Abstract
Background
Renovascular hypertension (RVH) leads to left ventricular (LV) hypertrophy and diastolic dysfunction, associated with increased cardiovascular mortality. Intra-renal delivery of endothelial progenitor cells (EPCs) and mesenchymal stem cells (MSCs) improves kidney function in porcine RVH, and the potent anti-inflammatory properties of MSCs may serve to blunt inflammatory mediators in the cardio-renal axis. However, their relative efficacy in attenuating cardiac injury and dysfunction remains unknown. This study tested the hypothesis that the cardio-protective effect of EPCs and MSCs delivered into the stenotic-kidney in experimental RVH are comparable.
Methods
Pigs (n=7 per group) were studied after 10 weeks of RVH or control untreated or treated with a single intra-renal infusion of autologous EPCs or MSCs 4 weeks earlier. Cardiac and renal function (fast-CT) and stenotic-kidney release of inflammatory mediators (ELISA) were assessed in-vivo, and myocardial inflammation, remodeling, and fibrosis ex-vivo.
Results
After 10 weeks of RVH, blood pressure was not altered in cell-treated groups, yet stenotic-kidney glomerular filtration rate (GFR), blunted in RVH, improved in RVH+EPC and normalized in RVH+MSC. Stenotic-kidney release of monocyte chemoattractant protein (MCP)-1 and its myocardial expression were elevated in RVH+EPC, but normalized only in RVH+MSC pigs. RVH-induced LV hypertrophy was normalized in both EPC and MSC-treated pigs, while diastolic function (E/A ratio) was restored to normal levels exclusively in RVH+MSC. RVH-induced myocardial fibrosis and collagen deposition decreased in RVH+EPC, but further decreased in RVH+MSC-treated pigs.
Conclusions
Intra-renal delivery of EPCs or MSCs attenuates RVH-induced myocardial injury, yet MSCs restore diastolic function more effectively than EPCs, possibly by greater improvement in renal function or reduction of MCP-1 release from the stenotic-kidney. These observations suggest a therapeutic potential for EPCs and MSCs in preserving the myocardium in chronic experimental RVH.
Keywords: renal hypertension, myocardium, stem cells, progenitor cells
Introduction
Renovascular hypertension (RVH), an increase in systemic arterial pressure secondary to renal artery stenosis (RAS), is associated with major cardiac abnormalities and increased cardiovascular morbidity and mortality (3). We have recently shown that despite similar antihypertensive treatment, cardiac structure and diastolic function are impaired in RVH patients compared with essential hypertensive patients (21). Furthermore, renal revascularization does not prevent major adverse cardiovascular events compared to medical therapy alone (4), underscoring the need to identify more effective therapies to protect the damaged myocardium.
Inflammation is a key mediator in cardio-renal cross-talk. We have previously shown that porcine RVH induces myocardial inflammation, diastolic dysfunction, and fibrosis, which are partly mediated by the inflammatory cytokine monocyte chemoattractant protein (MCP)-1 (26). Furthermore, we have demonstrated that the post-stenotic human kidney with reduced glomerular filtration rate (GFR) and renal blood flow (RBF) releases inflammatory mediators that aggravate renal injury, and may serve as therapeutic targets to attenuate RVH-induced renal and myocardial damage (10).
Experimental studies suggest that both endothelial progenitor cells (EPCs) and mesenchymal stem cells (MSCs) hold promise to protect the kidney and myocardium in RVH. In porcine RVH intra-renal injection of autologous EPCs during renal revascularization decreases stenotic-kidney release of inflammatory cytokines and improves renal function (15). In addition, delivery of EPCs into the stenotic-kidney of porcine atherosclerotic RVH preserves the myocardial microvasculature and improves cardiac diastolic function, by decreasing release of inflammatory and injury mediators (34). However, autologous EPCs are difficult to isolate and expand, limiting their translational capacity.
In contrast, MSCs can be easily isolated from several tissues including adipose tissue and possess unique immunomodulatory properties. We have previously shown in porcine RVH that intra-renal infusion of MSCs ameliorates renal injury and improves renal function with or without renal revascularization (13,14), but whether this treatment decreases myocardial injury remains unknown. Importantly, the potent anti-inflammatory properties of MSCs may serve to blunt inflammatory mediators in the cardio-renal axis. Therefore, this study assesses the cardio-protective effect of MSCs when delivered into the stenotic-kidney and tested the hypothesis that this therapeutic strategy offers similar cardio-protection compared to intra-renal delivery of EPCs in experimental RVH.
Methods
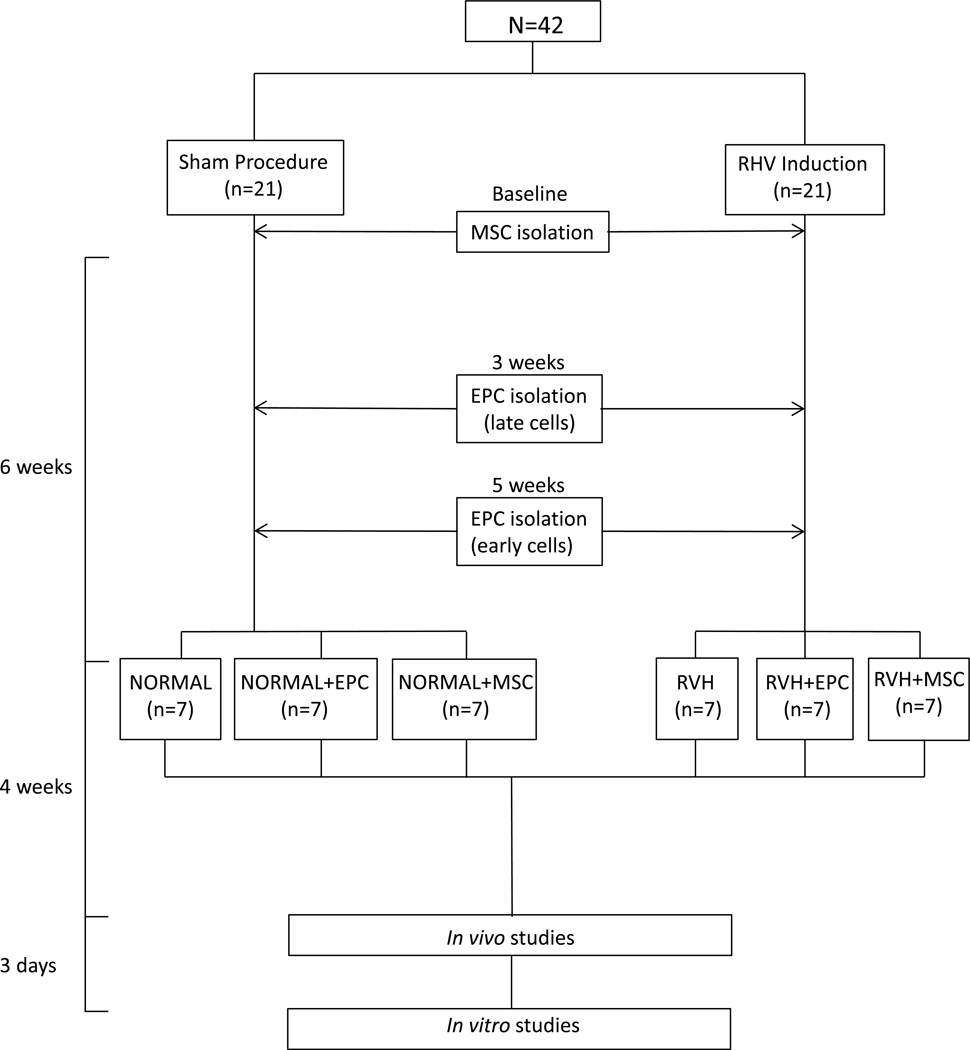
Forty-two domestic pre-menstrual female pigs (Manthei Hog Farm, Elk River, MN) were studied after 10 weeks of observation (Figure 1) and approval of the Mayo Clinic Institutional Animal Care and Use Committee. Unilateral RAS was induced in 21 RVH pigs by placing an irritant coil (Goodfellow Corporation, Coraopolis, PA) in the main renal artery under fluoroscopy (Siemens, Munich, Germany). This procedure produces a gradual narrowing of the renal artery and increases blood pressure within 3–7 days (25). A sham-procedure was performed in the other 21 normal pigs. Blood pressure was continuously measured using an implantable telemetry system (Data Sciences International, St. Paul, MN) (16).
