Abstract
Neural stem cells (NSCs) are defined by their ability to self-renew and to differentiate into mature neuronal and glial cell types. NSCs are the subject of intense investigation, owing to their crucial roles in neural development and adult brain function and because they present potential targets for gene and cell replacement therapies following injury or disease. Approaches to specifically genetically perturb or modulate NSC function would be valuable for either motivation. Unfortunately, most gene delivery vectors are incapable of efficient or specific gene delivery to NSCs in vivo. Vectors based on adeno-associated virus (AAV) present a number of advantages and have proven increasingly successful in clinical trials. However, natural AAV variants are inefficient in transducing NSCs. We previously engineered a novel AAV variant (AAV r3.45) capable of efficient transduction of adult NSCs in vitro. Here, to build upon the initial promise of this variant, we investigated its in vitro and in vivo infectivity. AAV r3.45 was more selective for NSCs than mature neurons in a human embryonic stem cell-derived culture containing a mixture of cell types, including NSCs and neurons. It was capable of more efficient and selective transduction of rat and mouse NSCs in vivo than natural AAV serotypes following intracranial vector administration. Delivery of constitutively active β-catenin yielded insights into mechanisms by which this key regulator modulates NSC function, indicating that this engineered AAV variant can be harnessed for preferential modulation of adult NSCs in the hippocampus. The capacity to rapidly genetically modify these cells might greatly accelerate in vivo investigations of adult neurogenesis.
Keywords: Adeno-associated virus, Gene delivery, Neural stem cell
SUMMARY: The adeno-associated virus variant r3.45 is used to efficiently and selectively transduce adult mouse, rat, and human neural stem cells both in vitro and in vivo.
INTRODUCTION
Neural stem cells (NSCs) are characterized by the capacity for self-renewal and differentiation into different neural cell types, including neurons, astrocytes and oligodendrocytes (Gage, 2000; Temple, 2001). Within the adult brain, active NSC populations exist in the subventricular zone (SVZ), the striatum (in humans) and the subgranular zone of the dentate gyrus of the hippocampus (Ernst et al., 2014; Gage, 2000). In the subgranular zone, neurogenesis begins with the activation and division of quiescent Type 1 NSCs (which express nestin, Sox2 and Gfap) to generate Type 2a mitotic NSCs (which express nestin and Sox2, but not Gfap) (Lugert et al., 2010; Suh et al., 2009). As differentiation proceeds, Type 2a NSCs develop into Type 2b neuronal precursors (expressing Sox2 and Dcx), which later mature into Type 3 neuroblasts (expressing Dcx but not Sox2), migrate into the granule cell layer, differentiate into mature neurons [expressing NeuN (Rbfox3)] and integrate into the neural network (Lugert et al., 2010; Mira et al., 2010; Suh et al., 2009). Type 1 NSCs are also capable of differentiating into mature hippocampal astrocytes (expressing Gfap and S100β) (Bonaguidi et al., 2011). Adult neurogenesis has been shown to play key roles in learning and memory in mammals, including hippocampal-dependent spatial navigation learning, spatial pattern discrimination and contextual fear conditioning (Deng et al., 2010; Ming and Song, 2011).
Efficient and preferential gene delivery would offer a versatile and rapid means to study regulatory mechanisms of NSC quiescence, proliferation, self-renewal and differentiation. Nestin-CreERT2 transgenic mice, which express tamoxifen-inducible Cre recombinase under the control of the nestin promoter, have been heavily utilized to track NSCs and their progeny in vivo (Ashton et al., 2012; Bonaguidi et al., 2011; Lagace et al., 2007). These mouse lines have enabled a number of basic advances in NSC investigations; however, deriving a new line to study each new gene is highly time- and labor-intensive, taking months to years (Haruyama et al., 2009). In addition to basic studies, gene delivery could be harnessed for gene or cell replacement therapies to treat neurodegenerative disease or injury; for example, via the overexpression or knockdown of genes that modulate the generation of new neurons. Also, gene delivery to NSCs has been harnessed to express neurotrophic factors for protection from neurodegenerative diseases (Blesch et al., 2002), and restoration of fragile X mental retardation protein expression specifically in adult NSCs rescued mice from learning deficits in a murine model of fragile X syndrome (Guo et al., 2011).
There have been several efforts to deliver genes to adult NSCs in vivo. Hashimoto and Mikoshiba used replication-defective adenoviral vectors to deliver genes to progenitor cells in the developing brains of mice at embryonic days 10.5-14.5; these vectors enabled tracking of the differentiation of the progenitor cells, but delivery to adult NSCs has not been demonstrated (Hashimoto and Mikoshiba, 2004). Falk et al. administered polyethyleneimine (PEI) complexes, containing plasmids driving reporter gene expression via enhancer elements from the second intron of the human nestin gene, to the lateral ventricle of mice and showed some selective delivery to NSCs in the SVZ, although the efficiency was limited (Falk et al., 2002). In another study, Lemkine et al. used PEI-DNA complexes and showed low specificity towards mouse SVZ NSCs as compared with globular cells following delivery to the lateral ventricle (Lemkine et al., 2002). Additionally, van Hooijdonk et al. (van Hooijdonk et al., 2009) used a vesicular stomatitis virus G glycoprotein-pseudotyped lentivirus to target neural progenitor cells and immature neurons in the subgranular zone of the dentate gyrus of the mouse hippocampus. Although the lentivirus preferentially transduced neuronal progenitor cells and immature neurons, only 11% of cells infected with the virus were nestin+ 1 week after administration (van Hooijdonk et al., 2009). Finally, retroviral vector administration to the mouse hippocampus is useful for targeting mitotic neural progenitors and neuroblasts (Jessberger et al., 2008), but early stage stem cells rarely divide (Bonaguidi et al., 2011).
Adeno-associated virus (AAV) is a nonpathogenic, non-enveloped virus that is a member of the parvovirus family. The AAV icosahedral protein capsid encloses a 4.7 kb single-stranded DNA genome that contains flanking inverted terminal repeats (ITRs), which serve as the origin of replication and signal for the genome to be packaged (Knipe and Howley, 2007). Between the ITRs, the rep open reading frame (ORF) encodes four nonstructural proteins that are responsible for viral replication in the presence of a helper virus, transcriptional regulation of the rep and cap ORFs, site-specific integration into the AAVS1 locus and virion assembly (Knipe and Howley, 2007). The cap ORF encodes three structural proteins (VP1, VP2 and VP3) that assemble to form the 60-mer viral capsid (Knipe and Howley, 2007). The amino acid sequence translated from the cap ORF determines the gene delivery properties of AAV, including antibody binding, cell surface receptor binding, glycan binding and endosomal escape, and currently eleven naturally occurring serotypes and over 100 variants of the AAV capsid have been identified (Kotterman and Schaffer, 2014; Schaffer et al., 2008; Wu et al., 2006).
