Abstract
Significance: Diabetes is a disorder that is well known to delay wound repair resulting in the formation of colonized chronic wounds. Over their lifetime, diabetic patients have a 25% incidence of foot ulcers (DFUs), which contribute to increased risk of morbidity, including osteomyelitis and amputations, and increased burden to the healthcare system.
Recent Advances: The only active product approved for the treatment of diabetic ulcers, Regranex®, is not widely used due to minimal proven efficacy and recent warnings added to the Instructions for Use. A novel topical agent that accelerates healing and increases the proportion of fully healed DFUs, DSC127 [aclerastide; active ingredient, NorLeu3-angiotensin (1-7) (NorLeu3-A(1-7))], is recruiting patients in Phase III clinical trials (NCT01830348 and NCT01849965). NorLeu3-A(1-7) is an analog of the naturally occurring peptide, angiotensin 1-7. The mechanisms of action include induction of progenitor proliferation, accelerated vascularization, collagen deposition, and re-epithelialization.
Critical Issues: Current modalities for the treatment of DFUs include strict offloading, bandaging, debridement and, on a limited basis, application of Regranex. Novel potent therapies are needed to combat this significant burden to the diabetic patient and the healthcare system.
Future Direction: Preclinical and clinical research shows that DSC127 is highly effective in the closure of diabetic wounds and is superior to Regranex in animal studies. Clinical development of DSC127 as a topical agent for the healing of DFU is underway. Further investigation into the mechanisms by which this product accelerates healing is warranted.

Kathleen E. Rodgers, PhD
Scope and Significance
This review will summarize the development and current status of a novel topical agent for the treatment of diabetic foot ulcers (DFUs), DSC127 (aclerastide, pINN). Phase I studies in DFU patients showed tolerability and lack of systemic exposure to the active ingredient, NorLeu3-A(1-7). NorLeu3-A(1-7) was superior to Regranex® in preclinical studies and DSC127 accelerated wound closure and increased the proportion of wounds fully closed and remaining closed for up to 20 weeks after 4 weeks of treatment in Phase II clinical studies. Patients are now being recruited to pivotal Phase III trials.
Translational Relevance
The preclinical development and currently known mechanism of action of DSC127 will be reviewed. In the preclinical optimization of the product, it was noted that the wound first filled in with the extracellular matrix that was similar to normal skin and then the wound was re-epithelialized presenting histologically as nearly scarless healing.1,2 Similarly, clinical studies showed a reduction in the volume of the wound, followed by the reduction in area, and then closure confirming the translation of preclinical pharmacology studies into clinical benefit.
Clinical Relevance
Currently, the majority of modalities to accelerate the closure of DFU involve maintenance of a moist wound environment, optimization of bandaging, and off-loading, as well as debridement and infection control. The only active therapy for the treatment of DFU, Regranex, has been recently reintroduced into the market with close oversight of the patients receiving this active growth factor. This review provides insight into a novel, active topical agent for the treatment of DFU, which is not a topical growth factor.
Background
Wounds result in tissue destruction and loss of vascularization at the site of injury. Normal healing processes at the wound site involve proliferation, migration, and differentiation of keratinocytes, fibroblasts, and endothelial cells. Fibroblasts repair the dermal matrix, endothelial cells populate it with new blood vessels (angiogenesis), and then keratinocytes cover it with fresh epithelium (epithelialization). Although soft tissue healing usually follows a similar orderly process, complications of diabetes, a metabolic disorder with dysregulated glucose and lipid metabolism, include delayed wound healing and impaired infection control.