Figure 1.
Schematic of the experimental protocol. RVH: renovascular hypertension, MSCs: mesenchymal stem cells, EPCs: endothelial progenitor cells.
Six weeks after induction of RVH or sham, animals were anesthetized with 0.25g of intramuscular tiletamine hydrochloride/zolazepam hydrochloride (Telazol®, Fort Dodge Animal Health, New York, NY) and 0.5g of xylazine, anesthesia maintained with intravenous ketamine (0.2mg/kg/min, Ketaset®, Zoetis, Bethesda, MD) and xylazine (0.03mg/kg/min, Anased®, Lloyd Laboratories, Walnut, CA), and the degree of RAS determined by angiography (Pinnacle Studio version 12.0.0.6163, Pinnacle Systems, Mountain View, CA). Normal and RVH pigs were either untreated or treated with a single infusion of autologous EPCs or MSCs, as previously described (2,13).
Four weeks later, animals were similarly anesthetized, the degree of RAS determined by angiography, and single-kidney GFR (5) and cardiac function (6) assessed by multi-detector computed-tomography (MDCT). Inferior vena cava (IVC) samples were collected for plasma renin activity (PRA) levels (Gamma-Coat® PRA kit; DiaSorin, Stillwater, MN). In addition, IVC and stenotic-kidney renal vein (RV) levels of interferon (IFN)-γ (Kingfisher, VS0259S-002), tumor necrosis factor (TNF)-α (Invitorgen, Cat# KSC3011), MCP-1 (Kingfisher Biotech, Cat# VS0081S-002), and interleukin (IL)-10 (Invitrogen, Cat# KSC0101) were measured by enzyme-linked immunosorbent assay (ELISA). Then, we estimated cytokine gradient (RV-IVC) and net renal release (gradientxRBF) for each measured marker.
Three days later, animals were euthanized with sodium pentobarbital (100mg/kg, Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI) (26). The heart and kidneys were removed, dissected, and samples from the left ventricle (LV) frozen or preserved in formalin for ex-vivo studies. In addition, the lungs, liver, and spleen were visually inspected for tumor or abnormal growth formations.
In vivo MDCT studies
To evaluate cardiac and renal function and structure, animals were scanned using MDCT (Somatom Sensation 64; Siemens Medical Solutions, Forchheim, Germany) under intravenous ketamine and xylazine anesthesia, as described above. Briefly, a 50-s flow study was performed in selected LV sections following a bolus injection of nonionic, low osmolar contrast medium (Iopamidol-370, 0.33 ml/kg, Omnipaque®, Novalplus, USA) into the right atrium. For cardiac systolic and diastolic functions and LV muscle mass (LVMM), the entire LV was scanned 20 times throughout the cardiac cycle. LVMM, cardiac output, end-diastolic volume (EDV), early (E) and late (A) ventricular filling rates were calculated using Analyze™ (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). Myocardial perfusion index was calculated at baseline and after a 5min intravenous infusion of adenosine (400µg/kg/min, Sigma-Aldrich, St. Louis, MO) (34).
Stenotic and contralateral (CLK) kidney GFR and RBF were measured using MDCT images acquired following a central venous injection of iopamidol (0.5mL/kg) and calculated from time density curves, as described (5,9).
Ex-vivo studies
EPCs were obtained from mononuclear cells isolated from peripheral blood (100ml) by density gradient method (38). “Late” EPCs were obtained from cells collected 3 weeks after RAS induction, and subsequently cultured for 3 weeks, while “early” EPCs were obtained from cells collected and cultured 1 week before infusion (2).
EPCs were characterized by fluorescence activated cell sorting (FACS) analysis after immunostaining with monoclonal antibodies against the progenitor markers CD133 (R&D Systems, MN, Cat# AF3890, NS0-derived rpCD34 1:50) and kinase-insert domain receptor (KDR, Santa-Cruz, CA, Cat# sc-504, Clone: C-1158 1:50), as previously described (10).
MSCs were isolated from adipose tissue (5–10g) collected from pigs during RVH induction or sham. Tissue was processed for MSC isolation with standard protocol (32), and cultured with advanced minimum-essential-medium (Gibco/Invitrogen) supplemented with 5% platelet lysate (Mayo Clinic Transfusion Medicine) in 37°/5% CO2. FACS was used to determine cellular phenotype for the MSC markers CD44 (abcam Cat#: ab10558 1:100) and CD90 (BD Pharmigen Cat#:55593 1:100). Before delivery EPCs and MSCs were labeled with a fluorescent membrane dye (CM-DiI, CellTracker™, Catalog #: C7001, Life Technologies) and kept in cell recovery medium at −80°C for transplantation. Then, 6 weeks after RVH or sham induction, 10^6 cells/mL of EPC (an equal mix of early and late) or MSCs suspended in 10ml of PBS (Life Technologies, # 10010-023) were injected slowly through a balloon catheter (OPTA® Pro PTA Dilatation Catheter, Cordis, New Jersey) placed in the renal artery proximal to the stenosis.
Fluorescent-labeled cells were subsequently manually counted ex-vivo under fluorescence microscopy (ZEN® 2012 blue edition, Carl ZEISS SMT, Oberkochen, Germany) in 5µm LV cross-sections and 5µm renal tissue sections stained with cytokeratin (AbD Serotec, Cat# MCA1907), and their number per field averaged (7,8,34). Furthermore, EPC and MSC distribution was evaluated by immunofluorescence staining with the distal tubular marker peanut agglutinin (PA, Vector Lab, Cat# FL-1071, 1:500), the proximal tubular marker phaseolus vulgaris erythroagglutinin (PHA-E, Vector Lab, Cat# FL-1121, 1:500), the endothelial marker CD31 (AbD Serotec, Cat# MCA 1747, dilution 1:50), and the proliferating cell nuclear antigen (PCNA, Abcam, Cambridge, MA; Cat# ab29,1:100).
Myocardial injury
Myocardial inflammation was evaluated in 5-µm sections of the LV fluorescently-double stained with connexin-43 (Abcam; Cat# ab79010) and either MCP-1 (MyBioSource, 1:7500), TNF-α (Santa Cruz, 1:200), (IF)-γ (Santa Cruz 1:200), or IL-10 (Santa Cruz 1:200). Images were semiautomatically quantified using ZEN® in 20 random fields, and expressed as percentage of staining of total surface area. Myocyte cross-sectional area (H&E), myocardial fibrosis (trichrome), and interstitial collagen deposition (Sirius red) was assessed in mid-LV cross-sections.