In the recombinant versions of AAV used for gene delivery, rep and cap are replaced by a gene of interest that is inserted between the ITRs. To produce the gene delivery vector encoding the gene of interest, a plasmid containing rep and cap and additional helper viral genes are provided to the packaging cells (Flotte, 2004). Recombinant AAV vectors are capable of transducing both dividing and non-dividing cells, and stable transgene expression is possible for years in postmitotic tissue. To date, no natural AAV has been associated with any human disease, which, along with their high efficiency on some cell types, is a key reason why recombinant AAV has emerged as an attractive vector for gene therapy (Knipe and Howley, 2007).
Unfortunately, the use of naturally occurring AAV serotypes has revealed a number of challenges to their widespread use in clinical gene therapy. These include significantly lower transduction in the presence of neutralizing antibodies (Jaski et al., 2009; Manno et al., 2006), lack of specific and/or efficient distribution to many potential target tissues (Zincarelli et al., 2008), lack of efficiency (Manno et al., 2003; Moss et al., 2007; Wagner et al., 2002) and incapacity for targeted delivery to specific cell types. These issues arise because the properties that mediate successful natural viral infections are distinct from those required for success in basic biological or biomedical applications, and viruses did not evolve for the latter. In particular, none of the natural AAV serotypes is capable of efficient gene delivery to NSCs (Jang et al., 2011) and many instead show highly specific tropism for mature neurons (Bartlett et al., 1998; Kaspar et al., 2002; Ortinski et al., 2010).
Directed evolution is a high-throughput molecular engineering approach that has been successfully harnessed to generate AAV variants with altered receptor binding, neutralizing antibody-evasion properties and novel cell tropism (Asuri et al., 2012; Excoffon et al., 2009; Koerber et al., 2008; Maheshri et al., 2006). As is the case with natural evolution, directed evolution utilizes an iterative process in which genetic variants undergo cycles of additional diversification and increasing selective pressure to allow for the emergence of key mutations that improve function for a specific application. The coupling of random diversification and highly tailored selection enables the generation of significantly improved functionality even if the mechanism of action is unknown. Recently, we applied directed evolution to isolate an AAV variant capable of efficient NSC transduction in vitro (Jang et al., 2011). Specifically, selection for the capacity to infect cultured adult rat hippocampal NSCs yielded AAV r3.45, an AAV2 variant with a seven-amino-acid peptide insertion at position 588. AAV r3.45 demonstrated 50-fold increased transduction of rat NSCs in vitro as compared with wild-type AAV2 and AAV5. This variant AAV was also capable of significantly increased transduction of murine NSCs, human fetal NSCs and human embryonic stem cell (hESC)-derived neural progenitor cells compared with AAV2 (Jang et al., 2011). In addition to improved transduction of NSCs, AAV r3.45 significantly improved homologous recombination-based gene correction: its use resulted in a fivefold increase in targeted gene correction in NSCs compared with wild-type AAV2 and AAV5 (Jang et al., 2011). When AAV r3.45 was immobilized onto elastin-like peptides, delivery to human NSCs was further enhanced (Kim et al., 2012b).
Although the majority of evolved AAV variants have been created using in vitro selections, some of these variants have demonstrated success when translated to an in vivo model. For example, two variants evolved by Koerber et al. for the ability to infect primary human astrocytes in culture also transduced 3.3- and 5.5-fold more astrocytes, relative to neurons, than AAV2 within the striatum following intracranial injection in rats (Koerber et al., 2009). Furthermore, in vivo analysis revealed that another variant from the astrocyte selection was capable of highly specific and efficient infection of Müller glia when compared with AAV2 and AAV6 (Klimczak et al., 2009). The success of AAV variants created through in vitro selection to evade neutralization by human antibodies has also translated to increased antibody evasion in a mouse model of immunity (M.A.K., Bum-Yeol Hwang, Daniel Stone, James T. Koerber and D.V.S., unpublished).
Based on these successes, we investigated the in vivo transduction properties of AAV r3.45 and demonstrated its utility for targeted genetic modification of adult NSCs in vivo. In particular, AAV r3.45 exhibited efficient and selective transduction of adult mouse, rat and human NSCs, both in vitro and in vivo. In addition, to investigate its utility for basic biological investigation, AAV r3.45 was harnessed to deliver constitutively active β-catenin to NSCs in the mouse hippocampus in order to study the mechanisms by which β-catenin signaling increases neurogenesis.
RESULTS
AAV r3.45 enables increased selectivity towards hESC-derived NSCs in vitro
Jang et al. previously described the directed evolution of a novel AAV2 variant, AAV r3.45, which contained a LATQVGQKTA peptide insertion at amino acid 587 with a V719M mutation (Jang et al., 2011). The variant mediated enhanced gene delivery to rat, mouse and human NSCs in vitro compared with several wild-type AAV serotypes (Jang et al., 2011). However, efficiency is distinct from selectivity, and in vitro is different from in vivo. To initially assess selectivity, an hESC-derived culture containing a mixture of cells, including neural progenitor cells, neurons and astrocytes, was infected at an MOI of 10,000 with GFP-encoding AAV r3.45, its parental wild-type AAV2 serotype, or wild-type AAV6 (the most effective natural serotype tested on NSCs in vitro) (Jang et al., 2011). Forty-eight hours later, the percentage of infected cells in each culture that stained positive for the neural stem and progenitor cell marker nestin was significantly higher for AAV r3.45 compared with wild-type AAV2 and AAV6 (Fig. 1). Furthermore, AAV r3.45 was the only virus to transduce a higher proportion of NSCs relative to neurons. In conjunction with the in vitro data reported by Jang et al., this experiment indicates that AAV r3.45 is capable of general and selective neural stem and progenitor cell infection.
Fig. 1.
