Abstract
Background
Brain magnetic resonance image (MRI) registration alters structure orientation, size, and/or shape.
Purpose
To determine whether linear registration methods (image transformation to 6, 9, and 12 degrees of freedom) alter structural volume and cognitive associations, we examined transformation alterations to the caudate nucleus within individuals diagnosed with Parkinson’s disease (PD) and demographically matched non-PD peers.
Hypotheses
Volumes from native and six were expected be significantly different from nine and 12 degree of freedom methods. Caudate nucleus volumes were expected to associate with measures of processing speed and mental flexibility, but the strength of the association based on transformation approach was unknown.
Methods
MRI brain scans from individuals with Parkinson’s disease (n=40) and age-matched controls (n=40) were transformed using 6, 9, and 12 degrees of freedom to an average brain template. Correlations controlling for total intracranial volume assessed expected structural-behavioral associations.
Results
Volumetric: Raw nine and 12-degree transformed volumes were significantly larger than native and 6-degree volumes. Only nine and 12-degree volumes revealed group differences with PD less than controls. Intracranial volume considerations were essential for native and six-degree between group comparisons. Structural-Behavioral: The 9 and 12-degree caudate nucleus volume transformations revealed the expected brain-behavioral associations.
Conclusion
Linear registration techniques alter volumetric and cognitive-structure associations. The study highlights the need to communicate transformation approach and group intracranial volume considerations.
Keywords: Parkinson’s disease, linear image registration, caudate nucleus, degrees of freedom, intracranial volume, template
Introduction
Brain magnetic resonance (MR) imaging research in the behavioral sciences typically involves the comparison of brain regions among groups of individuals or between multiple MR scans of the same individual. Owing to advances in freeware applications (e.g., FreeSurfer, FSL; Fischl, 2012), researchers are now able to use various image techniques to study different brain structures. MR transformation models commonly include global linear and non-linear transformations.
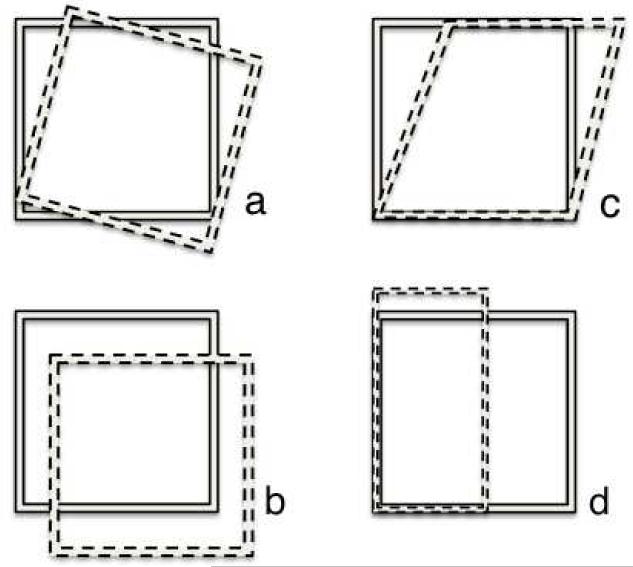
Linear transformations are frequently used in cognitive-behavioral volumetric analyses and are consequently the focus of the present study. Linear registration alters a natively acquired image (i.e., image from the MRI scanner) to fit a preselected coordinate system template or to fit a reference image from the study population (Ashburner & Friston, 2000) with all natively acquired MRI images in a sample transformed to the same template. Briefly, templates are chosen based on considerations for age, gender, hemispheric asymmetry, anatomical correspondence, spatial normalization methodology and disease-specificity (Evans, Janke, Collins & Baillet, 2012). In many instances it is appropriate to employ a template that is a part of a widely used software package (e.g., SPM, FSL) or available standardized templates (e.g., Talairach, MNI); however, these templates are less appropriate for pediatric or disease-affected populations (Evans et al., 2012). In these cases, a brain template may be created by averaging all the brain images in a study sample together to develop a unique template more fitting to the population under study. Individual images are transformed and aligned to the chosen template using 6, 9, or 12 degree of freedom (DOF) transformations. A 6 DOF linear transformation method is a form of rigid body registration that involves three translations and three rotations. The natively acquired image is aligned to the reference image by shifts and rotations in the x, y, and z planes. The term rigid body refers to the fact that this method does not alter the dimensions of the native space image, but rather adjusts its position in space to match that of the reference image. A 9 DOF transformation performs the same alterations as the 6 DOF transformation but can also stretch the image to fit the template image in x, y, and z directions, thus changing the geometry within the image. A 12 DOF transformation adds shearing to this process and can result in the greatest degree of warping in an attempt to fit the template image. See Figure 1 for a schematic of the alterations associated with the 6, 9, and 12 degree transformations.
Figure 1.
The solid square represents the template. The dotted square represents the structure of interest relative to the template based on (a) rotation, (b) translation, (c) shear, (d) stretch/zoom.
Registering an image with 6, 9, or 12 degrees of transformation can inherently alter its appearance and associated metrics, including volumetric measurements. It has yet to be agreed upon, however, if degree of transformation should be a major consideration for brain-behavior based analyses and brain structural volumetric comparisons. It is unclear which transformation approach is most preferred; some studies report on 6 DOF alignment approaches (e.g., Allen, Damasio, & Grabowski, 2002; Hefkemeijer et al., 2012; Moller et al., 2013; Singh et al., 2012), while others report 12 DOF setting (e.g., Gitelman et al., 2013). Still, some publications do not report any degree of transformation (e.g., Jiji, Smitha, Gupta, Pillai, & Jayasree, 2013; Lee et al., 2013; Lubin et al., 2013; Martinot et al., 2013; Serra-Blasco et al., 2013; Winston et al., 2013). This methodological issue is particularly germane to the modern behavioral scientist studying brain-behavior relationships.
Our current proof-of-principle study examined whether linear registration from a native brain image transformed to 6, 9, and 12 DOF would alter volumetric quantification and volumetric-cognitive associations. We focused our examination on one structure: the caudate nucleus. Subcortical structures like the caudate nucleus are ideal for volumetric analyses due to the ease with which they can be automatically or manually segmented from both clinical and research-quality MR images.