CLK injury
Tubulo-interstitial fibrosis (trichrome staining) was assessed in 5µm CLK sections, as previously described (11).
Statistical analysis
Data were analyzed using JMP 9.0 (SAS Institute, Cary, NC) and results expressed as mean±standard deviation for normally distributed variables and median (range) for non-Gaussian distributed data. One-way ANOVA and unpaired Student’s t-test were used the former, while non-parametric (Wilcoxon and Kruskal-Wallis) tests were used the latter. The post-hoc Bonferroni correction was used after ANOVA and Kruskal-Wallis for multiple comparisons. Statistically significance was accepted for p<0.05.
Results
After a 10-week observation, body weight was similar among the groups, but mean arterial pressure and degree of stenosis were higher in all RVH groups compared to normal (Table 1, all p<0.05 vs. normal). PRA was not significantly different among the groups, whereas stenotic-kidney GFR was lower in RVH was improved in RVH+EPC compared to normal single-kidney GFR, yet restored to normal levels only in RVH+MSC-treated pigs (p=0.01 vs. RVH and p=0.17 vs. normal).
Table 1.
Systemic characteristics of normal, normal+endothelial progenitor cells (EPCs), normal+mesenchymal stem cells (MSCs), renovascular hypertension (RVH), RVH+EPC, and RVH+MSC pigs (n=7 each) at 10 weeks.
| NORMAL | NORMAL+EPC | NORMAL+MSC | RVH | RVH+EPC | RVH+MSC | |
|---|---|---|---|---|---|---|
| Body weight (Kg) | 49.2±3.0 | 48.0±8.2 | 47.8±3.4 | 49.1±4.1 | 50.4±6.4 | 48.9±5.4 |
| Degree of stenosis (%) | 0 (0-0) | 0 (0-0) | 0 (0-0) | 60 (60–99)* | 60 (75–80)* | 65 (60–99)* |
| Mean arterial pressure (mmHg) | 95.3 (71.0–134.7) | 95.7 (79.7–117.0) | 96.2 (85.0–113.3) | 131.0 (96.7–150.7)* | 127.3 (93.3–150.0)* | 136.7 (90.3–149.7)* |
| PRA (ng/ml/hr) | 0.1 (0.07–0.3) | 0.2 (0.09–0.3) | 0.1 (0.02–0.3) | 0.2 (0.06–0.3) | 0.2 (0.02–0.3) | 0.1 (0.1–0.3) |
| Single-kidney GFR (ml/min) | 97.0±8.1 | 95.5±23.4 | 91.6±12.0 | 49.7±7.1*#† | 68.5±7.9* | 80.6±20.1 |
| Heart rate (bpm) | 94.6±25.4 | 98.7±23.8 | 96.5±4.2 | 97.9±22.0 | 99.9±11.9 | 95.2±15.9 |
| Ejection Fraction (%) | 58.9±7.8 | 56.0±5.7 | 58.1±6.3 | 63.8±13.0 | 63.6±5.7 | 58.9±5.5 |
| Stroke volume (ml) | 43.9±7.6 | 46.4±8.2 | 51.0±3.4 | 49.1±8.7 | 43.1±10.5 | 48.3±7.3 |
| LVMM (g/kg body weight) | 0.9±0.1 | 1.0±0.1 | 0.9±0.2 | 1.3±0.1*#† | 0.8±0.1 | 0.9±0.1 |
| E/A ratio | 1.1±0.2 | 1.1±0.2 | 1.2±0.2 | 0.8±0.1*† | 0.9±0.2* | 1.0±0.2 |
| End diastolic volume (ml) | 84.3±8.4 | 84.6±11.6 | 88.5±12.4 | 65.8±3.2*#† | 77.3±3.0*† | 88.2±6.1 |
| Myocardial perfusion (ml/min/g) | ||||||
| Baseline | 0.8 (0.6–1.4) | 0.8 (0.7–1.1) | 0.8 (0.5–1.0) | 0.6 (0.5–0.7)*† | 0.7 (0.6–0.9)*† | 1.0 (0.8–1.0) |
| Response to adenosine (Δ) | 0.1±0.1 | 0.1±0.1 | 0.2±0.1 | −0.1±0.1 | 0.2±0.1 | 0.1±0.1 |
p<0.05 vs. Normal;
p<0.05 vs. RVH+EPC,
p<0.05 vs. RVH+MSC;
p<0.05 vs. Baseline.
PRA: Plasma renin activity, GFR: glomerular filtration rate, E/A: Early (E) and atrial (A) ventricular filling, LVMM: Left ventricular muscle mass.
Cardiac function
Heart rate, ejection fraction, and stroke volume were similar among the groups (Table 1, p>0.05, ANOVA). LVMM was elevated in RVH, but restored to normal levels in both RVH+EPC and RVH+MSC groups. E/A ratio was lower in both RVH and RVH+EPC compared to normal, and restored to normal levels in RVH+MSC animals, although it was not different from RVH+EPC. EDV was lower in RVH compared to normal, improved in RVH+EPC, but did not differ from normal levels in RVH+MSC-treated animals. Likewise, baseline myocardial perfusion was similarly blunted in RVH and RVH+EPC, but restored to normal levels in RVH+MSC pigs (p=0.002 vs. RVH and p=0.49 vs. normal). After Bonferroni adjustment, E/A ratio remained lower in both RVH and RVH+EPC compared to normal (p=0.003 and p=0.014, respectively), and EDV and basal myocardial perfusion remained lower in RVH+EPC compared to RVH+MSC (p=0.003 and p=0.011, respectively). Myocardial perfusion response to adenosine did not differ among the groups (Table 1).