Selectivity towards hESC-derived NSCs in vitro. (A) Representative images of areas of hESC-derived neuronal cultures containing neural stem and progenitor cells (top row, red) or mature neurons (bottom row, red), 48 h post-infection with recombinant AAV2, AAV6 or AAV r3.45 vectors expressing GFP (green). Representative examples of infected cells of each type are marked with arrowheads. Scale bar: 100 μm. (B) The percentage of GFP+ cells co-staining for nestin or MAP2B was quantified to determine the selectivity of each viral vector. Error bars indicate s.d. (n=3); *P<0.01, **P<0.005 (ANOVA).
AAV r3.45 enables increased selectivity towards and infectivity of adult NSCs in the rodent brain
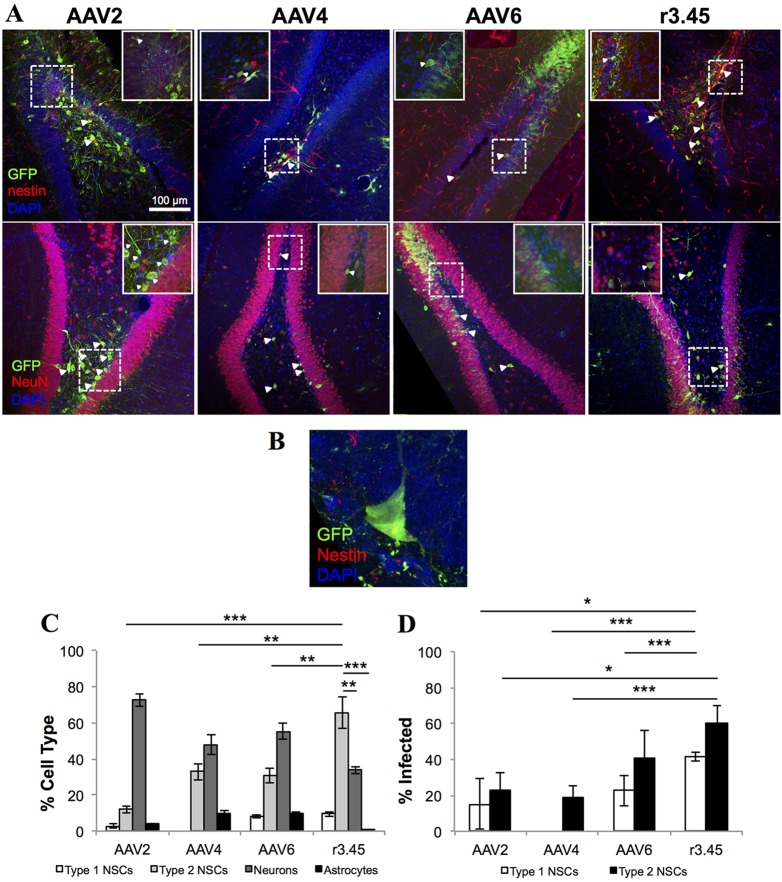
Several AAV variants generated via in vitro directed evolution systems have proved successful when translated to an in vivo model (Klimczak et al., 2009; Koerber et al., 2009), and we therefore investigated the transduction properties of AAV r3.45 in vivo. This AAV variant encoding GFP was initially administered via intracranial injection to the dentate gyrus of the rat hippocampus. Consistent with previous reports, AAV2 showed strong tropism for mature neurons (Bartlett et al., 1998; Kaspar et al., 2002; Ortinski et al., 2010) in the hilar region. In clear contrast to wild-type AAV2, AAV4 and AAV6, a two- to fivefold higher fraction of cells infected by AAV r3.45 expressed the markers nestin and Sox2 (Fig. 2A-C; supplementary material Fig. S1A). Specifically, ∼65% of the cells infected by AAV r3.45 expressed nestin and Sox2, as compared with ∼33% NeuN-expressing neurons and 1% Gfap- and S100β-expressing glia (Fig. 2B), demonstrating selective infectivity in the brain. AAV r3.45 also infected a 1.5- to threefold larger fraction of the resident Type 1 (nestin+/radial morphology) and Type 2a (nestin+/Sox2+) neural stem and progenitor cells in the subgranular zone (∼41% and ∼60%, respectively) than any wild-type AAV serotypes tested, indicating that it is also capable of efficient NSC transduction in vivo (Fig. 2D).
Fig. 2.
Selectivity towards and infectivity of adult NSCs in the rat brain. (A) Representative images at low (main, 20×) and high (inset, 100×) magnification of the rat dentate gyrus 3 weeks post-injection of recombinant AAV2, AAV4, AAV6 or AAV r3.45 vectors expressing GFP (green). Brain sections were co-stained for nestin (top row, red) or NeuN (bottom row, red) along with DAPI (blue), and infected cells of each type are marked with arrowheads. Dashed rectangles indicate the regions magnified in the insets. Scale bar: 100 μm. (B) Representative image of a Type 1 NSC infected with AAV r3.45 vector. (C) The percentage of GFP+ cells co-staining for markers of each cell type was quantified to determine the selectivity of each viral vector. (D) The percentage of nestin+/Gfap+ (Type 1) or nestin+/Sox2+ (Type 2a) cells infected by each viral vector was quantified to determine NSC infectivity. Error bars indicate s.d. (n=3); *P<0.05, **P<0.01, ***P<0.005 (ANOVA).
To extend these results to a murine model, GFP-encoding AAV r3.45 was administered to the mouse hippocampus. Three weeks post-administration, ∼82% of cells infected by AAV2 and 67% of cells infected by AAV6 were neurons (Fig. 3A,B; supplementary material Fig. S1B). By comparison, AAV r3.45 selectively transduced murine Type 2a cells relative to wild-type AAV2 and AAV6, although it appeared to transduce neurons and NSCs with similar selectivity (Fig. 3A,B). However, NSCs continuously undergo proliferation and differentiation into neurons over a period of days to weeks (Ashton et al., 2012; Kim et al., 2012a), and it is thus possible that GFP+ neurons visualized 3 weeks after injection originated from cells that were NSCs at the time of AAV administration. To determine whether the difference in NSC versus neuronal transduction between mouse and rat could be attributed to the more rapid timescale for stem cell differentiation in mice (Duan et al., 2008), a second cohort of animals received BrdU to label proliferating stem cells following injection of AAV r3.45. Of the infected neurons present 3 weeks post-injection, a significantly higher percentage were BrdU+ in mice administered with AAV r3.45 than in mice administered AAV2 or AAV6 (Fig. 3C), indicating that these cells may have been stem and progenitor cells at the time of infection.