Caudate Nuclei
The caudate nuclei are a set of bilateral subcortical gray matter structures that are part of the basal ganglia and an integral element of a broader frontal-striatal network. The caudate nucleus has been implicated in motor, affective, and cognitive functioning, and it is a crucial structure in the study of cognition in subcortical diseases such as Huntington’s and Parkinson’s disease (PD), as well as schizophrenia (Grahn, Parkinson & Owen, 2009).
The role of the caudate nucleus in higher human cognition is now well established (for a review see Middleton & Strick, 2000). Specifically, the caudate nucleus is involved in higher cognitive processes via the “Dorsolateral Prefrontal Circuit” as classically described by Alexander, DeLong and Strick in 1986. The dorsal prefrontal convexity (Brodmann’s areas 9, 10) projects to the dorsolateral head of the caudate nucleus with a continuous rostrocaudal expansion that extends to the caudate nucleus tail. The caudate nucleus is engaged in an active feedback loop (Postuma & Dagher, 2006) associated with directing attention, switching attention between processes, processing speed and mental flexibility (Grahn, Owen & Parkinson, 2008). Disruption to this connection is associated with frontal systems impairment and cognitive-executive difficulties such as those observed in Parkinson’s disease (Owen et al., 1998; Dagher et al., 2001; Cools et al., 2002).
Caudate Nucleus in Parkinson’s Disease
Parkinson’s disease (PD) is characterized by a loss of dopamine projections to the striatum. Dopaminergic depletions in this area result in a distinct pattern of motor impairments, commonly in association with mild cognitive impairment, especially within the executive domain. The regions that cause the significant motor impairments observed in a patient with PD (i.e. basal ganglia and striatum) are the same interconnected areas that contribute to slowed speed of thinking, impaired attention and other executive deficits (Marie et al., 1999). Specifically, evidence suggests that the pattern of cognitive impairments seen in individuals with PD may be explained by the spatiotemporal progression of dopamine depletion in the striatum with greatest depletion observed within the caudate nucleus head (to a maximum of about 90%) (Rinne et al., 1989). This is an area heavily connected with dorsolateral regions of the frontal lobe via terminal cortical afferents and the dorsolateral prefrontal circuit described by Alexander, DeLong and Strick (1986). PD-related cognitive deficits have resembled impairments similar to those in individuals with prefrontal cortex lesions (Taylor, Saint-Cyr & Lang, 1986; Owen et al., 1992; Dubois et al., 1994).
Proof of Principle
The current study examined whether MR linear transformation approach altered caudate nucleus volumetric measurements and associated structure-function correlations within a sample of individuals diagnosed with idiopathic PD and non-PD control peers. For all individuals, the caudate nucleus volumes segmented in native space were compared to those segmented and transformed to 6, 9, and 12 DOF. Given that a 6 DOF transformation is a form of rigid body registration, as opposed to the 9 and 12 DOF, we hypothesized that the latter two techniques would significantly alter calculated volumes of the caudate nucleus, whereas the 6 DOF transformation would result in little or no change in calculated volumes from the native-based measurements. We expected caudate nucleus volumes to be smaller for PD participants relative to the non-PD controls, regardless of transformation space. Using data from all participants (PD and non-PD controls) we expected caudate nucleus volumes to positively associate with better processing speed and mental flexibility, although the strength of the associations relative to transformation approach was unknown. We incorporated measures of general cognition, naming, and vocabulary into our structure-function analyses to examine dissociations in caudate nucleus functions (i.e., structural specificity to processing speed and mental flexibility, rather than explicit general cognition/naming abilities).
Methods
Participants
The investigation was conducted with approval from the University of Florida’s Institutional Review Board and in compliance with the Declaration of Helsinki. A priori hypotheses were examined using data acquired from a federally funded investigation. Participants had completed a brain MRI and also a comprehensive neuropsychological assessment. Inclusion criteria required 1) ≥ 60 years of age, 2) able to read and write, 3) native English speakers, 4) intact instrumental activities of daily living (Lawton & Brody, 1969), 5) non-demented via DSM-IV criteria (APA, 2000), 6) successful T1-weighted MRI sequence acquired on a MRI 3T scanner, and 7) neuropsychological data available for analysis. Participants within the PD group had an idiopathic disease onset, were on medication, and showed no signs of dementia based on a neuropsychological assessment. Individuals with PD were diagnosed by a movement disorder fellowship trained neurologist, met criteria outlined by the UK Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria (Hughes, Ben-Shlomo et al. 1992) and had a Hoehn and Yahr scale (Hoehn and Yahr 1967) ranging from 1-3. Medical exclusions included the presence of any underlying medical disease likely to limit lifespan or confound outcome analysis: cancer requiring treatment in past 5 years (exception: non-melanoma skin cancer), serious infectious diseases (e.g., self-reported HIV), myocardial infarction or cerebrovascular accident in the last six months, congestive heart failure, chronic hepatitis, history of organ transplantation, and any other medical condition likely to limit lifespan, seizure disorders, head trauma resulting in intensive care. Surgical exclusion included having undergone Deep Brain Stimulation or recent major surgery requiring anesthesia in the last three months. Neurodegenerative exclusions included evidence of secondary or atypical Parkinsonism as suggested by the presence of any of the following: 1) history of major stroke(s), 2) exposure to toxins or neuroleptics, 3) history of encephalitis, 4) neurological signs of upper motor neuron disease, cerebellar involvement, supranuclear palsy.
MRI Parameters and Structure Registration
Data were acquired from a Siemens 3T Verio scanner using an 8-channel head coil. For gray-white matter sequencing purposes, the protocol included two separate MPRAGE T1-weighted protocols with the following parameters: 176 contiguous slices, 1x1x1mm voxel, TR/TE = 2500/3.77ms, sagittal acquisition.