EPC and MSC phenotype and engraftment
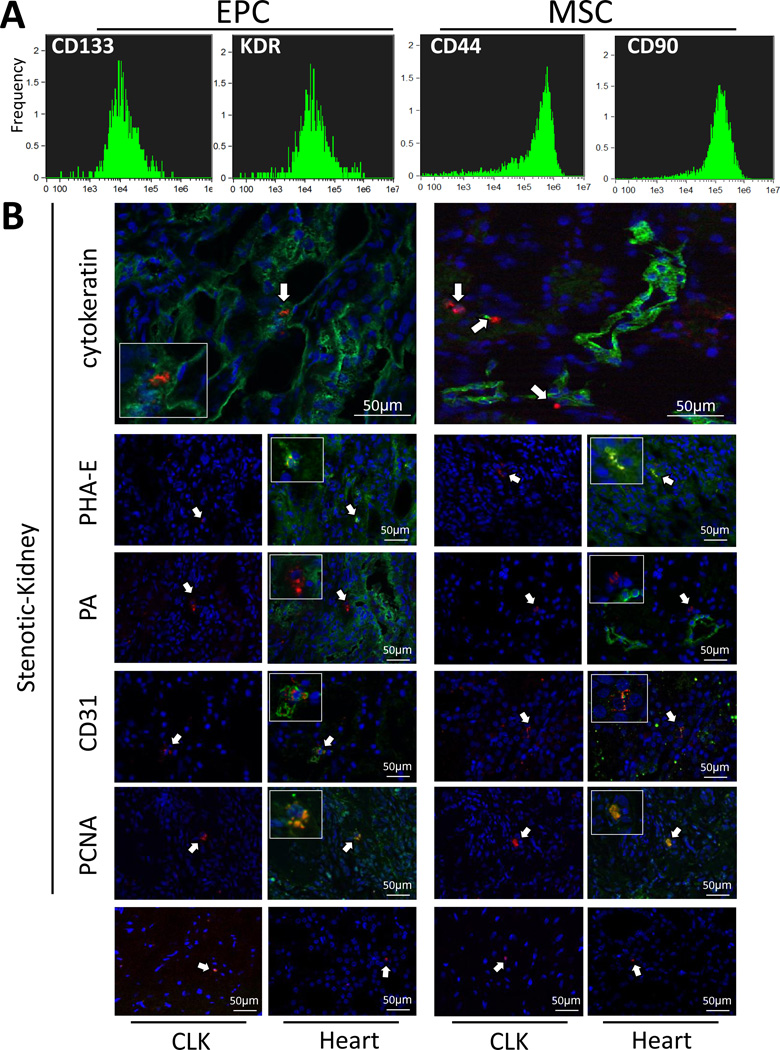
EPCs expressed the endothelial and progenitor markers CD133 (95.8±1.4%) and KDR (94.2±2.1%), while MSCs expressed the MSC markers CD44 (98.1±1.7%) and CD90 (89.2±2.2%) (Figure 2A). Isolated MSCs transdifferentiated into adipocytes, osteocytes, and chondrocytes, as previously shown (14,41).
Figure 2.
A: Fluorescence-activated cell sorting analysis performed on in-vitro-cultured endothelial progenitor cells (EPCs) and mesenchymal stem cells (MSCs) to determine cell surface expression of CD133, kinase-insert domain receptor (KDR), CD44, and CD90. B: Representative immunofluorescence staining (40×) of CM-Dil (red) EPCs and MSCs in the stenotic-kidney, contralateral kidney (CLK), and left-ventricle sections. Cytokeratin, phaseolus vulgaris erythroagglutinin (PHA-E), peanut agglutinin (PA), CD31, and proliferating cell nuclear antigen (PCNA): Green, DAPI: Blue.
EPCs and MSCs showed a similar retention rate in the post-stenotic kidneys 4 weeks after delivery (10–15%, 1–2 cells per 40× field or 800–1,000 cells/slide), while only 1–2 EPCs or MSCs were observed per CLK and myocardial slide (Figure 2B). In the kidney, MSCs were observed in equal proportions in the interstitium and engrafted in renal tubules (47–48%), while only 3–4% engrafted in blood vessels (CD31 staining). Contrarily, most EPCs integrated into tubules (50–55%), followed by interstitial (30–35%) and vascular (18–20%) engraftment. EPCs and MSCs integrated only into proximal (PHA-E positive), but not distal (PA positive) tubules, and co-stained with PCNA, suggesting in-situ proliferation.
Inflammation
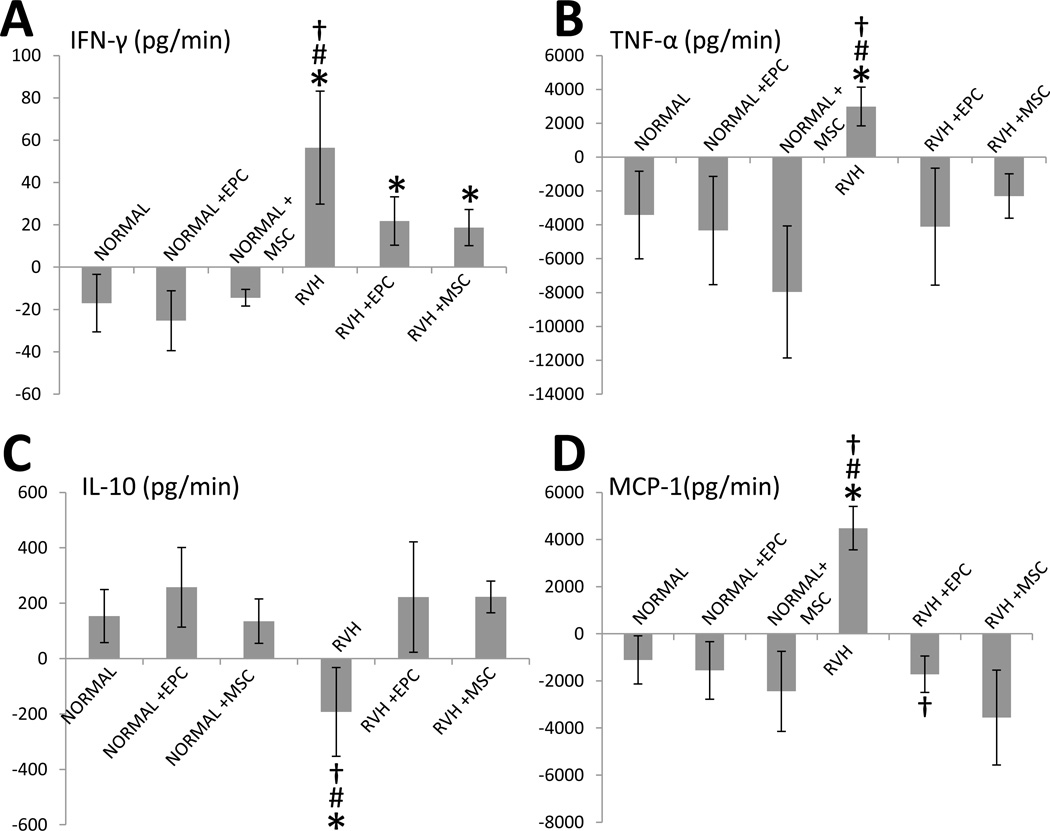
Net renal release of IFN-γ and TNF-α was higher in RVH compared with normal, but similarly decreased or normalized, respectively, in both RVH+EPC and RVH+MSC pigs (Figure 3A–B). Conversely, IL-10 release was lower in RVH compared to normal and was preserved in both RVH+EPC and RVH+MSC (Figure 3C). Renal release of MCP-1, higher in RVH compared with normal, fell in RVH+EPC, but significantly decreased further in RVH+MSC pigs (Figure 3D).
Figure 3.
Stenotic-kidney net release of interferon (IF)-γ (A), tumor necrosis-factor (TNF)-α (B), interleukin (IL)-10 (C), and monocyte-chemoattractant-protein (MCP)-1 (D) in normal, normal+EPC, normal+MSC, RVH, RVH+EPC, and RVH+MSC. *p<0.05 vs. Normal; #p<0.05 vs. RVH+EPC; †p<0.05 vs. RVH+MSC.
