Abstract
Lifetime risk estimation for cardiovascular disease (CVD) has been proposed as a useful strategy to improve risk communication in the primary prevention setting. However, the perception of lifetime risk for CVD is unknown. We included 2,998 individuals from the Dallas Heart Study. Lifetime risk for developing CVD was classified as high (≥39%) vs. low (<39%) according to risk factor burden as described in our previously published algorithm. Perception of lifetime risk for myocardial infarction was assessed via a 5-point scale. Baseline characteristics were compared across levels of perceived lifetime risk. Multivariable logistic regression analyses were performed to determine the association of participant characteristics with level of perceived lifetime risk for CVD and with correctness of perceptions. 64.8% (1942/2998) of participants were classified as high predicted lifetime risk for CVD. There was significant discordance between perceived and predicted lifetime risk. After multivariable adjustment, family history of premature MI, high self-reported stress, and low perceived health were all strongly associated with high perceived lifetime risk (OR [95% CI]: 2.37 [1.72–3.27], 2.17 [1.66–2.83], and 2.71 [2.09–3.53]). However, the association between traditional CVD risk factors and high perceived lifetime risk was more modest. In conclusion, misperception of lifetime risk for CVD is common and frequently reflects the influence of factors other than traditional risk factor levels. These findings highlight the importance of effectively communicating the significance of traditional risk factors in determining the lifetime risk for CVD.
Keywords: Lifetime Risk for Cardiovascular Disease, Predicted Lifetime Risk for Cardiovascular Disease, Perceived Lifetime Risk for Cardiovascular Disease
INTRODUCTION
Although a majority of the US population is at low risk for cardiovascular disease (CVD) in the short-term (10–year estimate), most of these individuals are actually at high risk for developing cardiovascular disease during their remaining lifespan (1, 2). Physicians have routinely used short-term CVD risk estimation in primary prevention to guide decisions to treat blood pressure and cholesterol levels and encourage therapeutic lifestyle changes for those at the highest risk (3). However, short-term risk estimates have important limitations, classifying most adults < 50 years of age and many women as low risk regardless of risk factor burden (2, 3). Therefore, national guidelines have recently encouraged the use of long-term or lifetime risk as an adjunct to short-term risk communication in the primary prevention setting (3–5). Prior studies have observed that knowledge of short-term risk has been associated with healthy lifestyle patterns (6) and the effectiveness of cholesterol (7) and blood pressure lowering therapy (8). However, little is known about the perception of lifetime risk for CVD in the general population. Therefore, we sought to determine the perception of lifetime risk for CVD by comparing Dallas Heart Study participants’ perceived lifetime risk with their predicted lifetime risk for CVD using our previously published algorithm (1, 9, 10).
METHODS
The Dallas Heart Study (DHS) is a multiethnic, population-based probability sample of adult residents of Dallas County age 18–65 years enrolled between July 2000 and January 2002 (11). All participants provided informed consent to participate in the study and the protocol was approved by the Institutional Review Board of University of Texas Southwestern Medical Center. In the initial home visit, 6,101 participants underwent extensive household interviews. A subset of 3,557 participants age 30–65 years participated in a follow-up visit, providing fasting blood and urine specimens and serial blood pressure measurements. Details of the study design, including collection of medical history, blood pressure, anthropometric measurements, and laboratory measurements have been described previously in detail (11). In the present study, we included 2,998 subjects who participated in the follow-up visit of the DHS after excluding participants with a self-report of prior myocardial infarction and/or stroke (n=181), a non-fasting blood sample (n=94), and those missing measured baseline covariates (n=284).