Caudate Segmentation
For each individual, the left and right caudate nuclei were automatically segmented in native space using FreeSurfer software (version 5.3; Fischl, 2012). Using these segmented volumes, structure registration was completed to 6, 9 and 12 DOF using FMRIB’s Linear Image Registration Tool (FLIRT; Jenkinson & Smith, 2001; http://fsl.fmrib.ox.ac.uk/fsl/flirt). FLIRT applies voxel intensity from grayscale native scans to co-register images. FLIRT is used for automated intra- and inter-individual and intra- and inter-modal registration of three-dimensional images. It may also be employed for the correction of the effects of motion during an MRI scan. Our image processing utilized a correlation ratio cost function with trilinear interpolation for whole brain registration and nearest neighbor interpolation for binary masks. A total of four caudate nuclei volume measurements were acquired for each participant: native space and each registration space (6, 9, and 12 DOF). Analyses were completed on total volumes (sum of left and right caudate nuclei). Caudate nucleus volume differences across the transformation states (native, 6, 9, 12) were assessed as a raw volume and as a proportion of native space total intracranial volume.
Total Intracranial Volume (TICV)
In order to address potential differences in head size on structural transformation associations, we applied the FSL Brain Extraction Tool (version 5.0.3; BET; Smith, 2002) to extract the intracranial region including the cerebellum as seen in native space for each participant. These 3D total intracranial masks were then visually inspected by two trained raters and were corrected using ITK-SNAP (Yushkevich et al., 2006; www.itksnap.org). Rater reliability for final TICV volumes was excellent [DSC intra- and inter-rater reliability > 0.99, sample of 10 randomly selected de-identified brains completed on two separate occasions].
Brain Template
All 80 subjects were included to create an average brain (‘template’) representative of the sample using FreeSurfer (make_average_subject). FLIRT was applied to transform the bilateral caudate nuclei from each individual into alignment with the averaged aggregate template brain.
Cognitive Behavioral Indices
Motor and general cognitive functions involved outcome variables from
Unified Parkinson’s Disease Rating Scale (UPDRS) (Fahn et al., 1987; Goetz et al., 2008)
This assessment involves multiple components intended to track the course and severity of PD symptoms. Evaluation is based on five subscales in which a higher score equates to increased impairment. The UPDRS administration was videotaped and scored by trained and reliable raters blinded to group category. The primary dependent variable (DV) was UPDRS Section III - motor subscale (higher score = poorer motor functioning). This measure was chosen to assess the relationship between PD-specific motor functioning and caudate nucleus volume.
Dementia Rating Scale-2 (DRS-2) (Jurica, Leitten & Mattis, 2001)
This measure assesses the overall cognitive status of older adults over several domains: attention, initiation/perseveration, construction, conceptualization, and memory (DV = sum of all raw scores on each domain; total possible correct = 144 points; analyses completed on age and education based standardized scores). The DRS-2 is typically utilized as a general cognitive screener of intact mental status in older adults.
Frontal-subcortical functions were quantified from the following metrics
Processing Speed Index
The Processing Speed Index standardized composite of the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III; Wechsler, 1997) was used to assess visual-motor processing speed. This is a composite of the Digit Symbol subtest (a test requiring rapid matching of symbols and numbers shown randomly in horizontal arrays across a portrait 8x11 inch page; DV=total correct within 120 seconds) and Symbol Search (requiring the recognition of correctly matched symbols; DV = total correct in 120 seconds; WAIS-III standardized index score). We expected larger caudate nucleus volumes to associate with better processing speed scores.
Trail Making Test, Part B (Reitan, 1969)
A timed test requiring individuals to rapidly alternate between ascending numbers and letters distributed randomly throughout a page (DV = standardized z-score conversion of the total time to completion; score based on age and education based normative comparisons (Heaton, Miller, Taylor, Grant, 2004)). This cognitive test is often used to assess frontal systems function. We expected larger caudate nucleus volume to associate with scores indicating faster time to completion.
Neuropsychological dissociation measure
Boston Naming Test (BNT; Kaplan, Goodglass, & Weintrab, 1983)
This measure of confrontation naming requires individuals to name objects shown as line drawings (DV = total correct; total score = 60 possible points; final score based on age and education based normative comparisons; Heaton, Miller, Taylor, Grant, 2004). Research has indicated that there is increased variability in raw scores in normal older adult populations (Lezak, 2004). Task performance has been positively associated with temporal lobe gray matter (Baldo, Arevale, Patterson, Dronkers, 2013) and not highly dependent upon caudate nucleus volume.
Wechsler Abbreviated Scale of Intelligence (WASI), Vocabulary subtest (Wechsler, 1999)
The Vocabulary subtest of the WASI assesses one’s ability to succinctly define words of increasing difficulty. A score of 0, 1, or 2 is assigned based on the degree specificity and accuracy of responses (DV= age and education standardized total score). Test performance is associated with education and is typically resistant to changes in subcortical structures such as the caudate nucleus (Lezak, 2004).
Statistical Analysis
Data were examined for normality of score distributions and requirements for various statistical tests. Due to skew, Mann-Whitney U test was used to examine differences in UPDRS-III score for PD versus controls. For both raw caudate nucleus volumes and caudate nuclei as a proportion of TICV (caudate/TICV), we conducted separate mixed model Analyses of Variance for Transformation (Native, 6, 9, 12) by Group type (PD, Control). This allowed us to assess the main effect of transformation on the entire sample and an interaction between transformation and structure volume size by group type (PD, Control). Follow-up planned pair-wise comparisons were conducted with Least Significant Differences. In order to maximize both range and power, we combined both the PD and non-PD peers to assess relationships between caudate nuclei volumes and standardized cognitive-behavioral variables. For these analyses, we used two-tailed Pearson Product Moment Correlations. Due to violations of normative assumptions, analyses with UPDRS-III scores were examined with non-parametric correlation analyses (Spearman’s rho, ρ). Spearman correlations were used to assess caudate nuclei volumes relative to UPDRS-III scores. A separate bivariate correlation examined associations between processing speed and trail making test while controlling for motor severity as measured by the UPDRS. For neuroanatomical-cognitive associations, Fisher’s r-to-z transformation (Meng, Rosenthal & Rubin, 1992) assessed for statistical differences in native versus 12 DOF correlation coefficients.