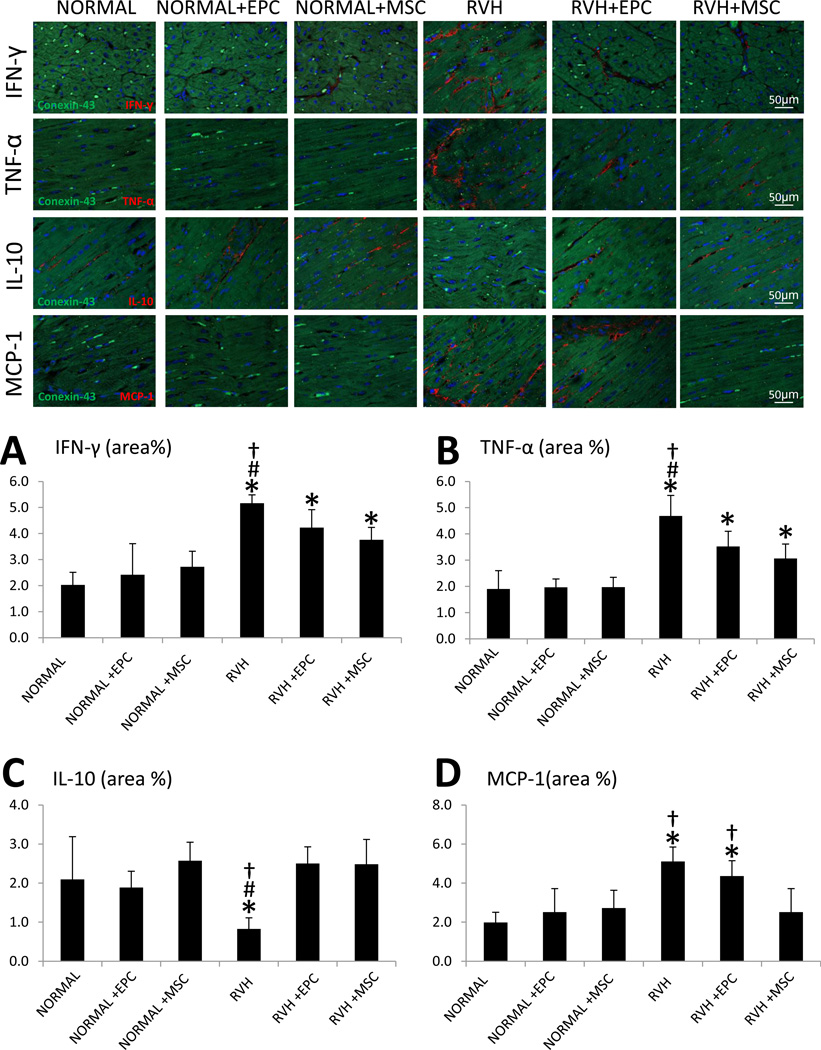
IFN-γ and TNF-α myocardial immunoreactivity was upregulated in all RVH hearts compared to normal, but ameliorated in RVH pigs treated with either EPCs or MSCs (Figure 4A–B). Moreover, myocardial expression of IL-10 was downregulated in RVH, but did not differ from normal levels in EPC or MSC-treated pigs (Figure 4C). However, myocardial expression of MCP-1 was similarly elevated in RVH and RVH+EPC compared to normal and to RVH+MSC, and normalized only in MSC-treated pigs (Figure 4D). None of the inflammatory markers co-stained with the myocyte marker connexin-43, arguing against cardiomyocyte expression.
Figure 4.
Myocardium double immunofluorescence staining of connexin-43 (green) and the inflammatory mediators (red): interferon (IF)-γ (A), tumor necrosis-factor (TNF)-α (B), interleukin (IL)-10 (C), and monocyte-chemoattractant-protein (MCP)-1 (D) and their quantification. Blue: DAPI. *p≤0.05 vs. Normal; #p<0.05 vs. RVH+EPC; †p<0.05 vs. RVH+MSC.
Notably, differences in MCP-1 net renal release between RVH+EPC and RVH+MSC persisted upon Bonferroni adjustment (p=0.010). Similarly, MCP-1 myocardial immunoreactivity remained upregulated in RVH and RVH+EPC compared to normal and to RVH+MSC, and normalized only in MSC-treated pigs (p=0.011).
Myocardial remodeling
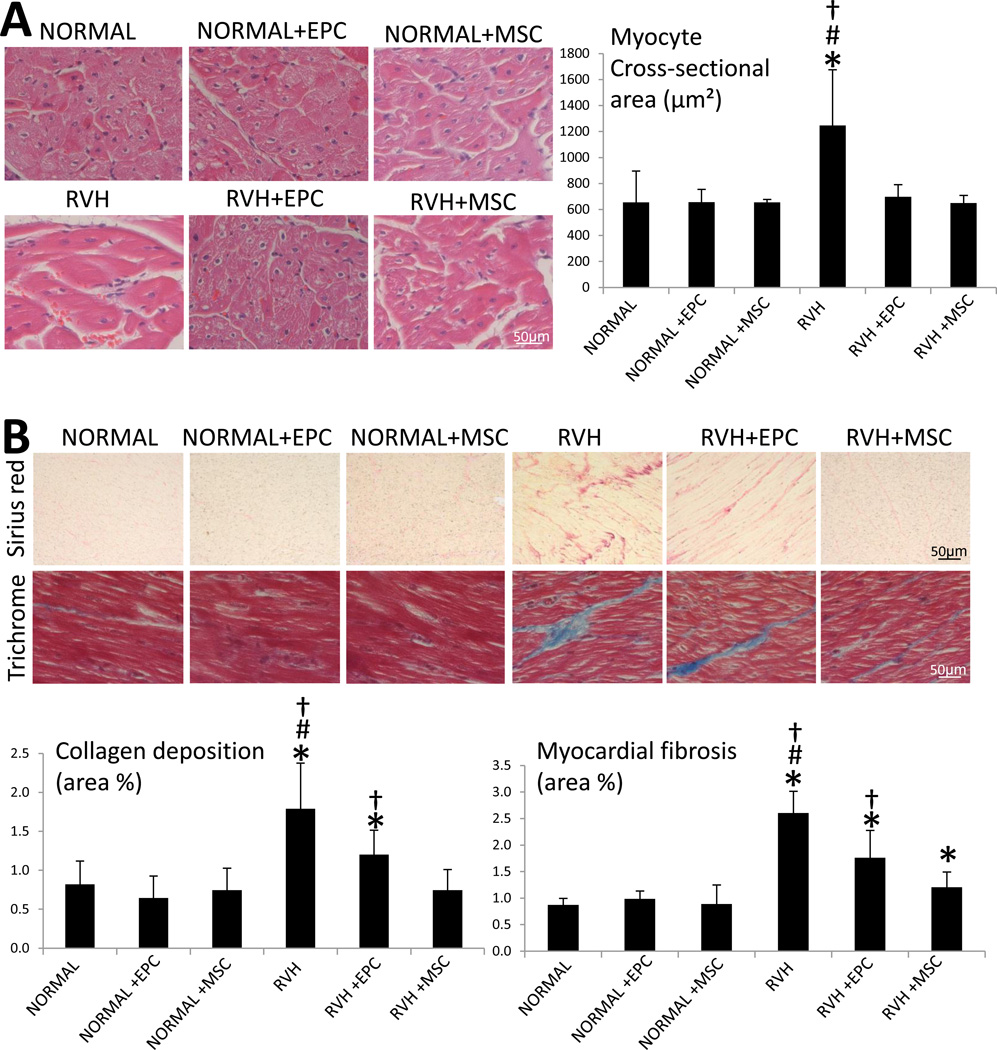
Myocyte cross-sectional area was increased in RVH, but equally decreased to normal levels in RVH+EPC and RVH+MSC pigs (Figure 5A). Myocardial collagen deposition (Sirius-red) was increased in RVH compared to normal, but restored to normal levels in RVH+EPC and RVH+MSC. Consequently, myocardial fibrosis (Trichrome) was greater in RVH, improved in RVH+EPC, and further improved in RVH+MSC (Figure 5B).
