred framework. It is defined as “a client-centred, performance-based, problem solving approach that enables skill acquisition through a process of strategy use and guided discovery.”13 The clinical objectives are skill acquisition, cognitive strategy use, and generalization and transfer of learning. Elements from the motor domain are used primarily to meet the clinical objective of skill acquisition; these include practicing specific, functional tasks (ie task-specific training), usually as a whole task, such as dressing, cutting food, or walking outdoors, rather than task components such as reaching, grasping, or balance training in isolation from the task.
A limitation of task-specific training, when used alone, is that generalization to other situations and transfer to other tasks, are generally not demonstrated,9 and improvements gained in therapy are not consistently maintained once therapy stops.10,14 In CO-OP, retention, generalization and transfer have consistently been reported.11,12,15,16 This may, in part, be because of the cognitive and metacognitive elements that are superimposed on the task-based training. In CO-OP, a global cognitive strategy, adapted from Meichenbaum’s GOAL-PLAN-DO-CHECK,17 teaches participants to problem solve and self-monitor their own task performance. Additionally, within each task performance, therapists use a conversation-based teaching and feedback technique known as guided discovery,18 in which participants are guided through questions, cues, and coaching to solve task performance problems on their own, rather than being given explicit instrutions. Through this process they learn to analyze their own performance and to subsequently develop performance strategies to overcome issues. This differs markedly from traditional approaches, in which it is the therapist who does the analysis of performance breakdowns, develops performance strategies and explicitly teaches the patient how to use those performance strategies.
Preliminary evidence to support CO-OP’s benefits for individuals post stroke was first demonstrated in people more than a year post stroke.11,12,19 Two single case experimental series showed not only improved activity performance and retention of learning following CO-OP intervention,11 but also improvement of untrained activities, suggesting the occurrence of transfer to new skills.12 Because it is relatively well established that stroke outcomes are improved if rehabilitation occurs in the first few months following the event,8,20,21 and because past CO-OP participants recommended the strategies be taught much earlier in the rehabilitation process,22 we sought to investigate the efficacy of the approach in sub-acute stroke. Therefore, the primary objective of this exploratory trial was to estimate, in people less than 3 months post stroke, the effect of CO-OP compared to usual occupational therapy on immediate and longer-term activity performance and participation.
Methods
A single blind, exploratory, randomized controlled trial with a usual care control arm was conducted. Participants referred to outpatient stroke rehabilitation programs at two university-affiliated, freestanding rehabilitation centres were randomized to receive either the usual outpatient program, which included occupational therapy (Usual Care), or the usual outpatient program with COOP replacing usual occupational therapy (CO-OP).
Sampling and Randomization Procedures
Patients who had sustained an ischemic stroke (ICD-10 codes I63 and I64) referred to outpatient rehabilitation at Sunnybrook-St. John’s Rehab in Toronto, ON, Canada or The Rehabilitation Institute of St. Louis, St. Louis, MO, USA between March 2011 and March 2013 were included. Exclusion criteria were: more than 3 months post-stroke when starting outpatient rehabilitation, hemorrhagic stroke, other neurological diagnoses, major psychiatric illness, moderate or severe aphasia (Combined scores of 6 or less on Canadian Institute of Health Information 23 items 64 and 66) or cognitive impairment (Montreal Cognitive Assessment 24 scores of 21 or less). It was estimated a priori that a sample size of 14 per group would provide 82% power to detect a between-group difference of 1.3 units on the Canadian Occupational Performance Measure (COPM).25 To ensure balanced group sizes, a consulting statistician prepared a blocked randomization procedure stratified by site. A block size of 6 and an allocation ratio of 1:1 were used to ensure equal assignment to the treatment and control groups for every 6 patients entered in the study at each centre. The random number function in Excel®i was used to create a random sort order within each block. To ensure allocation concealment, an administrative assistant at each site, not associated with the study, created sequentially numbered sealed opaque envelopes for the study coordinator at each site. The study coordinator was not involved in the assessment or the treatment of any of the participants. Treatment allocation was not completed until after consent was obtained and all inclusion/exclusion criteria had been reviewed. Because knowledge of blocking reduces the unpredictability of the next assignment, the study investigators, project coordinators, and treating therapists were all blinded to the randomization procedure and block size.
Assessment and Intervention Procedures
At Time 1 participants underwent a baseline assessment battery conducted by a research assistant who was blinded to group allocation. Following that, a research occupational therapist, not involved with delivering either intervention and also blinded, conducted a goal-setting interview using the COPM. During this interview, participants selected the four to six personally meaningful functional activity goals that were the most important to them. The participants then had a third baseline assessment session in which they were video-recorded performing those self-selected activity goals.
Following the Time 1 assessments, both the CO-OP and Usual Care groups received usual outpatient stroke rehabilitation with specific services based on their individual needs, such as physical therapy, speech-language pathology, or nursing. The Usual Care group received usual outpatient occupational therapy from therapists employed by the participating sites, whereas the CO-OP group received occupational therapy from CO-OP trained occupational therapists that were part of the research team. Treatments were generally twice per week; sessions were 45 minutes long for the CO-OP group and ranged from 45–60 minutes long for the Usual Care group. Because the participants had a range of stroke severity and rehabilitation needs, and as is typical in usual outpatient rehabilitation, the number of sessions varied; the number of treatment sessions attended by an individual participant depended on his or her needs, the clinical judgment of the treating occupational therapist, and the institutional policies. The CO-OP group received a maximum of 10 CO-OP intervention sessions, and any additional sessions more complex patients needed were conducted as usual care. These additional usual care sessions were tracked and counted as part of the total number of CO-OP treatments. A limitation on the number of sessions received by the Usual Care group was institutional policy. In Toronto, clients were limited to a maximum of 32 treatment sessions, and in St. Louis, clients were limited by the number of sessions covered by their insurance provider or other means of payment.
