Abstract
Silencing specific gene expression by RNA interference (RNAi) has rapidly become a standard tool for reverse-genetic analysis of gene functions. It also has a tremendous potential in the treatment of diseases for which currently effective treatment is not available or is suboptimal. However, the poor cellular uptake of synthetic small interfering RNAs (siRNAs) is a major impediment for their clinical use. Great progress has been made in recent years to overcome this barrier and several methods have been described for in vivo delivery of siRNA. Moreover, latest advances have focused on achieving targeted siRNA delivery restricted to relevant tissues and cell types in vivo. These approaches are expected to reduce the dose requirement as well as minimize siRNA-induced toxicities, thereby advancing the field of siRNA therapy towards clinical use.
Keywords: RNA interference, small interfering RNA, therapeutic siRNA, delivery system, systemic delivery
Introduction
RNA interference (RNAi) is a recently discovered phenomenon where small double-stranded RNAs (dsRNAs) regulate specific gene expression. The biology and mechanism of RNAi have been extensively reviewed.1–3 Essentially, RNAi can be induced either by endogenously encoded small RNAs called microRNAs (miRNAs) or exogenously introduced small interfering RNAs (siRNAs). In either case, the 21–23 nucleotide dsRNAs associate in the cytoplasm with a protein complex called RNA-induced silencing complex (RISC), whereupon one of the two RNA strands is degraded and the other guide strand guides the RISC to mediate sequence-specific degradation of the corresponding mRNA (in the case of siRNAs) and/or translational repression by binding to the 3’ untranslated region (UTR) (in the case of miRNAs). In plants and worms, siRNAs can be generated by the processing of long double-stranded RNAs generated within the cell (for example following viral infection) by the cytoplasmic enzyme Dicer. However the main purpose of RNAi machinery in mammalian cells appears to be to generate small non-coding regulatory miRNAs, although endogenous siRNAs has also been reported to be produced in certain cell types such as mouse oocytes and embryonic stem cells.4, 5 However, the existence of RNAi machinery also makes it possible for exotic designer small RNAs [synthetic siRNA orsmall hairpin RNA (shRNA) - see Box1] to be used for silencing virtually any gene of interest in a sequence-specific manner. Ever since externally introduced double-stranded siRNAs were shown to silence specific gene expression in mammalian cells, there has been a tremendous interest in using them as a research tool as well as applying them as potential novel drugs for the treatment of diseases.6
Box 1. Synthetic siRNA versus vector driven shRNA.
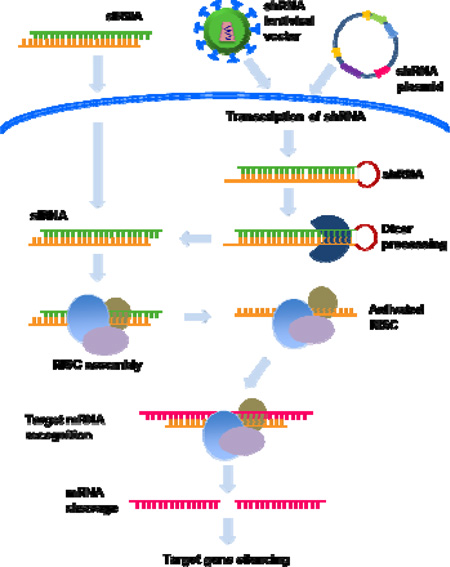
RNAi can be induced by the introduction of synthetic siRNA or by intracellular generation of siRNA from vector driven expression of the precursor small hairpin (sh) RNAs. In the latter method, an oligonucleotide containing the siRNA sequence followed by a ~9 nt loop and a reverse complement of the siRNA sequence is cloned in plasmid or viral vectors to endogenously express shRNA which is subsequently processed in the cytoplasm to siRNA. While synthetic siRNA is introduced to cells by transfection, shRNA can be introduced by transfection or transduction via viral vectors. Because of ease of delivery particularly in primary cells, non-replicating, recombinant viral vectors (such as adeno, retro and lentiviral vectors) are commonly used for shRNA expression. Since the viral DNA gets incorporated in the host genome, the main advantage of this method is the long-term expression of shRNAs and gene silencing.
Both shRNA and siRNA have their own advantages and limitations. Because the shRNA is endogenously produced, the gene-silencing effects are long lasting (weeks to months) whereas the synthetic siRNA effects are short lived (generally ~3–5 days) because of dilution with cell division and intracellular degradation. While long-term expression may be advantageous in chronic diseases, it may also be associated with several disadvantages including the vector induced immune response and interference with endogenous miRNA pathway leading to toxic effects. Moreover, retroviral integration into the host genome also enhances the risk of insertional mutagenesis, exemplified by the development of leukemia in patients undergoing retroviral-based therapy for severe combined immunodeficiency.77, 78 On the other hand synthetic siRNA, similar to drug treatment, provides a way to achieve transient gene silencing without the risks associated with shRNAs. The major disadvantage is delivery to cells in vivo. Also, as mentioned earlier, the silencing effect generally fades after 4–5 days in dividing cells, in non-dividing cells such as macrophages and neurons, siRNA silencing has been observed for at least 3 weeks.79, 80
Thus, while siRNA appears to be ideal for situations where short-term gene silencing is needed like in acute viral infection, shRNA may be useful to treat chronic viral infections and cancer. See Box Figure 1.
A number of studies have established the proof of concept that RNAi could be potentially used in the treatment of a variety of diseases, including cancer, viral infections, autoimmune diseases and neurodegenerative diseases.6, 7 Recent results from phase I and phase II clinical studies of siRNAs for age-related macular degeneration (AMD) and respiratory syncytial virus (RSV) infection have also demonstrated their therapeutic potential,8–10 although the specificity of siRNA-mediated anti-angiogenic effect in AMD has recently been questioned.11 Despite these rapid advances and their great potentiality, applying RNAi to humans in a clinical setting is significantly limited by the short serum half-life and poor cellular uptake of siRNA.12 Thus, delivering effective quantities of siRNAs into the right target cells in vivo through clinically feasible methods represents a major challenge for the successful development of RNAi-based therapeutics.13 In recent years, several delivery platforms have been developed that could revolutionize siRNA therapeutics. In this review, we will discuss the obstacles for siRNA delivery and the progress made in overcoming this barrier, focusing on the novel targeted delivery approaches that might facilitate the eventual clinical use of siRNAs. Although significant progress has also been made in the design and delivery of shRNA, we will confine our discussion in this review to highlight advances in synthetic siRNA delivery because the advances in shRNA delivery has been recently reviewed.14 We lay particular emphasis to discuss targeted in vivo siRNA delivery approaches which have the greatest potential for translation to human therapy.