Figure 5.
A: Myocyte cross-sectional area (H&E, 40×) and its quantification. B: Representative immunostaining and quantification of Sirius red and trichrome (40×). *p<0.05 vs. Normal; #p<0.05 vs. RVH+EPC; †p<0.05 vs. RVH+MSC.
Bonferroni-adjusted collagen deposition and myocardial fibrosis remained significantly different between EPC and MSC-treated groups (p=0.008 and p=0.011, respectively), underscoring superior MSC cardio-protection in chronic experimental RVH.
Effect of EPC and MSC on the CLK
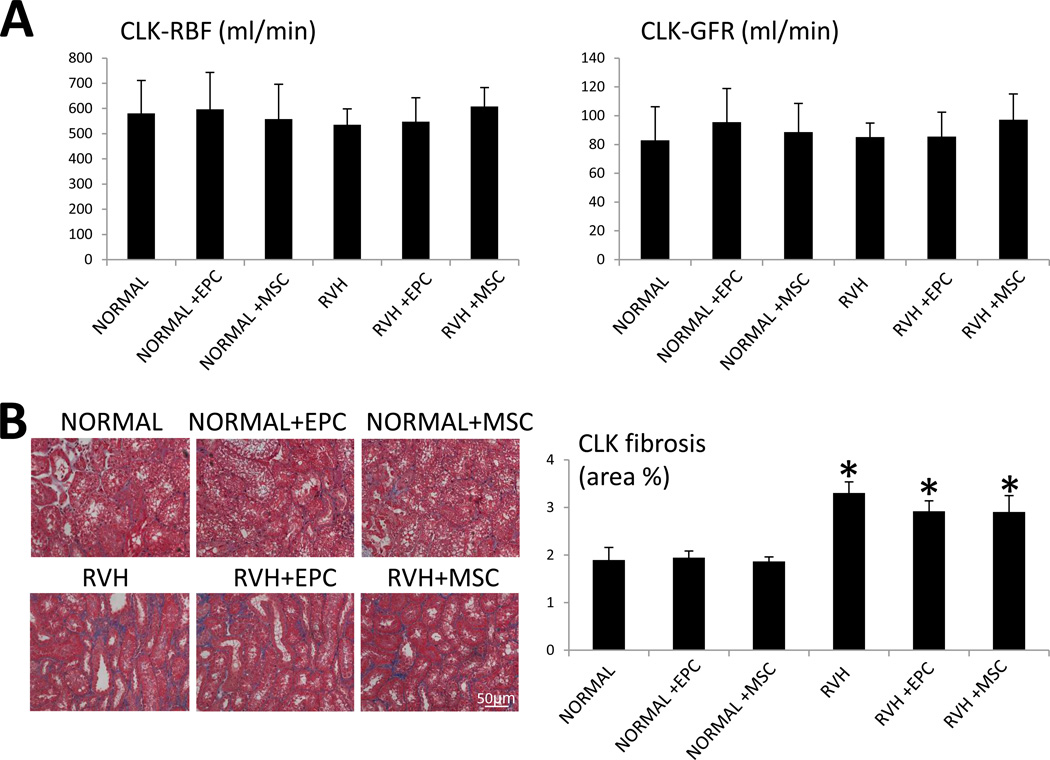
CLK RBF and GFR did not differ among the groups (Figure 6A). However, the CLK showed the same elevation of tubulo-interstitial fibrosis in all RVH groups compared to normal (Figure 6B).
Figure 6.
A: Renal blood flow (RBF), and glomerular filtration rate (GFR) in the contralateral kidney (CLK) of NORMAL, NORMAL+EPC, NORMAL+MSC, RVH, RVH+EPC, and RVH+MSC pigs. B: Representative images of CLK tubulo-interstitial trichrome staining (left) and quantification of tubulo-interstitial fibrosis (right). *p<0.05 vs. Normal.
Discussion
This study shows that intra-renal delivery of either EPCs or MSCs can improve myocardial injury in experimental RVH. Nevertheless, MSCs showed slightly superior potential to improve diastolic function and ameliorate myocardial fibrosis. This might be related to a greater capacity of MSCs to improve renal function and decrease stenotic-kidney release and myocardial expression of the inflammatory mediator MCP-1. These observations underscore the central role of inflammation in cardiorenal signaling and support a role for regenerative therapies to preserve the myocardium in chronic experimental RVH.
RVH, a frequent cause of hypertension in elderly individuals, induces myocardial injury and diastolic dysfunction. Cross-sectional echocardiographic studies have shown that patients with RVH have an increased incidence of LV hypertrophy and diastolic dysfunction (39), which progress to left ventricular dilatation over the first year after diagnosis (40). In agreement, we have recently shown that cardiac structure and diastolic function are impaired in RVH patients compared with essential hypertensive patients (21,22). These studies underscore the need to identify more effective strategies to attenuate myocardial remodeling and preserve diastolic function in patients with RVH.
Over recent years, experimental studies have uncovered the potential therapeutic value of cell-based therapy for renovascular diseases. We have previously shown that intra-renal delivery of either EPCs (3) or MSCs (2) during renal revascularization reduces renal dysfunction beyond the stenotic lesion, and intra-renal delivery of either EPCs (34) or MSCs (13) ameliorates renal injury without any vascular intervention. The current study extends our previous observations demonstrating that despite sustained hypertension, intra-renal delivery of both EPCs and MSCs confer cardio-protection in chronic experimental RVH. Possibly, renal cell delivery might have impacted myocardial injury by either preservation of renal function per se, and/or amelioration of kidney-derived inflammation.
a) Preservation of stenotic-kidney function
Decreased GFR is an important predictor of cardiovascular morbidity and mortality, independent of other cardiovascular risk factors (18). In patients with renal insufficiency, coexistent cardiac dysfunction is associated with poor prognosis (29), while kidney transplantation increases ejection fraction, improves functional status, and increases survival (36). Taken together, these observations suggest continuous crosstalk between the kidney and the heart. Nevertheless, the precise mechanisms by which decrease GFR adversely affects cardiac function remain elusive. Contrarily, we found that despite unchanged RBF and GFR, tubulointerstitial fibrosis remained elevated in RAS+EPC and RAS+MSC CLK, likely because EPC and MSC injection in the stenotic-kidney had no effect on blood pressure.
Here we found that stenotic-kidney GFR improved in RVH+EPC and RVH+MSC-treated pigs. Similarly, diastolic function (E/A ratio and EDV), which was blunted in RVH, improved, confirming the potential of interventions that preserve renal function to attenuate cardiac abnormalities and dysfunction in experimental RVH.
b) Amelioration of kidney-derived inflammation
We have previously shown that the human stenotic-kidney releases inflammatory markers that portend kidney injury and reduced function (10). Furthermore, we have shown in porcine atherosclerotic RVH that attenuation of renal-derived inflammation, preserves remote myocardial microvascular function (34). Hence, therapies that abrogate renal inflammation and injury may attenuate cardiac impairment.