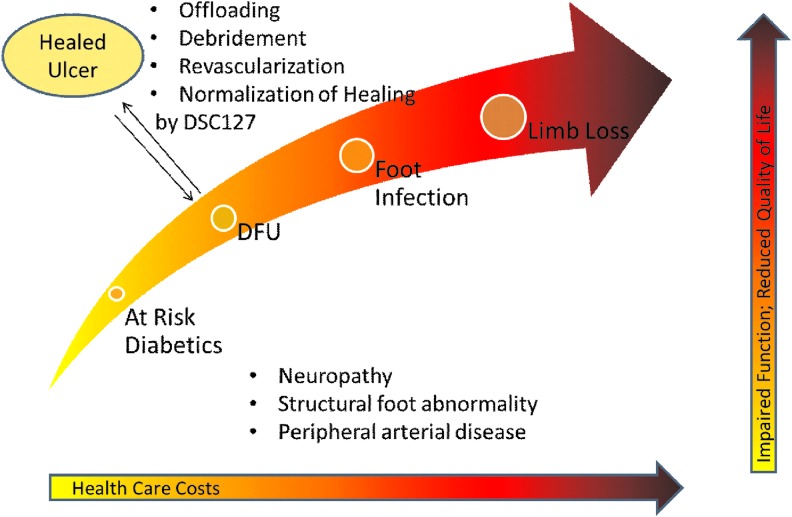
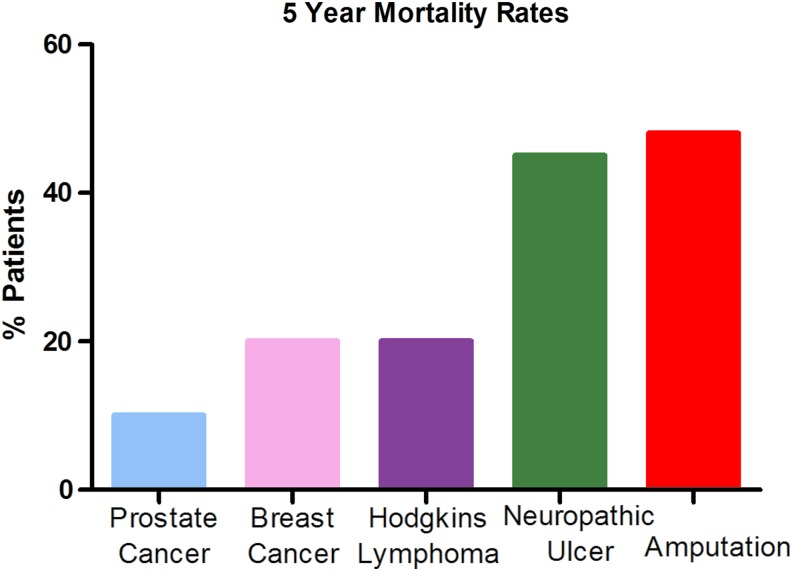
An estimated 347 million people worldwide have diabetes, with incidence increasing rapidly. Diabetes mellitus occurs throughout the world, but is more common (especially type 2) in more developed regions. Persons with diabetes are at high risk for infection and amputation of the lower extremity (Fig. 1).3 DFUs constitute a significant, growing global healthcare concern and exert an enormous toll in terms of health, economic, and social costs. Along with pain and morbidity, DFUs account for as much as 20% of total expenditures on diabetes in North America and Europe.4 Approximately 900,000 people a year in the United States suffer from DFUs, which can take years to heal and there is generally a high rate of wound recurrence, infection, and amputation. Each year, ∼90,000 people (∼1 every 6 min) in the United States must undergo amputations as a result of their wounds. The 5-year survival of patients, after amputation, is very low compared with other widespread diseases, such as breast and prostate cancer (Fig. 2). In developed countries, lower limb amputations are at least 10 times more common in people with diabetes.5 A pre-existing DFU is the major risk factor for amputation due to delayed healing of the wound.6,7 DFUs must be brought to closure as quickly as possible to reduce the risk of infection and amputation.3,7 Once a patient has undergone amputation for chronic vascular disease, including diabetes, their life expectancy is between 2 and 5 years.8 The current standard treatment for DFU includes debridement, maintenance of a moist wound environment, management of any infection, revascularization, and off-loading of the ulcer, but novel therapies are needed to improve outcomes for these patients.
Figure 1.
Amputation associated with diabetic foot ulcer (DFU). The costs to the healthcare system and to the patient increase as the DFU progresses to amputation. Healing of the ulcer avoids the complications associated with chronic nonhealing wounds. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 2.
Five-year mortality rates associated with amputation. The 5-year survival rate associated with amputation secondary to a DFU is very low compared with other common chronic diseases, such as breast and prostate cancer. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The only pharmacological agent approved by the U.S. Food and Drug Administration (FDA, 1997) to treat DFUs is the human recombinant platelet-derived growth factor in an aqueous carboxymethylcellulose topical gel (Regranex). This drug is indicated for treatment of lower extremity diabetic neuropathic ulcers. The drug was approved based on the results of four large clinical studies in which Regranex was applied topically. The primary endpoint of the studies was complete ulcer closure within 20 weeks. Review of the development of Regranex is in an accompanying chapter.