Barriers to the delivery of siRNA in vivo
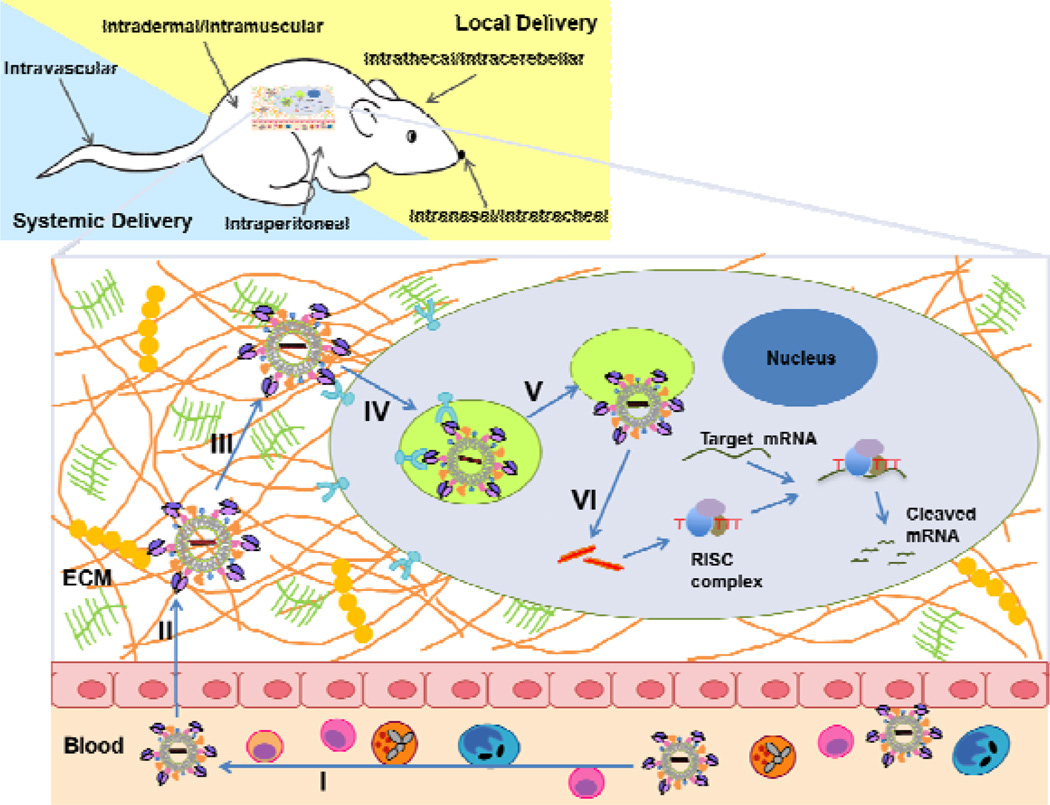
To mediate gene-silencing activity, intact double-stranded siRNAs have to be introduced into the cellular cytoplasm, where they can be recognized by the endogenous RNAi machinery and loaded onto RISC. Although small by nucleic acid standards, siRNAs are much larger molecules compared with typical small-molecule drugs (~13 kDa, about 50 times larger by molecular mass. The length of 19 base-paired siRNA with two-base overhangs is about 7 nm).15 Moreover, siRNAs also have a strong negative charge (~40 negative phosphate charges on the siRNA backbone) and thus they cannot readily cross biological membranes to be taken up by cells.16 Although some cell and tissue types spontaneously uptake siRNA without carriers via caveolin-mediated endocytosis at a very low efficiency,17 poor cellular uptake is the first major barrier for the use of siRNA, which limits its use even for local administration. In addition, many tissues in vivo can only be reached through systemic administration of siRNA via the blood stream. For an effective systemic delivery, the siRNA has to remain intact in the blood stream, extravasate through the vessels, diffuse through the extracellular matrix (ECM), penetrate the cellular membranes and be released into the cytoplasm. Thus, in addition to the poor cellular uptake, a series of other biological barriers stand between the systemically administered siRNAs and their target site inside the cells.18 (Fig. 1)
Figure 1.
In vivo application routes and key barriers of systemic siRNA delivery. SiRNA can be delivered in vivo by many routes either local administrations (intradermal, intramuscular, intrathecal, intracerebellar, intranasal, and intratracheal) or systemic administrations (intravascular, intraperitoneal) based on the disease types and targeted tissues. The in vivo application, especially systemic delivery of siRNA, is facing more challenges from multiple hurdles in the extracellular environment and various barriers for the intracellular uptake. The figure lists key barriers to effective in vivo delivery of siRNA: (I) rapid excretion via the kidney, degradation by serum and tissue nucleases, uptake by phagecytes, and immunogenicity in the blood stream, (II) failure to cross the capillary endothelium due to poor capillary permeability, (III) slow diffusion in the extracellular matrix, (IV) inefficient endocytosis and poor cellular uptake, (V) inefficient release from endosomes, and (VI) inefficient dissociation/decomplexation and release siRNA from delivery carrier. Addressing these issues is crucial for efficient in vivo delivery of siRNA for clinical use of RNAi as potential therapeutics.
As the main goal of in vivo delivery is to have intact and active siRNAs delivered to the cytoplasm of target cells, the stability of siRNA in the extracellular and intracellular environments is crucial. Naked siRNAs have a very short half-life of a few minutes in serum owing to degradation by ribonucleases (RNAses), rapid renal excretion, uptake by the reticuloendothelial system (RES) and aggregation with serum proteins.19 Some of these issues such as degradation by ribonucleases can be overcome by introducing chemical modifications such as a phosphorotioate backbone and 2’-sugar modifications that resist nuclease degradation, although their actual benefit for therapy has yet to be demonstrated.20 Even if they survive a while in the plasma, the next major barrier of siRNA delivery is the tight vascular endothelial wall. Generally, molecules larger than 5 nm in diameter do not readily cross the capillary endothelium.21 However, liver, spleen and some tumors have enhanced vascular permeability that allows the egress of macromolecules and nanoparticles up to approximately 200 nm in diameter, known as the enhanced permeation and retention (EPR) effect.22 After the siRNA complex passes through the vasculature, it must diffuse through the ECM, which is a dense network of polysaccharides and fibrous proteins that can hinder siRNA complex diffusion.23 Finally, when the siRNA complex reaches the target cells, cellular uptake of siRNA by endocytosis and exit from endosomes to reach the cytoplasm are the last barriers.24
Although successful in vivo delivery of siRNA has been reported using hydrodynamic injection in mice, it is not suitable for clinical application to humans because this harsh treatment requires the rapid injection of solutions two-and-a-half times the blood volume.25 Moreover, siRNAs can also induce toxicities (see later) and other side effects that can be reduced by limiting siRNA delivery to specific cell types. Thus, therapeutic applications of siRNAs require more effective delivery systems that allow siRNA stabilization, specific cell recognition, internalization and subcellular localization to the cytoplasm of target tissues and cells in vivo.26 These considerations would also minimize potential siRNA-induced toxicities.