Results
Participant demographics
Table 1. Data from 80 participants were acquired (40 idiopathic non-demented PD; 40 non-demented, non-PD control participants). We evaluated the sample of mixed PD and non-PD participants as a whole as well as seperately in their respective groups. The focus of the main analyses, however, involved the combined group sample as a whole in order to provide a greater range of values for both volumetric measurements of the caudate nucleus and scores on test measures. Individuals with PD and the non-PD matched controls were similar in age (t(78)=.33, p=0.74) and education (t(78)=0.44, p=0.44). Individuals with PD were in the mild stage of the disease (H&Y range 1-3; mean UPDRS, part III= PD 17.58±10.74; Controls 2.75± 3.36; U = 83.50, p<.001), and included a mixture of left (n=25) and right (n=14) side symptom dominance and axial symptom dominance (n=1). Cognitively, individuals with PD were statistically similar to the non-PD controls on the DRS-2 general cognitive screening measure (t(78)=1.23, p=0.22), confrontation naming (t(78)=0.58, p=0.56) and vocabulary (t(78)=1.07, p=0.29. As expected, PD performed lower relative to controls on the WAIS-III Processing Speed Index (t(77)=5.32, p<.001) and Trail Making Test Part B (t(78)=4.30, p<.001).
Table 1. Demographic and Cognitive Variable Means, Standard Deviations, and Minimum to Maximum scores (shown in parentheses) by Total Sample and Group.
| Total | PD | Controls | |
|---|---|---|---|
| Age (years) | 67.99±5.03 (60-79) | 67.80±5.44 (60-79) | 68.18±4.64 (62-79) |
| Sex (n) | M=65, F=15 | M=32, F=8 | M=33, F=7 |
| Education (years) | 16.51±2.70 (10-22) | 16.28±3.03 (10-22) | 16.75±2.35 (12-20) |
| UPDRS Part III* | 10.16± 0.87 (0-46) | 17.58±10.74 (3-46) | 2.75±3.36 (0-15) |
| DRS-2 total raw | 139.81±2.84 (131-144) | 139.43±3.13 (131-144) | 140.20±2.49 (133-144) |
| DRS-2 z-score | 0.18±0.73 (−1.00-1.67) | 0.16±0.82 (−1.00-1.67) | 0.20±0.64 (−0.67-1.67) |
| PS Index Score* | 105.99±12.55 (79-137) | 99.44±10.43 (79-122) | 112.38±11.16 (93-137) |
| TMT B raw time* | 86.84±38.78 (31-218) | 103.54±44.02 (46-218) | 70.14±23.18 (31-134) |
| TMT B z-score* | −0.03±1.03 (−1.9-2.5) | −0.48±0.94 (−1.9-1.4) | 0.42±0.93 (−1.6-2.5) |
| BNT raw | 57.18±2.84 (44-60) | 56.80±3.34 (44-60) | 57.55±2.21 (52-60) |
| BNT z-score | 0.71±0.98 (−1.3-2.6) | 0.65±0.97 (−1.2-2.3) | 0.77±1.00 (−1.3-2.6) |
| Vocab raw | 67.21±6.58 (47-77) | 66.40±7.56 (47-77) | 68.03±5.39 (55-77) |
| Vocab z-score | 1.08±0.62 (−0.6-2.1) | 1.00±0.70 (−0.6-2.1) | 1.16±0.53 (−0.1-2.10) |
Sample size was PD (n=40), control (n=40) unless indicated otherwise. Abbreviations: DRS-2: Dementia Rating Scale-2; UPDRS Part III: Unified Parkinson’s Disease Rating Scale, motor portion (part 3); PS Index: Processing Speed Index standardized score - note PD n=39 (due to stopwatch error); TMT B raw = Trail Making Text Part B raw time in seconds to completion; TMT B z-score = Trail Making Test Part B standardized z-score; BNT raw: Boston Naming Test raw score; BNT z-score: Boston Naming Text z-score; Vocab raw= WASI Vocabulary subtest raw score; Vocab z-score= WASI Vocabulary subtest z-score;
PD < Controls: p <.001
Transformation and Raw Caudate Nucleus Volume Differences
Table 2. A Mixed Model Analysis of Variance revealed a significant main effect for transformation approach [F(3, 234) = 304.57, p<.001] on raw caudate nucleus volumes. When collapsed across all participants regardless of group, the 9 and 12 DOF volumes were significantly larger than the native volumes and the 6 DOF (p’s < 0.01). Native and 6 DOF caudate nucleus volumes were similar in volume (p=0.39), as were the 9 and 12 DOF (p=0.18).
Table 2. Caudate Nucleus Volumes by Total Sample and Group (PD, Non-PD Controls).
| Native | 6-DOF | 9-DOF | 12-DOF | |
|---|---|---|---|---|
| Raw Volumes (mm3) | ||||
| Total (n=80) | ||||
| 7469.55 ± 1131.79 | 7468.11 ± 1132.72 | 9160.86 ± 1167.69 | 9174.74 ± 1157.06 | |
| PD (n=40) | ||||
| 7578.20 ± 1185.31 | 7576.78 ± 1187.95 | 8920.63 ± 1163.94 | 8941.70 ± 1146.95 | |
| Control (n=40) | ||||
| 7360.90 ± 1079.61 | 7359.45 ± 1078.68 | 9401.10 ± 1135.98 | 9407.78 ± 1133.58 | |
| TICV proportion (mm3)* | ||||
| Total (n=80) | ||||
| 0.0045 ± 0.00059 | 0.0045 ± 0.00059 | 0.0056 ± 0.00101 | 0.0056 ± 0.00101 | |
| PD (n=40) | ||||
| 0.0044 ± 0.00058 | 0.0044 ± 0.00058 | 0.0052 ± 0.00095 | 0.0052 ± 0.00095 | |
| Control (n=40) | ||||
| 0.0046 ± 0.00058 | 0.0046 ± 0.00058 | 0.0059 ± 0.00096 | 0.0059 ± 0.00096 | |
TICV = Total intracranial volume; decimals shown out to five places due to the smaller size of the corrected structure.