Race/ethnicity and current smoking status were defined by self-report. Education level was used as a surrogate for socioeconomic status instead of income level because many participants declined to provide financial information. High education level was defined as college degree or higher. Body mass index (BMI) was calculated from measured height and weight. Diabetes mellitus was defined by a fasting glucose ≥126 mg/dl, non-fasting glucose ≥200 mg/dl, or the use of glucose-lowering medications. Family history of premature myocardial infarction (MI) was defined as a first-degree male relative with a heart attack at age <50 years or a first-degree female relative with a heart attack at age <55 years in survey responses. Levels of stress and perceived health were determined by self-report to the following survey items in the DHS: “on a scale of 1–5, how would you rate your stress level?” (1 = No stress at all; 5 = Extremely high stress); and “How would you say your general health is?” (excellent, very good, good, fair, or poor). High perceived stress was defined as a score of 4–5; low perceived stress was defined as a score of 1–3. High perceived health was defined as excellent, very good or good; low perceived health was defined as fair or poor. Perceived lifetime risk for CVD was measured using the participants’ response to the following question: “On a scale of 1–5, how likely is it that you will have a heart attack in your lifetime? (1 = Least likely, 5 = Most likely" (12).
We estimated predicted lifetime risk according to our previously published algorithm (1, 9, 10) where we classified each participant into one of five mutually exclusive risk factor categories according to their level of measured traditional CVD risk factors: all optimal risk factors, ≥1 not-optimal risk factors, ≥1 elevated risk factors, 1 major risk factor or ≥ 2 major risk factors. Compared to individuals in the lowest two risk factor categories (i.e. all optimal or ≥1 not-optimal risk factor), individuals in the top three risk factor categories (i.e. at least one elevated risk factor) represent a unique subset, with a higher observed lifetime risks for CVD, the presence of at least one treatable risk factor, and a higher prevalence and progression of subclinical atherosclerosis (1, 9, 10). Therefore, in the present study we used this previously validated threshold to determine the predicted lifetime risk for CVD, classifying each individual as either “low predicted lifetime risk” or “high predicted lifetime risk” (supplemental Table 1).
Because perceived lifetime risk was measured on a relative (i.e. “least likely” to “most likely”) rather than a quantitative scale, we studied further those individuals at the extremes of perceived lifetime risk. Therefore, those individuals who selected “5-most likely” were defined as having “high perceived lifetime risk”; and those individuals who selected “1-least likely” were defined as having “low perceived lifetime risk”. We compared baseline characteristics across levels of perceived lifetime risk [1 (least likely) to 5 (most likely)] using linear trend tests and the Cochrane-Armitage trend test for continuous and categorical variables, respectively.
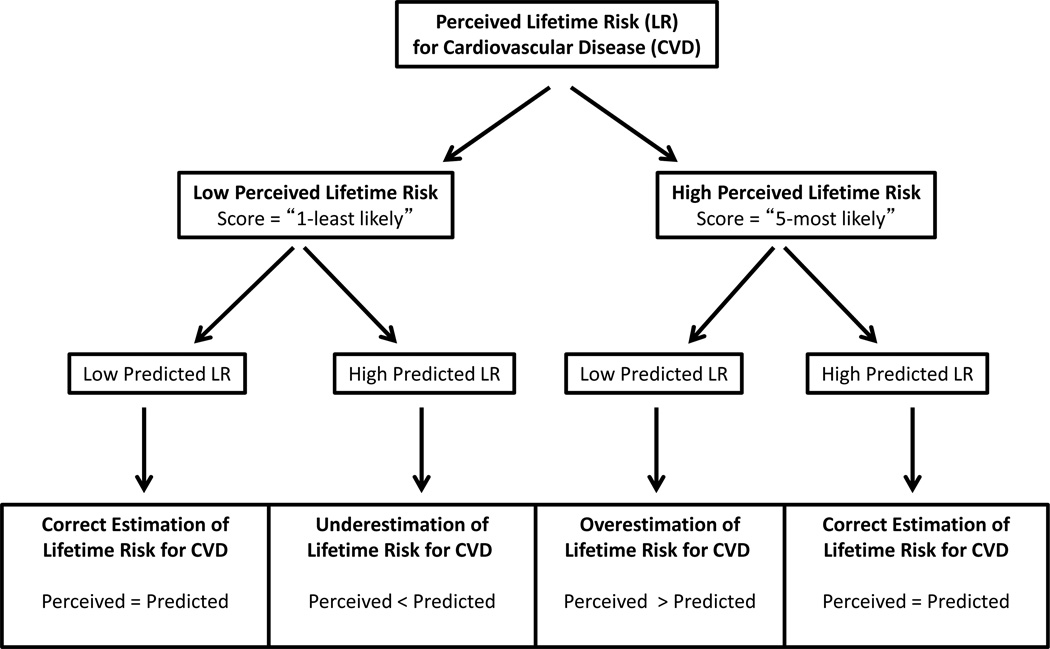