Fig. 3.
Selectivity towards and infectivity of adult NSCs in the mouse brain. (A) Representative images at low (main, 20×) and high (inset, 100×) magnification of the mouse dentate gyrus 3 weeks post-injection of recombinant AAV2, AAV6 or AAV r3.45 vectors expressing GFP (green). Brain sections were co-stained for nestin (top row, red) or NeuN (bottom row, red) along with DAPI (blue), and infected cells of each type are marked with arrowheads. Dashed rectangles indicate the regions shown at high magnification in the insets. Scale bar: 100 μm. (B) The percentage of GFP+ cells co-staining for markers of each cell type was quantified to determine the selectivity of each viral vector. (C) The percentage of GFP+ cells co-staining for NeuN (a neuronal marker) or NeuN and BrdU (a cell division marker) were analyzed to determine the proportion of GFP+ neurons that had differentiated from infected NSCs prior to sacrifice. (D) The percentage of GFP+ cells co-staining for markers of each cell type was quantified to determine the selectivity of variant AAV r3.45 at 3, 7 and 14 days post-injection. (E) The percentage of nestin+/Sox2+ (Type 2a) cells infected by each viral vector was quantified to determine the infectivity of NSCs. Error bars indicate s.d. (n=3); **P<0.005, ***P<0.001 (ANOVA).
To investigate the timing of AAV transgene expression and NSC differentiation in greater detail, hippocampi were analyzed 3 days, 1 week and 2 weeks post-injection with AAV r3.45. At each of these three earlier time points, a significantly higher percentage of NSCs was infected relative to neurons (Fig. 3D), offering additional evidence that AAV r3.45 preferentially infects NSCs in the mouse hippocampus. Consistent with the results from administration to rats, AAV r3.45 infected ∼38% of mouse NSCs in the subgranular zone in vivo, a larger percentage than with the wild-type AAV serotypes tested, showing that efficient transduction of NSCs is conserved across rodent species (Fig. 3E).
β-catenin increases neurogenesis through both proliferation and differentiation of NSCs
NSCs are regulated by cues from the environment, including growth factors, morphogens, neurotransmitters and other signals (Faigle and Song, 2013). For example, the Wnt pathway generally functions in cell-cell communication in both the embryo and adult and has been shown to play a role in stem cell proliferation and differentiation during development and healing (Logan and Nusse, 2004). The canonical Wnt pathway involves the stabilization of β-catenin, which then translocates to the nucleus to act as a transcriptional co-factor of key transcriptional targets. Previous work determined that the canonical Wnt pathway elevates the number of newborn neurons, although the study did not investigate whether this pathway did so via regulation of proliferation or differentiation of NSCs (Lie et al., 2005). Subsequent studies showed through lineage tracing of Wnt-responsive NSCs in Axin2CreERT2 transgenic mice, Wnt7a knockout mice, and lentivirus-mediated delivery of a β-catenin inhibitor to mouse NSCs in vivo that the Wnt pathway regulates the proliferation of NSCs (Bowman et al., 2013; Qu et al., 2010). However, recent work demonstrates that ephrin B2 signals through β-catenin, the activation of which induces neuronal lineage commitment of NSCs in vivo (Ashton et al., 2012).
Based on the ability of AAV r3.45 to efficiently and selectively infect NSCs in the mouse hippocampus, we delivered constitutively active β-catenin (CA β-catenin) to NSCs in the mouse hippocampus to study its mechanism of action. Mice were injected with EdU to label proliferating cells 3 days post-AAV administration, at the approximate time when gene expression from the AAV r3.45 vectors is initiated (based on GFP expression in Fig. 3D). Analysis 18 days later showed significant increases in the number of infected cells per hippocampus that were EdU+ (15.00±1.12 cells), EdU+/nestin+ (7.17±0.17 cells), EdU+/Dcx+ (4.56±0.25 cells) and EdU+/NeuN+ (3.28±0.69 cells) (Fig. 4) in the mice administered AAV r3.45 expressing CA β-catenin, as compared with the number of infected cells per hippocampus that were EdU+ (6.89±0.94 cells), EdU+/nestin+ (4.17±0.17 cells), EdU+/Dcx+ (2.28±0.59 cells) and EdU+/NeuN+ (0.44±0.19 cells) when administered control, GFP-encoding AAV r3.45. The EdU+ counts indicate that CA β-catenin acts to increase proliferation of the NSC population. Furthermore, the increase in the number of infected cells that were EdU+/NeuN+ was much greater than the increase in infected cells that were EdU+/nestin+ and EdU+/Dcx+, which indicates that β-catenin may also induce differentiation (Fig. 4C).
Fig. 4.

Proliferation and differentiation of NSCs induced by CA β-catenin. (A,B) Representative images at low (20×) magnification of the mouse dentate gyrus 3 weeks post-injection of recombinant AAV r3.45 vectors expressing (A) GFP (green) or (B) CA β-catenin-2×FLAG (green). Brain sections were co-stained for NeuN (blue) and EdU (red), and infected cells co-staining for NeuN and EdU are marked with arrowheads. Scale bar: 100 μm. (C) Infected cells (FLAG+, β-catenin condition; GFP+, control condition) co-staining for EdU (a cell division marker), nestin (an NSC marker), Dcx (an immature neuronal marker) and/or NeuN (a neuronal marker) were analyzed to determine the degree to which β-catenin stimulates the proliferation and differentiation of NSCs. Error bars indicate s.d. (n=3); *P<0.001 (ANOVA).
These data suggest that the Wnt pathway functions through both proliferation and differentiation mechanisms to induce neurogenesis, consistent with reports that propose a proliferative and a differentiative role (Ashton et al., 2012; Chen et al., 2013; Israsena et al., 2004; Otero et al., 2004). In addition, this study establishes proof-of-principle that AAV r3.45 can be used to deliver transgenes to study NSC regulation in the hippocampus.
