Abstract
Aim: This observational study evaluated the use of a novel, ultraportable, mechanically powered topical negative pressure device in promoting healing in chronic wounds, including venous and mixed etiology leg ulcers and neuropathic foot ulcers.
Materials and Methods: Evaluable patients (n=37) received treatment with the SNaP® Wound Care System for up to 6 weeks. The primary objective was percentage change in wound size, with secondary objectives of clinical assessment of wound parameters, ease of use, and impact on quality of life.
Results: A 42.64% mean percentage decrease in wound area was observed, with an overall decrease for each wound etiology subgroup. Increased granulation tissue, decreased exudate levels, and decreased wound pain were reported. Quality-of-life scores increased overall, and the device was easy to use, comfortable, portable, and inconspicuous.
Conclusion: The SNaP Wound Care System has the potential to promote healing in chronic wounds of different etiologies.

Neal Walkley, PhD, BSc
Introduction
Topical negative pressure (TNP) therapy, an approach that employs continuous suction to the wound site to promote healing, is increasingly being used in the management of acute and chronic wounds.1,2 Since the introduction of the concept, there have been a number of devices that have been developed to provide continuous suction. However, the basic principles of TNP, using a foam or gauze wound contact layer in which tubing that is connected to the suction device is placed under an occlusive dressing seal, remains fundamentally similar for all devices.3
TNP removes excess moisture from the wound bed and in doing so reduces local interstitial fluid pooling. In chronic wounds, theoretically, this is associated with the removal of proinflammatory cytokines, which are believed to be important in maintaining a nonhealing chronic state.4–6 Moreover, rather akin to the heated suction cups on intact skin used in the early part of the last century, the use of TNP on wounds is associated with an increase in local blood flow.3,7,8 Studies in animals3,9 and humans8 have demonstrated between a four- and five-fold increase in tissue blood perfusion by laser Doppler with TNP, although at suction pressures of 400 mmHg and above, tissue blood perfusion may be inhibited. Many mesenchymal cells, including endothelial cells (cells that give rise to blood vessels), respond to mechanical forces, and therefore, it is assumed that this then drives blood vessel growth in tissues in response to TNP.10,11 However, direct evidence to support TNP-associated angiogenesis through induction of angiogenic factor, such as vascular endothelial growth factor, is lacking.
The most notable clinical effect of TNP on wound healing has been the rapid accumulation of granulation tissue within the wound bed.1,2 TNP therapy has revolutionized the treatment of open abdominal wounds to achieve delayed primary closure with fascia or to accelerate granulation tissue formation before skin grafting.12 But there remain clinical challenges to its use in this setting, such as retention of dressing13 and formation of fistulae.14 Yet, there is widespread acceptance of the use of TNP in this setting, due to the rapid accumulation of granulation tissue and reduction in wound volume and surface area.15 Similarly, TNP is used to promote rapid granulation tissue after trauma resulting in loss of soft tissue as well as other surgical acute wound settings.1,2
Over 1% of the population has an open wound at any one time. A proportion of these will be associated with diseases that hinder healing and render them chronic.16 At any one time, there are an estimated 200,000 UK individuals with chronic wounds, including 1 in 7 diabetics who develop diabetic foot ulceration, 0.3% of the population with chronic lower leg ulceration, and ∼5% of hospital inpatients who develop pressure ulcers.17–20 While many of these patients respond to conventional care, approximately one quarter have persisting wounds that take months or even years to heal, if they heal at all, regardless of the etiological basis of their chronic ulcerating disease.21 While overall healing rates compared to conventional care may not differ, where TNP has been clinically found to be useful is the rate at which healthy granulation tissue can be induced in the wound when compared to conventional care.22 However, TNP has not to date demonstrated sufficient evidence that shows greater efficacy at healing chronic wounds compared to conventional treatments.23,24 As alluded to earlier, TNP appears very effective at inducing wound bed granulation and tissue perfusion but may act as a hindrance to re-epithelialization. Hence, the benefits of TNP in chronic wounds need still to be characterized. One obvious hypothesis would be that TNP in chronic wounds can induce healthy granulation tissue that is free of the detrimental effects of inflammation-associated proteases.
Classical TNP devices are powered by battery sources and as such are quite bulky and expensive. Recently, a new TNP device was launched that creates suction through a novel spring-loaded mechanism, resulting in a relatively simpler, fully disposable, and easily transportable device (Fig. 1). The device, called the SNaP® Wound Care System (Spiracur, Sunnyvale, CA), thus far has demonstrated similar efficacy in acute and chronic wounds as conventional TNP devices and when compared to standard modern dressing therapies.25–28 A multicenter RCT29,30 comparing the effectiveness of mechanical versus electrically powered negative pressure wound therapy devices demonstrated similar wound healing outcomes, such as wound area reduction, closure, and incidence of infection, between the two systems. Since many wounds start small and become larger, the SNaP device was specifically designed to actively treat small wounds before they increased in size and become more difficult to manage.
Figure 1.
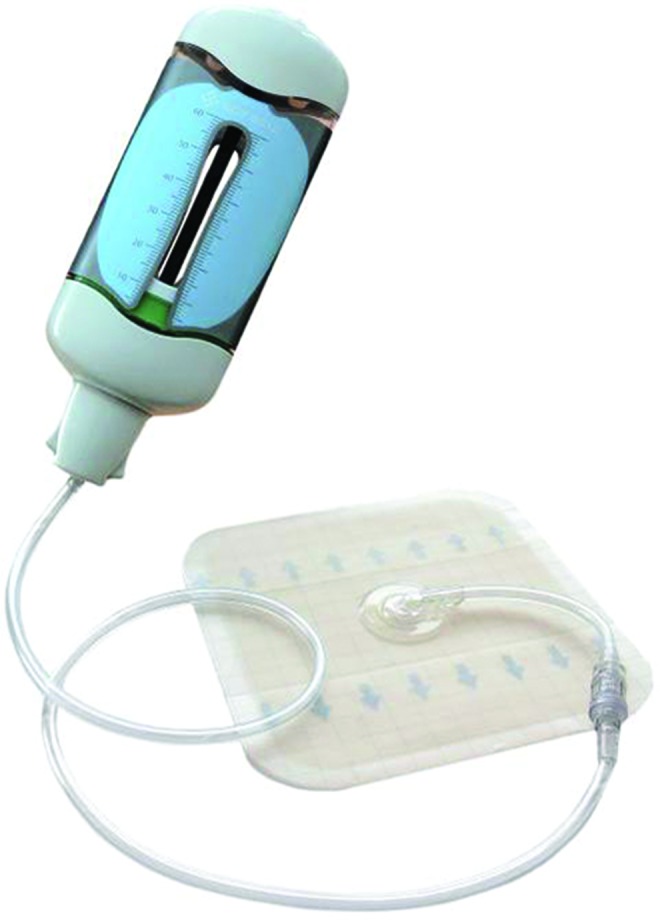
Topical negative pressure device that is battery independent, called the SNaP® Wound Care System. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
In this observational study to explore the effects of the system on healing and other aspects of chronic wounds, we aimed to test the efficacy of the SNaP Wound Care System TNP in the management of chronic wounds: venous leg ulcers, mixed etiology leg ulcers, and diabetic or nondiabetic neuropathic foot ulcers. South East Wales Research Ethics Committee gave approval on May 20, 2011.