Side effects of siRNA therapy
Although siRNAs have beneficial effects, they can also induce toxicities such as activation of innate immunity through induction of interferon responses as well as off-target gene silencing. siRNAs can potentially elicit interferon responses either through the cytosolic dsRNA-activated protein kinase, PKR, or binding to toll-like receptors (TLRs) 3 and 7 that recognize RNA on the cell surface or in endosomes.27 Certain nucleotide motifs such as 5'-UGUGU-3' or 5'-GUCCUUCAA-3' within siRNAs appear to be responsible for inducing interferon and interleukin production by plasmacytoid dendritic cells.27 Apart from immunostimulation, siRNAs can also induce off-target effects. The majority of off-target effects are caused by small regions of seed sequence homology to the 3' untranslated region (UTR) of cellular mRNAs. Large amounts of siRNAs can also potentially affectcellular miRNA activity by saturating the RISC. Some of the toxicities can be avoided by better design of siRNAs to reduce the amounts required and by restricting the delivery to only the desired tissue and/or specific cell types. In addition, non-selective systemic delivery approaches result in nonspecific distribution of siRNAs throughout the body, thereby significantly decreasing the local concentration in the desired tissue and thus requiring large amounts of siRNA for gene silencing in vivo. In addition to the siRNA-induced toxicities, systemic administration of siRNA carriers could also induce cytotoxic effects and vector-directed immune responses that could jeopardize therapy. Thus, there has been a great deal of interest in developing targeted delivery systems that reduce the effective dose as well as toxicities in bystander cells of non-target tissues.28
Targeted siRNA delivery
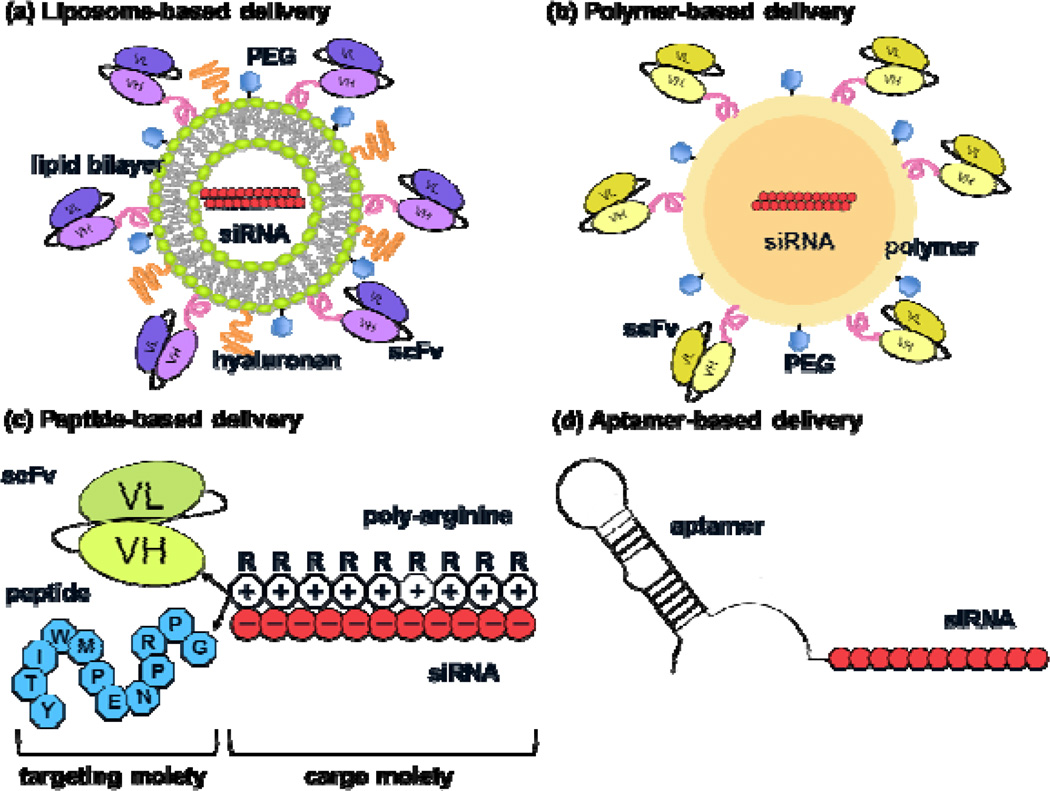
Specific tissues or cell types can be selectively targeted using cell-type-specific affinity ligands such as antibodies, peptides or aptamers. Recent advances have led to the identification of various tissue- and cell-specific markers that can be exploited for siRNA delivery.29 The key here is that the cellular receptors should be readily internalized after ligand binding as well as rapidly re-expressed on the cell surface to allow repeated targeting as well as to avoid prolonged disruption of their normal ligand binding functions. Another important consideration for using cell surface receptors for siRNA delivery is to engineer the ligands to enable siRNA ‘piggybacking’ without disrupting the receptor binding properties. Finally, the carrier should stabilize the siRNA to enable sufficient circulation half-life. Several novel approaches have been developed to achieve this, and generally these delivery vehicles comprise a targeting and a cargo moiety (Fig. 2). SiRNAs can be packaged inside nanoparticles made of liposomes or other polymers and the surface of these particles can be modified to incorporate specific targeting ligands. Alternatively, specific ligands or cell penetrating peptides such as HIV tat peptide can be covalently bound to siRNAs (Fig. 2).30 The details of different approaches for siRNA delivery are discussed below (Table 1).
Figure 2.
Schematic of strategies for targeted in vivo siRNA delivery. Essentially, these delivery vehicles comprise a targeting and a cargo moiety. In a liposome-based delivery vehicle (a), the siRNA is encapsulated within the aqueous core of a uni- or multi-lamellar lipid bilayer, and the outer surface of the lipid is conjugated with stabilizing molecules such as PEG and a targeting agent such as an antibody to a specific cell-surface antigen. Liposome-based delivery vehicle showed a superior capacity to entrap ~4000 siRNA molecules per particle as compared to an antibody-protamine fusion protein, which carried five siRNA molecules per fusion protein.39 In a polymeric delivery vehicle (b), the siRNA is condensed within different kinds of cationic polymers such as PEI, chitosan and cyclodextrin that form nanoparticles, and the surface of the nanoparticles is decorated with PEG and targeting moieties. In peptide-based delivery systems (c), the siRNA is either covalently conjugated with or noncovalently bound to a positively charged peptide such as poly-arginine, protamine or TAT and additional targeting peptide or antibody moieties conjugated to the cationic peptide. In aptamer-based vehicles (d), the cell-targeting aptameric RNA is directly conjugated with the siRNA.