DISCUSSION
Adult NSCs, as defined by their capacity for self-renewal and differentiation into mature neural cell types of the brain, contribute to neurogenesis throughout mammalian life. Selective and efficient gene delivery to NSCs in vivo offers an opportunity to more rapidly study the basic mechanisms that regulate the quiescence, proliferation, self-renewal and differentiation of NSCs in their in vivo environment, and opens potential future avenues to harness NSCs to treat CNS injury or disease. The development of transgenic mouse lines has led to advances in adult neurogenesis research (Ashton et al., 2012; Bonaguidi et al., 2011; Lagace et al., 2007). However, such efforts are labor intensive (Haruyama et al., 2009), and gene delivery by comparison is more rapid and offers translational opportunities (Kotterman and Schaffer, 2014). Prior work in the field established some transduction of neural progenitors, but efficiency was limited and did not include Type 1 NSCs (Falk et al., 2002; Lemkine et al., 2002; van Hooijdonk et al., 2009). AAV vectors have gathered increasing momentum for basic biological investigation (Oh et al., 2014) and for clinical gene delivery (Bainbridge et al., 2008; Kotterman and Schaffer, 2014; MacLaren et al., 2014; Maguire et al., 2008, 2009; Nathwani et al., 2011; Ojala et al., 2015). AAV r3.45 is reportedly capable of efficient transduction of rat, mouse and human NSCs in vitro (Jang et al., 2011). This work further establishes that AAV variants engineered in vitro can also be successful when translated to in vivo models (Klimczak et al., 2009; Koerber et al., 2009).
In an hESC-derived culture containing a mixture of cells, including NSCs, neurons and astrocytes, the percentage of cells infected by AAV r3.45 that were NSCs was significantly higher than with wild-type AAV. Upon characterization of the in vivo properties of AAV r3.45, it was discovered that this variant was also capable of efficient and preferential infection of NSCs in adult rat and mouse brain. Approximately 65% of the cells infected in the rat hippocampus by AAV r3.45 were Type 2a NSCs, and 9% were Type 1 NSCs 3 weeks post-injection. Furthermore, overall 60% of Type 2a NSCs and 41% of Type 1 NSCs were transduced. This trend continued in the mouse brain, where ∼38% of Type 2a NSCs were transduced in the hippocampus 3 weeks post-injection. This level of selectivity already offers utility for investigating NSC function, and could be even further refined with promoters or miRNA-binding elements (Brown and Naldini, 2009; Jessberger et al., 2008).
Previous work to elucidate the mechanism of β-catenin-mediated increased neurogenesis indicated two possible effects: (1) the neuronal differentiation of stem cells and (2) the proliferation of later stage transit amplifying cells. Using AAV r3.45 to deliver CA β-catenin to mouse NSCs in vivo enabled the study of these pathways. Analysis showed significant increases in the number of infected NSCs, neural progenitor cells and neurons, suggesting that the Wnt pathway functions through both proliferation and differentiation mechanisms to induce neurogenesis, consistent with reports demonstrating each role (Ashton et al., 2012; Chen et al., 2013; Israsena et al., 2004; Otero et al., 2004).
In conclusion, AAV r3.45 exhibits efficient and selective transduction of human NSCs in vitro and adult mouse and rat NSCs in vivo. This characterization of AAV r3.45 revealed its potential utility in further studies of neurogenesis in the adult brain and in novel gene therapy and cell replacement therapy applications.
MATERIALS AND METHODS
Virus production
HEK293T cells, obtained from the American Type Culture Collection (Manassas), were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Invitrogen) at 37°C and 5% CO2. Recombinant AAV vectors expressing green fluorescent protein (GFP) or CA β-catenin under the control of a CMV promoter were packaged in HEK293T cells using the calcium phosphate transfection method as described, and the viruses were purified by iodixonal gradient centrifugation and Amicon filtration (Excoffon et al., 2009; Maheshri et al., 2006). DNase-resistant genomic titers were determined by quantitative PCR as previously described (Lai et al., 2002).
In vitro infection of human embryonic stem cell-derived mixed neuronal cultures
H1 human embryonic stem cells (WiCell) were cultured on Matrigel-coated cell culture plates (BD Biosciences) in mTeSR1 maintenance medium (Stem Cell Technologies) for growth and expansion. To initiate cortical differentiation of hESCs, cells were seeded in adherent conditions at a density of 5×104 cells/cm2 in growth medium. At 50% confluence, the medium was gradually changed to NeuroBasal medium (Invitrogen) containing N2 and B27 supplements (Invitrogen). SMAD inhibitors LDN193189 (1 µM, Stemgent) and SB432542 (10 µM, Tocris Biosciences) were added for the first week of neural induction. Cyclopamine (400 ng/ml, Calbiochem) and FGF2 (10 ng/ml, Peprotech) were added on days 3-14 of differentiation. After 12-14 days, cells were mechanically passaged into poly-L-ornithine (Sigma-Aldrich) and laminin (20 µg/ml, Invitrogen) coated plates and allowed to mature for 3-6 weeks. BDNF (10 ng/ml, Peprotech) was added to cultures 1 week after initiation of neuronal maturation.
Cells were infected at a multiplicity of infection (MOI) of ∼10,000 with recombinant AAV2, AAV6 or AAV r3.45 vectors encoding GFP. Forty-eight hours post-infection, cells were fixed in 4% paraformaldehyde for 15 min, washed three times with phosphate-buffered saline (PBS), and blocked with 1% BSA and 0.1% Triton X-100 in PBS for 30 min. Cells were incubated overnight at 4°C with a mouse anti-nestin (1:500; Abcam, ab6142) or mouse anti-MAP2 (1:500; BD Biosciences, 610460) primary antibody. Cells were then washed three times with PBS and incubated with a fluorescent-conjugated donkey anti-mouse secondary antibody (1:250; Invitrogen, 715-545-150) for 2 hours. Cells were imaged using an Axio Observer.A1 inverted microscope (Zeiss). Quantification of infected cells was performed using the Cell Counter function in ImageJ (NIH).
Stereotaxic injections
Animal protocols were approved by the UC Berkeley Animal Care and Use Committee and conducted in accordance with National Institutes of Health guidelines. Recombinant AAV2, AAV4, AAV6 or AAV r3.45 vectors encoding GFP were stereotaxically injected into the hippocampus (AP, −3.5; ML, ±2.0; V/D, −3.5) of 12-week-old female Fischer 344 rats. Animals were anesthetized with ketamine (Butler Animal Health Supply; 68 mg/kg body weight) and xylazine (Lloyd Laboratories; 38 mg/kg body weight) prior to injection, and 3 μl of 5×108 viral genomes (vg)/μl AAV vector per hippocampus was injected using a Hamilton syringe as described (Lai et al., 2002).