Materials and Methods
A single-center prospective clinical study was performed with the primary objective of evaluating the effect of the SNaP Wound Care System on the percentage change in wound size for all treated patients over a 6-week period. Secondary objectives included ease of application and removal, assessment of clinical features such as wound bed condition, levels of exudate, odor and pain, and condition of surrounding skin, plus impact on quality of life.
As this was a pilot study looking at the effect of TNP patients with neuropathic foot ulceration, venous or mixed etiology lower limb ulceration of >30 days of duration and with a surface area between 1 and 100 cm2 were included in the trial. The wound had to be located in an area amenable to creation of an airtight seal around it using an adhesive dressing. Patients with wound infection on the day of inclusion, uncontrolled diabetes (HbA1C >12%), ulcers due to inflammatory diseases, or untreated osteomyelitis were excluded. The SNaP Wound Care System was applied two to three times weekly at the center's research clinic, and the time to apply the device was recorded. The device remained in place 24 h a day until the next clinic visit. Appropriate standard care was continued during the study period, such as compression for venous leg ulcer disease and offloading for the neuropathic foot ulceration. Depending on the level of arterial disease, an appropriate level of compression was applied to patients with a mixed venous–arterial etiology. The subjects were included in the study for 8–6 weeks of treatment with TNP and a 2-week follow-up visit. TNP was only applied to the wound while, in the opinion of the treating clinician, further treatment was required. If the wound became too small, an appropriate primary dressing was applied in place of the SNaP system and the patient followed up at the end of week 6.
The SNaP Wound Care System is able to deliver three different levels of negative pressure (−75, −100, and −125 mmHg) depending on the cartridge selected. The selection of the cartridge level was made by the treating clinician on an individual patient basis with consideration primarily of wound type and pain levels. The highest level of cartridge that was considered tolerable for the patient was initially applied. As negative pressure should never be painful for the patient, if any discomfort was experienced, a lower negative pressure cartridge was applied or treatment with SNaP discontinued if already on the −75 mmHg cartridge. Dressing changes were performed two to three times weekly depending on exudate levels and condition of the surrounding skin. When necessary, a barrier film (SurePrep) was applied to the surrounding skin before application of the device as protection from the adhesive dressing. While subjects could not be entered into the study if they had active infection, if the patient developed an infection during the study period, oral antibiotic therapy and/or topical antibacterial/antimicrobial agents were permitted. The SNaP TNP therapy could be discontinued temporarily if required while there was active infection and then restarted once infection had resolved.
Wound size was measured once weekly at the assessment visit using the Visitrak™ wound measurement device. Digital photographs were also taken at these visits. Pain experience was measured at each assessment visit using a numerical rating scale (NRS), whereby subjects were asked for a number between 1 and 10 (where 1=No pain and 10=Extreme pain), which best described the level of pain they were currently experiencing. All assessments were conducted by experienced wound care specialist research nurses. The photographs were used by an independent monitor to verify the clinician's measurements, such as wound size and condition of wound bed.
The Cardiff Wound Impact Schedule (CWIS) questionnaire was used as the data collection tool for the assessment of quality of life, along with a study exit questionnaire completed by the patient at the end of the treatment period, which covered areas such as function, comfort, and ease of use.
Results
A total of 38 patients were recruited to the study over a period of 20 months, of which 37 were evaluable (22 males and 15 females). An evaluable was defined as anyone who had received 2 weeks of topic TNP treatment. Thirty-four evaluable patients were followed for up to 6 weeks. However, four discontinued treatment shortly after the 2-week evaluable period and exited the study. The mean age for the entire cohort was 64.35 years (range 33–87 years, standard deviation [SD] 14.225). Of the evaluable patients, 33 subjects (89.2%) completed the full study and 4 (10.8%) subjects were withdrawn. One subject withdrew themselves, two subjects were withdrawn by the physician (one to be referred to vascular surgery and one due to maceration), and one withdrew for medical reasons (allergic to dressing). Analysis of the results was carried out using the Intention-to-Treat approach, hence all patients' data were included.
Of the evaluable patients, 15 had venous ulcers, 13 had mixed etiology ulcers, and 9 had neuropathic foot ulcers. The mean wound age for the entire cohort was 18.8 months (range 1–252 months).
Primary outcome
The mean surface area measurement of the target ulcer at baseline was 9.5 cm2 (range 1.2–39.0 cm2, SD 8.231). A breakdown of the mean ulcer area by wound type can be seen in Table 1.
Table 1.
Mean ulcer area and range by wound type
| Ulcer Area (cm2) | |||||
|---|---|---|---|---|---|
| Mean | Min | Max | Range | Standard Deviation | |
| Neuropathic foot ulcer | 11.1 | 1.7 | 20.7 | 19.0 | 8.076 |
| Mixed etiology ulcer | 11.9 | 1.2 | 39.0 | 37.8 | 10.341 |
| Venous ulcer | 6.4 | 1.3 | 17.2 | 15.9 | 5.292 |
| Total | 9.5 | 1.2 | 39.0 | 37.8 | 8.231 |
Tables 2 and 3 indicate the changes in wound size for the whole group and stratified according to etiology. In terms of the primary outcome, there was a 42.64% decrease in wound area overall, and an overall mean reduction in area across each of the subgroups.
Table 2.
Breakdown of wounds that increased or decreased in size, or remained the same, by wound type between week 1 and week 8 (or the final visit if sooner)
| Decreased | Increased | Remained Same | Total | |
|---|---|---|---|---|
| Neuropathic foot ulcer | 8 | 0 | 1 | 9 |
| Mixed etiology ulcer | 9 | 4 | 0 | 13 |
| Venous ulcer | 13 | 2 | 0 | 15 |
| Total | 30 | 6 | 1 | 37 |
Table 3.