CO-OP Intervention Description
Complete details about the theoretical underpinnings and the implementation of the CO-OP approach have been published in a textbook.13 In this study, CO-OP treatment occurred separately from the rest of the outpatient team, to avoid contamination. During the first session, the CO-OP therapist reviewed the goals previously established in the COPM interview, and worked with the participant to decide on which 3 of those would become the focus of intervention sessions. Also in the first session, the CO-OP therapist used a visual presentation to teach the participant the global cognitive strategy, GOAL-PLAN-DO-CHECK.17 In subsequent sessions the GOAL-PLAN-DO-CHECK strategy was used iteratively as the main problem-solving framework to facilitate activity acquisition. The participant would work one or more of the 3 GOALs set guided by the therapist to discover a PLAN to achieve the goal. The participant would then DO the plan, and subsequently CHECK to see if the plan was implemented and if it worked, i.e. the goal was achieved. If the goal was not achieved, the participant was guided to analyze the performance breakdown and modify or create a new PLAN. Within the PLAN phase, the therapist used guided discovery to help the participant analyze the performance breakdown and discover domain-specific strategies to overcome the particular performance problems of that client with that activity. Thus, the PLAN-DO-CHECK process was repeated until the performance breakdown was successfully overcome, repeatedly; then going to the next performance breakdown until all were overcome and the GOAL was achieved, repeatedly. It is important to note that although in CO-OP the focus is on performing the task to be learned, there is no particular emphasis on the number of repetitions. Repetitions are never stipulated; rather, the number of times a particular task is practiced depends on the quality and consistency of the performance. Once the participant is satisfied with the performance on the particular part of the task being worked on, as determined by the CHECK in the global cognitive strategy, the next breakdown is identified and a new learning cycle begins. This is repeated until the GOAL is achieved.
Usual Care Description
Participants randomized to the Usual Care group received usual outpatient occupational therapy with one modification. As described above, a non-treating research occupational therapist blinded to group allocation administered the COPM to assist participants to self-select personally meaningful goals, prior to beginning therapy. This was a departure from usual administration of the COPM, as it tends to be conducted by the treating occupational therapist if done at all. The COPM results were made available to the treating occupational therapist, but no instructions were given regarding what to do with the information.
A survey given during the preparation for this study indicated that Usual Care consisted of a combination of functional, task-based training and component-based training as deemed necessary by the treating therapists.
CO-OP Intervention Fidelity
Two therapists, one in Toronto and one in St. Louis, were trained in CO-OP with a standard 2-day CO-OP workshop. For ongoing training, consolidation, and fidelity purposes, the two CO-OP therapists then were video recorded treating a series of pilot participants and received feedback until they were consistently scoring full marks on an intervention fidelity checklist. Once the intervention began, videos from sessions 3, 6, and 10 were reviewed and scored using the same intervention fidelity checklist to ensure ongoing fidelity.
Outcomes Measures
Table 1 provides an overview of instrument characteristics and timing of their administration. Research staff blinded to group allocation conducted assessments prior to the intervention starting (Time 1), after discharge from occupational therapy or after 10 sessions (Time 2), and 3 months after Time 2 (Time 3). Because the number of intervention sessions varied among participants based on the severity of their stroke and their individual rehabilitation needs, representatives from Usual Care, either therapists or administrative staff, were asked to inform the study coordinator when the participant was discharged from occupational therapy or when 10 sessions were completed, whichever came first. Time 2 assessments were performed at this point, as an attempt to assess initial outcomes after a similar treatment dosage. Because many of the participants had been discharged to home from acute care hospital only a few days before the Time 1 assessment, and because the questions posed in the participation measures are designed for people who have been in the community for at least for a few weeks, these were administered only at Time 2 and Time 3 to ensure their validity.
Table 1.
| OUTCOME | DESCRIPTION AND PROPERTIES | TIMING |
|---|---|---|
| Performance Quality Rating Scale (PQRS) |
The PQRS rates video recorded performance of participant-selected activities on a 10-point scale, with a score of 1 indicating “can’t do the skill at all” and 10 indicating “does the skill very well”.15 The activities performed and video recorded are determined using the COPM, and most, but not all, goals selected by participants are amenable to video recording. The PQRS has substantial test-retest reliability and good internal responsiveness.26 |
Times 1, 2, & 3 |
| Canadian Occupational Performance Measure (COPM) |
The COPM is a standardized instrument for eliciting performance issues from the client perspective, and for capturing perceived changes in performance over time.25 The COPM was used to elicit 4–6 participant-selected goals, as well as for rating self- perceived performance and performance satisfaction for each goal on a 10-point scale, for each participant. The COPM has demonstrated test-retest reliability of 0.89 in people with stroke.27 A change of 2 points or more on the COPM is considered clinically significant.25 |
Times 1, 2 & 3 |
| Community Performance Indicators (CPI) |
The CPI is a complex self-report measure of community participation. In this study, we analyzed two enfranchisement factors, importance of participation (14 items) and control over participation (13 items). Participants rate items on a 5 point scale, which are converted using a Rasch-based key form to a score of 0- 100 28,29 There is good evidence of validity and reliability for these factors. |
Times 2 & 3 |
| Stroke Impact Scale (SIS) |
The SIS30 is a 59-item questionnaire based measure of the perceived impact of stroke on function and everyday life. The SIS evaluates eight domains including participation. Each item is scored on a 5-point Likert scale related to the degree of difficulty the person with stroke is experiencing. The SIS is widely used in stroke intervention studies as an outcome measure and the psychometric properties of the instrument are well- defined.30–32 |
SIS Participation Domain, Times 2 & 3. All other domains, Times 1,2 & 3. |
| Self-Efficacy Gauge (SEG) |
The SEG was designed to measure an individual’s confidence in his or her ability to perform daily occupations that span a range of self-care, productivity, and leisure activities. Participants are asked to rate their confidence in their ability to perform 28 items, each on a 10-point scale, with 1 representing “not confident at all” and 10 representing “completely confident”. The SEG has very high internal consistency (0.94) and test-retest reliability (0.90).33 |
Times 1,2, & 3 |
The primary study outcome was change in actual performance quality of self-selected activities, measured with the Performance Quality Rating Scale (PQRS).15 The PQRS is a 10-point scale used to rate video recorded performances of participant-selected activities. The participants perform the activities selected in the COPM interview in a safe environment but unaided by physical support or verbal cueing. The video recordings are rated on a scale of 1 to 10 in which 1 indicates, “can’t do the activity at all” and 10 indicates, “does the activity very well”. In this study, an independent blinded observer viewed the videos in randomized, non-chronological order. The PQRS has substantial test-retest reliability and good internal responsiveness.26
The COPM was included as an indicator of perceived performance quality and satisfaction with performance quality. The secondary study outcome was participation, assessed with the Stroke Impact Scale participation domain and the Community Participation Index. Additionally, self-efficacy was measured using the Self-Efficacy Gauge.