In addition, recombinant AAV2, AAV6 or AAV r3.45 vectors encoding GFP were stereotaxically injected into the hippocampus (AP, −2.12; ML, ±1.5; V/D, −1.55) of 9-week-old female BALB/c mice. The animals were anesthetized with ketamine (Butler Animal Health Supply; 50 mg/kg body weight) and xylazine (Lloyd Laboratories; 50 mg/kg body weight) prior to injection, and 1 μl of 1.5×109 vg/μl AAV vector per hippocampus was injected using a Hamilton syringe as described (Lai et al., 2002). Mice were injected with 50 mg/kg 5-bromo-2′-deoxyuridine (BrdU) for 3 consecutive days pre-stereotaxic injection, then injected with 50 mg/kg BrdU every other day until perfusion or injected with 50 mg/kg 5-ethynyl-2′-deoxyuridine (EdU) on days 6-9 post-stereotaxic injection. Three days to 3 weeks post-injection, animals were transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. Brains were post-fixed in 4% paraformaldehyde overnight at 4°C and stored in 30% sucrose for cryoprotection.
Histological processing and immunohistochemistry of brain tissue
Brains were mounted onto a Series 8000 sliding microtome (Bright) with Clear Frozen Section Compound (VWR) and frozen with dry ice. Coronal sections (40 μm) were cut, and sections containing the hippocampus were stored at −20°C prior to immunostaining. Brains were stained as floating sections in a 12-well dish. Tissue sections were washed three times for 15 min each in PBS, then blocked in a solution containing 3% donkey serum and 0.3% Triton X-100 for 2 h at room temperature. After blocking, tissue sections were incubated with primary antibodies for 72 h at 4°C. The primary antibodies and dilutions used were: mouse anti-nestin (1:500; Abcam, ab6142), rabbit anti-Sox2 (1:250; Millipore, AB5603), mouse anti-NeuN (1:100; Millipore, MAB377), guinea pig anti-Dcx (1:1000; Millipore, AB2253), mouse anti-Gfap (1:1000; Advanced ImmunoChemical, 2-GFAP), rabbit anti-Gfap (1:1000; Abcam, ab7260), rabbit anti-S100β (1:1000; Abcam, ab52642) and chicken anti-GFP (1:1000; Abcam, ab13970). Tissue sections were washed again three times for 15 min each in PBS, then blocked in a solution containing 3% donkey serum and 0.3% Triton X-100 for 1 h at room temperature. After blocking, tissue sections were incubated with AffiniPure donkey anti-mouse, rabbit, guinea pig and chicken secondary antibodies (1:250; Jackson ImmunoResearch, 715-545-150, 711-545-152, 706-605-148, 703-605-155, respectively) and 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain (Invitrogen) for 2 h at room temperature. Tissue sections were washed three more times in PBS, then mounted onto slides and coverslipped. Images of the sections were taken using an LSM 710 laser scanning confocal microscope (Zeiss). Quantification of infected cells within the sections was performed using an Axio Imager.M1 microscope and analysis system (Zeiss) and Stereo Investigator analysis software (version 8.26, MBF Bioscience). Cells were scored using the following markers: Type 1 NSCs, nestin+/Gfap+; Type 2a NSCs, nestin+/Sox2+; neurons, NeuN+; astrocytes, Gfap+/S100β+.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceived and designed experiments: M.A.K., T.V. and D.V.S. Performed the experiments: M.A.K. Analyzed the data: M.A.K. and D.V.S. Contributed reagents/materials: T.V. Wrote and edited the manuscript: M.A.K. and D.V.S.
Funding
This work was funded by the National Institutes of Health [R01 NS074831 to D.V.S.] and by a National Science Foundation (NSF) Graduate Fellowship (to M.A.K.). Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.115253/-/DC1
References
- Ashton R. S., Conway A., Pangarkar C., Bergen J., Lim K.-I., Shah P., Bissell M. and Schaffer D. V. (2012). Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat. Neurosci. 15, 1399-1406. 10.1038/nn.3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuri P., Bartel M. A., Vazin T., Jang J.-H., Wong T. B. and Schaffer D. V. (2012). Directed evolution of adeno-associated virus for enhanced gene delivery and gene targeting in human pluripotent stem cells. Mol. Ther. 20, 329-338. 10.1038/mt.2011.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge J. W. B., Smith A. J., Barker S. S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G. E., Stockman A., Tyler N. et al. (2008). Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 358, 2231-2239. 10.1056/NEJMoa0802268 [DOI] [PubMed] [Google Scholar]
- Bartlett J. S., Samulski R. J. and McCown T. J. (1998). Selective and rapid uptake of adeno-associated virus type 2 in brain. Hum. Gene Ther. 9, 1181-1186. 10.1089/hum.1998.9.8-1181 [DOI] [PubMed] [Google Scholar]
- Blesch A., Lu P. and Tuszynski M. H. (2002). Neurotrophic factors, gene therapy, and neural stem cells for spinal cord repair. Brain Res. Bull. 57, 833-838. 10.1016/S0361-9230(01)00774-2 [DOI] [PubMed] [Google Scholar]
- Bonaguidi M. A., Wheeler M. A., Shapiro J. S., Stadel R. P., Sun G. J., Ming G.-l. and Song H. (2011). In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145, 1142-1155. 10.1016/j.cell.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A. N., van Amerongen R., Palmer T. D. and Nusse R. (2013). Lineage tracing with Axin2 reveals distinct developmental and adult populations of Wnt/β-catenin-responsive neural stem cells. Proc. Natl. Acad. Sci. USA 110, 7324-7329. 10.1073/pnas.1305411110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. D. and Naldini L. (2009). Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat. Rev. Genet. 10, 578-585. 10.1038/nrg2628 [DOI] [PubMed] [Google Scholar]
- Chen B.-Y., Wang X., Wang Z.-Y., Wang Y.-Z., Chen L.-W. and Luo Z.-J. (2013). Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/β-catenin signaling pathway. J. Neurosci. Res. 91, 30-41. 10.1002/jnr.23138 [DOI] [PubMed] [Google Scholar]