Breakdown of mean increase or decrease in wound area by wound type between week 1 and week 8 (or the final visit if sooner)
| Mean% Decrease | Min (%) | Max (%) | |
|---|---|---|---|
| Neuropathic foot ulcer | 55.14 | 0.00 | 95.28 |
| Mixed etiology ulcer | 9.60 | −141.67 | 95.58 |
| Venous ulcer | 63.76 | −21.05 | 100.00 |
| Total | 42.64 | −141.67 | 100.00 |
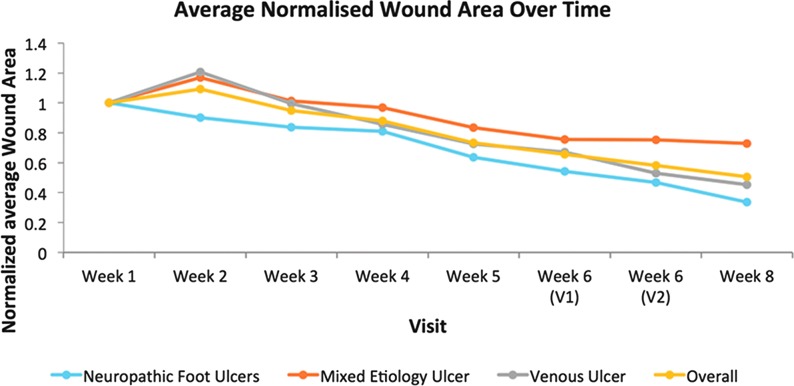
The overall change in the normalized average wound size is shown in Fig. 2, both for the whole cohort and stratified according to etiology. This shows clearly the trend for decreasing wound size throughout the study for all groups.
Figure 2.
Chart showing the normalized average wound size for all study groups over the 8-week study period. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Secondary outcomes
Clinical assessment of wound healing parameters
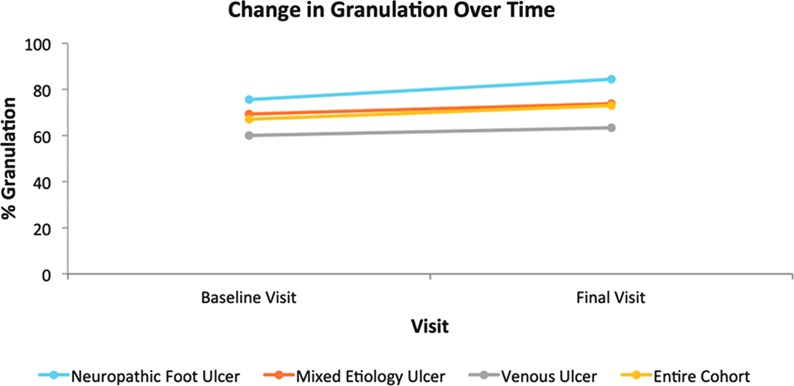
The mean percentage change in granulation tissue present in the wound bed at the baseline visit to the final visit shows an overall increase for the entire cohort (Fig. 3). The biggest mean percentage increase in granulation tissue by cohort was seen in the neuropathic foot ulcer group.
Figure 3.
Schematic representation of the change in percentage granulation tissue over the 8-week treatment period. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The venous leg ulcer group displayed little mean change in the percentage of granulation tissue across the whole group from initial to final visits, although it should be remembered that three patients in this subgroup achieved complete epithelialization and therefore would not have had percentage granulation tissue captured on their final visit. The assessment of granulation tissue was determined, subjectively, as a percentage of the tissue present in the wound bed and was calculated independently by two experienced wound care nurses and the mean value recorded.
Infection
Fifteen patients experienced wound infection and treatment was suspended until the infection resolved. The maximum suspension of treatment was 2 weeks. Although this affected 41% of the evaluable patients, the findings are reflective of the incidence of infection generally observed in patients with chronic hard to heal wounds.
Pain
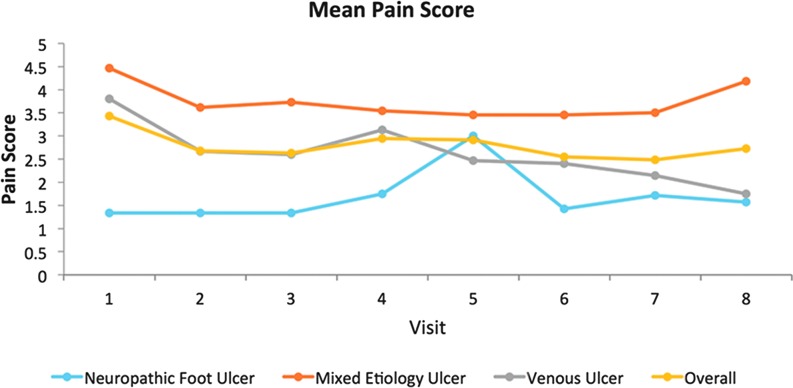
The results of the NRS performed by each patient at their weekly assessment visit generally indicate that mean pain scores decreased for the entire cohort from baseline to week 8 (Fig. 4). The only group to record an increase in mean pain score during the study was the neuropathic foot ulcer group, although the difference at weeks 1 and 8 was minimal. Variations occurred in pain scores throughout the study, as would be expected when there are incidences of infection, for example. The graph indicates that the mixed etiology group experienced the most pain on average of all the groups, with an increase in pain in the final week, but it is important to note that this reported pain occurred once the device had been removed at week 6.
Figure 4.
Graph depicting change in mean pain score from baseline to the end of the study. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
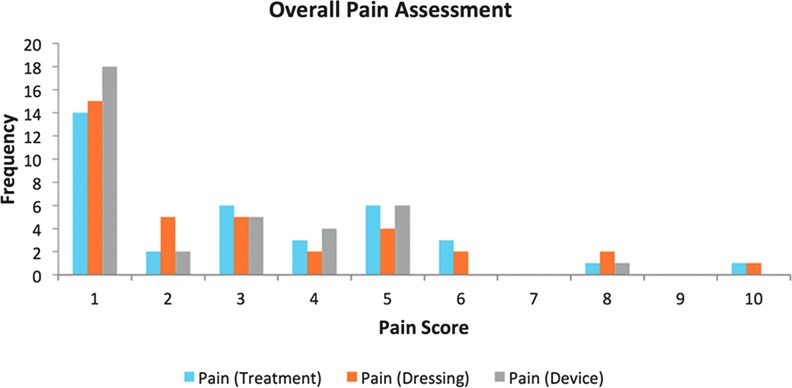
Results of the exit survey regarding pain during treatment, at dressing change, and with general device wear highlight that by far the largest number of patients reported no pain (Pain Score 1) for all three subgroups during the study overall (Fig. 5). One patient recorded a pain score of 10 on the exit survey during treatment and dressing changes, but not for general device wear.
Figure 5.
Pain score during treatment, dressing change, and general device wear as reported on exit survey. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Levels of exudate
Exudate was recorded as none, mild, moderate, or severe during assessment visits. Changes in exudate levels varied during the study, as would be expected, but results suggest when comparing recorded exudate level at the first and last visit that the use of the SNaP device did not cause a significant increase (Table 4). The majority of subjects either experienced decreased or unchanged exudate levels when using the device.