The current study extends our previous observations demonstrating that intra-renal delivery of EPCs and MSCs improved renal function and decreased stenotic-kidney release of IFN-γ and TNF-α, but preserved renal release of IL-10. IFN-γ and TNF-α are cytokines with important inflammatory properties in the myocardium (35), whereas IL-10 possesses important anti-inflammatory properties, and contributes to myocardial infarction-induced EPC mobilization and survival (23).
Interestingly, we found that renal release of MCP-1, higher in RVH compared with normal, fell in RVH+EPC, but decreased further in RVH+MSC pigs. MCP-1 is a key inflammatory cytokine that mediates monocyte recruitment to the site of injury, and was indeed expressed mostly in the myocardial interstitium, possibly by infiltrating inflammatory cells. We have previously shown that porcine RVH is associated with elevated expression of MCP-1 and increased number of pro-inflammatory macrophages populating the myocardium (12), and that its inhibition decreases LV remodeling and diastolic dysfunction (26). Hence, the capability of MSCs to ameliorate MCP-1-driven myocardial inflammation may at least partly account for the improvement in cardiac structure and function in RVH+MSC pigs.
MSCs vs. EPCs
MSCs possess important advantages over EPCs. For example, harvesting autologous EPCs requires large amounts of peripheral blood, which restricts their clinical application. In contrast, MSCs can be easily harvested from multiples tissues (1), differentiate into a broad spectrum of cell lineages, and possess potent immunomodulatory and tissue-trophic properties that promote tissue repair and decrease inflammation (27). We have previously shown that MSCs more effectively suppress inflammatory cytokines in-vitro compared to EPCs, and produced greater improvement in renal function in-vivo (41), as confirmed in the current study.
Furthermore, MSCs improved the damaged myocardium slightly better than EPCs. Despite similar attenuation of LV hypertrophy, MSCs decreased fibrosis more efficiently than EPCs. Consequently, diastolic function, which was blunted in RVH, slightly improved in RVH+EPC, but normalized only in RVH+MSC-treated pigs, underscoring the potential of this intervention to attenuate cardiac abnormalities in experimental RVH. This efficacy might be attributed to not only the greater improvement in renal function in MSC-treated compared to RVH+EPC pigs, but also the ability of MSCs to attenuate renal release and myocardial expression of MCP-1.
Limitations
Limitations in our study include the use of young animals, short duration of the disease, and lack of comorbidities. Nevertheless, the strengths of the current investigation include many human-like features of the pig model, with similar myocardial injury and cardiac dysfunction compared to humans. Previous reports have raised safety concerns about tumors, malformation, or microinfarctions after MSC therapy (24,33) or pathological vessel wall remodeling induction by EPC (17,20). However, several clinical studies have shown that EPC and MSC are well tolerated in patients with cardiovascular diseases and have an excellent safety record (28,37). In the current study, no structural changes (tumor or abnormal growth formations) were detected in the lung, liver, spleen, or kidneys by either imaging in-vivo or visual inspection ex-vivo. Furthermore, histology showed no evidence of such abnormalities in renal tissue sections 4 weeks after cell injection. Nevertheless, additional long-term follow-up studies are needed to determine the safety of EPC and MSC therapy, as well as the optimal dose and timing of cell delivery. Notably, we found that an important number of EPCs and MSCs were retained in the stenotic kidney 4 weeks after delivery resembling engraftment rates reported using renal progenitors (31), stem cells (30), and MSCs (19) in different models of kidney injury. Contrarily, their low engraftment in the myocardium argues against a major contribution of direct local effects to amelioration of chronic injury. Further studies are needed to determine different regimens and validate the clinical efficacy EPC and MSC-based reparative therapies to preserve the myocardium in RVH.
Conclusions
RVH induces myocardial injury and diastolic dysfunction, partly mediated by renal release of inflammatory mediators and impaired renal function. This study demonstrates that intra-renal delivery of both EPCs and MSCs confers cardio-protection in chronic experimental RVH. Yet, MSCs preserve the myocardium more efficiently than EPCs, possibly due to a greater improvement in renal function and amelioration of stenotic-kidney-induced inflammation. Therefore, our observations suggest EPCs and MSCs as novel therapeutic options to preserve cardiac structure and function in RVH.
Acknowledgments
Partly supported by NIH grants: DK73608, DK104273, UL1-RR000135, DK100081, DK102325, AG31750, and HL123160.
Footnotes
Disclosures
None.
References
- 1.Asanuma H, Meldrum DR, Meldrum KK. Therapeutic applications of mesenchymal stem cells to repair kidney injury. J Urol. 2010;184(1):26–33. doi: 10.1016/j.juro.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 2.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119(4):547–557. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrysochou C, Kalra PA. Atherosclerotic renovascular disease and the heart. J Ren Care. 2010;36(Suppl 1):146–153. doi: 10.1111/j.1755-6686.2010.00161.x. [DOI] [PubMed] [Google Scholar]
- 4.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370(1):13–22. doi: 10.1056/NEJMoa1310753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology. 2007;243(2):405–412. doi: 10.1148/radiol.2432060655. [DOI] [PubMed] [Google Scholar]
- 6.Daghini E, Primak AN, Chade AR, Zhu X, Ritman EL, McCollough CH, Lerman LO. Evaluation of porcine myocardial microvascular permeability and fractional vascular volume using 64-slice helical computed tomography (CT) Invest Radiol. 2007;42(5):274–282. doi: 10.1097/01.rli.0000258086.78179.90. [DOI] [PubMed] [Google Scholar]
- 7.Ebrahimi B, Eirin A, Li Z, Zhu XY, Zhang X, Lerman A, Textor SC, Lerman LO. Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PLoS One. 2013;8(7):e67474. doi: 10.1371/journal.pone.0067474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebrahimi B, Li Z, Eirin A, Zhu XY, Textor SC, Lerman LO. Addition of endothelial progenitor cells to renal revascularization restores medullary tubular oxygen consumption in swine renal artery stenosis. Am J Physiol Renal Physiol. 2012;302(11):F1478–F1485. doi: 10.1152/ajprenal.00563.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eirin A, Ebrahimi B, Zhang X, Zhu XY, Tang H, Crane JA, Lerman A, Textor SC, Lerman LO. Changes in glomerular filtration rate after renal revascularization correlate with microvascular hemodynamics and inflammation in Swine renal artery stenosis. Circ Cardiovasc Interv. 2012;5(5):720–728. doi: 10.1161/CIRCINTERVENTIONS.112.972596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eirin A, Gloviczki ML, Tang H, Gossl M, Jordan KL, Woollard JR, Lerman A, Grande JP, Textor SC, Lerman LO. Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J. 2013;34(7):540–548a. doi: 10.1093/eurheartj/ehs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eirin A, Li Z, Zhang X, Krier JD, Woollard JR, Zhu XY, Tang H, Herrmann SM, Lerman A, Textor SC, et al. A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension. 2012;60(5):1242–1249. doi: 10.1161/HYPERTENSIONAHA.112.199919. [DOI] [PubMed] [Google Scholar]