Table 1.
Characteristics of non-viral siRNA delivery vehicles
| Vehicle | Aptamer | Cationic peptide | Polymer | Liposome |
|---|---|---|---|---|
| Capacity (siRNA/delivery molecule) | 1 | Several | ~several thousands | ~several thousands |
| Cellular internalization | +/− | + | + | + |
| Transfection efficiency | +/− | + | + | + |
| Potential cytotoxicity | − | − | +/− | +/− |
| Protection against degradation | − (chemical modification needed) | + | + | + |
| Surface modification | − | − | ++ | ++ |
| Mode of complexation | Direct conjugation | Ionic interaction/condensation | Condensation/encapsulation | Encapsulation |
| Ease of production | ++ | ++ | + | + |
| Biodegradability | ++ | ++ | +/− | ++ |
| Controlled release | − | − | +/− | − |
| Targeted delivery | yes | yes | yes | Yes |
| Duration of silencing | Transient | Transient | Transient | Transient |
| Immunogenicity | low | low | low | Low |
−, low; +/−, moderate; +, high; ++, very high
In vivo siRNA delivery systems
Ideally, a delivery system should have the following characteristics: 1) be biocompatible (non-cytotoxic and non-immunogenic) and biodegradable, 2) allow protection from nucleases during transit through the circulation and on release into endosomes, 3) avoid rapid clearance by the RES, and 4) be capable of binding siRNAs in a reversible manner to ensure subsequent efficient release of the siRNAs at the target site.31 A number of delivery carriers have been developed for improved in vivo delivery of siRNA (Table 2). As siRNAs are negatively charged and readily bind to cationic molecules, delivery carriers usually consist of cationic polymers,32 peptides33, 34 or liposomes35 that form complexes by ionic interactions. The resulting complexes can provide excellent protection for siRNA from nuclease attack and facilitate cellular uptake via the endocytic pathway. The global positive charge of carrier–siRNA complexes improves the cellular uptake through interactions with the negative charges on cell membranes.36 However, many cationic agents used for condensing siRNAs have often exhibited significant cytotoxicity, which can limit clinical applications and the cause of eliciting cytotoxicity has not been understood yet.37 By contrast, direct conjugation of siRNA to various carriers such as peptides, aptamers and cholesterol has also been used to avoid toxicity.38
Table 2.
Targeted siRNA delivery in vivo
| Formulation | Targeting moiety | Target gene product |
Delivery route |
Disease model | Reference |
|---|---|---|---|---|---|
| Liposomes | |||||
| Cationic liposome | Antibody to transferrin receptor | Her-2 | Intravascular | Breast cancer, Pancreatic cancer | 45, 46 |
| PEGylated liposome | Antibody to insulin and transferrin receptor | Luciferase, EGF receptor | Intravascular | Brain cancer | 44 |
| Stabilized liposome | Antibody to beta7 integrin | Cyclin D1 | Intravascular | Gut inflammation | 39 |
| DOTAP:Chol liposome | Transferrin | Cy3-siRNA, Luciferase | Intracerebellar | – | 81 |
| Polymers | |||||
| PEI nanoparticle | Folate | Survivin | Cell mediated | Nasopharyngeal epidermal carcinoma | 55 |
| Cyclodextrin nanoparticle | Antibody to transferrin receptor | Ews-Fli1 | Intravascular | Ewing’s sarcoma | 65 |
| Peptides | |||||
| Protamine | HIV envelope gp160, ErbB2 antibody Fab | c-myc, VEGF, MDM2, Ku70 | Intratumoral, intravascular | gp160-expressing melanoma cells, breastcancer | 68 |
| Protamine | LFA-1 antibody scFv | Cy3-siRNA | Intravascular | K562 xenograft | 69 |
| Nona-D-arginine | RVG | JEV envelope | Intravascular | JEV infection | 33 |
| Nona-D-arginine | CD7 antibody scFv | vif, tat, ccr5 | Intravascular | HIV infection | 34 |
| Aptamers | |||||
| Aptamer | PSMA | Plk1, Bcl-2 | Intratumoral | Prostate cancer | 73, 74 |
Abbreviations: Chol, cholesterol; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; EGF, epidermal growth factor; MDM2, murine double minute 2; VEGF, vascular endothelial growth factor.
Liposomes and lipid-like materials
The most common approach for nucleic acid delivery to cells in vitro is to use lipid-based transfection reagents. Cationic lipids in these reagents provide a suitable platform for incorporating negatively charged siRNA with a superior payload compared with that of other delivery materials.39 Some of the conventional transfection reagents have also been used for siRNA delivery in vivo, particularly for delivery at local and mucosal sites. For example, intranasal administration of siRNA complexed with oligofectamine in mice resulted in effective delivery to the lungs for protection against influenza virus infection.40 Similarly, intravaginal application of siRNA mixed with oligofectamine effectively induced protection against herpes simplex virus (HSV) infection in mice.41 Brain-compatible cationic liposomes, i-FECT and JetSI/DOPE have also been used for intracranial administration of siRNA to protect mice from viral encephalitis.42 However, the cationic lipid-based reagents are generally considered too toxic for systemic siRNA delivery in vivo. Despite this, liposomes offer a number of advantages, such as protection from nucleases and easy penetration into cell membranes, making them a promising siRNA delivery vehicle. Recent interest in this field has been focused on developing novel non-toxic liposomal delivery vehicles. Liposomes also offer the additional advantage of incorporating molecules on their surface such as polyethylene glycol (PEG) to enhance circulation half-life by reducing aggregation and elimination by RES. In addition, incorporation of cell-targeting agents such as antibodies or other ligands can enable targeted delivery to desired cell types. This approach has been demonstrated by the use of PEGylated liposomes coated with an antibody against receptors for transferrin and/or insulin for targeted siRNA delivery to brain in the mouse and monkey models.43, 44 In addition, an immunoliposome coated with antibody to transferrin has been used to deliver siRNA against human epidermal growth factor receptor-2 (HER-2, also known as ERBB2) to tumor cells in vivo, which resulted in re-sensitizing pancreatic and breast cancer cells to chemotherapeutic drugs.45, 46
Another significant advance in this field has been the development of stable nucleic acid–lipid particles (SNALPs) consisting of a mixture of cationic and fusogenic lipids that enables the cellular uptake and endosomal release of the particle's nucleic acid payload.47 ApoB-specific siRNAs encapsulated within SNALPs were effective in reducing ApoB and serum cholesterol levels in mice and monkeys. Moreover, this delivery method required a clinically acceptable dose of 2.5 mg/kg siRNA. SNALPs have also been used to suppress hepatitis B virus (HBV) and Ebola virus infection in animal models.47, 48 Further advancement of liposomal carriers of siRNA has been achieved by the use of neutral phospholipids to circumvent the potential toxicity common to cationic lipids for systemic siRNA delivery. In an interesting study, these liposomes were stabilized with a hyaluronan coating and were also coated with an antibody against beta7 integrin for targeting mucosal leukocytes.39 Intravenous injection of siRNA targeting the cell cycle regulator cyclin D1 entrapped within this delivery vehicle effectively silenced cyclin D1, suppressed leukocyte proliferation and reversed experimentally induced colitis in mice. A new chemistry to synthesize lipid-like molecules called lipidoids has also been described recently to overcome the disadvantages of cationic liposomes.49 In this, a library of lipidoids was generated through the conjugation of alkyl-acrilates and amides to primary and secondary amines that can be screened for siRNA delivery in vivo. Using this technology, some lead candidates that can effectively deliver siRNA to liver and lungs in mice and monkeys have been identified. These advances offer new hopes for developing liposomes as clinically viable delivery vehicles.