Discussion
Renin–angiotensin system in dermal tissue
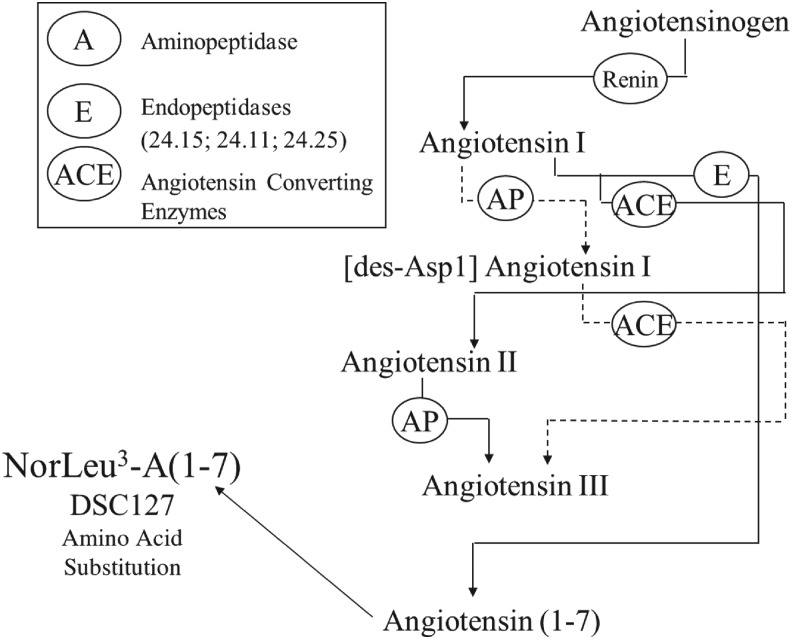
Active peptides of the rennin–angiotensin system (RAS), angiotensin II and angiotensin 1-7 (A(1-7)), have been shown to accelerate the healing of injuries (Fig. 3).9–14 A complete RAS has been shown to reside within human skin.15,16 Studies by multiple laboratories have reported alterations in the level of RAS components by injury and the contribution of the RAS to wound healing through increased collagen deposition and transactivation of the epidermal growth factor receptor.15–24 In human skin, AT1 and AT2 receptors were found in the epidermis and in dermal vessel walls.15 Recently, an upregulation of the Mas receptor, the functional receptor for A(1-7), in the skin after injury has been shown.25
Figure 3.
Renin–angiotensin system.
Once the activity of A(1-7) was identified, a series of analogs were generated and tested for activity in both in vitro and in vivo models of healing. In these studies, NorLeu3-A(1-7) was found to be most active in the acceleration of wound repair and had the novel mechanism of restoration of skin architecture, for example, regeneration.1,2 NorLeu3-A(1-7), the active ingredient in DSC127, accelerates healing in multiple animal models. In the rat full-thickness excision model, NorLeu3-A(1-7) reduced the wound size by more than 60% compared to placebo controls. A(1-7) was less effective, causing a 45% reduction in wound size. Regranex reduced the wound size by only 20%. Thus, NorLeu3-A(1-7) was three times more effective in wound healing than the FDA-approved drug in this model. In the diabetic mouse dorsal full-thickness excision model, NorLeu3-A(1-7) was again more effective in wound healing than either A(1-7) or Regranex. By 18 days after injury, about 60% of NorLeu3-A(1-7)-treated excisions completely healed. Complete healing of the wound is the endpoint required by the FDA for approval. The wound area reduced from baseline by over 80%.1 In contrast, none of the diabetic animals that received Regranex was fully healed and the wound area was reduced from baseline by ∼20%. Furthermore, ∼20% of the wounds were fully healed with A(1-7) at day 18. NorLeu3-A(1-7) is stable in the presence of common wound enzymes and fragments of NorLeu3-A(1-7) remain active.14 Thus, NorLeu3-A(1-7) formulated as a preserved clinical gel (DSC127) is superior to the FDA-approved drug (Regranex) in animal models of wound healing and is undergoing Phase III clinical trials.