Data Analysis
Data analysis was conducted using SPSS version 21ii and Microsoft Excel.iii The data were cleaned, checked for accuracy and missing values, and checked for normal distribution using the Shapiro-Wilk test. Descriptive statistics were compiled for all variables and baseline comparisons between sites and between the groups were made.
Time 1 to Time 2 and Time 1 to Time 3 mean change scores and standard deviations were calculated for normally distributed data, and Cohen’s d effect sizes and confidence intervals were calculated. For non-normally distributed data, medians were determined, and a nonparametric effect size r was calculated using the formula r=z/N.34
CO-OP therapist logs and institutional patient records were reviewed to establish which self-selected activities were trained during the outpatient rehabilitation program. Records from occupational therapy sessions were reviewed by occupational therapists from the research team (DC and MD) for evidence of training. A self-selected activity was considered trained if there was any indication of practicing all or part of it, or any indication of discussions or education concerning the activity. If no evidence of training was found, it was considered untrained. In 4 cases, only partial records were available, in which case trained and untrained goals were confirmed by examining typical therapy activities by the same therapist with other participants. For COPM and PQRS scoring and analysis, trained and untrained activity scores were grouped, summed, and averaged separately to give a single trained and a single untrained score for each participant.
The ethics review boards at the relevant institutions approved this study.
RESULTS
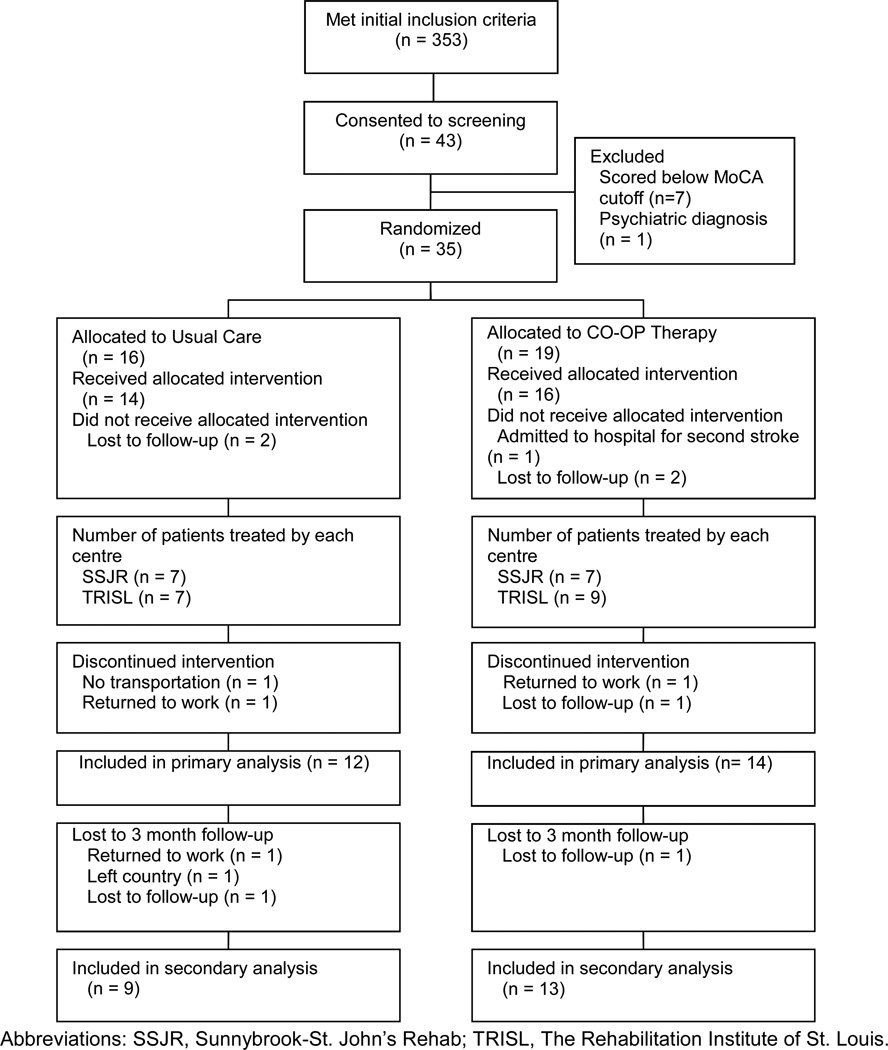
Figure 1 is the CONSORT diagram depicting participant flow through the study. Thirty-five (35) eligible participants were randomized, 20 from St. Louis and 15 from Toronto. Of those, 26 completed the intervention. Table 2 provides summary scores for participant demographics by site and by group. Participants in Toronto had more years of education (p=0.03), and participants in St. Louis began outpatient rehabilitation approximately 17 days sooner following their stroke than did participants in Toronto (p=0.04). Otherwise, there were no significant between-site differences at baseline. The two treatment groups were equivalent at baseline on all variables examined. After excluding two extreme outliers, who had over 100 treatment sessions, one in each treatment group and both from St. Louis, those in the Usual Care group who completed the study had an average of 13.3 occupational therapy sessions (range 3–30); those in the CO-OP group had an average of 12.2 occupational therapy sessions (range 5–33). CO-OP therapists scored an average of 80% accuracy across on CO-OP fidelity checklists.