Cationic polymers
Similar to liposomes, cationic polymers can also serve as efficient transfection reagents because they can bind and condense nucleic acids into stabilized nanoparticles. Polyethyleneimine (PEI) is probably the most studied and characterized polymer for nucleic acid delivery. PEI is a synthetic polymer that has been used in branched or linear forms of different lengths for nucleic acid delivery.50 PEI has also been used for in vivo siRNA delivery at local sites as well as systemically. Intrathecal delivery of PEI-conjugated siRNA has been used for effectively knocking down the pain receptor for N-methyl-D-aspartate (NMDA) in rats.51 Similarly, PEI-conjugated siRNA has achieved suppression of influenza virus infection in mice.52 PEI has also been employed for siRNA delivery by injection into breast tumors in mice.53 The free amino groups on PEI also allow modifications with PEG and targeting moieties. Thus, a number of studies have used PEI conjugated with ligands for molecules highly expressed on cancer cells such as the folate receptor (FOLR1),54, 55 transferrin receptor (TFR1),56 ERBB2/HER-257 or epidermal growth factor receptor (EGFR).58 Systemic administration of siRNA targeting survivin packaged within PEI nanoparticles, conjugated with PEG and folate inhibited growth of transplanted human epidermal carcinoma cells in mice.55 Similarly, PEGylated RGD peptide (recognizing integrins) conjugated to PEI has been used for siRNA delivery to cancer cells.59 However, as with liposomes, PEI is also considered too toxic for systemic delivery, although efforts are being made to reduce such toxicities by modifying the structure of PEI such as using oleic or stearic acid modified branched PEI or using linear instead of branched PEI.60, 61
Chitosan, a natural polysaccharide, also has many advantages as a siRNA carrier, including positive charge and biodegradability.62 Effective in vivo silencing of green fluorescent protein (GFP) has been achieved in bronchiolar epithelial cells of transgenic GFP mice after intranasal administration of chitosan–siRNA formulations.63 Another study also showed efficient knockdown of tumor necrosis factor-α (TNF-α) expression in peritoneal macrophages by intraperitoneal administration of chitosan–siRNA nanoparticles in mice to downregulate systemic and local inflammation.62 In another study, intranasal administration of chitosan polymeric nanoparticles was used for siRNA delivery to the lungs for protection against RSV infection.64
The cyclic oligosaccharide, cyclodextrin-based nanoparticles provide another non-toxic polymeric delivery vehicle for siRNA delivery. These nanoparticles can be coated with PEG and targeting ligands. In a recent study, cyclodextrin nanoparticles were conjugated with transferrin to target cells bearing the transferrin receptor. Intravenous administration of these nanoparticles containing a siRNA to target the Ewing sarcoma Ews-Fli1 oncogene product resulted in a dramatic reduction of tumor growth in a metastatic Ewing’s sarcoma model in mice.65
Another major advance in the field is the development of d-glucan-based micrometer-sized particles suitable for oral delivery of siRNA.66 Here, porous 2–4 µm-sized shells comprising primarily beta1,3-d-glucan were generated by treating baker's yeast with a series of alkaline, acid and solvent extractions and siRNAs condensed with PEI were packed inside the shells. Upon oral administration of this material in mice, macrophages within the intestine efficiently phagocytosed the particles and circulated throughout the body. Moreover, siRNA in as small a dose as 20 µg/kg (100 fold less than the doses generally used in other systemic administration studies) efficiently silenced TNF production and protected mice from lipopolysaccharide (LPS)-induced lethality. These advances in nanoparticle formulations also appears to have greatscope for systemic siRNA delivery.