To determine the independent association between risk factors and the perception of lifetime risk for CVD, we first constructed multivariable logistic regression models for all study participants to test the association between demographics, traditional CVD risk factors, and other personal characteristics and the perception of lifetime risk for MI, with high perceived lifetime risk (i.e. score = 5) as the outcome variable. To identify factors associated with correct or incorrect perception of lifetime risk of MI, we constructed multivariable logistic regression models separately for individuals with low predicted lifetime risk (i.e. all optimal or ≥1 not-optimal risk factor), and for those with high predicted lifetime risk (i.e. at least one elevated risk factor). In both high and low predicted lifetime risk subgroups, incorrectly perceived lifetime risk was the outcome variable, and correctly perceived lifetime risk was the referent (i.e. perceived = predicted). Among participants with low predicted lifetime risk, we determined the association between participant characteristics and overestimation of lifetime risk for CVD (perceived > predicted). Similarly, among participants with high predicted lifetime risk, we determined the association between participant characteristics and underestimation of lifetime risk for CVD (perceived < predicted). (Figure 1)
Figure 1.
Categories of Perceived and Predicted Lifetime Risk for Cardiovascular Disease in the Dallas Heart Study. This figure provides a schematic reflecting the stratification of perceived and predicted lifetime risk, allowing participants to be categorized as having correctly estimated risk, underestimated risk (perceived < predicted), or overestimated risk (perceived > predicted).
All models were adjusted for age, gender, race, education level, BMI, systolic blood pressure, total cholesterol, current smoking, presence or absence of diabetes, presence or absence of family history of premature MI, and perceived levels of stress and health. These variables were selected in an effort to compare basic demographics, components of the Framingham and Lifetime Risk for CVD estimates, or because they were significantly different across levels of perceived lifetime risk. All reported p-values are two sided at a significance level of 5%. Statistical analyses were performed using SAS for Windows (release 9.2; SAS Institute, Inc., Cary, NC). Chi-square statistics were included to facilitate comparison of both categorical and continuous variables in determining risk perception.
RESULTS
Most of the study participants had a high predicted lifetime risk for CVD. Out of the 2,998 participants, 64.8% (n=1,942) had a high predicted lifetime risk for CVD, while 35.2% (n=1,056) had a low predicted lifetime risk for CVD (Supplemental Table 1). The average perceived lifetime risk for CVD in the Dallas Heart Study was 2.6 on a scale from 1 (least likely) to 5 (most likely) (Table 1). There was a significant discordance between perceived and predicted lifetime risk for CVD. For example, among 736 participants with the lowest perceived lifetime risk (Score = 1, least likely), 42% had a high predicted lifetime risk and therefore appeared to underestimate their lifetime risk (perceived < predicted). Similarly, among 312 participants with the highest perceived lifetime risk (Score = 5, most likely), about half (49%) actually had a low predicted lifetime risk and therefore overestimated their lifetime risk for CVD (perceived > predicted) (Table 1). Factors associated with higher perceived lifetime risk for CVD were older age, traditional CVD risk factors, positive family history of premature MI, higher levels of perceived stress, and lower levels of perceived health (Table 1).
Table 1.
