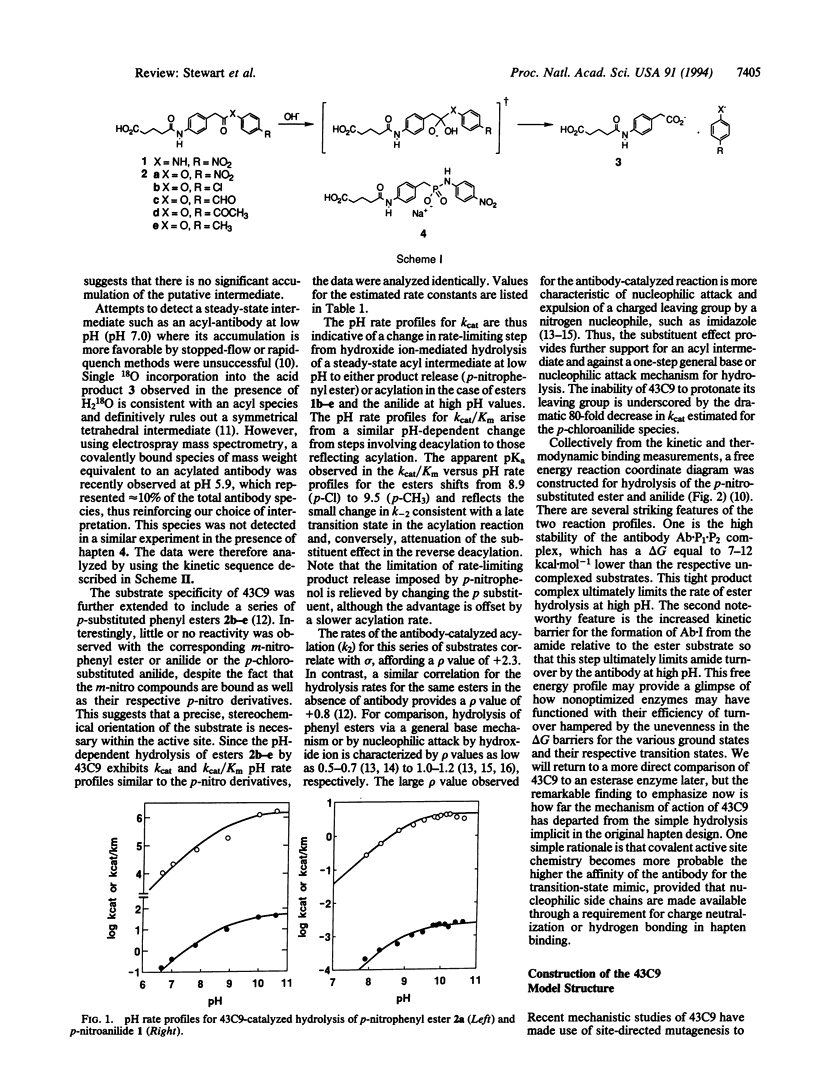
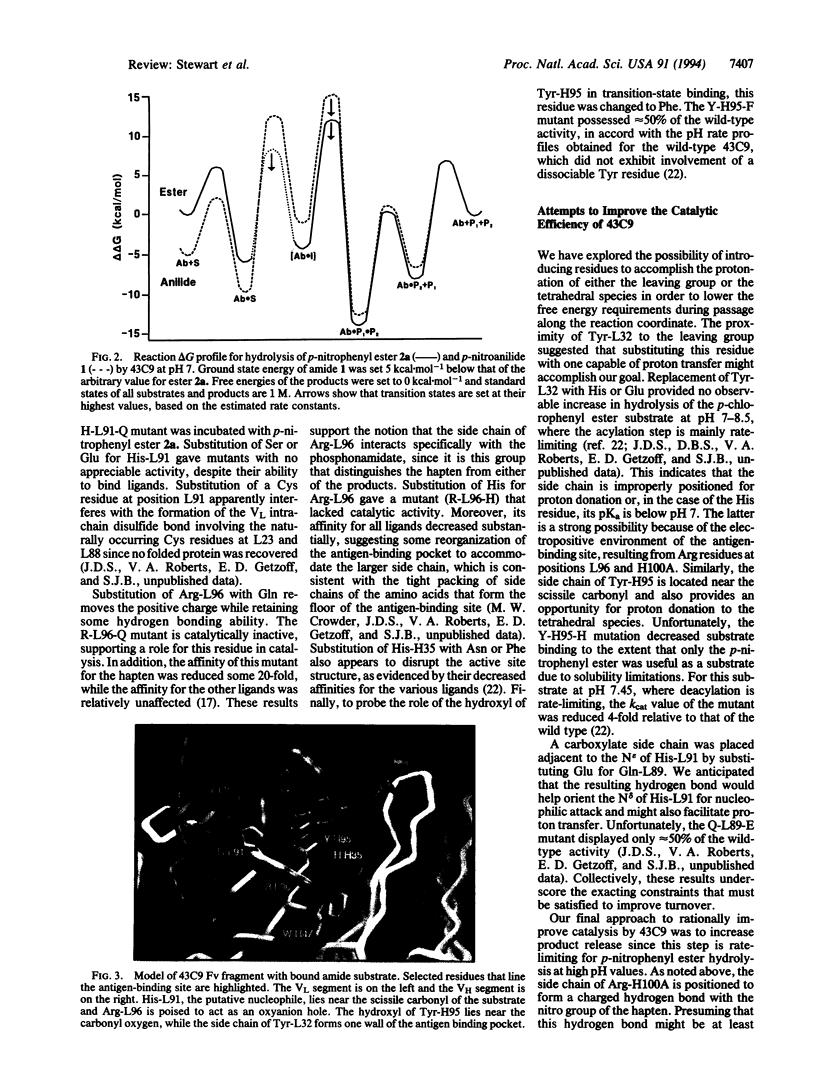
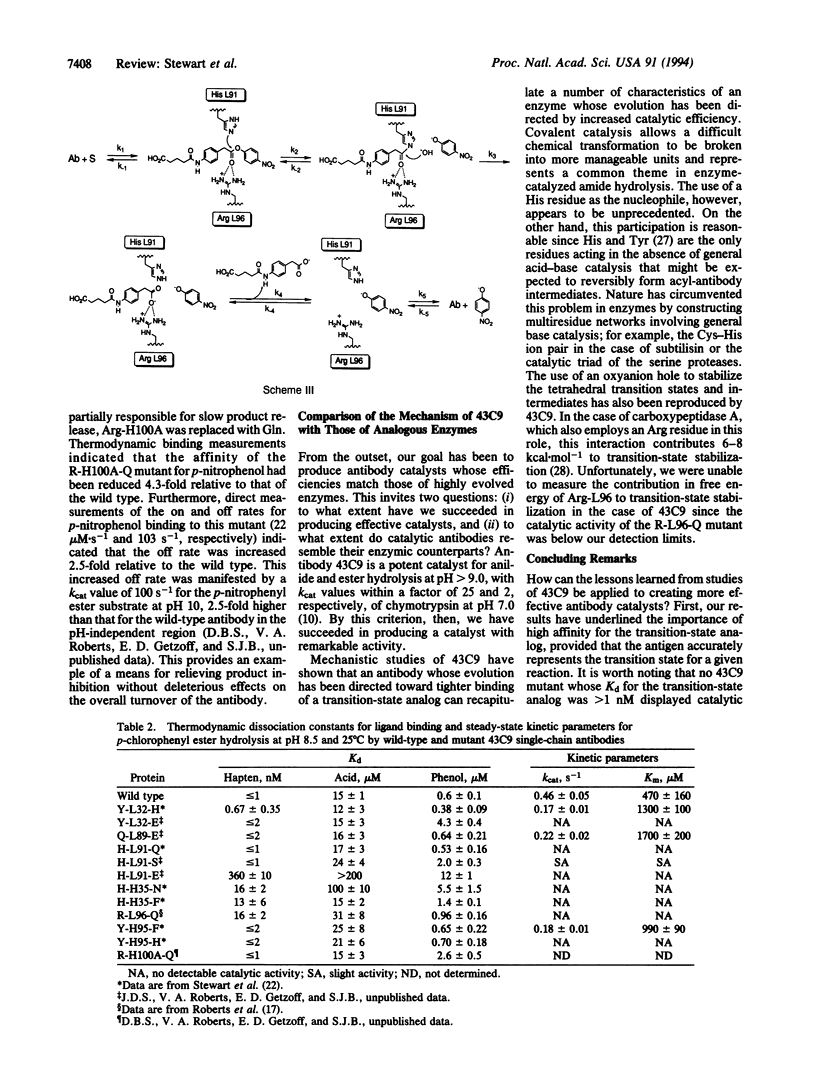
Abstract
Antibody 43C9 accelerates the hydrolysis of a p-nitroanilide by a factor of 2.5 x 10(5) over the background rate in addition to catalyzing the hydrolysis of a series of aromatic esters. Since this represents one of the largest rate accelerations achieved with an antibody, we have undertaken a series of studies aimed at uncovering the catalytic mechanism of 43C9. The immunogen, a phosphonamidate, was designed to mimic the geometric and electronic characteristics of the tetrahedral intermediate that forms upon nucleophilic attack by hydroxide on the amide substrate. Further studies, however, revealed that the catalytic mechanism is more complex and involves the fortuitous formation of a covalent acyl-antibody intermediate as a consequence of complementary side chain residues at the antibody-binding site. Several lines of evidence indicate that the catalytic mechanism involves two key residues: His-L91, which acts as a nucleophile to form the acyl-antibody intermediate, and Arg-L96, which stabilizes the anionic tetrahedral moieties. Support for this mechanism derives from the results of site-directed mutagenesis experiments and solvent deuterium isotope effects as well as direct detection of the acyl-antibody by electrospray mass spectrometry. Despite its partial recapitulation of the course of action of enzymic counterparts, the reactivity of 43C9, like other antibodies, is apparently limited by its affinity for the inducing immunogen. To go beyond this level, one must introduce additional catalytic functionality, particularly general acid-base catalysis, through either improvements in transition-state analog design or site-specific mutagenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benkovic S. J., Adams J. A., Borders C. L., Jr, Janda K. D., Lerner R. A. The enzymic nature of antibody catalysis: development of multistep kinetic processing. Science. 1990 Nov 23;250(4984):1135–1139. doi: 10.1126/science.2251500. [DOI] [PubMed] [Google Scholar]