Figure 1.
CONSORT Diagram
Table 2.
Demographics
| St. Louis | Toronto | Total | ||||
|---|---|---|---|---|---|---|
| Variable | Group | n | Mean (SD) | Mean (SD) | t | Mean (SD) |
| Days since stroke | UC | 15 | 41.6 (17.1) | 52.0 (25.4) | −2.50 | 46.5 (21.3) |
| CO-OP | 19 | 30.5 (10.7) | 53.1 (23.9) | 40.1 (20.4) | ||
| Therapy sessions (#) | UC | 11 | 10.2 (2.8) | 17.0 (13.0) | −0.96 | 13.3 (9.2) |
| CO-OP | 14 | 10.0 (5.4) | 14.4 (8.7) | 12.2 (7.3) | ||
| Age | UC | 16 | 50.7 (14.5) | 59.3 (12.7) | −0.85 | 54.4 (14.0) |
| CO-OP | 19 | 57.4 (15.5) | 57.6 (12.7) | 57.5 (14.0) | ||
| Years of Education | UC | 16 | 12.2 (1.5) | 14.4 (1.9) | −1.33 | 13.2 (2.0) |
| CO-OP | 19 | 14.3 (3.0) | 15.3 (5.6) | 14.7 (4.2) | ||
| n (%) | n (%) | χ2 | n (%) | |||
| Sex (female) | UC | 16 | 5 (44.4) | 2 (28.6) | 1.23 | 7 (43.8) |
| CO-OP | 19 | 4 (36.4) | 2 (25.0) | 6 (31.6) | ||
| Stroke side (right) | UC | 16 | 7 (77.8) | 6 (85.7) | 0.10 | 13 (81.3) |
| CO-OP | 18 | 6 (54.5) | 5 (62.5) | 11 (57.9) | ||
| Handedness (right) | UC | 16 | 8 (88.9) | 6 (85.7) | 0.59 | 14 (87.5) |
| CO-OP | 19 | 9 (81.8) | 8 (100) | 17 (89.5) | ||
| Living Arrangement (with others) |
UC | 16 | 8 (88.9) | 7 (100) | 2.03 | 15 (93.8) |
| CO-OP | 19 | 7 (63.6) | 7 (87.5) | 14 (73.7) | ||
| Ethnicity | ||||||
| Caucasian | UC | 2 (25.0) | 5 (71.4) | 7 (46.7) | ||
| CO-OP | 5 (45.5) | 4 (50.0) | 9 (47.4) | |||
| African American | UC | 6 (75.0) | 0 | 6 (40.0) | ||
| CO-OP | 6 (54.4) | 4 (50.0) | 10 (52.6) | |||
| Asian | UC | 0 | 1 (14.3) | 1 (6.7) | ||
| CO-OP | 0 | 0 | 0 | |||
| Other | UC | 0 | 1 (14.3) | 1 (6.7) | ||
| CO-OP | 0 | 0 | 0 | |||
Table 3 provides an overview of the 178 goals selected during the COPM interviews. Twenty-three (23) of the 178 goals, such as “weight management” or “traveling to see family”, could not be video-recorded, and thus did not have PQRS scores associated with them. As an individual’s PQRS score was based on an average of scores from all his or her self-selected activities, the loss of some activities that couldn’t be video recorded did not cause missing PQRS data; all participants who completed the intervention had at least one trained and one untrained activity that could be included in the analysis.
Table 3.
Summary of participant-selected goals
| Goal Category | Number | Examples |
|---|---|---|
| Activity and Participation Goals | ||
| Handyman work | 5 | Repairing car; Using hedge clippers |
| Cleaning | 14 | Housework; Helping with dishes: Making bed |
| Laundry | 6 | Laundry; Folding laundry |
| Cooking | 19 | Cooking; Getting items from kitchen |
| Eating | 10 | Cutting food; Using a knife and fork to eat |
| Dressing | 15 | Dressing; Putting on socks; Using a zipper |
| Personal hygiene | 5 | Hygiene after toileting; Hair care |
| Opening medicine bottles | 2 | |
| Using a door knob | 1 | |
| Walking | 16 | Walking outdoors; Walking without device |
| Climbing stairs | 1 | |
| Transfers | 8 | Bathtub; Toilet; Car |
| Caregiving Roles | 3 | Caregiving for husband; Playing with kids |
| Work | 7 | Return to work; Apply for job; School; Volunteering |
| Manage finances | 2 | |
| Communicating over the phone | 1 | |
| Keyboarding/Computer Use | 8 | Keyboarding; Typing email; Use computer mouse |
| Handwriting | 3 | Write legibly |
| Office activities | 6 | Folding paper and putting it in envelopes |
| Drive | 5 | |
| Use public transportation | 2 | |
| Attending outings with friends/family | 2 | Travelling to see family |
| Grocery shopping | 7 | Groceries; Shop without getting lost |
| Recreation | 16 | Go to casino; Floor hockey; Play drums; Fishing; Dancing |
| Sitting and standing more independently for synagogue |
1 | |
|
TOTAL ACTIVITY & PARTICIPATION |
164 | |
| Impairment Goals | ||
| Concentration/memory/multitasking | 3 | Improve memory for day to day activities |
| Balance | 4 | Balance for walking and gardening |
| Endurance | 2 | Increase endurance for traveling |
| Weight management | 1 | |
| Strengthening | 3 | Strength for manual labour at work |
| TOTAL IMPAIRMENT | 14 | |
Table 4 displays summary statistics, change scores, and effect sizes. Cohen’s d effect size can been interpreted that 0.2 represents a small effect, 0.5 represents a medium effect, and 0.8 represents a large effect.35 CO-OP’s effect over Usual Care at Time 2 was medium for PQRS trained activities (d=0.5) and large for PQRS untrained activities (d=1.2). Large effects for Time 3 change scores were found for both PQRS trained (d=1.6) and untrained activities (d=1.1). There was no effect of CO-OP over Usual Care on Time 2 COPM change scores, and a small effect at Time 3 for changes in COPM performance and satisfaction of untrained goals, and a small effect for changes in performance of untrained goals. At Time 3, CO-OP had a medium effect over Usual Care for change in CPI perceived control (d=0.7) and the SEG (d=0.7).