Baseline characteristics of Dallas Heart Study participants stratified according to level of Perceived Lifetime Risk* for Cardiovascular Disease (N=2,998)
| Least Likely | Most likely | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| (n = 736) | (n = 772) | (n = 806) | (n = 372) | (n=312) | P-value | |
| Variable: | ||||||
| Age (years) | 42 ± 10 | 43 ± 10 | 44 ± 10 | 45 ± 9 | 44 ± 10 | <0.001 |
| Men | 339 (46%) | 317 (41%) | 363 (45%) | 175 (47%) | 128 (41%) | 0.665 |
| Race | ||||||
| Black | 464 (63%) | 340 (44%) | 347 (43%) | 167 (45%) | 162 (52%) | <.0001 |
| White | 110 (15%) | 239 (31%) | 298 (37%) | 167 (45%) | 97 (31%) | <.0001 |
| Hispanic | 147 (20%) | 170 (22%) | 145 (18%) | 34 (9%) | 44 (14%) | <.0001 |
| Other | 15 (2%) | 23 (3%) | 16 (2%) | 4 (1%) | 9 (3%) | 0.926 |
| Family history of premature MI | 52 (7%) | 62 (8%) | 73 (9%) | 56 (15%) | 66 (21%) | <0.001 |
| Systolic Blood Pressure (mm Hg) | 123 ± 18 | 122 ± 17 | 124 ± 18 | 127 ± 18 | 127 ± 20 | <0.001 |
| Diabetes Mellitus | 66 (9%) | 62 (8%) | 73 (9%) | 56 (15%) | 53 (17%) | <0.001 |
| Total cholesterol (mg/dl) | 176 ± 38 | 180 ± 37 | 182 ± 38 | 184 ± 41 | 183 ± 46 | 0.001 |
| Body mass index (kg/m2) | 30 ± 7 | 30 ± 8 | 30 ± 7 | 31 ± 7 | 32 ± 8 | 0.0002 |
| Current smoker | 191 (26%) | 178 (23%) | 234 (29%) | 130 (35%) | 100 (32%) | 0.0001 |
| Low predicted lifetime risk for CVD | 427 (58%) | 425 (55%) | 419 (52%) | 182 (49%) | 153 (49%) | 0.0002 |
| High predicted lifetime risk for CVD | 309 (42%) | 347 (45%) | 387 (48%) | 190 (51%) | 159 (51%) | 0.0002 |
| Stress level | ||||||
| None | 132 (18%) | 62 (8%) | 40 (5%) | 15 (4%) | 16 (5%) | <.0001 |
| Extremely high | 44 (6%) | 46 (6%) | 73 (9%) | 45 (12%) | 69 (22%) | <.0001 |
| Perceived health | ||||||
| Excellent | 162 (22%) | 100 (13%) | 81 (10%) | 26 (7%) | 19 (6%) | <.0001 |
| Poor | 7 (1%) | 15 (2%) | 16 (2%) | 22 (6%) | 34 (11%) | <.0001 |
| Education Level | ||||||
| < High School | 179 (24%) | 153 (20%) | 128 (16%) | 51 (14%) | 74 (24%) | 0.013 |
| High School | 247 (34%) | 214 (28%) | 232 (29%) | 115 (31%) | 112 (36%) | 0.656 |
| Some College | 202 (27%) | 221 (29%) | 223 (28%) | 114 (31%) | 82 (26%) | 0.905 |
| College + | 107 (15%) | 184 (24%) | 223 (28%) | 92 (25%) | 44 (14%) | 0.078 |
Perceived Lifetime Risk was determined by the response to survey question: “On a scale of 1-5, how likely is it that you will have a heart attack in your lifetime?” Those who selected “1-least likely” were defined as Low Perceived Lifetime Risk for CVD, whereas those who selected “5-most likely” were defined as High Perceived Lifetime Risk for CVD.