Table 4.
Wound exudate over time by wound type as a percentage of the total number that experienced exudate (N = 37) (first and last visits compared)
| Increased | % of Total | Decreased | % of Total | Same | % of Total | |
|---|---|---|---|---|---|---|
| Neuropathic foot ulcer | 0 | 0.0 | 5 | 13.5 | 4 | 10.8 |
| Mixed etiology ulcer | 1 | 2.7 | 4 | 10.8 | 8 | 21.6 |
| Venous ulcer | 1 | 2.7 | 8 | 21.6 | 6 | 16.2 |
| Total | 2 | 5.4 | 17 | 45.9 | 18 | 48.6 |
Odor
One patient was recorded as having odor at the baseline visit, which was resolved at the final visit. Only one patient was recorded as having odor present at the final visit when there had previously been no odor. Eight patients developed odor while using the device between 1 and 5 weeks, but this was resolved before discontinuation of SNaP therapy.
Condition of surrounding skin
Of the 66 adverse events recorded as having at least a possible relationship with the device, 32 (48%) were related to the surrounding skin (allergic reaction, blistering, eczema, maceration). Also, 10 of the adverse events related to the development of new wounds possibly attributed to the device were often secondary to the initial event, such as blistering or eczema.
Overall, the results indicate that the condition of the surrounding skin was much more likely to deteriorate in the leg ulcer groups than the foot ulcer group (26 adverse events [AEs] vs. 6 AEs). There was a much higher incidence of eczema in particular.
Ease of application
Ease of application of the SNaP Wound Care System was assessed through documentation of the time taken to apply the device. This was the time taken from application of the SurePrep skin barrier film to achieving a complete and consistent seal with the SNaP device. A mean device application time was calculated at 7 min and 11 s for the overall cohort, with the neuropathic foot ulcers taking the longest time on average (Table 5).
Table 5.
Mean dressing application times for each cohort
| Mean Dressing Application Duration (h:min:s) | |
|---|---|
| Neuropathic foot ulcer | 00:10:55 |
| Mixed etiology ulcer | 00:06:05 |
| Venous ulcer | 00:05:58 |
| Overall | 00:07:11 |
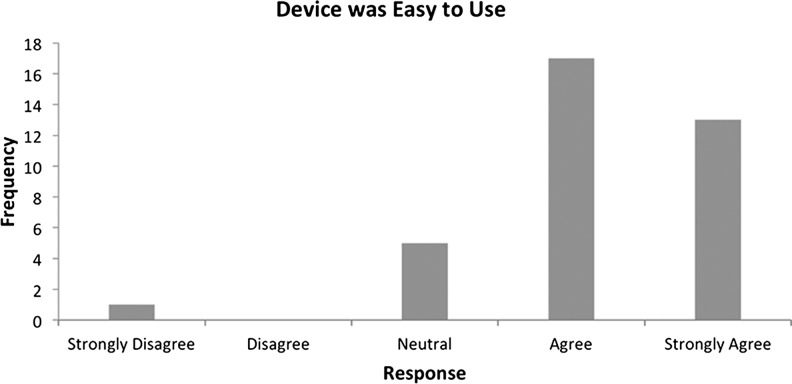
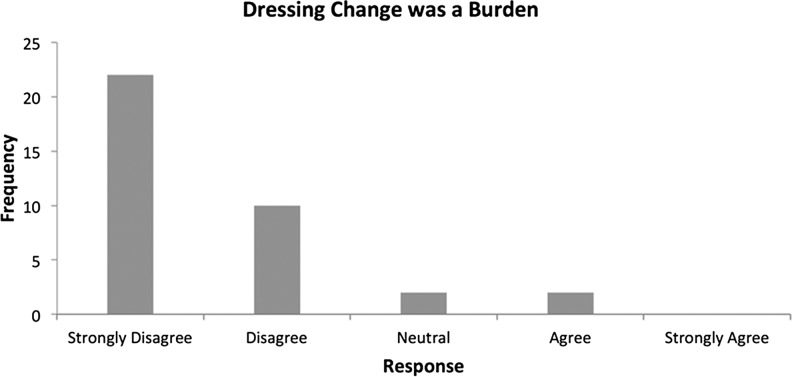
From the patient's perspective, questions related to the ease of use of the device on the exit survey indicated that patient's generally found the device easy to use and found the dressing changes straightforward and not burdensome (Figs. 6 and 7).
Figure 6.
Exit survey (ease of use).
Figure 7.
Exit survey (dressing changes).
Quality of life
Results of the CWIS demonstrate that over 60% of the scores across all three domains (Well Being, Physical Symptoms and Daily Living and Social Life) increased during the study, indicating that the majority of patients experienced an improved quality of life after using the SNaP device. This was also reflected in the results of the etiology subsets.
The NRS completed, where 0 was the worst possible quality of life and 10 the best possible, indicated that 46% of patients scored their quality of life higher at the end of the study than at the beginning, while 30% of patients scored their quality of life the same. In addition, 43% scored their satisfaction with their quality of life as higher at the final visit than at baseline, with 30% scoring the same.
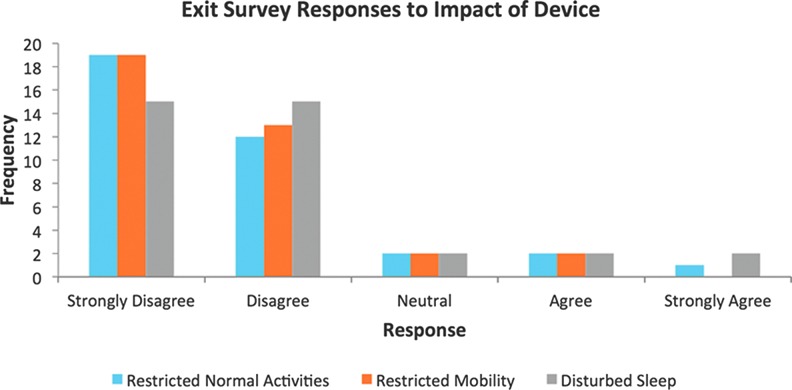
The exit survey completed alongside the CWIS questionnaire at week 6 also provided data on quality-of-life issues, such as sleep, mobility, and activities of daily living. Subjects were questioned on whether using the SNaP device impacted on various areas of their daily lives. Figure 8 clearly highlights that the majority of patients disagreed or strongly disagreed that the device negatively impacted on their normal activities, sleep, or mobility.
Figure 8.