Mechanism of action: NorLeu3-A(1-7), the active ingredient in DSC127
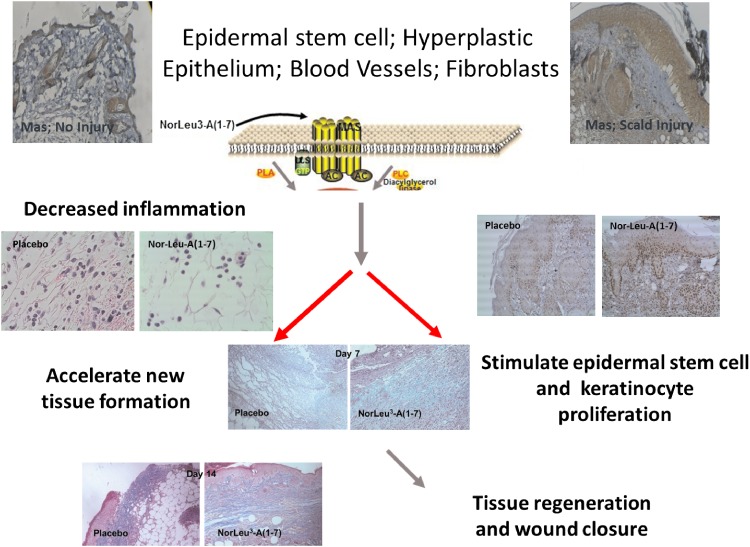
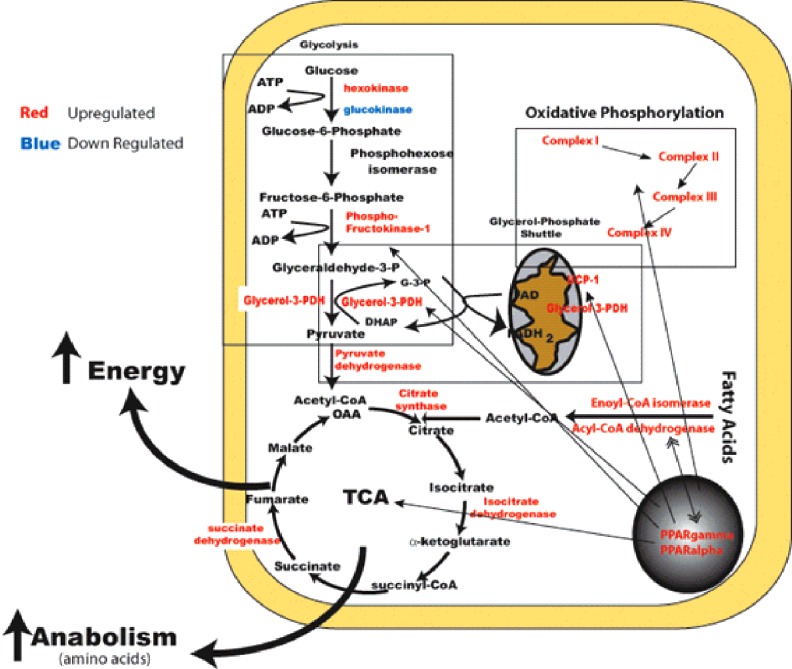
Preclinical studies showed that NorLeu3-A(1-7) accelerated healing through a receptor of the protective arm of the RAS, Mas, as A779, a Mas antagonist, blocked the effects of NorLeu3-A(1-7) on wound repair.2 To further investigate the cellular mechanisms by which NorLeu3-A(1-7) accelerated healing, we evaluated the quality of tissue repair in the same model in diabetic mice (Fig. 4).1,2,26 One factor essential in wound healing is the deposition of collagen in the wound area to allow for restructuring of the tissue. The natural wound repair process in diabetic mice involves a slow gradual increase in collagen in the diabetic wounds. NorLeu3-A(1-7), in a model of full-thickness excision in a mouse model of diabetes [db/db], accelerated the deposition of histologically and biochemically normal collagen, compared to vehicle controls, to speed up the wound repair process through rapid formation of normal granulation tissue. Further histological examination revealed that the collagen present was more mature at later time points with a basket-weave appearance consistent with a more regenerative process. NorLeu3-A(1-7) was either equivalent or superior to AII and A(1-7) in accelerating collagen deposition. The peptide also greatly accelerated dermal re-epithelialization at the wound site. Epidermal regrowth, assessed histologically, was seen as early as 4 days after the treatment started. NorLeu3-A(1-7) caused four- to sixfold increases in epidermal growth and accelerated the maturation of wound healing such that by 18 days after NorLeu3-A(1-7) treatment, most wounds were fully healed and fully re-epithelialized. Unpublished studies show that administration of NorLeu3-A(1-7) to diabetic full-thickness excision wounds upregulated the expression of 50 of 291 genes in the mitochondria associated with energy metabolism compared with vehicle-treated wounds at the same postoperative time point (day 7; Fig. 5). This possible restoration of mitochondrial function may underpin the observed increased tissue responses.