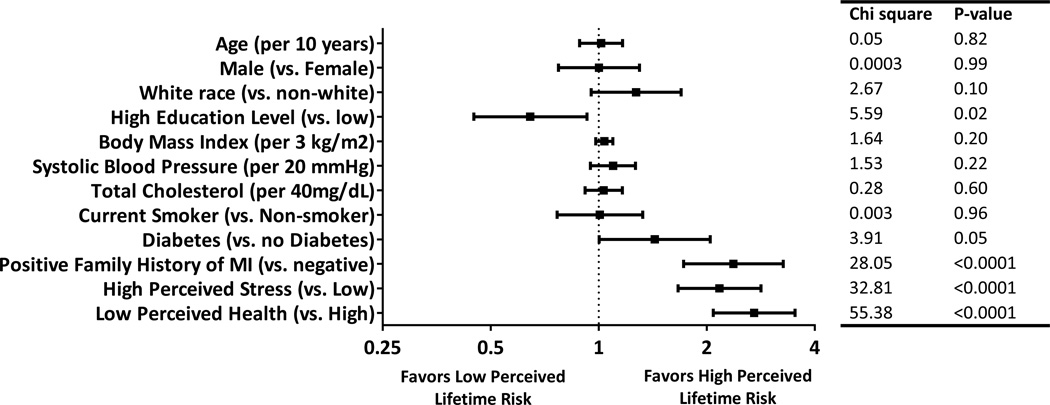
After multivariable adjustment, these associations persisted, with particularly strong associations for family history of premature MI, high perceived stress, and low perceived health. However, the association between traditional CVD risk factors and high perceived lifetime risk was more modest (Figure 2).
Figure 2.
Multivariable-adjusted odds ratios comparing participant characteristics and the probability of having high perceived lifetime risk for cardiovascular disease (N = 2,998). In this figure, high perceived lifetime risk is defined as having a perceived lifetime risk score of 5; lower perceived lifetime risk is defined as having a perceived lifetime risk score of 1-4 (see Methods for details). Odds ratios are multivariable adjusted for all covariates listed.
We further examined the factors that were associated with misperception of lifetime risk for CVD. Among participants with a low predicted lifetime risk for CVD (N= 1,056), 751 did not perceive their risk level to be low or “1-least likely”, but instead rated their perceived risk level to be higher with scores from 2–5. The determinants of overestimated risk (perceived > predicted) included older age (OR [95% CI]: 1.23 [1.03–1.47), white race (OR [95% CI]: 2.42 [1.66–3.50]), high education level (OR [95% CI]: 1.56 [1.09–2.23]), elevated BMI (OR [95% CI]: 1.10 [1.02–1.18]), family history of premature MI (OR [95% CI]: 2.16 [1.15–4.05]), high perceived stress (OR [95% CI]: 1.87 [1.27–2.76]), and low perceived health (OR [95% CI]: 2.05 [1.34–3.12]).
Similarly, among participants with high predicted lifetime risk for CVD (N = 1,942), 1,704 did not perceive their risk level to be high or “5-most likely”, but instead rated their perceived risk level to be lower with scores from 1–4. The determinants of underestimated risk (perceived < predicted) included participants without a family history of premature MI (OR [95% CI]: 1.76 [1.20–2.58]), with low perceived stress (OR [95% CI]: 2.13 [1.57–2.90]), and with high perceived health (OR [95% CI]: 2.68 [1.99–3.62]).
Finally, because perceived lifetime risk was measured on a relative scale of survey responses, we chose a priori to study those at the extremes of perceived risk (ie. Score of 1 vs. 5). After additional sensitivity analyses in which we varied the threshold for “low perceived lifetime risk” (i.e. a score of 1, 1–2, and 1–3 were considered to be “low perceived lifetime risk” in separate analyses), we observed a consistent pattern of results, suggesting that our findings were independent of the threshold (data not shown).
DISCUSSION
To our knowledge, the present study represents the first report of the perceived lifetime risk for CVD in the general population. Our findings demonstrate that the perception of lifetime risk for CVD varies considerably and is often inaccurate. In addition, our findings demonstrate that the perception of CVD risk is influenced more by personal factors (i.e. subjective perception of stress and personal health) than traditional CVD risk factors. Therefore patients and physicians have different perspectives regarding estimation of lifetime risk for CVD. These findings have implications for primary prevention practice, emphasizing the importance of more effective risk communication regarding the role of established, traditional risk factors in determining the lifetime risk for CVD.