- Benkovic S. J. Catalytic antibodies. Annu Rev Biochem. 1992;61:29–54. doi: 10.1146/annurev.bi.61.070192.000333. [DOI] [PubMed] [Google Scholar]
- Benkovic S. J., Napper A. D., Lerner R. A. Catalysis of a stereospecific bimolecular amide synthesis by an antibody. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5355–5358. doi: 10.1073/pnas.85.15.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummel C. L., Lee I. N., Zhou Y., Benkovic S. J., Winograd N. A mass spectrometric solution to the address problem of combinatorial libraries. Science. 1994 Apr 15;264(5157):399–402. doi: 10.1126/science.8153627. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Padlan E. A., Sheriff S. Antibody-antigen complexes. Annu Rev Biochem. 1990;59:439–473. doi: 10.1146/annurev.bi.59.070190.002255. [DOI] [PubMed] [Google Scholar]
- Getzoff E. D., Tainer J. A., Lerner R. A., Geysen H. M. The chemistry and mechanism of antibody binding to protein antigens. Adv Immunol. 1988;43:1–98. doi: 10.1016/s0065-2776(08)60363-6. [DOI] [PubMed] [Google Scholar]
- Gibbs R. A., Posner B. A., Filpula D. R., Dodd S. W., Finkelman M. A., Lee T. K., Wroble M., Whitlow M., Benkovic S. J. Construction and characterization of a single-chain catalytic antibody. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4001–4004. doi: 10.1073/pnas.88.9.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glockshuber R., Stadlmüller J., Plückthun A. Mapping and modification of an antibody hapten binding site: a site-directed mutagenesis study of McPC603. Biochemistry. 1991 Mar 26;30(12):3049–3054. doi: 10.1021/bi00226a010. [DOI] [PubMed] [Google Scholar]