Cationic peptides
The natural cationic protein protamine (which nucleates DNA in the sperm) has long been used to deliver DNA into cells because of its ability to bind with and condense the negatively charged nucleic acids.67 Recently a protamine-antibody fusion protein has been used for targeted siRNA delivery to specific cell types in vivo.68 In this study, the protamine moiety was linked to a Fab antibody heavy chain fragment against extracellularly displayed human immunodeficiency virus 1 (HIV-1) envelope glycoprotein gp160 to selectively deliver siRNA to HIV-infected cells. This recombinant protein was able to non-covalently bind siRNA by charge interaction. Moreover, antiviral siRNAs delivered using this vehicle inhibited HIV replication in the hard-to-transfect HIV-infected primary T cells. In the same study, cell-specific delivery and gene silencing were also obtained with the protamine–single-chain-antibody fusion protein that targeted ErbB2-expressing breast cancer cells. Systemic delivery of a cocktail of anti-tumor siRNAs targeting c-myc, MDM2 and VEGF, bound to the delivery vehicle effectively suppressed the growth of transplanted tumors in mice. Similarly, a single chain antibody variable fragment (scFv)–protamine fusion protein targeting the leukocyte-specific lymphocyte function-associated antigen-1 (ITGAL/LFA-1) integrin also delivered siRNAs to various populations of primary blood cells including lymphocytes, monocytes and dendritic cells in vivo in mice.69
Another promising group of cationic peptides is the so-called cell-penetrating peptides (CPPs) that can enhance cellular uptake of a wide range of macromolecules. Various CPPs, including TAT and MPG proteins from HIV-1, penetratin and polyarginine, have been used for the delivery of proteins as well as nucleic acid payloads into cells.70, 71 In a recent study, cholesterol-conjugated ninemer arginine (9R) peptide was shown to deliver siRNA to a transplanted tumor in mice.72 Using 9R peptide as a siRNA carrier, a common platform has recently been developed for targeted siRNA delivery to specific tissues and cell types in vivo. In this, a peptide or an antibody fragment that binds to a specific cell-surface protein is conjugated to the 9R peptide, with the idea that the former will permit specific cell targeting and the latter will allow piggybacking of the siRNA for internalization following ligand binding of the cellular receptor. In one study, a synthetic peptide was used derived from the Rabies viral envelope glycoprotein (RVG) that binds to the acetylcholine receptor expressed by (both mouse and human) neuronal cells as a neuronal-cell-targeting ligand.33 This peptide was fused to 9R residues for siRNA binding. Systemic administration of antiviral siRNA bound to this chimeric RVG-9R peptide efficiently protected mice from fatal Japanese encephalitis virus (JEV) infection. Another study, this time targeting human T cells, conjugated the 9R peptide to a scFv that binds to the CD7 antigen.34 Systemic administration of a mixture of siRNAs targeting the cellular coreceptor CCR5 and the HIV tat and vif genes using this reagent was able to protect humanized mice from HIV-1 infection by effectively suppressing HIV viremia and T-cell depletion. The CD7-specific antibody appears to be well suited for clinical development because this antibody has already been used in clinical studies to target toxins to T-cell lymphomas and leukemias.12 In these systems, siRNA binding is achieved simply by mixing the siRNA with the reagent and this non-covalent binding is enough to protect siRNA against degradation by serum nucleases. Thus, the simplicity of peptide-based methods would be a great advantage for systemic siRNA delivery compared to the other methods described.
Aptamers
Another technology for targeted delivery is based on aptamer–siRNA chimeric RNAs.73 Aptamers are synthetically prepared small, highly structured nucleic acid molecules that bind to specific target molecules by providing a limited number of specific contact points embedded in a larger, defined three-dimensional structure. Aptamers have been linked to siRNA, on the premise that the aptamer carries the siRNA into the cell after binding with a specific cellular receptor and subsequent internalization. For example, an aptamer that binds to prostate-specific membrane antigen (PSMA, a cell-surface antigen overexpressed in prostate cancer cells and tumor vascular endothelium) was covalently linked to siRNA targeting pro-survival genes Plk1 and Bcl-2.73 Intratumoral injection of this aptamer effectively delivered siRNA specifically to tumor cells in a mouse xenograft model, resulting in triggering of apoptosis, growth inhibition and tumor regression. In a related study, a modular streptavidin bridge was used for connecting siRNAs against lamin A/C or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to the PSMA-targeting aptamer.74 This system also induced silencing of the targeted genes only in cells expressing the PSMA receptor.
An aptamer-based delivery system has also been used to suppress HIV infection. In this case, anti-gp120 RNA aptamer was covalently conjugated with one strand of a 27-mer anti-HIV siRNA, and the second siRNA strand was annealed to the first strand. This vehicle was able to deliver the attached siRNA to HIV-infected cells and, moreover, the siRNA was efficiently processed by Dicer and silenced HIV replication in cell lines.75 In a recent development, a 'sticky' sequence has been introduced to another gp120 aptamer that facilitates attachment of multiple siRNAs to a single aptamer.76 A key advantage of the aptamer-based systems is that the targeting reagents can be produced in a simple invitro transcription reaction and would therefore be free of contaminating cell or bacterial products that can be a problem with protein-based delivery systems.
Concluding remarks
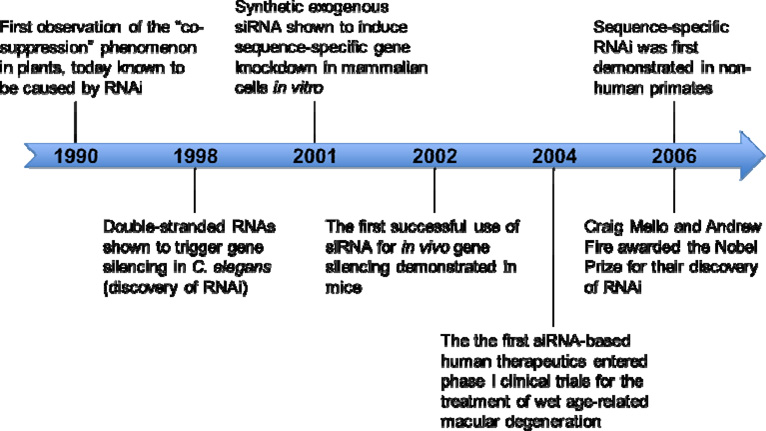
The field of RNAi is moving forward at a remarkable pace (See Box 2). Because of its ability to induce transient and reversible effects, siRNAs offer a drug-like approach for disease treatment and, thus, several clinical trials are being conducted to assess the safety and efficacy of this approach (Table 3). However, currently the siRNAs being tried are mostly aimed at delivering siRNAs at local sites, such as the eye and lungs. The success of systemic siRNA treatment can only truly be realized after effective delivery strategies are developed that are acceptable for human use.
Box 2. Timeline of RNAi discovery.
This timeline highlights some of the most important milestones in the discovery of RNAi. See Box Figure 2.
Table 3.