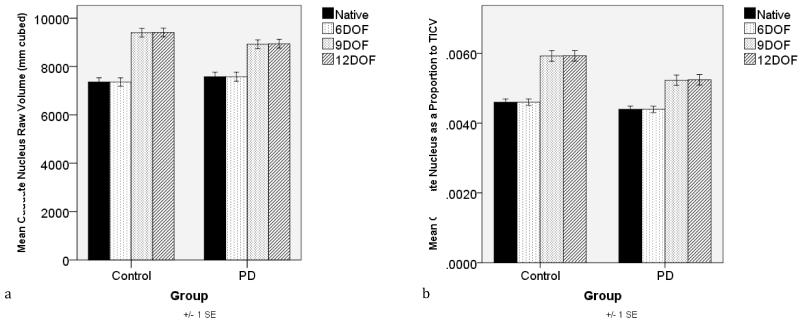
There was a significant interaction between Transformation DOF (Native, 6, 9, 12) and Group (PD, Control) [F(3, 234)=12.58, p<0.001]. In the native and 6 DOF, the control and PD groups had statistically similar raw caudate volumes [native: t(78)=0.86, p=0.394; 6DOF: t(78)=0.86, p=0.394], with a review of the means showing the controls having lower volumes on average. By contrast, raw caudate nuclei from 9 and 12 DOF demonstrated a trend for group differences in the expected direction with the PD volumes less than the control volumes [9DOF: t(78)=1.87, p=0.07; 12DOF: t(78)=1.83, p=0.07]. Figure 2a.
Figure 2.
Group (PD, Control) raw (2a) and total intracranial corrected caudate volumes (2b) by degree of freedom (DOF) transformation approach (native, six, nine, twelve).
Transformation and Caudate Nucleus Volume Corrected for Intracranial Volume
Table 2. Individuals with PD had significantly larger native space TICVs than non-PD age and education matched peers (t(78)= −3.59, p=0.001). Transformation approach analyses were re-analyzed using TICV corrected volumes.
For caudate/TICV, a Mixed Model Analysis of Variance revealed a significant main effect for Transformation DOF [F(3,234) = 239.57, p<.001]. When collapsed across all participants regardless of group, the 9 and 12 DOF volumes were significantly larger than the TICV corrected native and the 6 DOF volumes (p’s < 0.01). Native and 6 DOF caudate nucleus volumes were statistically similar in volume between the groups (p=0.36), as were the 9 and 12 DOF (p=0.21).
There was a significant interaction between Transformation DOF (Native, 6, 9, 12) and Group (PD, Control) [F(3, 234)=12.01, p<0.001]. For the TICV corrected native and 6 DOF, the control and PD groups had statistically similar TICV caudate nucleus volumes [native: t(78)=1.58, p=0.12; 6DOF: t(78)=1.58, p=0.12], but with PD volumes smaller than controls on average. The 9 and 12 DOF analyses demonstrated significant group differences with the PD volumes less than the control volumes [9DOF: t(78)=3.25, p=0.002; 12DOF: t(78)=3.22, p=0.002]. Figure 2b.
Total Caudate Nuclei Corrected for TICV and Cognitive/Behavioral Associations
Table 3. Native and 6 DOF: There were no significant associations between the behavioral or cognitive measures and TICV corrected caudate volumes. 9 and 12 DOF: Analyses showed the expected positive association between caudate nucleus volume and higher WAIS-III Processing Speed (p<0.01) and Trail Making Part B (p=0.05) standardized scores, and lower (better) scores on the UPDRS-Part III p<0.01). For Processing Speed Index (PSI), relationships remained significant after controlling for motor function (p<0.01).
Table 3. Caudate Nucleus and Cognitive Associations for Total Sample (n=80) by Transformation (native,6,9,12).
| UPDRS Pt. 3 | DRS-2 | PSI | TMT B z | BNT z | Vocab z | |
|---|---|---|---|---|---|---|
| Total (n=80): | ||||||
| Native | −0.11, p=0.33 | 0.07, p=0.53 | 0.18, p=0.10 | 0.17, p=0.13 | 0.00, p=0.99 | 0.03, p=0.79 |
| 6 DOF | −0.11, p=0.33 | 0.07, p=0.52 | 0.19, p=0.10 | 0.17, p=0.13 | 0.00, p=0.99 | 0.03, p=0.80 |
| 9 DOF | −0.31, p<0.01 * | 0.16, p=0.17 | 0.32, p<0.01 * | 0.21, p=0.06+ | −0.01, p=0.97 | −0.02, p=0.89 |
| 12 DOF | −0.30, p<0.01 * | 0.15, p=0.18 | 0.32, p<0.01 * | 0.22, p=0.06+ | −0.00, p=0.98 | −0.02, p=0.92 |
UPDRS III = Unified Parkinson’s Disease Rating Scale – Part III; DRS-2 = Dementia Rating Scale Second Edition standardized z-score; TMT B z = Trail Making Test standardized z-score; BNT z = Boston Naming Test standardized z score; Vocab z= WASI Vocabulary subtest standardized z score; Note: Correlations reflect the total caudate nucleus volume as a proportion to total intracranial volume (TICV).
Significant correlations at (p<.01, two-tailed);
Trending correlations
Fisher’s r-to-z transformation revealed the native based caudate-UPDRS correlation coefficient as significantly weaker relative to the 12 DOF caudate-UPDRS correlation coefficient (p=0.02). The native based caudate-Processing speed Index coefficient was statistically similar to the 12 DOF caudate-Processing Speed Index coefficient (p=0.06), but notable for a trend.
The Boston Naming Test and WASI Vocabulary subtest scores did not significantly associate with caudate nucleus volumes for any of transformation. No associations were observed with the Dementia Rating Scale.
Discussion
This proof of principle investigation shows that region of interest transformation approaches alter volumetric and structure-function correlations. Only the 9 and 12 degree of freedom transformations demonstrated 1) the expected volume pattern between PD and control participants, and 2) the expected structure-function associations. The findings also demonstrate the necessity for considering total intracranial volume when examining the relationship of neuroanatomical structures to cognitive or motor functioning, as previously emphasized by Bigler and Tate (2001). We offer considerations for how 9 and 12 DOF provide an alternative metric when total intracranial volume is not available, and comment upon how differing brain templates may alter volumes.
Volumetric Considerations
We identified three major findings relative to transformation and volumetric differences. First, 9 and 12 DOF volumes were significantly different in size from native and 6 DOF volumes. On average, raw caudate nucleus volumes acquired natively and in six DOF transformed space were 18.5% smaller than the raw 9 and 12 DOF transformed volumes. A similar proportion of change occurred when there was additional correction for total intracranial volume. This volume change is explained by the nature of the 9 and 12 DOF transformation.