Figure 4.
Mechanisms by which DSC127 accelerates healing. NorLeu3-A(1-7) accelerates healing through reduction of inflammation and normalization of tissue healing. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 5.
NorLeu3-A(1-7) increases energy metabolism genes in the granulation tissue of full-thickness excision wounds at day 7 after surgery. Db/db mice underwent full-thickness excision injury at day 0 and granulation tissue was harvested on day 7. Genes in red were upregulated compared with vehicle controls. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The cumulative results of these extensive in vivo wound healing studies indicate that NorLeu3-A(1-7) is highly effective in dermal and epidermal repair. It was significantly superior to Regranex as well as to AII and A(1-7) in animal studies. In every model system tested, NorLeu3-A(1-7) significantly improved wound repair.
Formulation in development
Formulation work was conducted to optimize the delivery of NorLeu3-A(1-7) in a biocompatible vehicle with preservatives to allow multiple applications delivery from a single tube. Efficacy studies confirmed the suitability of hydroxyethyl cellulose as a vehicle for topical formulations in our full-thickness excision model in diabetic mice. The optimal formulation was chosen for clinical development.
Clinical development of DSC127
Phase I
Two Phase I studies were conducted. A Phase I safety study was conducted in normal volunteers using 0.3% NorLeu3-A(1-7) in hydroxyethyl cellulose gel with paraben preservatives. No adverse effects associated with drug use were observed. A second study was performed in patients with DFUs to assess circulating drug levels and the formation of antibodies to the drug substance. The open-label, single-center Phase I pharmacokinetic study enrolled diabetic subjects with at least one chronic nonhealing Wagner Grade 1 or 2 plantar neuropathic foot ulcer(s) on the mid- and forefoot that was at least 3.0 cm2. Eighteen subjects completed the trial. Subjects topically applied 0.03% DSC127 to the wound once each day for 28 days. The mean ulcer area at baseline was 9.5 cm2 (standard deviation 11.9 cm2). DSC127 was not detected (using an assay with a lower limit of quantitation of 0.200 ng/mL) in any serum samples at any time point. All samples were also considered to be nonreactive in an assay for antidrug antibodies. Study subjects showed no clinically significant changes in clinical chemistry, hematology, and electrocardiograms. The 0.03% DSC127 gel was well tolerated and there were no adverse events or serious adverse events considered related to the study drug. None of the wounds treated with the DSC127 gel deteriorated.
Phase II
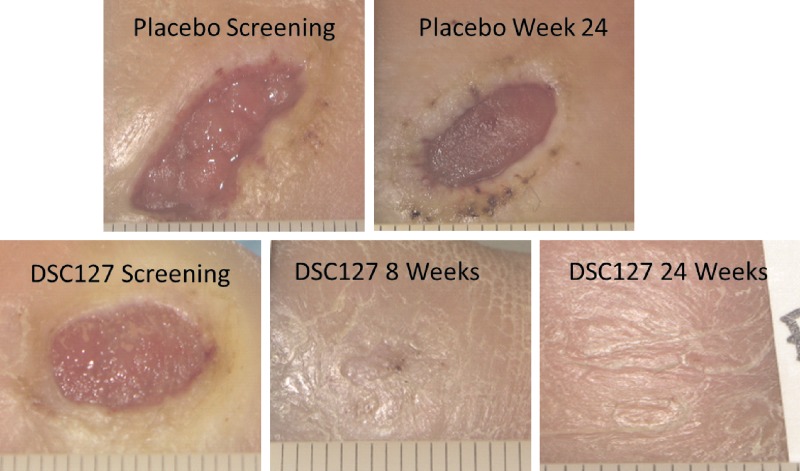
A Phase II clinical study evaluated the safety and preliminary efficacy of DSC127 in subjects with DFUs. In a double-blind, placebo-controlled multicenter clinical trial, 77 patients were randomized to receive one of two dose strengths of DSC127 (0.03% and 0.01%) or vehicle placebo control (Fig. 6).27 After 14 days of best standard-of-care (including off-loading) to evaluate ulcer healing and ensure the wounds were nonhealing, subjects randomized into the study received 4 weeks of once daily active treatment followed by 8 weeks of observation and assessment, and a further 12 weeks of follow up to assess durability of healed ulcers. Safety parameters among the intent-to-treat groups were comparable. Treatment with 0.03% DSC127 significantly reduced the area of the wound from baseline compared with placebo at 24 weeks. In the intent-to-treat (ITT) population, the results show that 54% of the diabetic wounds treated with the 0.03% dose (high dose) of DSC127 achieved 100% closure in 12 weeks or less, compared with 33% of subjects receiving placebo control and 30% of subjects receiving the 0.01% dose (low dose) of DSC127. By 24 weeks, 73% of wounds treated with the 0.03% dose (high dose) of DSC127 achieved 100% closure compared with only 46% in the placebo (p=0.058) and 48% in the 0.01% dose, respectively. Based on odds ratio