Several prior studies have examined the association between short-term perceived and predicted risk for CVD (13–16). Most of these studies use data derived from primary care physician practices and/or use self-reported risk factors to create predicted risk estimates. In spite of these methodological differences, these prior studies also observed that incorrect perception of short-term CVD risk was not uncommon and was associated with race, socioeconomic status, family history of CVD, and perceived health. Interestingly, a study of women’s awareness of CVD demonstrated that although general knowledge of CVD as a leading cause of death has increased over the last decade, this knowledge has not translated into an accurate perception of personal risk for CVD (17). There exists an “optimism bias,” in which people generally underestimate their own personal risk for CVD (18).
In the present study, we extend these prior observations in several important ways. First, we provide the first reported description of perceived lifetime, rather than short-term, risk for CVD and its associated demographic, personal, and risk factor characteristics in a large, multi-ethnic, population-based sample of US adults. Second, using measured baseline risk factors and our previously published lifetime risk prediction algorithm we were able to compare the perceived lifetime risk with a reliable estimate of the predicted lifetime risk for CVD.
We found that despite the fact that the majority of our study participants (64%) are at high predicted lifetime risk for CVD, most do not perceive themselves as high risk. And, on both ends of the spectrum, participants commonly misperceived their predicted lifetime risk level, with almost half in each of the extreme perceived risk groups, (42% of those rated “1-least likely” and 49% of those rated “5-most likely”) having predicted risk levels that were actually opposite of their perceived lifetime risk (Table 1). Interestingly, while general perception of lifetime risk tracked with the burden of traditional CVD risk factors, misperception of lifetime risk had almost no relation to risk factors. Perception of risk level was instead strongly associated with an individual’s family history of premature MI and their subjective perceptions of levels of stress and personal health.
It has been shown that patients’ awareness of cardiovascular risk level is a motivating factor for them to make lifestyle changes (6), and to take blood pressure (8) and cholesterol-lowering medications (7), resulting in a reduction in CVD risk factor burden (19). Self-perception of low cardiac risk, on the other hand, decreases motivation to engage in lifestyle modification (6). Patients, and in particular racial/ethnic minorities, have reported that encouragement from their physician is a motivating factor to make lifestyle changes (20). Although risk communication is not a guarantee for successful prevention of CVD, these prior findings suggest the importance of an individual’s risk perception and therapeutic lifestyle counseling by healthcare providers as motivators for change, and support the recent performance measures from the American Heart Association and the American College of Cardiology encouraging health care providers to provide dietary and physical activity counseling to promote CVD prevention (21).
Some limitations should be noted. First, it should be noted that we compared the perceived lifetime risk for a “heart attack” with the predicted lifetime risk for total atherosclerotic CVD. Although the lifetime risk for MI is smaller than total CVD, the association between traditional risk factors is similar across all endpoints and therefore is unlikely to have influenced our results (9, 22). Second, we have previously reported that premature family history was also associated with a higher lifetime risk for CVD (23). Had we considered premature family history as part of the definition of lifetime risk, it is likely that this variable would have been associated more strongly with correctly estimated lifetime risk. However, in the present paper, we define lifetime risk strata according to traditional risk levels alone as this represents the most well established approach to lifetime risk estimation (1, 5, 9, 10).
Supplementary Material
Acknowledgments
Sources of Funding:
Dr. Berry receives funding from (1) the Dedman Family Scholar in Clinical Care endowment at UT Southwestern Medical Center, (2) grant K23 HL092229 from the National Heart, Lung, and Blood Institute, and (3) grant 13GRNT14560079 from the American Heart Association. Grant support for the Dallas Heart Study was provided by the Donald W. Reynolds Foundation at UT Southwestern Medical Center, Dallas, TX. Dr. Powell-Wiley is supported by the Division of Intramural Research at the NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures:
Dr. Berry reports receiving financial compensation from Merck in the form of speaker’s bureau fees. Dr. de Lemos reports receiving speaker honoraria and consulting income from Astra Zeneca and consulting income from Janssen Pharmaceuticals. No other authors report relevant financial disclosures.