Table 4.
Means, standard deviations, change scores and Cohen’s d effect size
| Outcome | Group | Time 1 | Time 2 | Time 1 – 2 Change Score |
Effect Size | Time 3 | Time 1 – 3 Change Score |
Effect Size |
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | d (95% CI) | Mean (SD) | Mean (SD) | d (95% CI) | ||
| PQRS Trained | UC | 5.7 (1.9) | 7.5 (2.0) | 1.8 (2.9) | 6.9 (1.4) | 1.5 (2.2) | ||
| CO-OP | 4.3 (2.0) | 7.1 (1.7) | 2.9 (1.4) | 0.5 (−0.4 – 1.4) |
8.3 (1.2) | 4.5 (1.7) | 1.6 (0.5 – 2.7) |
|
| PQRS Untrained | UC | 5.1 (1.7) | 5.6 (2.0) | 0.5 (2.5) | 7.1 (0.7) | 1.5 (2.0) | ||
| CO-OP | 4.8 (1.8) | 7.7 (2.0) | 2.9 (1.4) | 1.2 (0.1 – 2.2) |
8.3 (1.7) | 3.6 (2.3) | 1.1 (−0.1 – 2.3) |
|
| COPM-Performance Trained |
UC | 4.2 (1.9) | 6.8 (2.4) | 2.3 (2.1) | −0.1 (−0.8 – 0.7) |
6.6 (3.0) | 2.3 (2.5) | |
| CO-OP | 4.6 (1.8) | 6.2 (2.5) | 1.5 (3.0) | 7.7 (2.2) | 2.9 (1.8) | 0.4 (−0.5 – 1.2) |
||
| COPM-Performance Untrained |
UC | 3.8 (1.5) | 5.9 (2.5) | 1.9 (2.3) | −0.1 (−0.9 – 0.7) |
6.7 (2.7) | 2.4 (2.5) | |
| CO-OP | 4.7 (2.0) | 6.1 (2.9) | 1.3 (3.1) | 7.8 (2.2) | 3.1 (2.7) | 0.2 (−0.7 – 1.0) |
||
| COPM-Satisfaction Trained |
UC | 3.9 (2.5) | 6.0 (2.2) | 1.8 (2.3) | 7.3 (2.5) | 3.7 (3.3) | ||
| CO-OP | 3.8 (2.1) | 5.8 (3.0) | 1.9 (2.8) | 0.1 (−0.7 – 0.9) |
7.2 (2.9) | 3.2 (1.8) | 0.1 (−1.0 – 0.8) |
|
| COPM-Satisfaction Untrained |
UC | 3.5 (1.7) | 5.6 (2.7) | 1.7 (2.1) | 6.8 (2.7) | 2.8 (2.3) | ||
| CO-OP | 4.0 (1.9) | 5.7 (3.0) | 1.5 (3.3) | 0.0 (−0.8 – 0.7) |
7.5 (2.6) | 3.2 (3.0) | 0.2 (−0.7 – 1.1) |
|
| CPI-Importance of Participation |
UC | - | 53.3 (12.5) | - | - | 50.7 (8.1) | −0.5 (5.2) | |
| CO-OP | - | 52.1 (8.1) | - | 52.4 (10.5) | 1.2 (5.9) | 0.3 (−0.6 – 1.2) |
||
| CPI-Control over Participation |
UC | - | 64.1 (16.5) | - | - | 61.9 (13.2) | −3.0 (11.4) | |
| CO-OP | - | 58.4 (9.3) | - | 63.8 (12.6) | 3.4 (7.2) | 0.7 (−0.2 – 1.6) |
||
| CPI-Satisfaction with Participation* |
UC | 69.0 | 140.0 | 20.7 | ||||
| CO-OP | 70.3 | 85.0 | 13.5 | 0.2 | ||||
| SIS-Participation | UC | - | 54.6 (13.7) | - | - | 56.9 (13.3) | 1.9 (12.5) | |
| CO-OP | - | 47.9 (16.8) | - | 56.6 (16.7) | 8.0 (9.9) | 0.5 (−0.4 – 1.4) |
||
| SEG | UC | 211.7 (51.7) | 227.5 (42.0) | 3.8 (48.9) | 228.3 (28.1) | 9.4 (41.8) | ||
| CO-OP | 198.4 (45.3) | 224.6 (45.2) | 23.2 (38.9) | 0.4 (−0.3 – 1.2) |
239.2 (36.3) | 38.8 (38.1) | 0.7 (−0.2 – 1.6) |
Abbreviations: UC, usual care; CO-OP, Cognitive Orientation to daily Occupational Performance; SD, standard deviation; CI, confidence interval; PQRS, Performance Quality Rating Scale; COPM, Canadian Occupational Performance Measure; SIS, Stroke Impact Scale; SEG, Self-Efficacy Gage.