analysis, subjects treated with DSC127 0.03% were 2.3 times more likely to have their wounds heal completely by 12 weeks and 3.1 times more likely by 24 weeks compared with subjects treated with placebo/standard of care. In the Per-Protocol (PP) population, the results show that 65% of the diabetic wounds treated with the 0.03% dose of DSC127 achieved 100% closure in 12 weeks or less, compared with 38% of subjects receiving placebo control, and 28% of subjects receiving the 0.01% dose of DSC127. By 24 weeks 85% of wounds treated with the 0.03% dose (high dose) of DSC127 achieved 100% closure compared with only 52% in the placebo (p=0.032) and 50% in the 0.01% dose respectively. Based on odds ratio analysis, subjects treated with DSC127 0.03% were 3.0 times more likely to have their wounds heal completely by week 12 and 5 times more likely by week 24 compared with subjects treated with placebo/standard-of-care. Ulcers in the 0.03% dose group healed on average within 10 weeks in the ITT population compared with ulcers treated with placebo, which took on average 23 weeks to heal. In the PP population, the 0.03% group healed in 8.5 weeks compared to the placebo group at 22 weeks (p=0.04). Covariate analysis compared reduction in the ulcer area, depth, and volume from baseline, and reductions in the 0.03% DSC127 group were greater at weeks 12 and 24.
Figure 6.
DSC127 accelerated healing that was durable. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Preclinical safety and tolerability
A series of pharmacology and toxicology studies have been performed with NorLeu3-A(1-7) and its formulated drug product DSC127. Dermatotoxicology, chronic toxicology after dermal exposure in miniswine, systemic toxicology studies in dogs and rats, and reproductive toxicology studies were performed and demonstrated safety in support of long-term use in clinical populations. Carcinogenicity studies are ongoing.
Summary
NorLeu3-A(1-7) is an analog of the active member of the RAS, A(1-7). The peptide accelerates and normalizes the healing of dermal injuries in multiple animal models. In Phase I and II clinical studies, NorLeu3-A(1-7) increased full closure of DFUs at 12 weeks. DermaSciences is now recruiting patients to participate in the pivotal Phase III studies to confirm the efficacy of DSC127 to increase the proportion of DFUs fully healing in larger populations. The efficacy of this product was seen as early as 4 weeks with a reduction in wound volume. This observation is consistent with that observed in preclinical studies where normal granulation tissue was rapidly deposited. By week 12, there was an absolute increase in full wound closure in the Phase II trial of 27%. The benefit continued over the durability phase of the trial where at 24 weeks after initiation of treatment (20 weeks since the last exposure), the absolute increase in complete wound closure was 27–33% (52% of PP placebo subjects or 85% of 0.03% DSC127 PP subjects healed; 46% of ITT placebo subjects or 73% of 0.03% DSC127 ITT subjects healed) even in the face of less rigorous clinical oversight and off-loading requirements after the first 12 weeks of study participation. This therapy has the potential to be an improvement over the current active therapy available to accelerate healing of DFU, Regranex.
Based on the homology of RAS expression in cutaneous tissue of all mammalian systems studied to date, including humans, topical administration of NorLeu3-A(1-7) should fulfill a wide variety of unmet clinical needs. Clinical studies have shown the pharmaceutical formulation of NorLeu3-A(1-7) delivered in an easily administered multiple use tube to be safe. Additional preclinical studies in other indications, including scar formation and radiation dermatitis, are now ongoing.
TAKE-HOME MESSAGES.
• DFUs are a significant clinical issue contributing to the healthcare system's economic and resource costs and reducing patient's quality of life.
• Significant morbidity is associated with the nonhealing DFU, including amputation and shortened life expectancy after amputation.