Exit survey responses to questions of the impact of the device on various quality-of-life issues. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Customer satisfaction
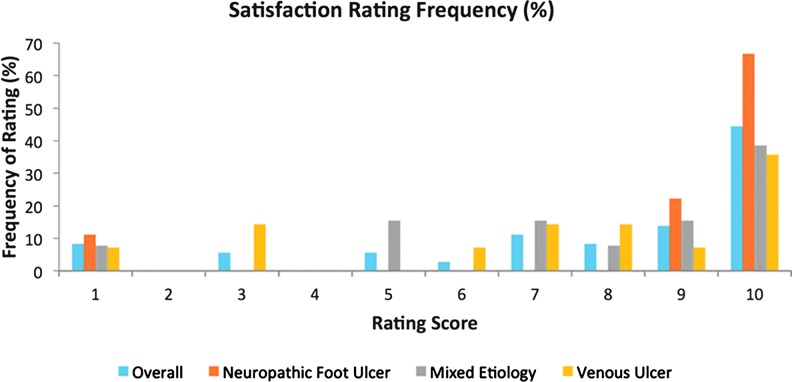
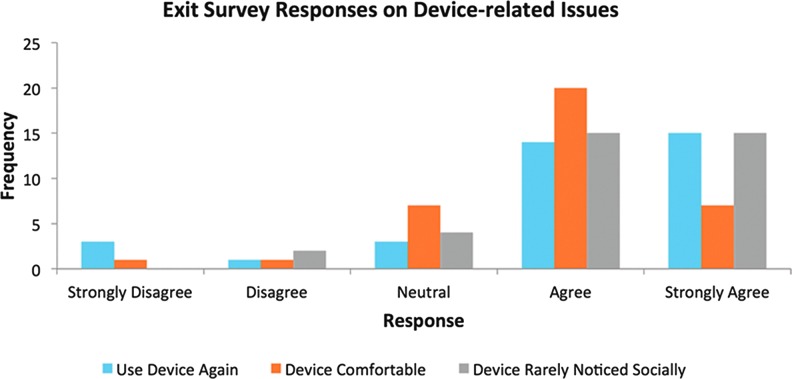
The exit survey results generally demonstrated that patients were highly satisfied with the SNaP device overall (Fig. 9) across all etiology subsets. Device-related responses also indicated that the majority of patients found the device comfortable, socially acceptable, and indicated they would definitely use the device again (Fig. 10). No patients found the device to be noisy, and only one patient reported the device as not being portable. This was mainly due to personal issues where they felt conscious that the device was visible under their clothing.
Figure 9.
Satisfaction rating for subjects by wound type. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 10.
Exit survey responses on device-related issues. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
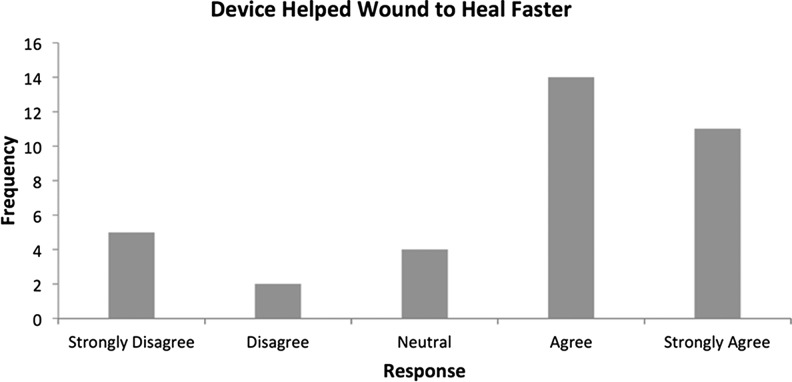
The responses regarding the patient's attitudes toward whether the device actually increased the speed of healing were slightly more widespread. However, 69.4% of the sample population positively felt that the device had encouraged faster healing (Fig. 11).
Figure 11.
Exit survey (wound healing).
Discussion
The demographics for the total study population were generally reflective of those in each wound type subset. In the foot ulcer group, there was a much higher proportion of male (n=8) to female (n=1). The mean age of subjects was 64.35 years, which would be expected with this patient population. The mean wound duration for the overall patient group was 18.8 months, indicating the chronic nature of the wounds included in this study. The mixed etiology group had a much longer mean duration of 33.9 months compared to each of the other groups and to the overall mean average, which could indicate the particular difficulties encountered in healing these types of leg ulcers.
Primary endpoint
The average target ulcer size at baseline was 9.5 cm2, with the foot ulcers and mixed etiology having a similar mean size of 11.1 and 11.9 cm2, respectively, and the venous ulcers averaging smaller at 6.4 cm2.
The mean percentage change in wound area across the study population between weeks 1 and 8 indicated a decrease of 42.64%. This is also represented by the normalized average wound size graph indicating the decrease in wound size for the overall group and for each etiology subset over the 8-week period. These results suggest that use of the SNaP Wound Care System may promote healing in different types of chronic wounds in terms of reduction in wound size.
All the wound etiologies experienced a mean reduction in wound size overall, although this was particularly notable for the venous ulcer and foot ulcer groups with mean decreases of 64% and 55%, respectively. Three patients in the venous ulcer group went on to achieve complete re-epithelialization of their wounds during the course of the study. For the mixed etiology group, the mean percentage decrease was much smaller at 9.6%, although over double the number of patients experienced a decrease in wound size compared to an increase in wound size for the group overall. The smaller reduction in wound size in this group could be attributed to the increased difficulty in healing wounds with an ischemic component due to the reduced blood supply in the affected limb. It could also be a reflection of the lower pressure cartridges generally tolerated in this group due to preexisting wound pain. These findings indicate an overall positive outcome for the use of the SNaP Wound Care System in promoting healing in chronic wounds of various etiologies, as suggested by previous research25,29,30 but particularly neuropathic foot ulcerations and venous leg ulcerations. As several RCTs have demonstrated that the use of an electrically powered TNP system is superior to standard care for diabetic foot and venous leg ulcers,22,24 and RCT evidence suggests that SNaP is comparable to conventional TNP systems,29,30 this is another positive indication for the efficacy of the SNaP system and its prospective use in clinical practice. Comparison of the efficacy of the SNaP system compared to standard care cannot be made from this pilot clinical evaluation.
Secondary endpoints
Appearance of wound bed
Previous research into the effect of TNP wound therapy has particularly noted stimulation of granulation tissue formation as a positive outcome.1,2 Recent published data have suggested that granulation tissue development can be used as a predictor of wound healing.31
The mean percentage change in granulation tissue present in the wound bed at the baseline visit to the final visit shows an overall increase for the entire cohort. The mixed etiology group also experienced an overall increase in granulation tissue, which is encouraging as this group demonstrated the smallest decrease in wound area: the increase in healthy tissue is a positive sign of the effect of the negative pressure therapy.