Data were not normally distributed therefore median and non-parametric effect size r are reported.
Effect Sizes: .2, small effect; .5 medium effect; .8 large effect.
DISCUSSION
Incorporating the CO-OP approach as part of an outpatient rehabilitation program is associated with a large effect at follow up on actual performance of trained and untrained self-selected functional activities compared to programs incorporating usual occupational therapy. This suggests not only improved performance on skills trained in rehabilitation, but also transfer of cognitive strategy training to permit those living with the effects of stroke to learn new skills outside of the rehabilitation setting as the need arises. This discussion elaborates on the features of the CO-OP approach that may contribute to transfer of learning to untrained activities, on the small effect of perceived performance, and on study limitations.
Transfer of skills learned in rehabilitation to novel skills is necessary to achieve optimal long-term functional health, as rehabilitation programs are unable to teach clients all the activities they may need at home and in the community, or even all variations of a single activity. Measurement of transfer of cognitive strategy training to real-world situations has been accomplished by the assessment of untrained tasks, by standardized assessment of daily tasks, or by participant or proxy self-report of daily life situations.36 In this study, the primary indicator of transfer was change in performance on self-selected tasks or activities not addressed during the intervention sessions with the therapist (untrained tasks). This type of far transfer (transfer to a completely unrelated task) is expected with cognitive strategy training, because the therapist’s primary emphasis is on teaching problem solving skills, rather than on teaching the particular functional skill itself.36
Therapists trained to use the CO-OP approach are explicitly taught to work towards generalization and transfer.13 Other CO-OP features may also contribute to transfer, including guided discovery, performance analysis, and self-discovery of performance strategies. Transfer is reported to be more closely linked to variable practice than blocked practice.37 One theory for explaining this phenomena is the learned-variability model of skill transfer, in which learning a skill involves learning how to do the skill in different ways and learning when to alter it.38 In CO-OP, learned variability occurs through the use of guided discovery combined with performance analysis within the PLAN and CHECK phases of the GOAL-PLAN-DO-CHECK framework. The client is taught to CHECK or self-analyze his or her performance breakdowns and is guided to identify different PLANs or strategies until settling on one that works best to perform the task at hand; learning different ways to do the skill as part of the process. Additionally, clients are guided to identify their own strategies rather than being assigned strategies by the therapist. There is experimental evidence to suggest that providing time to attend to performance issues and subsequently self-select a strategy is more strongly associated with transfer than being given a strategy.39
Evidence from a neuroimaging study suggests that the combined training of motor and cognitive systems has a positive impact on transfer. Olsson and colleagues conducted an experiment comparing motor practice, mental practice, and combined motor and mental practice to learn a finger tapping task, and unexpectedly discovered improvements on a novel transfer task only in the combined training group.40 Functional magnetic imaging (fMRI) data indicated overall broader cortical activity in the combined training group, and showed the posterior cerebellar hemisphere was involved in transfer.
Transfer is also linked to high self-efficacy,41 and high self-efficacy is linked to better functional outcomes in stroke.42 Stevens and colleagues demonstrated that practice of an easier task rather than a more difficult task was associated with higher self-efficacy, and subsequently that higher self-efficacy predicted better ability on a transfer task41. Those authors speculated that self-efficacy, rather than implicit learning, is a mediator of transfer to a similar but more difficult task, although they are cautious to emphasize that further research is required to confirm this finding. Interestingly, in this CO-OP study, an unexpected medium effect of CO-OP on self-efficacy was shown. We speculate that following CO-OP, self-efficacy in a broad range of daily activities likely comes from having experienced success with the self-selected activities practiced in therapy, the attribution of that success to the newly-learned ability to problem solve performance issues, and the subsequent willingness to try new activities at home independently. Thus it is plausible that improved self-efficacy is an outcome of CO-OP, and self-efficacy then mediates transfer.
Self-efficacy and the ability to transfer new learning from rehabilitation to the real world may both be mediators of participation. It may be that improvements in self-efficacy need to be in place before measurable improvements in participation are seen. For example, the changes in the Community Participation Index for the CO-OP group were higher for the perceived control over participation domain than for involvement in living situations domain, suggesting a degree of confidence in the ability to participate, but less actual involvement. This may be because the transition from confidence and ability to actual doing takes more time.
Limitations
This was an exploratory study with the objective of estimating CO-OP’s effect relative to a control treatment in preparation for a larger, more definitive trial. As such, the sample size was too small to find statistically significant differences for most outcomes, or to stratify groups on potential key confounders, such as stroke severity. The sample size also limited the statistical analysis to univariate procedures without the capacity to control for the effect of potential confounders, such as site and dosage.
The decision to have a usual care control meant the comparison treatment was unstandardized. Both control programs were in university-affiliated hospitals linked to well-regarded academic programs, and the control treatment is believed to have been close to current best practice, i.e. interdisciplinary treatment, largely based on repetitive functional task training, with treatment of impairments and components when deemed appropriate by the therapist.
For the purposes of blinding and consistency between the two treatment groups, the COPM was administered by a research therapist with no clinical relationship with the patient, rather than by the treating therapist as is usually done. This may have resulted in a disconnect between the participant and the treating therapist regarding the self-selected goals, may have had implications for the relatively smaller effect of CO-OP on COPM, and may be something to reconsider in future studies
A final important limitation of this study was the relatively short follow-up period, which was just 3 months after the post-intervention assessment, and an average of 7 months following the stroke. To get a better estimate of the stability of the treatment effects and the impact on participation, future studies should follow participants for at least one year after discharge.
Conclusion
CO-OP, incorporated as part of a usual outpatient stroke rehabilitation program, was associated with a large treatment effect compared to usual outpatient rehabilitation alone on follow up performances of both trained and untrained self-selected activities. Results also suggest a medium effect on changes in participation from post-intervention to follow-up and on, self-efficacy. A larger scale trial is warranted.