- Deng W., Aimone J. B. and Gage F. H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339-350. 10.1038/nrn2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X., Kang E., Liu C. Y., Ming G.-l. and Song H. (2008). Development of neural stem cell in the adult brain. Curr. Opin. Neurobiol. 18, 108-115. 10.1016/j.conb.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A., Alkass K., Bernard S., Salehpour M., Perl S., Tisdale J., Possnert G., Druid H. and Frisén J. (2014). Neurogenesis in the striatum of the adult human brain. Cell 156, 1072-1083. 10.1016/j.cell.2014.01.044 [DOI] [PubMed] [Google Scholar]
- Excoffon K. J. D. A., Koerber J. T., Dickey D. D., Murtha M., Keshavjee S., Kaspar B. K., Zabner J. and Schaffer D. V. (2009). Directed evolution of adeno-associated virus to an infectious respiratory virus. Proc. Natl. Acad. Sci. USA 106, 3865-3870. 10.1073/pnas.0813365106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faigle R. and Song H. (2013). Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta 1830, 2435-2448. 10.1016/j.bbagen.2012.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk A., Holmström N., Carlén M., Cassidy R., Lundberg C. and Frisén J. (2002). Gene delivery to adult neural stem cells. Exp. Cell Res. 279, 34-39. 10.1006/excr.2002.5569 [DOI] [PubMed] [Google Scholar]
- Flotte T. R. (2004). Gene therapy progress and prospects: recombinant adeno-associated virus (rAAV) vectors. Gene Ther. 11, 805-810. 10.1038/sj.gt.3302233 [DOI] [PubMed] [Google Scholar]
- Gage F. H. (2000). Mammalian neural stem cells. Science 287, 1433-1438. 10.1126/science.287.5457.1433 [DOI] [PubMed] [Google Scholar]
- Guo W., Allan A. M., Zong R., Zhang L., Johnson E. B., Schaller E. G., Murthy A. C., Goggin S. L., Eisch A. J., Oostra B. A. et al. (2011). Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat. Med. 17, 559-565. 10.1038/nm.2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruyama N., Cho A. and Kulkarni A. B. (2009). Overview: engineering transgenic constructs and mice. Curr. Protoc. Cell Biol. Chapter 19, Unit 19.10 10.1002/0471143030.cb1910s42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M. and Mikoshiba K. (2004). Neuronal birthdate-specific gene transfer with adenoviral vectors. J. Neurosci. 24, 286-296. 10.1523/JNEUROSCI.2529-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israsena N., Hu M., Fu W., Kan L. and Kessler J. A. (2004). The presence of FGF2 signaling determines whether beta-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev. Biol. 268, 220-231. 10.1016/j.ydbio.2003.12.024 [DOI] [PubMed] [Google Scholar]
- Jang J.-H., Koerber J. T., Kim J.-S., Asuri P., Vazin T., Bartel M., Keung A., Kwon I., Park K. I. and Schaffer D. V. (2011). An evolved adeno-associated viral variant enhances gene delivery and gene targeting in neural stem cells. Mol. Ther. 19, 667-675. 10.1038/mt.2010.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaski B. E., Jessup M. L., Mancini D. M., Cappola T. P., Pauly D. F., Greenberg B., Borow K., Dittrich H., Zsebo K. M. and Hajjar R. J. (2009). Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J. Card. Fail. 15, 171-181. 10.1016/j.cardfail.2009.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S., Toni N., Clemenson G. D. Jr, Ray J. and Gage F. H. (2008). Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat. Neurosci. 11, 888-893. 10.1038/nn.2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar B. K., Vissel B., Bengoechea T., Crone S., Randolph-Moore L., Muller R., Brandon E. P., Schaffer D., Verma I. M., Lee K.-F. et al. (2002). Adeno-associated virus effectively mediates conditional gene modification in the brain. Proc. Natl. Acad. Sci. USA 99, 2320-2325. 10.1073/pnas.042678699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. R., Christian K., Ming G.-L. and Song H. (2012a). Time-dependent involvement of adult-born dentate granule cells in behavior. Behav. Brain Res. 227, 470-479. 10.1016/j.bbr.2011.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-S., Chu H. S., Park K. I., Won J.-I. and Jang J.-H. (2012b). Elastin-like polypeptide matrices for enhancing adeno-associated virus-mediated gene delivery to human neural stem cells. Gene Ther. 19, 329-337. 10.1038/gt.2011.84 [DOI] [PubMed] [Google Scholar]
- Klimczak R. R., Koerber J. T., Dalkara D., Flannery J. G. and Schaffer D. V. (2009). A novel adeno-associated viral variant for efficient and selective intravitreal transduction of rat Müller cells. PLoS ONE 4, e7467 10.1371/journal.pone.0007467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M. and Howley P. M. (2007). Fields’ Virology. Philadelphia, PA, USA: Lippincott Williams & Wilkins. [Google Scholar]
- Koerber J. T., Jang J.-H. and Schaffer D. V. (2008). DNA shuffling of adeno-associated virus yields functionally diverse viral progeny. Mol. Ther. 16, 1703-1709. 10.1038/mt.2008.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber J. T., Klimczak R., Jang J.-H., Dalkara D., Flannery J. G. and Schaffer D. V. (2009). Molecular evolution of adeno-associated virus for enhanced glial gene delivery. Mol. Ther. 17, 2088-2095. 10.1038/mt.2009.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotterman M. A. and Schaffer D. V. (2014). Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 15, 445-451. 10.1038/nrg3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace D. C., Whitman M. C., Noonan M. A., Ables J. L., DeCarolis N. A., Arguello A. A., Donovan M. H., Fischer S. J., Farnbauch L. A., Beech R. D. et al. (2007). Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J. Neurosci. 27, 12623-12629. 10.1523/JNEUROSCI.3812-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K., Kaspar B. K., Gage F. H. and Schaffer D. V. (2002). Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 6, 21-27. 10.1038/nn983 [DOI] [PubMed] [Google Scholar]
- Lemkine G. F., Mantero S., Migné C., Raji A., Goula D., Normandie P., Levi G. and Demeneix B. A. (2002). Preferential transfection of adult mouse neural stem cells and their immediate progeny in vivo with polyethylenimine. Mol. Cell. Neurosci. 19, 165-174. 10.1006/mcne.2001.1084 [DOI] [PubMed] [Google Scholar]