• Treatment options for the accelerated healing of DFUs currently on the market are limited.
• DSC127 and its active ingredient, NorLeu3-A(1-7), are potent stimulators of tissue repair and possibly regeneration leading to increased proportions of complete full-thickness wound healing.
• To date, DSC127 has been shown to be well tolerated in both preclinical and clinical studies. The Phase I clinical study showed no circulating levels of NorLeu3-A(1-7).
• DFU patients are being recruited into Phase III clinical trials for DSC127 (NCT01830348 and NCT01849965).
Abbreviations and Acronyms
- A(1-7)
angiotensin 1-7
- DFU
diabetic foot ulcer
- DSC127
clinical formulation of NorLeu3-A(1-7) in a preserved viscoelastic gel
- FDA
U.S. Food and Drug Administration
- ITT
intent-to-treat
- NorLeu3-A(1-7)
NorLeu3-angiotensin (1-7)
- PP
Per-Protocol
- RAS
rennin–angiotensin system
Acknowledgments and Funding Sources
This work was supported by the U.S. Department of Health and Human Services, National Institutes of Health 2R44DK076425, RC3 DK 088638, and Derma Sciences, Inc.
Author Disclosure and Ghostwriting
The authors are inventors on patents that protect the use of DSC127 in DFUs and/or consult for DermaSciences regarding clinical development. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Kathleen E. Rodgers, PhD, is an Associate Professor in the Titus Family Department of Clinical Pharmacy and Pharmacoeconomics, School of Pharmacy at the University of Southern California. Laura L. Bolton, PhD, is an Adjunct Associate Professor, Department of Surgery, Rutgers Robert Wood Johnson University Medical School, New Brunswick, New Jersey, USA, and co-chair, Association for the Advancement of Wound Care Guideline Task Force, Malvern, Pennsylvania, USA. Shelagh Verco, PhD, is the owner of Shelton Consulting who has experience in the management of preclinical and clinical development of products in several clinical indications, including dermal healing and DFUs. Gere S. diZerega, MD, is Professor in the Department of Obstetrics and Gynecology at the Keck School of Medicine at the University of Southern California.
References
- 1.Rodgers KE, Roda N, Felix JE, Espinoza T, Maldonado S, diZerega G. Histological evaluation of the effects of angiotensin peptides on wound repair in diabetic mice. Exp Dermatol 2003;12;784–790 [DOI] [PubMed] [Google Scholar]
- 2.Rodgers KE, Espinoza T, Felix J, Roda N, Maldonado S, diZerega G. Acceleration of healing, reduction of fibrotic scar and normalization of tissue architecture by angiotensin analog, NorLeu3-A(1-7). Plast Recon Surg 2003;111:1195–1206 [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Consensus development conference on diabetic foot wound care. Diabetes Care 1999;22:1354–1360 [DOI] [PubMed] [Google Scholar]
- 4.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–1724 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization Global status report on noncommunicable diseases 2010, description of the global burden of NCDs, their risk factors and determinants. www.who.int/nmh/publications/ncd_report_chapter1.pdf (last accessed March19, 2012)
- 6.Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999;22:382–387 [DOI] [PubMed] [Google Scholar]
- 7.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care 1990;13:513–521 [DOI] [PubMed] [Google Scholar]
- 8.Bhuvaneswar CG, Epstein LA, Stern TA. Reactions to amputation: recognition and treatment. Prim Care Companion J Clin Psychiatry 2007;9:303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mrug M, Stopka T, Julian BA, Prchal JF, Prchal JT. Angiotensin II stimulates proliferation of normal early erythroid progenitors. J Clin Invest 1997;100:2310–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodgers KE, Abiko M, Girgis W, St. Amand K, Campeau J, diZerega G. Acceleration of dermal tissue repair by angiotensin II. Wound Repair Regen 1997;5:175–183 [DOI] [PubMed] [Google Scholar]