Second, contrary to the native and six degree transformations, the 9 and 12 DOF transformations revealed less caudate nucleus volume in the PD relative to non-PD control group. The 9 and 12 DOF findings were at trend level for the raw values, but statistically significant after correcting for total intracranial volume. These findings demonstrate the importance of reporting not only transformation approach but also intracranial volume size for samples where templates or individual comparisons are made.
An unexpected third finding involved our discrepant intracranial volume in our sample; individuals with PD had significantly larger intracranial volumes than the non-PD matched controls. Raw native and 6 DOF transformed caudate nuclei volumes were consequently larger on average for PD group relative to the controls. Only after correcting for total intracranial volume did the native and 6 DOF transformed volumes suggest an expected volume pattern (i.e., PD < Controls). Correcting for intracranial volume therefore appears essential for the native and rigid (6DOF) body methods. The 9 and 12 DOF caudate nucleus transformations, by contrast, consistently showed the expected group differences (PD < control). Controlling for intracranial volume after in 9 and 12 DOF transformation may serve as an overcorrection.
Increased intracranial volume in PD has been reported by some (see Krabbe et al., 2005) and referenced as a potential genetic association (Taul, et al., 2012). Others, however, report no intracranial differences in PD relative to peers (see O’Neill, et al., 2002; Camicioli, et al., 2003). Our intracranial volume findings may therefore represent random sampling variation. This underscores previous assertions that intracranial correction is highly relevant for group volumetric analyses (Bigler & Tate, 2001). It also appears particularly relevant in native and rigid (6DOF) space.
Caudate Nuclei-Function Considerations
Caudate nuclei and function considerations were conducted with all 80 participants. This maximized score and volumetric ranges. We found that the Wechsler Adult Intelligence Scale, Third Edition Processing Speed Index (PSI) positively correlated with intracranial volume corrected caudate nucleus volumes transformed to 9 and 12 DOF but not native and 6 DOF transformed volumes; this cognitive-structure association was expected (for a review see Zgaljardic et al, 2003). The association remained significant even after controlling for motor functioning. Similarly, we found the motor subscale (part III) of the Unified Parkinson’s Disease Rating Scale (UPDRS) to be negatively correlated with volumes transformed to 9 and 12 DOF but not to native and 6 DOF transformed volumes. This is consistent with the literature suggesting caudate nucleus contributes to motor function (Middleton & Strick, 2000). Overall, the expected structure-function associations appeared to be more robust for the greater DOF transformations. On average, the 9 and 12 DOF transformed volumes explained 10.2% of the variance in PSI and 9% of the variance in UPDRS motor score compared to 3.2% and 2% variance explained for the native and 6 DOF volumes. It is worth noting that there was a trending relationship between a measure of mental flexibility, the Trail Making Test Part B, and caudate nucleus volumes transformed to 9 and 12 DOF (for both: p=0.06). It is possible that we did not have ample score range for this measure to reveal the hypothesized associations since all of the participants in our sample were cognitively well.
Taken together, the 9 and 12 DOF transformations appear to be more sensitive in detecting structure-function relationships. With this these transformations we observed associations between smaller caudate nuclei relative to intracranial volume and worse motor and cognitive function on measures of Parkinson’s disease-related motor function, speed of thinking and timed mental flexibility. The native space and 6 DOF volumes did not result in any significant associations with our cognitive variables of interest. The neuropsychological measures chosen for this study were selected based on a well-established theoretical framework of the function of the caudate nucleus (Owen et al., 1998; Middleton & Strick, 2000; Dagher et al., 2001; Cools et al., 2002; Grahn, Owen & Parkinson, 2008). Consequently, we do not believe these findings represent random associations; rather, we found associations that were expected based on past research into human neuropsychological functioning. Closer examinations in transformation methodology are warranted. The inconsistent use (or non-use) of transformations between investigations may account for differences in the results of studies examining brain-behavior relationships.
Regarding study limitations, we recognize the PD intracranial volume difference relative to non-PD controls may have exacerbated group level differences in the 9 and 12 degree transformation relative to the study template. We used FreeSurfer (Fischl, 2012) to create and register the brains in our sample to an average or aggregate image of all the participants’ brains. This approach allowed us to consider variations within group age, gender, hemispheric asymmetry and disease-specificity (Evans, Janke, Collins & Baillet, 2012). Our resulting template was 4.68% larger than the MNI152 template (distributed with FSL) reflecting the larger intracranial volume of the PD sample. This template size is, in turn, reflected in the value of the 9 and 12 DOF transformations. Given the group intracranial volume differences, the template may have artificially reduced the volume of the structures for the PD group and increased the volume of the control group. We encourage a separate investigation to document how template types (MNI, Talairach, representative brain from a sample, average brain) alter volumes, group profiles, and brain-behavior associations.
Overall, this proof-of-principle investigation demonstrated that transformation nuances can alter structural volumes and brain-behavioral patterns. We encourage additional investigations on this topic. At minimum, we suggest that researchers clearly define how anatomical regions of interest and resulting volumes were acquired (via what transformation, if any). This appears particularly relevant for brain-behavioral investigations. We also encourage studies more studies examining the interaction between group intracranial volume differences relative to template and transformation choices. We hope these studies will improve methodological communication between investigations thereby avoiding contradicting research results in the literature.
Acknowledgements and Funding
This work was completed in partial fulfillment of Ms. Schwab’s Master of Science degree in the Department of Clinical and Health Psychology, University of Florida, Gainesville, Florida. Supported by National Institute for Neurological Disorders and Stroke (NINDS) K23NS60660 (C.P.), NINDS RO1NS082386 (C.P.), NINDS R01NR014181 (C.P.), and in part by the National Institutes of Health/National Center for Advancing Translational Sciences (NIH/NCATS) Clinical and Translational Science Award to the University of Florida UL1TR000064. We are most thankful to the participants who provided us with the data making this study possible, Sylvia Orosco, John Collazo, and Stephen Towler for their time assisting with the original study concept and institutional review board requirements, and Jade Ward, B.S., for her expertise with participant recruitment and study coordination. We acknowledge William Perlstein, Ph.D., Associate Professor, Clinical and Health Psychology, University of Florida, for his statistical advice.