REFERENCES
- 1.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marma AK, Berry JD, Ning H, Persell SD, Lloyd-Jones DM. Distribution of 10-Year and Lifetime Predicted Risks for Cardiovascular Disease in US Adults: Findings From the National Health and Nutrition Examination Survey 2003 to 2006. Circ Cardiovasc Qual Outcomes. 2010;3:8–14. doi: 10.1161/CIRCOUTCOMES.109.869727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Expert Panel on Detection E. Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 4.Mosca L, Banka CL, Benjamin EJ, Berra K, Bushnell C, Dolor RJ, Ganiats TG, Gomes AS, Gornik HL, Gracia C, Gulati M, Haan CK, Judelson DR, Keenan N, Kelepouris E, Michos ED, Newby LK, Oparil S, Ouyang P, Oz MC, Petitti D, Pinn VW, Redberg RF, Scott R, Sherif K, Smith SC, Jr, Sopko G, Steinhorn RH, Stone NJ, Taubert KA, Todd BA, Urbina E, Wenger NK Expert Panel/Writing Group; American Heart Association; American Academy of Family Physicians; American College of Obstetricians and Gynecologists; American College of Cardiology Foundation; Society of Thoracic Surgeons; American Medical Women's Association; Centers for Disease Control and Prevention; Office of Research on Women's Health; Association of Black Cardiologists; American College of Physicians; World Heart Federation; National Heart, Lung, and Blood Institute; American College of Nurse Practitioners. Evidence-Based Guidelines for Cardiovascular Disease Prevention in Women: 2007 Update. Circulation. 2007;115:1481–1501. [Google Scholar]
- 5.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson J, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. Epub 2013 Nov 12. [Google Scholar]
- 6.Mosca L, Mochari H, Christian A, Berra K, Taubert K, Mills T, Burdick KA, Simpson SL. National study of women's awareness, preventive action, and barriers to cardiovascular health. Circulation. 2006;113:525–534. doi: 10.1161/CIRCULATIONAHA.105.588103. [DOI] [PubMed] [Google Scholar]
- 7.Grover SA, Lowensteyn I, Joseph L, Kaouache M, Marchand S, Coupal L, Boudrea G. Cardiovascular Health Evaluation to Improve Compliance and Knowledge Among Uninformed Patients (CHECK-UP) Study Group. Patient Knowledge of Coronary Risk Profile Improves the Effectiveness of Dyslipidemia Therapy: The CHECK-UP Study: A Randomized Controlled Trial. Arch Intern Med. 2007;167:2296–2303. doi: 10.1001/archinte.167.21.2296. [DOI] [PubMed] [Google Scholar]
- 8.Grover S, Lowensteyn I, Joseph L, Kaouache M, Marchand S, Coupal L, Boudrea G. Discussing Coronary Risk with Patients to Improve Blood Pressure Treatment: Secondary Results from the CHECK-UP Study. J Gen Intern Med. 2009;24:33–39. doi: 10.1007/s11606-008-0825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, Leip EP, Larson MG, D'Agostino RB, Beiser A, Wilson PWF, Wolf PA, Levy D. Prediction of Lifetime Risk for Cardiovascular Disease by Risk Factor Burden at 50 Years of Age. Circulation. 2006;113:791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 10.Berry JD, Liu K, Folsom AR, Polak JF, Lewis CE, Shea S, Sidney S, O'Leary DH, Chan C, Lloyd-Jones DM. Prevalence and Progression of Subclinical Atherosclerosis in Younger Adults with Low 10-Year Risk but High Lifetime Risk for Cardiovascular Disease: Findings from the CARDIA and MESA Studies. Circulation. 2009;119:382–389. doi: 10.1161/CIRCULATIONAHA.108.800235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH. Dallas Heart Study Investigators. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 12.Patel MJ, de Lemos JA, Philips B, Murphy SA, Vaeth PC, McGuire DK, Khera A. Implications of family history of myocardial infarction in young women. Am Heart J. 2007;154:454–460. doi: 10.1016/j.ahj.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Avis NE, Smith KW, McKinlay JB. Accuracy of perceptions of heart attack risk: what influences perceptions and can they be changed? Am J Public Health. 1989;79:1608–1612. doi: 10.2105/ajph.79.12.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreuter MW, Strecher VJ. Changing Inaccurate Perceptions of Health Risk: Results From a Randomized Trial. Health Psychol. 1995;14:56–63. doi: 10.1037//0278-6133.14.1.56. [DOI] [PubMed] [Google Scholar]