- Jacobs J. W. New perspectives on catalytic antibodies. Biotechnology (N Y) 1991 Mar;9(3):258–262. doi: 10.1038/nbt0391-258. [DOI] [PubMed] [Google Scholar]
- Janda K. D., Schloeder D., Benkovic S. J., Lerner R. A. Induction of an antibody that catalyzes the hydrolysis of an amide bond. Science. 1988 Sep 2;241(4870):1188–1191. doi: 10.1126/science.3413482. [DOI] [PubMed] [Google Scholar]
- Kirsch R., Schmidt H., Schmidt D. Der Säure-Basen-Haushalt in Extremhyperthermie beim Menschen. Arch Phys Ther (Leipz) 1968 Mar-Apr;20(2):127–132. [PubMed] [Google Scholar]
- Lerner R. A., Benkovic S. J., Schultz P. G. At the crossroads of chemistry and immunology: catalytic antibodies. Science. 1991 May 3;252(5006):659–667. doi: 10.1126/science.2024118. [DOI] [PubMed] [Google Scholar]
- Martin M. T., Napper A. D., Schultz P. G., Rees A. R. Mechanistic studies of a tyrosine-dependent catalytic antibody. Biochemistry. 1991 Oct 8;30(40):9757–9761. doi: 10.1021/bi00104a027. [DOI] [PubMed] [Google Scholar]
- Phillips M. A., Kaplan A. P., Rutter W. J., Bartlett P. A. Transition-state characterization: a new approach combining inhibitor analogues and variation in enzyme structure. Biochemistry. 1992 Feb 4;31(4):959–963. doi: 10.1021/bi00119a003. [DOI] [PubMed] [Google Scholar]
- Posner B., Lee I., Itoh T., Pyati J., Graff R., Thorton G. B., La Polla R., Benkovic S. J. A revised strategy for cloning antibody gene fragments in bacteria. Gene. 1993 Jun 15;128(1):111–117. doi: 10.1016/0378-1119(93)90161-u. [DOI] [PubMed] [Google Scholar]
- Posner B., Smiley J., Lee I., Benkovic S. Catalytic antibodies: perusing combinatorial libraries. Trends Biochem Sci. 1994 Apr;19(4):145–150. doi: 10.1016/0968-0004(94)90273-9. [DOI] [PubMed] [Google Scholar]