Footnotes
Disclosures: Nadine Schwab, Jared Tanner, Peter T. Nguyen, Ilona M. Schmalfuss, Dawn Bowers, Michael Okun, and Catherine C. Price declare that they have no conflict of interest.
Consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
References
- Alegret M, Junque C, Pueyo R, Valldeoriola F, Vendrell P, Mercader JM. MRI atrophy parameters related to cognitive and motor impairments in Parkinson’s disease. Neurologia. 2001;16:63–69. [PubMed] [Google Scholar]
- Alexander GE, Delong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Allen JS, Damasio H, Grabowski TJ. Normal neuroanatomical variation in the human brain: An MRI-volumetric study. American Journal of Anthropology. 2002;118:341–358. doi: 10.1002/ajpa.10092. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington, DC: 2000. text rev. [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Arevalo A, Patterson JP, Dronkers NF. Grey and white matter correlations of picture naming: Evidence from a voxel based lesion analysis of the Boston Naming Test. Cortex. 2013;49(3):658–667. doi: 10.1016/j.cortex.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone P. Neurotransmission in Parkinson’s disease: Beyond dopamine. European Journal of Neurology. 2009;17:364–376. doi: 10.1111/j.1468-1331.2009.02900.x. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Tate DF. Brain volume, intracranial volume, and dementia. Investigations in Radiology. 2001;36:539–546. doi: 10.1097/00004424-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL. White matter lesions in Parkinson disease. Nature Reviews Neurology. 2011;7:229–236. doi: 10.1038/nrneurol.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Tredici KD. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Research. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: A comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2003;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA. Parkinson’s disease is associated with hippocampal atrophy. Movement Disorders. 2003;18(7):784–790. doi: 10.1002/mds.10444. [DOI] [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high level cognition in Parkinson’s disease: The role of the prefrontal cortex revealed by PET. Brain. 2002;125:584–594. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- Crum WR, Modo M, Vernon AC, Barker GJ, Williams SCR. Registration of challenging pre-clinical brain images. Journal of Neuroscience Method. 2013;216:62–77. doi: 10.1016/j.jneumeth.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A, Owen AM, Boecker H, Brooks DJ. The role of the stiatum and hippocampus in planning: A PET activation study in Parkinson’s disease. Brain. 2001;124:1020–1032. doi: 10.1093/brain/124.5.1020. [DOI] [PubMed] [Google Scholar]
- Evans AC, Janke AL, Collins DL, Baillet S. Brain templates and atlases. NeuroImage. 2012;62:911–922. doi: 10.1016/j.neuroimage.2012.01.024. [DOI] [PubMed] [Google Scholar]
- Fahn SRLE, Elton R, UPDRS Development Committee Unified Parkinson’s disease rating scale. Recent developments in Parkinson’s disease. 1987;2:153–163. [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. doi:10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattellaro G, Minati L, Grisoli M, Mariani C, Carella F, Bruzzone MG. White matter involvement in idiopathic Parkinson disease: A diffusion tensor imaging study. Americal Journal of Neuroradiology. 2009;30:1222–1226. doi: 10.3174/ajnr.A1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Klein-Gitelman MS, Ying J, Sagcal-Gironella ACP, Zelko F, Beebe DW, Brunner HI. Brain morphometric changes associated with childhood-onset systemic lupus erythematosus and neurocognitve deficit. Arthritis and Rheumatism. 2013;65:2190–2200. doi: 10.1002/art.38009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Movement Disorders. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The role of the basal ganglia in learning and memory: Neuropsychological studies. Behavioural Brain Research. 2009;199:53–60. doi: 10.1016/j.bbr.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults (HRB) Psychological Assessment Resources; Lutz, Florida: 2004. [Google Scholar]
- Hefkemeijer A, Altmann-Schneider I, Oleksik AM, van de Wiel L, Middelkoop HAM, Buchem MA, Rombouts SA. Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connectivity. 2012;2:A1–A156. doi: 10.1089/brain.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Ben-Shlomo Y, et al. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology. 1992;42(6):1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jiji S, Smitha KA, Gupta AK, Pillai VPM, Jayasree RS. Segmentation and volumetric analysis of the caudate nucleus in Alzheimer’s disease. European Journal of Radiology. 2013;82:1525–1530. doi: 10.1016/j.ejrad.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, Mattis S. Psychological Assessment Resources Eds. FI; Odessa: 2001. DRS-2: Dementia Rating Scale-2. [Google Scholar]
- Kaplan E, Goodglass H, Weintrab S. The Boston Naming Test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe K, Karlsborg M, Hansen A, Werdelin L, Mehlsen J, Larsson HBW, Paulson OB. Increased intracranial volume in Parkinson’s disease. Journal of the Neurological Sciences. 2005;239:45–52. doi: 10.1016/j.jns.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- Lee JH, Han YH, Kang BM, Mun CW, Lee SJ, Balk SK. Quantitative assessment of subcortical atrophy and iron content in progressive supranuclear palsy and parkinsonian variant of multiple system atrophy. Journal of Neurology. 2013;260:2094–2101. doi: 10.1007/s00415-013-6951-x. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological assessment. 4th ed. University Press; Oxford: 2004. [Google Scholar]
- Lisanby SH, McDonald WM, Massey EW, Doraiswamy PM, Rozear M, Nemeroff C. Diminished subcortical nuclei volumes in Parkinson’s disease by MR imaging. Journal of Neural Transmission. Supplementa. 1993;40:13–21. [PubMed] [Google Scholar]