- 15.Marteau TM. Ann-Louise Kinmonth, Stephen Pyke, Simon G Thompson, Family Heart Study Group. Readiness for lifestyle advice: self-assessments of coronary risk prior to screening in the British family heart study. Br J Gen Pract. 1995;45:5–8. [PMC free article] [PubMed] [Google Scholar]
- 16.van der Weijden T, Bos LB, Koelewijn-van Loon MS. Primary care patients' recognition of their own risk for cardiovascular disease: implications for risk communication in practice. Curr Opin Cardiol. 2008;23:471–476. doi: 10.1097/HCO.0b013e32830b35f6. [DOI] [PubMed] [Google Scholar]
- 17.Kling JM, Miller VM, Mankad R, Wilansky S, Wu Q, Zais TG, Zarling KK, Allison TG, Mulvagh SL. Go Red for Women cardiovascular health-screening evaluation: the dichotomy between awareness and perception of cardiovascular risk in the community. J Womens Health (Larchmt) 2013;22:210–218. doi: 10.1089/jwh.2012.3744. [DOI] [PubMed] [Google Scholar]
- 18.Webster R, Heeley E. Perceptions of risk: understanding cardiovascular disease. Risk Manag Healthc Policy. 2010;3:49–60. doi: 10.2147/RMHP.S8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benner JS, Erhardt L, Flammer M, Moller RA, Rajicic N, Changela K, Yunis C, Cherry SB, Gaciong Z, Johnson ES, Sturkenboom MC, Garcia-Puig J, Girerd X. REACH OUT Investigators. A novel programme to evaluate and communicate 10-year risk of CHD reduces predicted risk and improves patients' modifiable risk factor profile. Int J Clin Pract. 2008;62:1484–1498. doi: 10.1111/j.1742-1241.2008.01872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mochari-Greenberger H, Mills T, Simpson SL, Mosca L. Knowledge, preventive action, and barriers to cardiovascular disease prevention by race and ethnicity in women: an American Heart Association national survey. J Womens Health (Larchmt) 2010;19:1243–1249. doi: 10.1089/jwh.2009.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redberg RF, Benjamin EJ, Bittner V, Braun LT, Goff DC, Jr, Havas S, Labarthe DR, Limacher MC, Lloyd-Jones DM, Mora S, Pearson TA, Radford MJ, Smetana GW, Spertus JA, Swegler EW. American Academy of Family Physicians, American Association of Cardiovascular and Pulmonary Rehabilitation, Preventive Cardiovascular Nurses Association. ACCF/AHA 2009 performance measures for primary prevention of cardiovascular disease in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Performance Measures for Primary Prevention of Cardiovascular Disease) developed in collaboration with the American Academy of Family Physicians, American Association of Cardiovascular and Pulmonary Rehabilitation; and Preventive Cardiovascular Nurses Association: endorsed by the American College of Preventive Medicine, American College of Sports Medicine, and Society for Women's Health Research. J Am Coll Cardiol. 2009;54:1364–1405. doi: 10.1016/j.jacc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd-Jones DM, Dyer AR, Wang R, Daviglus ML, Greenland P. Risk Factor Burden in Middle Age and Lifetime Risks for Cardiovascular and Non-Cardiovascular Death (Chicago Heart Association Detection Project in Industry) Am J Cardiol. 2007;99:535–540. doi: 10.1016/j.amjcard.2006.09.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachmann JM, Willis BL, Ayers CR, Khera A, Berry JD. Association between family history and coronary heart disease death across long-term follow-up in men: the Cooper Clinic Longitudinal Study. Circulation. 2012;125:3092–3098. doi: 10.1161/CIRCULATIONAHA.111.065490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.