siRNAtherapeutics in clinical trials
| Company | Product | Target | Disease | Delivery | Status |
|---|---|---|---|---|---|
| Acuity Pharmaceuticals | Bevasiranib | VEGF | Age-related macular degeneration | Intravitreal | Phase III |
| Acuity Pharmaceuticals | Bevasiranib | VEGF | Diabetic macular oedema | Topical | Phase II |
| Aegera Therapeutics | AEG35156 | X-linked inhibitor of apoptosis protein | Chronic lymphocytic leukemia, non-small cell lung cancer, pancreatic cancer | Systemic | Phase I/II |
| Allergan/Merck | AGN211745 | VEGF receptor-1 | Age-related macular degeneration | Intravitreal | Phase II |
| Alnylam Pharmaceuticals | ALN-RSV01 | Respiratory syncytial virus | Intranasal or inhalation | Phase II | |
| Benitec/City of Hope | sidrzT | HIV/AIDS | Lentiviral vector | Phase I | |
| Calando Pharmaceuticals | CALAA-01 | Ribonucleotide reductase M2 | Solid tumors | Systemic | Phase I |
| Merck/Sirna Therapeutics | Sirna-027 | Age-related macular degeneration | Phase II | ||
| Nucleonics | NUC B1000 | Hepatitis B virus | Systemic | Phase I | |
| QuarkPharma./Pfizer | RTP801i-14 | RTP801 | Age-related macular degeneration | Intravitreal | Phase I/II |
| Quark Pharma./Pfizer | RTP801i-14 | RTP801 | Diabetic macular oedema | Topical | Phase II |
| Quark Pharmaceuticals | AKIi-5 | p53 | Acute renal failure | Systemic | Phase I |
| Santaris Pharmaceuticals | SPC3649 | Hepatitis C virus | Phase I | ||
| Senetek PLC | ATN-RNA | Glioblastoma multiforme | Phase I | ||
| TransDerm Inc. | TD101 | Keratin 6a (K6a) N171K mutant | Pachyonychia congenita | Intradermal | Phase Ib |
Although the recent description of multiple targeted delivery systems heralds future therapeutic applications, there are still a number of concerns and scope for improvement. These include the efficacy levels of in vivo delivery and possible competition with endogenous cellular RNAi components. In addition, the possibility of toxicities and immune responses to the vehicle component as well as to the targeting component needs better evaluation. Many of the current delivery vehicles require many components and multiple, laborious assembly steps to complex siRNA in the vehicle. In this regard, peptide-based systems might have an edge as they can be easily synthesized and also, by being relatively small, are unlikely to induce an immune response. Although the mouse-derived targeting antibodies can themselves induce an immune response, this can possibly be minimized by ‘humanizing’ the antibody, and many such antibodies are in human use. The future will also see advances in the development of non-toxic liposomal and other forms of nanoparticle-based approaches, for, in this case, the vehicle allows relatively large amounts of siRNAs to be packaged. The recent description of an oral delivery system is a significant advance that needs to be evaluated further. In conclusion, the exciting advances reviewed here point to a bright future for siRNA-based therapeutics.
Box 3. Outstanding questions.
-
-
What is the mechanism and route by which siRNA is delivered into the cytoplasm of the cells?
-
-
Does thistechnology using antibody–receptor mediated targeting have a good enough specificity for target cell types with therapeutic relevance in vivo?
-
-
What is the required siRNA level needed for successful RNAi therapy and is it possible to reach the therapeutic levels in vivo?
-
-
How can the cytotoxicity of non-viral delivery vehicles (especially cationic agents) be minimized without disrupting the silencing effectiveness in vivo?
-
-
Will future improvements make the antibody-targeted siRNA delivery vehicles safe for long-term repeated administration? (Especially from potential immunogenicity of the antibody or antibody–siRNA complex)
-
-
From a commercial viewpoint, is it truly feasible to produce and use a drug consists of synthetic siRNA and nanoparticles conjugated with recombinant antibody fusion protein in a clinical setting?
Acknowledgements
This work was supported by NIH grant AI075419 to NM and KRF & MOEHRD grant KRF-2006-352-D00070 to SSK.
References
- 1.Kim DH, Rossi JJ. Overview of gene silencing by RNA interference. Curr Protoc Nucleic Acid Chem. 2009;Chapter 16(Unit 16):11. doi: 10.1002/0471142700.nc1601s36. [DOI] [PubMed] [Google Scholar]
- 2.Kim D, Rossi J. RNAi mechanisms and applications. Biotechniques. 2008;44:613–616. doi: 10.2144/000112792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Fougerolles A, et al. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe T, et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 2008;453:539–543. doi: 10.1038/nature06908. [DOI] [PubMed] [Google Scholar]
- 5.Babiarz JE, et al. Mouse ES cells express endogenous shRNAs, siRNAs, and other Microprocessor-independent, Dicer-dependent small RNAs. Genes Dev. 2008;22:2773–2785. doi: 10.1101/gad.1705308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457:426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manjunath N, et al. Interfering antiviral immunity: application, subversion, hope? Trends Immunol. 2006;27:328–335. doi: 10.1016/j.it.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Check E. A crucial test. Nat Med. 2005;11:243–244. doi: 10.1038/nm0305-243. [DOI] [PubMed] [Google Scholar]
- 9.DeVincenzo J, et al. Evaluation of the safety, tolerability and pharmacokinetics of ALN-RSV01, a novel RNAi antiviral therapeutic directed against respiratory syncytial virus (RSV) Antiviral Res. 2008;77:225–231. doi: 10.1016/j.antiviral.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Chappelow AV, Kaiser PK. Neovascular age-related macular degeneration: potential therapies. Drugs. 2008;68:1029–1036. doi: 10.2165/00003495-200868080-00002. [DOI] [PubMed] [Google Scholar]
- 11.Kleinman ME, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirchhoff F. Silencing HIV-1 In Vivo. Cell. 2008;134:566–568. doi: 10.1016/j.cell.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Grimm D, Kay MA. Therapeutic application of RNAi: is mRNA targeting finally ready for prime time? J Clin Invest. 2007;117:3633–3641. doi: 10.1172/JCI34129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manjunath N, et al. Lentiviral delivery of short hairpin RNAs. Adv Drug Deliv Rev. 2009 doi: 10.1016/j.addr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akhtar S, Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117:3623–3632. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59:75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Detzer A, et al. Phosphorothioate-stimulated cellular uptake of siRNA: a cell culture model for mechanistic studies. Curr Pharm Des. 2008;14:3666–3673. doi: 10.2174/138161208786898770. [DOI] [PubMed] [Google Scholar]
- 18.Juliano R, et al. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 2008;36:4158–4171. doi: 10.1093/nar/gkn342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dykxhoorn DM, et al. The silent treatment: siRNAs as small molecule drugs. Gene Ther. 2006;13:541–552. doi: 10.1038/sj.gt.3302703. [DOI] [PubMed] [Google Scholar]
- 20.Lewis DL, et al. Efficient delivery of siRNA for inhibition of gene expression in postnatal mice. Nat Genet. 2002;32:107–108. doi: 10.1038/ng944. [DOI] [PubMed] [Google Scholar]
- 21.Rippe B, et al. Transendothelial transport: the vesicle controversy. J Vasc Res. 2002;39:375–390. doi: 10.1159/000064521. [DOI] [PubMed] [Google Scholar]