- Lubin A, Rossi S, Simon G, Lanoe C, Leroux G, Poirel N, Houde O. Numerical transcoding proficiency in 10-year-old schoolchildren is associated with gray matter inter-individual differences: A voxel-based morphometry study. Frontiers in Psychology. 2013;4:1–7. doi: 10.3389/fpsyg.2013.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen SK, Ho AJ, Hua X, Saharan PS, Toga AW, Jack CR, Jr., Thompson PM. 3D maps localize caudate nucleus atrophy in 400 Alzheimer’s disease, mild cognitive impairment, and healthy elderly subjects. Neurobiology of Aging. 2010;31:1312–1325. doi: 10.1016/j.neurobiolaging.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DMA, Yates PO. Pathological basis for neurotransmitter changes in Parkinson’s disease. Neuropathology and Applied Neurobiology. 2008;9:3–19. doi: 10.1111/j.1365-2990.1983.tb00320.x. [DOI] [PubMed] [Google Scholar]
- Marie RM, Barre L, Dupuy B, Viader F, Defer G, Baron JC. Relationship between striatal dopamine denervation and frontal executive tests in Parkinson’s disease. Neuroscience Letters. 1999;260:77–80. doi: 10.1016/s0304-3940(98)00928-8. [DOI] [PubMed] [Google Scholar]
- Martinot MLP, Lemaitre H, Artiges E, Miranda R, Goodman R, Penttila J, IMAGEN Consortium White-matter microstructure and gray-matter volumes in adolescents with subthreshold bipolar symptoms. Molecular Psychiatry. 2013 doi: 10.1038/mp.2013.44. Advance online publication. doi: 10.1038/mp.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan RJ, Anderson TJ. Grey matter atrophy in cognitively impaired Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2012;83:188–194. doi: 10.1136/jnnp-2011-300828. [DOI] [PubMed] [Google Scholar]
- Meng XL, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Quantitative Methods in Psychology. 1992;111:172–175. [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia output and cognition: Evidence from anatomical, behavioral, and clinical study. Brain and Cognition. 2000;42:183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Moller C, Vrenken H, Jiskoot L, Versteeg A, Barkhof F, Scheltens P, van der Flier W. Different patterns of gray matter atrophy in early- and late-onset Alzheimer’s disease. Neurobiology of Aging. 2013;34:2014–2022. doi: 10.1016/j.neurobiolaging.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Naismith SL, Hickie I, Ward PB, Turner K, Scott E, Little E, Parker G. Caudate nucleus volume and genetic determinants of homocysteine metabolism in the prediction of psychomotor speed in older persons with depression. American Journal of Psychiatry. 2002;159:2096–2098. doi: 10.1176/appi.ajp.159.12.2096. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Schuff N, Marks WJ, Feiwell R, Aminoff MJ, Weiner MW. Quantitative 1H magnetic resonance spectroscopy and MRI of Parkinson’s disease. Movement Disorders. 2002;17(5):917–927. doi: 10.1002/mds.10214. [DOI] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Robbins TW. Frontostriatal cognitive deficits at different stages in Parkinson’s disease. Brain. 1992;115:1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC. Abnormal basal ganglia outflow in Parkinson’s disease identified with PET. Implications for higher cortical functions. Brain. 1998;121:949–965. doi: 10.1093/brain/121.5.949. [DOI] [PubMed] [Google Scholar]
- Piccini P, Pavese N, Canapicchi R, Paoli C, Del Dotto P, Bonuccelli U. White matter hyperintensities in Parkinson’s disease: Clinical correlations. Archives of Neurology. 1995;52:191–194. doi: 10.1001/archneur.1995.00540260097023. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Alexopoulos P, Wagenpfeil S, Bickel H, Kurz A. Head circumference, apolipoprotein E genotype and cognition in the Bavarian School Sisters Study. European Psychiatry. 2012;27:219–222. doi: 10.1016/j.eurpsy.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magentic resonance imaging publications. Cerebral Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Reisine TD, Fields JZ, Yamamura HI. Neurotransmitter receptor alterations in Parkinson’s disease. Life Sciences. 1977;21:335–343. doi: 10.1016/0024-3205(77)90514-8. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Manual for administration of neuropsychological test batteries for adults and children. Neuropsychology Laboratory; Indianapolis: 1969. [Google Scholar]
- Rinne JO, Rummukainen J, Paljarvi L, Rinne UK. Dementia in Parkinson’s disease is related to neuronal loss in the medial substantia nigra. Annals of Neurology. 1989;26:47–50. doi: 10.1002/ana.410260107. [DOI] [PubMed] [Google Scholar]
- Serra-Blasco M, Portella MJ, Gomez-Anson B, de Diego-Adelino J, Vives-Gilabert Y, Puigdemont D, Perez V. Effects of illness duration and treatment resistance on grey matter abnormalities in major depression. British Journal of Psychiatry. 2013;202:434–440. doi: 10.1192/bjp.bp.112.116228. [DOI] [PubMed] [Google Scholar]
- Singh S, Modi S, Bagga D, Kaur P, Shankar LR, Khushu S. Voxel-based morphometric analysis in hypothyroidism using diffeomorphic anatomic registration via an exponentiated lie algebra algorithm approach. Journal of Neuroendocrinology. 2012;25:229–234. doi: 10.1111/jne.12001. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taal HR, St Pourcain B, Thiering E, Das S, Mook-Kanamori DO, Warrington NM, Kaakinen M, Jaddoe VWV. Common variants at 12q15 and 12q24 are associated with infant head circumference. Nature Genetics. 2012;55:532–540. doi: 10.1038/ng.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA, Lang AE. Frontal lobe dysfunction in Parkinson’s disease. The cortical focus of neostriatal outflow. Brain. 1986;109:845–883. doi: 10.1093/brain/109.5.845. [DOI] [PubMed] [Google Scholar]
- Tessa C, Lucetti C, Gianelli M, Dicotti S, Poletti M, Toschi N. Progression of brain atrophy in the early stages of Parkinson’s disease: A longitudinal tensor-based morphometry study in de novo patients without cognitive impairment. Human Brain Mapping. 2014 doi: 10.1002/hbm.22449. Epub ahead of print, doi: 10.1002/hbm.22449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. Pearson; San Antonio, Texas: 1997. [Google Scholar]
- Winston GP, Stretton J, Sidhu MK, Symms MR, Thompson PJ, Duncan JS. Structural correlates of impaired working memory in hippocampal sclerosis. Epilepsia. 2013;54:1143–1153. doi: 10.1111/epi.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive and behavioral sequelae of Parkinson’s disease: Relationship to frontostriatal circuitry. Cognitive and Behavioral Neurology. 2003;16:193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Zijdenbos AP, Dawant BM, Margolin RA, Palmer AC. Morphometric analysis of white matter lesions in MR images: Method and validation. IEEE Transactions on Medical Imaging. 1994;13:716–724. doi: 10.1109/42.363096. [DOI] [PubMed] [Google Scholar]