The biggest mean percentage increase in granulation tissue by cohort was seen in the neuropathic foot ulcer group. Previous clinical trials have noted that the formation of healthy granulation tissue is an advantage of negative pressure therapy in neuropathic foot wounds.22 As this group also displayed a 55% mean decrease in wound area, these two results suggest positively that the SNaP Wound Care System promoted healing in these complex chronic wounds.
The venous leg ulcer group displayed little mean change in the percentage of granulation tissue across the whole group from initial to final visit, although it should be remembered that three patients in this group achieved complete re-epithelialization and therefore would not have percentage granulation tissue captured on their final visit.
Levels of exudate
Exudate levels over the 8-week period decreased in 45.9% (n=17) of patients compared to 5.4% (n=2) whose exudate levels increased. Exudate levels were unchanged in 18% (n=48.6) of the overall study group.
Removing excess moisture from the wound bed and thus the proinflammatory cytokines contained within it may prove beneficial to promoting healing in chronic wounds.4–6 On a clinical level, high exudate levels may be associated with damage to the surrounding skin of the wound if not adequately controlled, which can cause increased discomfort to the patient. Many patients also feel anxious about their wound dressings leaking, with associated odor and social embarrassment. The results indicated that over half of the study patients experienced decreased exudate levels during the study, suggesting that the SNaP Wound Care System can contribute to increased healing and patient comfort.
Odor
Results indicate that wound malodor was not a significant problem during the use of the SNaP device.
Ulcer-related pain
Anecdotal evidence suggests that pain is one of the main adverse effects associated with negative pressure wound devices,32 although Ozturk et al.33 found that negative pressure therapy may cause less pain than other modern wound therapies. Some comparative studies have suggested that gauze-based rather than foam-based interface dressings may cause less pain when using negative pressure wound therapy.34,35
Results of this pilot study using the SNaP Wound Care System show that 66.7% of the study participants who experienced pain indicated a decrease in their pain levels from baseline to the final visit. This suggests that the SNaP system may actually improve pain rather than exacerbate it. This supports the findings of a randomized controlled trial conducted by Vuerstaek et al.,24 using negative pressure therapy on chronic leg ulcer patients, showing reduced pain levels during the course of treatment.24
Four patients (16.7%) experienced an overall increase in their pain during the study, half of whom were in the neuropathic foot ulcer group. The increase in the mean pain score for this group as seen in Fig. 2 was a small margin from 1.3 to 1.6, indicating that the increase in pain level was small. The other two groups experienced an overall decrease in pain levels.
On the patient exit survey, the majority of patients who reported pain from the treatment at dressing change or generally from the device recorded it as 5 or lower on the NRS. Pain levels over 40 mm on a visual analogue scale (VAS) scale have been identified as moderate-to-severe intensity, which requires immediate review and intervention.36 Although this study used an NRS as opposed to a VAS, awareness of the potential for device-related pain needs to occur as with any wound intervention, even if all that is necessitated is a review of current analgesia.
There were six adverse events (5.7% of the total number of adverse events) recorded, which indicated a possible, probable, or definite relationship between the device and increased pain.
Condition of surrounding skin
The high incidence of eczema in the venous leg ulcer (VLU) group would be expected due to the increased incidence of varicose eczema related to the skin changes and increased skin sensitivities associated with venous disease.37
The clinical impression is that any deterioration in the surrounding skin when using the SNaP Wound Care System could mainly be attributed to the adhesive nature of the hydrocolloid dressing. Adhesive dressings are often avoided in patients with venous disease due to the increased risk of allergic contact dermatitis and varicose eczema commonly experienced by this patient group. It was therefore not an unexpected outcome for these patients to experience some decreased surrounding skin integrity during the course of the trial. Attempts were made to minimize this with the application of a skin barrier film before applying the adhesive dressing. Steroid ointments were also applied to the surrounding skin when indicated and left to absorb for 10 min before wiping away any excess before applying the SurePrep skin barrier film. Most adverse events related to surrounding skin were managed successfully in this way, as only two patients required withdrawal for skin-related events (one for allergy to adhesive dressing and one for persistent maceration).
Incidence of eczema could also be attributed to increased exudate on the surrounding skin tissue. There were only 10 adverse events of the 66 (15.1%) related to maceration of the surrounding skin, however, which would often be linked with skin damage from high volumes of exudate.
Ease of use
The documented benefits of negative pressure wound therapy are known to include reduction in the number of required dressing changes and in nursing time.38 A previous randomized controlled trial of the SNaP negative pressure system versus the commonly used VAC (KCI™) system indicated that the mean application time for SNaP was significantly less (p<0.0001) than that of the VAC system for all follow-up visits combined.29
The mean time taken for the application of the SNaP Wound Care System in this study was 7 min 11 s (range 01:00–50:00 min). This was not inclusive of the time taken to remove dressings, perform debridement if required, and to reapply compression bandages if necessary. As such, this could be viewed as the additional time required than that of a standard dressing change. It is evident from this time that the application of the SNaP device is not a lengthy process, which would suggest that the device is relatively easy to use. Consideration should also be given to the fact that it is only performed on average twice weekly. For highly exuding wounds, for example, dressing changes may be required daily or on alternate days, which may significantly increase the demand on nursing time and resources in the initial treatment period.
The time taken to apply the dressing and achieve a seal on the foot ulcer wounds was ∼5 min longer than that taken for the venous leg ulcer patients. This is understandable as the foot wounds are usually in a much more difficult position to dress than leg ulcers, meaning it could take longer to achieve an effective seal. Foot ulcers can often be time-consuming and difficult to dress with standard dressings. Also, the times for the neuropathic foot wounds would have increased the mean application time of the entire cohort; therefore, the ∼6 min taken for the venous leg ulcer patients is much more reflective of the short time needed to apply the SNaP device in this cohort of patients.
Effect on quality of life
These results suggest that use of the SNaP Wound Care System can positively impact on the quality of life experienced by patients with chronic wounds. This reflects outcomes displayed in the literature regarding the effect of using TNP therapy on the quality of life of patients with wounds,24,39 although the general consensus is that more research is required in this area. Results also need to be interpreted with caution as the CWIS has been validated to highlight changes in quality of life when performed at least 12 weeks apart, whereas here there was only a 6-week period between questionnaires. It has also been documented that purely participating in a clinical trial can lead patients to report an improvement in their state of health.40 It should be noted, however, that comparable results in terms of improvement in quality-of-life issues were found in the RCT, which compared the SNaP Wound Care System with an electrically powered NPWT device—patients also reported less impact on daily activities, mobility, social interactions, and sleep when using SNaP during this study30—although it is acknowledged that these data were gained purely from a comparable study exit survey rather than a validated QoL tool.