- Rahil J., Pratt R. F. Characterization of covalently bound enzyme inhibitors as transition-state analogs by protein stability measurements: phosphonate monoester inhibitors of a beta-lactamase. Biochemistry. 1994 Jan 11;33(1):116–125. doi: 10.1021/bi00167a015. [DOI] [PubMed] [Google Scholar]
- Roberts V. A., Iverson B. L., Iverson S. A., Benkovic S. J., Lerner R. A., Getzoff E. D., Tainer J. A. Antibody remodeling: a general solution to the design of a metal-coordination site in an antibody binding pocket. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6654–6658. doi: 10.1073/pnas.87.17.6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts V. A., Stewart J., Benkovic S. J., Getzoff E. D. Catalytic antibody model and mutagenesis implicate arginine in transition-state stabilization. J Mol Biol. 1994 Jan 21;235(3):1098–1116. doi: 10.1006/jmbi.1994.1060. [DOI] [PubMed] [Google Scholar]
- Schowen K. B., Schowen R. L. Solvent isotope effects of enzyme systems. Methods Enzymol. 1982;87:551–606. [PubMed] [Google Scholar]
- Stewart J. D., Benkovic S. J. Recent developments in catalytic antibodies. Int Rev Immunol. 1993;10(2-3):229–240. doi: 10.3109/08830189309061698. [DOI] [PubMed] [Google Scholar]
- Stewart J. D., Roberts V. A., Thomas N. R., Getzoff E. D., Benkovic S. J. Site-directed mutagenesis of a catalytic antibody: an arginine and a histidine residue play key roles. Biochemistry. 1994 Mar 1;33(8):1994–2003. doi: 10.1021/bi00174a004. [DOI] [PubMed] [Google Scholar]
- Tang Y., Hicks J. B., Hilvert D. In vivo catalysis of a metabolically essential reaction by an antibody. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8784–8786. doi: 10.1073/pnas.88.19.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubban K. S., Schowen R. L. The proton inventory technique. CRC Crit Rev Biochem. 1984;17(1):1–44. doi: 10.3109/10409238409110268. [DOI] [PubMed] [Google Scholar]