- Lie D.-C., Colamarino S. A., Song H.-J., Désiré L., Mira H., Consiglio A., Lein E. S., Jessberger S., Lansford H., Dearie A. R. et al. (2005). Wnt signalling regulates adult hippocampal neurogenesis. Nature 437, 1370-1375. 10.1038/nature04108 [DOI] [PubMed] [Google Scholar]
- Logan C. Y. and Nusse R. (2004). The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781-810. 10.1146/annurev.cellbio.20.010403.113126 [DOI] [PubMed] [Google Scholar]
- Lugert S., Basak O., Knuckles P., Haussler U., Fabel K., Götz M., Haas C. A., Kempermann G., Taylor V. and Giachino C. (2010). Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 6, 445-456. 10.1016/j.stem.2010.03.017 [DOI] [PubMed] [Google Scholar]
- MacLaren R. E., Groppe M., Barnard A. R., Cottriall C. L., Tolmachova T., Seymour L., Clark K. R., During M. J., Cremers F. P. M., Black G. C. M. et al. (2014). Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet 6736, 2117-2120. 10.1016/S0140-6736(13)62117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A. M., Simonelli F., Pierce E. A., Pugh E. N. Jr, Mingozzi F., Bennicelli J., Banfi S., Marshall K. A., Testa F., Surace E. M. et al. (2008). Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 358, 2240-2248. 10.1056/NEJMoa0802315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A. M., High K. A., Auricchio A., Wright J. F., Pierce E. A., Testa F., Mingozzi F., Bennicelli J. L., Ying G.-S., Rossi S. et al. (2009). Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet 374, 1597-1605. 10.1016/S0140-6736(09)61836-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshri N., Koerber J. T., Kaspar B. K. and Schaffer D. V. (2006). Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat. Biotechnol. 24, 198-204. 10.1038/nbt1182 [DOI] [PubMed] [Google Scholar]
- Manno C. S., Chew A. J., Hutchison S., Larson P. J., Herzog R. W., Arruda V. R., Tai S. J., Ragni M. V., Thompson A., Ozelo M. et al. (2003). AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 101, 2963-2972. 10.1182/blood-2002-10-3296 [DOI] [PubMed] [Google Scholar]
- Manno C. S., Pierce G. F., Arruda V. R., Glader B., Ragni M., Rasko J. J. E., Ozelo M. C., Hoots K., Blatt P., Konkle B. et al. (2006). Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 12, 342-347. 10.1038/nm1358 [DOI] [PubMed] [Google Scholar]
- Ming G.-l. and Song H. (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687-702. 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira H., Andreu Z., Suh H., Lie D. C., Jessberger S., Consiglio A., San Emeterio J., Hortigüela R., Marqués-Torrejón M. Á., Nakashima K. et al. (2010). Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7, 78-89. 10.1016/j.stem.2010.04.016 [DOI] [PubMed] [Google Scholar]
- Moss R. B., Milla C., Colombo J., Accurso F., Zeitlin P. L., Clancy J. P., Spencer L. T., Pilewski J., Waltz D. A., Dorkin H. L. et al. (2007). Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum. Gene Ther. 18, 726-732. 10.1089/hum.2007.022 [DOI] [PubMed] [Google Scholar]
- Nathwani A. C., Tuddenham E. G. D., Rangarajan S., Rosales C., McIntosh J., Linch D. C., Chowdary P., Riddell A., Pie A. J., Harrington C. et al. (2011). Adenovirus-associated virus vector–mediated gene transfer in Hemophilia B. N. Engl. J. Med. 365, 2357-2365. 10.1056/NEJMoa1108046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. W., Harris J. A., Ng L., Winslow B., Cain N., Mihalas S., Wang Q., Lau C., Kuan L., Henry A. M. et al. (2014). A mesoscale connectome of the mouse brain. Nature 508, 207-214. 10.1038/nature13186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala D. S., Amara D. P. and Schaffer D. V. (2015). Adeno-associated virus vectors and neurological gene therapy. Neuroscientist 21, 84-98. 10.1177/1073858414521870 [DOI] [PubMed] [Google Scholar]
- Ortinski P. I., Dong J., Mungenast A., Yue C., Takano H., Watson D. J., Haydon P. G. and Coulter D. A. (2010). Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat. Neurosci. 13, 584-591. 10.1038/nn.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero J. J., Fu W., Kan L., Cuadra A. E. and Kessler J. A. (2004). Beta-catenin signaling is required for neural differentiation of embryonic stem cells. Development 131, 3545-3557. 10.1242/dev.01218 [DOI] [PubMed] [Google Scholar]
- Qu Q., Sun G., Li W., Yang S., Ye P., Zhao C., Yu R. T., Gage F. H., Evans R. M. and Shi Y. (2010). Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat. Cell Biol. 12, 31-40. 10.1038/ncb2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer D. V., Koerber J. T. and Lim K.-l. (2008). Molecular engineering of viral gene delivery vehicles. Annu. Rev. Biomed. Eng. 10, 169-194. 10.1146/annurev.bioeng.10.061807.160514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H., Deng W. and Gage F. H. (2009). Signaling in adult neurogenesis. Annu. Rev. Cell Dev. Biol. 25, 253-275. 10.1146/annurev.cellbio.042308.113256 [DOI] [PubMed] [Google Scholar]
- Temple S. (2001). The development of neural stem cells. Nature 414, 112-117. 10.1038/35102174 [DOI] [PubMed] [Google Scholar]
- van Hooijdonk L. W. A., Ichwan M., Dijkmans T. F., Schouten T. G., de Backer M. W. A., Adan R. A. H., Verbeek F. J., Vreugdenhil E. and Fitzsimons C. P. (2009). Lentivirus-mediated transgene delivery to the hippocampus reveals sub-field specific differences in expression. BMC Neurosci. 10, 2 10.1186/1471-2202-10-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. A., Nepomuceno I. B., Messner A. H., Moran M. L., Batson E. P., Dimiceli S., Brown B. W., Desch J. K., Norbash A. M., Conrad C. K. et al. (2002). A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum. Gene Ther. 13, 1349-1359. 10.1089/104303402760128577 [DOI] [PubMed] [Google Scholar]
- Wu Z., Asokan A. and Samulski R. J. (2006). Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol. Ther. 14, 316-327. 10.1016/j.ymthe.2006.05.009 [DOI] [PubMed] [Google Scholar]
- Zincarelli C., Soltys S., Rengo G. and Rabinowitz J. E. (2008). Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 16, 1073-1080. 10.1038/mt.2008.76 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.