Patient satisfaction with treatment
The chart of overall customer satisfaction with using the SNaP device highlights a definite shift toward high satisfaction scores out of 10, both from the group overall and from the separate wound etiology subsets.
Patients agreed or strongly agreed (69.4%, n=25) that the device had helped their wound to heal faster and reported that they would wear the device in the future (81%, n=29). Patients also found the device comfortable (75%, n=27) and easy to use (83.3%, n=30). These results indicate a definite positive reaction from patients regarding using and wearing the SNaP Wound Care System.
The SNaP Wound Care System was designed to be small, lightweight, and ultraportable, not only allowing patients with small wounds access to negative pressure therapy but also to make use of the device more comfortable, more socially acceptable, and patient-friendly. Patients reported that the device was rarely noticed by others (83.3%, n=30), and only 5.6% (n=2) found the dressing changes burdensome, while nobody reported any problems with noise at all. Patients disagreed the device restricted their normal activities (86.1%, n=31), with the majority of patients strongly disagreeing that this was the case. Only two patients (5.6%) felt that the device interfered with their mobility, again with the majority of patients strongly disagreeing with this. Patients found the device portable (97.2%, n=35), with only four patients (11.1%) expressing that in their view the device could be more portable. Previous research also found that patients reported the SNaP system to interfere less with overall activity, sleep, and social interactions when compared to the VAC system, and comparable results for pain, perceived effectiveness, and patient satisfaction between the devices.29 These are all factors that can significantly affect a patient's psychological well-being, an important outcome to consider when evaluating any wound care intervention. Overall, these outcomes suggest that the novel design of the SNaP device achieved its aims for this patient population, and patients generally expressed high levels of satisfaction with the treatment across many areas.
Limitation of the study
This study was a pilot study and thus it is acknowledged that sample numbers are small, especially when subdivided into wound types. However, the aim was to evaluate the use of the device in chronic wounds—there was no attempt to offer comparisons with other devices or therapies, but merely to gain an overall perspective. Results have also only been displayed descriptively due to the small sample numbers so no statistical significance can be drawn from the results.
Further research
Further larger-scale research is required into the use of the SNaP Wound Care System with different wound types in terms of clinical effectiveness and quality of life to provide more definite outcomes. Adequately powered comparative trials with other negative pressure devices would be useful.
Conclusion
These study results indicate that the SNaP Wound Care System may promote healing in different chronic wound types not following the normal healing trajectory. The use of the SNaP device, in addition to standard care, reduced mean wound size for all the wound etiologies studied and appeared to encourage granulation tissue formation across the cohort. Wound exudate levels were well controlled, and odor was not a particular problem. The SNaP device can also be used in conjunction with standard care treatments, such as compression bandaging and offloading footwear, with minimal adjustment, ensuring the patient is receiving the best possible treatment to achieve ulcer healing.
Wound pain when using the SNaP device actually decreased for half of the study participants throughout the evaluation, and large increases in pain were not particularly reported.
Maintaining integrity of the surrounding skin was the most challenging aspect of using the SNaP device on this group of patients with complex chronic ulcers of the leg and foot normally susceptible to skin problems anyway. However, with the use of a skin barrier film and steroid ointments, most deterioration in skin condition was well managed.
The SNaP Wound Care System was easy to use and did not generally add a significant amount of time to an average dressing change. Results of quality-of-life questionnaires also suggest that the use of the SNaP device has the potential to improve quality of life for patients, and most patients would wear the device again. The small, portable, and relatively inconspicuous design of the device made wearing negative pressure therapy generally more comfortable, easy to manage, and socially acceptable for the patient.
Overall, the SNaP Wound Care System could be a very useful and effective tool for patients with small wounds who would otherwise benefit from the use of negative pressure wound therapy.
Abbreviations and Acronyms
- CWIS
Cardiff Wound Impact Schedule
- NRS
numerical rating scale
- TNP
topical negative pressure
Acknowledgment and Funding Source
This observational study was supported by funding from Spiracur Inc.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Sarah Bradbury, MSc, BSc (Hons), RGN, is a former research nurse from Cardiff University. Neal Walkley, PhD, BSc (Hons), is a graduate of Cardiff University and has molecular biology research experience within academic and commercial institutions. He has spent the last 6 years in the field of wound healing. Nicola Ivins, MSc, RGN, has worked in wound healing for 18 years. She trained at the University Hospital of Wales and worked in the Department of Surgery for 7 years. She now works in the Welsh Wound Innovation Centre. Her current role is Director of Clinical Research focusing on academic and commercial clinical trials in wound healing. Professor Keith Harding, CBE, FRCGP, FRCP, FRCS, is head of WHRU and Dean of Clinical Innovation at Cardiff University. He is medical director of the Welsh Wound Innovation Initiative.
References
- 1.World Union of Wound Healing Societies (WUWHS). A World Union of Wound Healing Societies' Consensus Document: Vacuum Assisted Closure: Recommendations for use. Medical Education Partnership (MEP) Ltd, London, 2008 [Google Scholar]
- 2.European Wound Management Association (EWMA). Position Document: Topical Negative Pressure in Wound Management. Medical Education Partnership (MEP) Ltd, London, 2007 [Google Scholar]
- 3.Morykwas MJ, Argenta LC, Shelton-Brown EI, et al. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–562 [DOI] [PubMed] [Google Scholar]
- 4.Trengove NJ, Stacey MC, Macauley S, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999;7:442–452 [DOI] [PubMed] [Google Scholar]
- 5.Greene AK, Puder M, Roy R, et al. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg 2006;56:418–422 [DOI] [PubMed] [Google Scholar]
- 6.Stechmiller JK, Kilapadi DV, Childress B, et al. Effect of vacuum-assisted closure therapy on the expression of cytokines and proteases in wound fluid of adults with pressure ulcers. Wound Repair Regen 2006;14:371–374 [DOI] [PubMed] [Google Scholar]