- 11.Rodgers KE, Xiong S, Steer R, diZerega GS. Effect of angiotensin II on hematopoietic progenitor cell proliferation. Stem Cells 2000;18:287–294 [DOI] [PubMed] [Google Scholar]
- 12.Rodgers KE, Xiong S, diZerega GS. Effect of angiotensin II and angiotensin (1-7) on hematopoietic recovery after intravenous chemotherapy. Cancer Chemother Pharmacol 2003;51:97–106 [DOI] [PubMed] [Google Scholar]
- 13.Ellefson DD, diZerega GS, Espinoza T, Roda N, Maldonado S, Rodgers KE. Synergistic effects of co-administration of angiotensin 1-7 and Neupogen on hematopoietic recovery in mice. Cancer Chemother Pharmacol 2004;53:15–24 [DOI] [PubMed] [Google Scholar]
- 14.Rodgers KE, Ellefson DD, Espinoza T, Maulhardt H, Roda N, Maldonado S, et al. Fragments of Nle3-Angiotensin(1-7) accelerate healing in dermal models. J Peptide Res 2005;66 Suppl 1:41–47 [Google Scholar]
- 15.Steckelings UM, Wollschläger T, Peters J, Hermes B, Artuc M. Human skin: source of and target organ for angiotensin II. Exp Dermatol 2004;13:148–154 [DOI] [PubMed] [Google Scholar]
- 16.Steckelings UM, Henz BM, Wiehstutz S, Unger T, Artuc M. Differential expression of angiotensin receptors in human cutaneous wound. Br J Dermatol 2005;153:887–893 [DOI] [PubMed] [Google Scholar]
- 17.Abiko M, Rodgers KE, Campeau JD, Nakamura RM, diZerega GS. Alterations in angiotensin II receptor levels in full thickness excisional wounds in rat skin. Wound Repair Regen 1996;4:363–367 [DOI] [PubMed] [Google Scholar]
- 18.Abiko M, Rodgers KE, Campeau JD, Nakamura RM, diZerega GS. Alterations in angiotensin II receptor levels in sutured wounds in rat skin. J Invest Surg 1996;9:447–453 [DOI] [PubMed] [Google Scholar]
- 19.Kimura B, Sumners C, Phillips MI. Changes in skin angiotensin II receptors in rats during wound healing. BBRC 1992;187:1083–1090 [DOI] [PubMed] [Google Scholar]
- 20.Viswanathan M, Saavedra JM. Expression of Angiotensin II AT2 receptors in rat skin during experimental wound healing. Peptides 1992;13:783–736 [DOI] [PubMed] [Google Scholar]
- 21.Kurosaka M, Suzuki T, Hosono K, Kamata Y, Fukamizu A, Kitasato H, et al. Reduced angiogenesis and delay in wound healing in angiotensin II type 1a receptor-deficient mice. Biomed Pharmacother 2009;63:627–634 [DOI] [PubMed] [Google Scholar]
- 22.Zhang GY, Li X, Yi CG, Pan H, He GD, Yu Q, et al. Angiotensin II activated connective tissue growth factor and induces extracellular matrix changes involving Smad/activation and p38 mitogen-activated protein kinase signaling pathways in human dermal fibroblasts. Exp Dermatol 2009;18:947–953 [DOI] [PubMed] [Google Scholar]
- 23.Tang HT, Cheng DS, Jia YT, Ben DF, Ma B, Lv KY, et al. Angiotensin II induces type I collagen gene expression in human dermal fibroblasts through an AP-1/TGF-beta 1-dependent pathway. Biochem Biophys Res Commun 2009;385:418–423 [DOI] [PubMed] [Google Scholar]
- 24.Wu HJ, Liu HW, Cheng B, Gu YF, Xie B, Xiao LL, et al. The change in angiotensin II production and its receptor expression during wound healing: possible role of angiotensin II in wound healing. Zhonghua Zheng Xing Wai Ke Za Zhi 2011;27:124–128 [PubMed] [Google Scholar]
- 25.Jadhav SS, Sharma N, Meeks CJ, Mordwinkin NM, Espinoza TB, Roda NR, et al. Effects of combined radiation and burn injury on the renin-angiotensin system. Wound Repair Regen 2013;21:131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodgers KE, Xiong S, Felix JC, Roda N, Espinoza T, Maldonado S, et al. Development of angiotensin (1-7) as an agent to accelerate dermal regeneration. Wound Repair Regen 2001;9:241–250 [DOI] [PubMed] [Google Scholar]
- 27.Balingit PP, Armstrong DG, Reyzelman AM, Bolton L, Verco SJ, Rodgers KE, et al. Norleu3-A(1-7) stimulation of diabetic foot ulcer healing: Results of a randomized, parallel-group, double-blind, placebo-controlled phase 2 clinical trial. Wound Rep Regen 2012;20:482–490 [DOI] [PMC free article] [PubMed] [Google Scholar]