Acknowledgements
This study was funded by a Canadian Institutes of Health Research Open Operating Grant (FRN#111200) and was supported by the St. John’s Rehab Hospital and The Rehabilitation Institute of St. Louis. The authors acknowledge the assistance of Alisa Grigorovich, Tanya Ramsey, Carin Roth, Tammy Craig, Kimberly Walker and Duana Russell-Thomas and the staff in the Performance, Participation, and Neurorehabilitation Laboratory in the Program in Occupational Therapy at Washington University in St. Louis for their assistance with assessment, evaluation, and treatment. Dr. McEwen’s salary support came from the St. John’s Rehab Foundation. Dr. Wolf received salary support from the National Center for Medical Rehabilitation Research (NCMRR) in the National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health under award numbers K12HD055931 & K23HD073190. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Microsoft Corporation, Microsoft Excel 2010, Version 14.0.
IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0
Microsoft Corporation, Microsoft Excel 2010, Version 14.0.
References
- 1.Appelros P, Samuelsson M, Karlsson-Tivenius S, Lokander M, Terent A. A national stroke quality register: 12 years experience from a participating hospital. Eur J Neurol. 2007;14(8):890–894. doi: 10.1111/j.1468-1331.2007.01826.x. [DOI] [PubMed] [Google Scholar]
- 2.Brock K, Black S, Cotton S, Kennedy G, Wilson S, Sutton E. Goal achievement in the six months after inpatient rehabilitation for stroke. Disabil Rehabil. 2008:1–7. doi: 10.1080/09638280802356179. [DOI] [PubMed] [Google Scholar]
- 3.Mayo NE, Wood-Dauphinee S, Cote R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months poststroke. Arch Phys Med Rehabil. 2002;83(8):1035–1042. doi: 10.1053/apmr.2002.33984. [DOI] [PubMed] [Google Scholar]
- 4.van der Zee CH, Visser-Meily JM, Lindeman E, Jaap Kappelle L, Post MW. Participation in the chronic phase of stroke. Top Stroke Rehabil. 2013;20(1):52–61. doi: 10.1310/tsr2001-52. [DOI] [PubMed] [Google Scholar]
- 5.Hackett ML, Glozier N, Jan S, Lindley R. Returning to paid employment after stroke: The psychosocial outcomes in StrokE (POISE) cohort study. PLoS One. 2012;7(7):e41795. doi: 10.1371/journal.pone.0041795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksson G, Aasnes M, Tistad M, Guidetti S, von Koch L. Occupational gaps in everyday life one year after stroke and the association with life satisfaction and impact of stroke. Top Stroke Rehabil. 2012;19(3):244–255. doi: 10.1310/tsr1903-244. [DOI] [PubMed] [Google Scholar]
- 7.Alzahrani MA, Ada L, Dean CM. Duration of physical activity is normal but frequency is reduced after stroke: An observational study. J Physiother. 2011;57(1):47–51. doi: 10.1016/S1836-9553(11)70007-8. [DOI] [PubMed] [Google Scholar]
- 8.Teasell RW, Foley NC, Salter KL, Jutai JW. A blueprint for transforming stroke rehabilitation care in Canada: The case for change. Arch Phys Med Rehabil. 2008;89(3):575–578. doi: 10.1016/j.apmr.2007.08.164. [DOI] [PubMed] [Google Scholar]
- 9.Veerbeek JM, van Wegen E, van Peppen R, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One. 2014;9(2):e87987. doi: 10.1371/journal.pone.0087987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.French B, Leathley M, Sutton C, et al. A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness. Health Technol Assess. 2008;12(30):1–117. doi: 10.3310/hta12300. iii, ix-x. [DOI] [PubMed] [Google Scholar]
- 11.McEwen SE, Polatajko HJ, Huijbregts MP, Ryan JD. Exploring a cognitive-based treatment approach to improve motor-based skill performance in chronic stroke: Results of three single case experiments. Brain Inj. 2009;23(13–14):1041–1053. doi: 10.3109/02699050903421107. [DOI] [PubMed] [Google Scholar]
- 12.McEwen SE, Polatajko HJ, Huijbregts MP, Ryan JD. Inter-task transfer of meaningful, functional skills following a cognitive-based treatment: Results of three multiple baseline design experiments in adults with chronic stroke. Neuropsychol Rehabil. 2010;20(4):541–561. doi: 10.1080/09602011003638194. [DOI] [PubMed] [Google Scholar]
- 13.Polatajko HJ, Mandich A. Enabiling occupation in children: The cognitive orientation to daily occupational performance (CO-OP) approach. First edition ed. Ottawa, Canada: CAOT Publications ACE; 2004. [Google Scholar]
- 14.Atler K, Malcolm M, Greife C. A follow-up study on the relationship among participation, activity and motor function in survivors of stroke following constraint-induced therapy. Disabil Rehabil. 2014;0(0):1–8. doi: 10.3109/09638288.2014.910560. [DOI] [PubMed] [Google Scholar]
- 15.Miller L, Polatajko HJ, Missiuna C, Mandich AD, Macnab JJ. A pilot of a cognitive treatment for children with developmental coordination disorder. Human Movement Science. 2001;20:183–210. doi: 10.1016/s0167-9457(01)00034-3. [DOI] [PubMed] [Google Scholar]
- 16.Dawson DR, Gaya A, Hunt A, Levine B, Lemsky C, Polatajko HJ. Using the cognitive orientation to occupational performance (CO-OP) with adults with executive dysfunction following traumatic brain injury. Can J Occup Ther. 2009;76:115–127. doi: 10.1177/000841740907600209. [DOI] [PubMed] [Google Scholar]
- 17.Meichenbaum DH, Goodman J. Training impulsive children to talk to themselves: a means of developing self-control. J Abnorm Psychol. 1971;77:115–126. doi: 10.1037/h0030773. [DOI] [PubMed] [Google Scholar]
- 18.Mayer RE. Should there be a three-strikes rule against pure discovery learning? The case for guided methods of instruction. Am Psychol. 2004;59:14–19. doi: 10.1037/0003-066X.59.1.14. [DOI] [PubMed] [Google Scholar]
- 19.Polatajko HJ, McEwen SE, Ryan JD, Baum CM. Pilot randomized controlled trial investigating cognitive strategy use to improve goal performance after stroke. Am J Occup Ther. 2012;66:104–109. doi: 10.5014/ajot.2012.001784. [DOI] [PubMed] [Google Scholar]
- 20.Salter K, Foley N, Teasell R. Social support interventions and mood status post stroke: a review. Int J Nurs Stud. 2010;47:616–625. doi: 10.1016/j.ijnurstu.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Ploughman M. A review of brain neuroplasticity and implications for the physiotherapeutic management of stroke. Physiotherapy Can. 2002;54:163–176. [Google Scholar]
- 22.McEwen SE, Polatajko HJ, Davis JA, Huijbregts MPJ, Ryan JD. “There’s a real plan here, and I am responsible for that plan:” participant experiences with a novel cognitive-based treatment approach for adults living with chronic stroke. Disabil Rehabil. 2010;32:540–550. doi: 10.3109/09638280903180189. [DOI] [PubMed] [Google Scholar]
- 23.Canadian Insitute for Health Information. CIHI national rehabilitation reporting system, listing of data elements. 2009 Available from http://www.cihi.ca/CIHI-extportal/pdf/internet/PDF_DATA_ELEMENTS_EN.