- 7.Bradford EH. The hyperaemia treatment of congested and inflamed tissues. N Engl J Med 1906;24:671–674 [Google Scholar]
- 8.Timmers MS, Le Cessie S, Banwell P, et al. The effects of varying degrees of pressure delivered by negative-pressure wound therapy on skin perfusion. Ann Plast Surg 2005;55:665–671; discussion 1097–1098 [DOI] [PubMed] [Google Scholar]
- 9.Fabian TS, Kaufman HJ, Lett ED, et al. The evaluation of subatmospheric pressure and hyperbaric oxygen in ischemic full-thickness wound healing. Am Surg 2000;66:1136–1143 [PubMed] [Google Scholar]
- 10.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res 1989;238:249. [PubMed] [Google Scholar]
- 11.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res 1989;239:263. [PubMed] [Google Scholar]
- 12.Swan M, Banwell PE. Topical Negative Pressure: Advanced Management of the Open Abdomen. Oxford, UK: Oxford Wound Healing Society, 2003 [Google Scholar]
- 13.Rao M, Burke D, Finan PJ, Sagar PM. The use of vacuum-assisted closure of abdominal wounds: a word of caution. Colerectal Dis 2007;9:266–268 [DOI] [PubMed] [Google Scholar]
- 14.Wild T, Goetzinger P, Telekey B. VAC and fistula formation. Colerectal Dis 2007;9:572–573 [DOI] [PubMed] [Google Scholar]
- 15.Open Abdomen Advisory Panel; Campbell A, Chang M, Fabian T, Franz M, Kaplan M, Moore F, Reed RL, Scott B, Silverman R. Management of the open abdomen: from initial operation to definitive closure. Am Surg 2009;75(11 Suppl):S1–S22 [PubMed] [Google Scholar]
- 16.Graham ID, Harrison MB, Nelson EA, Lorimer K, Fisher A. Prevalence of lower-limb ulceration: a systematic review of prevalence studies. Adv Skin Wound Care 2003;16:305–316 [DOI] [PubMed] [Google Scholar]
- 17.Harding KG, Morris HL, Patel GK. Science, medicine and the future: healing chronic wounds. BMJ 2002;324:160–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tennvall GR, Apelqvist J. Health Economic consequences of diabetic foot lesions. Clin Infect Dis 2004;39(supp):132–139 [DOI] [PubMed] [Google Scholar]
- 19.Callum MJ, Ruckley CV, Harper DR, Dale JJ. Chronic ulceration of the leg: extent of the problem and provision of care. BMJ 1985;290:1855–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanderwee K, Clark M, Dealey C, Gunningberg L, Defloor T. Pressure ulcer prevalence in Europe: a pilot study. J Eval Clin Pract 2007;13:227–235 [DOI] [PubMed] [Google Scholar]
- 21.Franks PJ, Moffatt CJ, Connolly M, Bosanquet N, Oldroyd MI, Greenhalgh RM, McCollum CN. Factors associated with healing leg ulceration with high compression. Age Ageing 1995;24:407–410 [DOI] [PubMed] [Google Scholar]
- 22.Armstrong DG, Lavery LA; Diabetic Foot Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–1710 [DOI] [PubMed] [Google Scholar]
- 23.Ford CN, Reinhard ER, Yeh D, et al. Interim analysis of a prospective, randomized trial of vacuum-assisted closure versus the Healthpoint system in the management of pressure ulcers. Ann Plast Surg 2002;49:55–61 [DOI] [PubMed] [Google Scholar]
- 24.Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State of art treatment of chronic leg ulcers: A randomised controlled trial comparing vacuum assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006;44:1029–1037 [DOI] [PubMed] [Google Scholar]
- 25.Lerman B, Oldenbrook L, Eichstadt SL, Ryu J, Fong KD, Schubart PJ. Evaluation of chronic wound treatment with the SNaP wound care system versus modern dressing protocols. Plast Reconstr Surg 2010;126:1253–1261 [DOI] [PubMed] [Google Scholar]
- 26.Landsman A. Analysis of the SNaP Wound Care System, a negative pressure wound device for treatment of diabetic lower extremity wounds. J Diabetes Sci Technol 2010;4:831–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lerman B, Oldenbrook L, Ryu J, Fong KD, Schubart PJ. The SNaP Wound Care System: a case series using a novel ultraportable negative pressure wound therapy device for the treatment of diabetic lower extremity wounds. J Diabetes Sci Technol 2010;4:825–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fong KD, Hu D, Eichstadt S, Gupta DM, Pinto M, Gurtner GC, Longaker MT, Lorenz HP. The SNaP system: biomechanical and animal model testing of a novel ultraportable negative-pressure wound therapy system. Plast Reconstr Surg 2010;125:1362–1371 [DOI] [PubMed] [Google Scholar]
- 29.Armstrong DG, Marston WA, Reyzelman DPM, Kirsner RS. Comparison of negative pressure wound therapy with an ultraportable mechanically powered device vs. traditional electrically powered device for the treatment of chronic lower extremity ulcers: A multicentre randomized-controlled trial. Wound Repair Regen 2011;19:173–180 [DOI] [PubMed] [Google Scholar]
- 30.Armstrong DG, Marston WA, et al. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: A multicenter randomized controlled trial. Wound Repair Regen 2012;20:332–341 [DOI] [PubMed] [Google Scholar]
- 31.Valenzuela-Silva CM, Tuero-Iglesias ÁD, García-Iglesias E, González-Díaz O, Del Río-Martín A, Yera Alos IB, Fernández-Montequín JI, López-Saura PA. Granulation response and partial wound closure predict healing in clinical trials on advanced diabetes foot ulcers treated with recombinant human epidermal growth factor. Diabetes Care 2013;36:210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fong KDD, Hu SL, Eichstadt E, et al. Initial clinical experience using a novel ultraportable negative pressure wound therapy device. Wounds 2010;22:230–236 [PubMed] [Google Scholar]
- 33.Ozturk E, Ozguc H, Yilmazlar T. the use of vacuum assisted closure therapy in the management of Fournier's gangrene. Am J Surg 2009;197:660–665 [DOI] [PubMed] [Google Scholar]
- 34.Fraccalvieri M, Ruka E, Bocchiotti MA, Zingarelli E, Brushi S. Patient's pain feedback using negative pressure wound therapy with foam and gauze. Int Wound J 2011;8:492–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorafshar AH, Franczyk M, Gottlieb LJ, Wroblewski KE, Lohman RF. A prospective randomized trial comparing subatmospheric wound therapy with a sealed gauze dressing and the standard vacuum-assisted closure device. Ann Plast Surg 2012;69:79–84 [DOI] [PubMed] [Google Scholar]
- 36.World Union of Wound Healing Societies (WUWHS). Principles of Best Practice: Minimising Pain at Wound-Dressing Related Procedures. A consensus document. London: Medical Education Partnership, 2004 [Google Scholar]
- 37.Cameron J. Dermatological changes associated with venous leg ulcers. Wound Essent 2007;2:60–66 [Google Scholar]
- 38.Dowsett C, Davis L, Henderson V, Searle R. The economic benefits of negative pressure wound therapy in community-based wound care in the NHS. Int Wound J 2012;9:544–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braakenburg A, Obdeijn MC, Feitz R, Van Rooij IA, Van Griethuysen AJ, Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg 2006;118:390–397 [DOI] [PubMed] [Google Scholar]
- 40.Verheggen FWSM, Nieman FH, Reerink E, Kok GJ. Patient satisfaction with clinical trial participation. Int J Qual Health Care 1998;10:319–330 [DOI] [PubMed] [Google Scholar]