- 12.Eirin A, Williams BJ, Ebrahimi B, Zhang X, Crane JA, Lerman A, Textor SC, Lerman LO. Mitochondrial targeted peptides attenuate residual myocardial damage after reversal of experimental renovascular hypertension. J Hypertens. 2014;32(1):154–165. doi: 10.1097/HJH.0b013e3283658a53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eirin A, Zhang X, Zhu X-Y, Tang H, Jordan KL, Grande JP, Dietz AB, Lerman A, Textor SC, Lerman LO. Renal vein cytokine release as an index of renal parenchymal inflammation in chronic experimental renal artery stenosis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29(2):274–282. doi: 10.1093/ndt/gft305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eirin A, Zhu XY, Krier JD, Tang H, Jordan KL, Grande JP, Lerman A, Textor SC, Lerman LO. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells. 2012;30(5):1030–1041. doi: 10.1002/stem.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eirin A, Zhu XY, Li Z, Ebrahimi B, Zhang X, Tang H, Korsmo MJ, Chade AR, Grande JP, Ward CJ, et al. Endothelial outgrowth cells shift macrophage phenotype and improve kidney viability in swine renal artery stenosis. Arterioscler Thromb Vasc Biol. 2013;33(5):1006–1013. doi: 10.1161/ATVBAHA.113.301164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eirin A, Zhu XY, Urbieta-Caceres VH, Grande JP, Lerman A, Textor SC, Lerman LO. Persistent kidney dysfunction in swine renal artery stenosis correlates with outer cortical microvascular remodeling. Am J Physiol Renal Physiol. 2011;300(6):F1394–F1401. doi: 10.1152/ajprenal.00697.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George J, Afek A, Abashidze A, Shmilovich H, Deutsch V, Kopolovich J, Miller H, Keren G. Transfer of endothelial progenitor and bone marrow cells influences atherosclerotic plaque size and composition in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2005;25(12):2636–2641. doi: 10.1161/01.ATV.0000188554.49745.9e. [DOI] [PubMed] [Google Scholar]
- 18.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 19.Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. International journal of molecular medicine. 2004;14(6):1035–1041. [PubMed] [Google Scholar]
- 20.Hu Y, Davison F, Zhang Z, Xu Q. Endothelial replacement and angiogenesis in arteriosclerotic lesions of allografts are contributed by circulating progenitor cells. Circulation. 2003;108(25):3122–3127. doi: 10.1161/01.CIR.0000105722.96112.67. [DOI] [PubMed] [Google Scholar]
- 21.Khangura KK, Eirin A, Kane GC, Misra S, Textor SC, Lerman A, Lerman LO. Cardiac function in renovascular hypertensive patients with and without renal dysfunction. American journal of hypertension. 2014;27(3):445–453. doi: 10.1093/ajh/hpt203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khangura KK, Eirin A, Kane GC, Misra S, Textor SC, Lerman A, Lerman LO. Extrarenal atherosclerotic disease blunts renal recovery in patients with renovascular hypertension. J Hypertens. 2014;32(6):1300–1306. doi: 10.1097/HJH.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnamurthy P, Thal M, Verma S, Hoxha E, Lambers E, Ramirez V, Qin G, Losordo D, Kishore R. Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium. Circulation research. 2011;109(11):1280–1289. doi: 10.1161/CIRCRESAHA.111.248369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunter U, Rong S, Boor P, Eitner F, Muller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L, et al. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. Journal of the American Society of Nephrology : JASN. 2007;18(6):1754–1764. doi: 10.1681/ASN.2007010044. [DOI] [PubMed] [Google Scholar]
- 25.Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC. Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol. 1999;10(7):1455–1465. doi: 10.1681/ASN.V1071455. [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Zhu X, Chade AR, Jordan KL, Lavi R, Daghini E, Gibson ME, Guglielmotti A, Lerman A, Lerman LO. Monocyte chemoattractant proteins mediate myocardial microvascular dysfunction in swine renovascular hypertension. Arterioscler Thromb Vasc Biol. 2009;29(11):1810–1816. doi: 10.1161/ATVBAHA.109.190546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marigo I, Dazzi F. The immunomodulatory properties of mesenchymal stem cells. Semin Immunopathol. 2011;33(6):593–602. doi: 10.1007/s00281-011-0267-7. [DOI] [PubMed] [Google Scholar]
- 28.Mathiasen AB, Haack-Sorensen M, Kastrup J. Mesenchymal stromal cells for cardiovascular repair: current status and future challenges. Future Cardiol. 2009;5(6):605–617. doi: 10.2217/fca.09.42. [DOI] [PubMed] [Google Scholar]
- 29.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109(8):1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 30.Rangel EB, Gomes SA, Dulce RA, Premer C, Rodrigues CO, Kanashiro-Takeuchi RM, Oskouei B, Carvalho DA, Ruiz P, Reiser J, et al. C-kit(+) cells isolated from developing kidneys are a novel population of stem cells with regenerative potential. Stem cells (Dayton, Ohio) 2013;31(8):1644–1656. doi: 10.1002/stem.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, et al. Regeneration of glomerular podocytes by human renal progenitors. Journal of the American Society of Nephrology : JASN. 2009;20(2):322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24(2):462–471. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- 33.Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem cells (Dayton, Ohio) 2007;25(2):371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 34.Urbieta-Caceres VH, Zhu XY, Jordan KL, Tang H, Textor K, Lerman A, Lerman LO. Selective improvement in renal function preserved remote myocardial microvascular integrity and architecture in experimental renovascular disease. Atherosclerosis. 2012;221(2):350–358. doi: 10.1016/j.atherosclerosis.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaddi K, Nicolini FA, Mehta P, Mehta JL. Increased secretion of tumor necrosis factor-alpha and interferon-gamma by mononuclear leukocytes in patients with ischemic heart disease. Relevance in superoxide anion generation. Circulation. 1994;90(2):694–699. doi: 10.1161/01.cir.90.2.694. [DOI] [PubMed] [Google Scholar]
- 36.Wali RK, Wang GS, Gottlieb SS, Bellumkonda L, Hansalia R, Ramos E, Drachenberg C, Papadimitriou J, Brisco MA, Blahut S, et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. Journal of the American College of Cardiology. 2005;45(7):1051–1060. doi: 10.1016/j.jacc.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 37.Wang X-X, Zhang F-R, Shang Y-P, Zhu J-H, Xie X-D, Tao Q-M, Zhu J-H, Chen J-Z. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. Journal of the American College of Cardiology. 2007;49(14):1566–1571. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 38.Winslow JM, Liesveld JL, Ryan DH, Dipersio JF, Abboud CN. CD34+ progenitor cell isolation from blood and marrow: a comparison of techniques for small-scale selection. Bone Marrow Transplant. 1994;14(2):265–271. [PubMed] [Google Scholar]
- 39.Wright JR, Shurrab AE, Cooper A, Kalra PR, Foley RN, Kalra PA. Left ventricular morphology and function in patients with atherosclerotic renovascular disease. J Am Soc Nephrol. 2005;16(9):2746–2753. doi: 10.1681/ASN.2005010043. [DOI] [PubMed] [Google Scholar]
- 40.Wright JR, Shurrab AE, Cooper A, Kalra PR, Foley RN, Kalra PA. Progression of cardiac dysfunction in patients with atherosclerotic renovascular disease. QJM. 2009;102(10):695–704. doi: 10.1093/qjmed/hcp105. [DOI] [PubMed] [Google Scholar]
- 41.Zhu XY, Urbieta-Caceres V, Krier JD, Textor SC, Lerman A, Lerman LO. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells. 2013;31(1):117–125. doi: 10.1002/stem.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]