- 24.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montréal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 25.Law M, Baptiste S, Carswell A, McColl MA, Polatajko H, Pollock N. Canadian Occupational Performance Measure. Fourth Edition ed. Ottawa, ON: CAOT Publications ACE; 2005. [Google Scholar]
- 26.Martini R, Rios J, Polatajko H, Wolf T, McEwen S. The Performance Quality Rating Scale (PQRS): reliability, convergent validity, and internal responsiveness for two scoring systems [published online April 28, 2014] Disabil Rehabil. doi: 10.3109/09638288.2014.913702. [DOI] [PubMed] [Google Scholar]
- 27.Cup EH, Scholte op Reimer WJ, Thijssen MC, van Kuyk-Minis MA. Reliability and validity of the Canadian Occupational Performance Measure in stroke patients. Clin Rehabil. 2003 Jul;17(4):402–409. doi: 10.1191/0269215503cr635oa. [DOI] [PubMed] [Google Scholar]
- 28.Heinemann AW, Lai JS, Magasi S, et al. Measuring participation enfranchisement. Arch Phys Med Rehabil. 2011;92:564–571. doi: 10.1016/j.apmr.2010.07.220. [DOI] [PubMed] [Google Scholar]
- 29.Heinemann AW, Magasi S, Bode RK, et al. Measuring enfranchisement: Importance of and control over participation by people with disabilities. Arch Phys Med. Rehabil. 2013;94:2157–2165. doi: 10.1016/j.apmr.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The Stroke Impact Scale version 2.0. evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 31.Duncan PW, Bode RK, Min Lai S, Perera S. Glycine Antagonist in Neuroprotection Americans Investigators. Rasch analysis of a new stroke-specific outcome scale: The Stroke Impact Scale. Arch Phys Med Rehabil. 2003;84:950–963. doi: 10.1016/s0003-9993(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 32.Edwards B, O'Connell B. Internal consistency and validity of the Stroke Impact Scale 2.0 (SIS 2.0) and SIS-16 in an Australian sample. Qual Life Res. 2003;12:1127–1135. doi: 10.1023/a:1026109920478. [DOI] [PubMed] [Google Scholar]
- 33.Gage M, Noh S, Polatajko HJ, Kaspar V. Measuring perceived self-efficacy in occupational therapy. Am J Occup Ther. 1994;48:783–790. doi: 10.5014/ajot.48.9.783. [DOI] [PubMed] [Google Scholar]
- 34.Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141:2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- 35.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 36.Geusgens CA, Winkens I, van Heugten CM, Jolles J, van den Heuvel WJ. Occurrence and measurement of transfer in cognitive rehabilitation: a critical review. J Rehabil Med. 2007;39:425–439. doi: 10.2340/16501977-0092. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt RA, Lee TD. Motor Control and Learning: A Behavioural Emphasis. 4th ed. Champaign, IL: Human Kinetics; 2005. [Google Scholar]
- 38.Stokes PD, Lai B, Holtz D, Rigsbee E, Cherrick D. Effects of practice on variability, effects of variability on transfer. J Exp Psychol Hum Percept Perform. 2008;34:640–659. doi: 10.1037/0096-1523.34.3.640. [DOI] [PubMed] [Google Scholar]
- 39.Lustig C, Flegal KE. Targeting latent function: encouraging effective encoding for successful memory training and transfer. Psychol Aging. 2008;23:754–764. doi: 10.1037/a0014295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olsson CJ, Jonsson B, Nyberg L. Learning by doing and learning by thinking: an fMRI study of combining motor and mental training. Front Hum Neurosci. 2008;2:5. doi: 10.3389/neuro.09.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens D, Anderson DI, O’Dwyer NJ, Mark Williams A. Does self-efficacy mediate transfer effects in the learning of easy and difficult motor skills? Conscious Cogn. 2012;21:1122–1128. doi: 10.1016/j.concog.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Korpershoek C, van der Bijl J, Hafsteinsdottir TB. Self-efficacy and its influence on recovery of patients with stroke: a systematic review. J Adv Nurs. 2011;67:1876–1894. doi: 10.1111/j.1365-2648.2011.05659.x. [DOI] [PubMed] [Google Scholar]