- 22.Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J Drug Target. 2007;15:457–464. doi: 10.1080/10611860701539584. [DOI] [PubMed] [Google Scholar]
- 23.Zamecnik J, et al. Extracellular matrix glycoproteins and diffusion barriers in human astrocytic tumours. Neuropathol Appl Neurobiol. 2004;30:338–350. doi: 10.1046/j.0305-1846.2003.00541.x. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira S, et al. Fusogenic peptides enhance endosomal escape improving siRNA-induced silencing of oncogenes. Int J Pharm. 2007;331:211–214. doi: 10.1016/j.ijpharm.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 25.Song E, et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- 26.Fattal E, Bochot A. State of the art and perspectives for the delivery of antisense oligonucleotides and siRNA by polymeric nanocarriers. Int J Pharm. 2008;364:237–248. doi: 10.1016/j.ijpharm.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Hornung V, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 28.Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genet. 2007;8:173–184. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- 29.Liu B. Exploring cell type-specific internalizing antibodies for targeted delivery of siRNA. Brief Funct Genomic Proteomic. 2007;6:112–119. doi: 10.1093/bfgp/elm015. [DOI] [PubMed] [Google Scholar]
- 30.Chiu YL, et al. Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells. Chem Biol. 2004;11:1165–1175. doi: 10.1016/j.chembiol.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Rossi JJ. Receptor-targeted siRNAs. Nat Biotechnol. 2005;23:682–684. doi: 10.1038/nbt0605-682. [DOI] [PubMed] [Google Scholar]
- 32.Urban-Klein B, et al. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12:461–466. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- 33.Kumar P, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 34.Kumar P, et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577–586. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmermann TS, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 36.Kawakami S, Hashida M. Targeted delivery systems of small interfering RNA by systemic administration. Drug Metab Pharmacokinet. 2007;22:142–151. doi: 10.2133/dmpk.22.142. [DOI] [PubMed] [Google Scholar]
- 37.Jeong JH, et al. siRNA conjugate delivery systems. Bioconjug Chem. 2009;20:5–14. doi: 10.1021/bc800278e. [DOI] [PubMed] [Google Scholar]
- 38.Soutschek J, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 39.Peer D, et al. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tompkins SM, et al. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc Natl Acad Sci U S A. 2004;101:8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palliser D, et al. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439:89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- 42.Kumar P, et al. A single siRNA suppresses fatal encephalitis induced by two different flaviviruses. PLoS Med. 2006;3:e96. doi: 10.1371/journal.pmed.0030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pardridge WM. shRNA and siRNA delivery to the brain. Adv Drug Deliv Rev. 2007;59:141–152. doi: 10.1016/j.addr.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, et al. Intravenous RNA interference gene therapy targeting the human epidermal growth factor receptor prolongs survival in intracranial brain cancer. Clin Cancer Res. 2004;10:3667–3677. doi: 10.1158/1078-0432.CCR-03-0740. [DOI] [PubMed] [Google Scholar]
- 45.Pirollo KF, et al. Tumor-targeting nanoimmunoliposome complex for short interfering RNA delivery. Hum Gene Ther. 2006;17:117–124. doi: 10.1089/hum.2006.17.117. [DOI] [PubMed] [Google Scholar]
- 46.Hogrefe RI, et al. Chemically modified short interfering hybrids (siHYBRIDS): nanoimmunoliposome delivery in vitro and in vivo for RNAi of HER-2. Nucleosides Nucleotides Nucleic Acids. 2006;25:889–907. doi: 10.1080/15257770600793885. [DOI] [PubMed] [Google Scholar]
- 47.Morrissey DV, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 48.Ma Z, et al. Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem Biophys Res Commun. 2005;330:755–759. doi: 10.1016/j.bbrc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 49.Akinc A, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shim MS, Kwon YJ. Controlled delivery of plasmid DNA and siRNA to intracellular targets using ketalized polyethylenimine. Biomacromolecules. 2008;9:444–455. doi: 10.1021/bm7007313. [DOI] [PubMed] [Google Scholar]
- 51.Tan PH, et al. Gene knockdown with intrathecal siRNA of NMDA receptor NR2B subunit reduces formalin-induced nociception in the rat. Gene Ther. 2005;12:59–66. doi: 10.1038/sj.gt.3302376. [DOI] [PubMed] [Google Scholar]
- 52.Thomas M, et al. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci U S A. 2005;102:5679–5684. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiu SJ, et al. Tumor-targeted gene delivery via anti-HER2 antibody (trastuzumab, Herceptin) conjugated polyethylenimine. J Control Release. 2004;97:357–369. doi: 10.1016/j.jconrel.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 54.Kim SH, et al. Comparative evaluation of target-specific GFP gene silencing efficiencies for antisense ODN, synthetic siRNA, and siRNA plasmid complexed with PEI-PEG-FOL conjugate. Bioconjug Chem. 2006;17:241–244. doi: 10.1021/bc050289f. [DOI] [PubMed] [Google Scholar]
- 55.Guo S, et al. Construction of folate-conjugated pRNA of bacteriophage phi29 DNA packaging motor for delivery of chimeric siRNA to nasopharyngeal carcinoma cells. Gene Ther. 2006;13:814–820. doi: 10.1038/sj.gt.3302716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pirollo KF, et al. Materializing the potential of small interfering RNA via a tumor-targeting nanodelivery system. Cancer Res. 2007;67:2938–2943. doi: 10.1158/0008-5472.CAN-06-4535. [DOI] [PubMed] [Google Scholar]
- 57.Park JW, et al. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8:1172–1181. [PubMed] [Google Scholar]
- 58.Mamot C, et al. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65:11631–11638. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- 59.Schiffelers RM, et al. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32:e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alshamsan A, et al. Formulation and delivery of siRNA by oleic acid and stearic acid modified polyethylenimine. Mol Pharm. 2009;6:121–133. doi: 10.1021/mp8000815. [DOI] [PubMed] [Google Scholar]
- 61.Zintchenko A, et al. Simple modifications of branched PEI lead to highly efficient siRNA carriers with low toxicity. Bioconjug Chem. 2008;19:1448–1455. doi: 10.1021/bc800065f. [DOI] [PubMed] [Google Scholar]
- 62.Howard KA, et al. Chitosan/siRNA nanoparticle-mediated TNF-alpha knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model. Mol Ther. 2009;17:162–168. doi: 10.1038/mt.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howard KA, et al. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol Ther. 2006;14:476–484. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 64.Zhang W, et al. Inhibition of respiratory syncytial virus infection with intranasal siRNA nanoparticles targeting the viral NS1 gene. Nat Med. 2005;11:56–62. doi: 10.1038/nm1174. [DOI] [PubMed] [Google Scholar]
- 65.Hu-Lieskovan S, et al. Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing's sarcoma. Cancer Res. 2005;65:8984–8992. doi: 10.1158/0008-5472.CAN-05-0565. [DOI] [PubMed] [Google Scholar]
- 66.Aouadi M, et al. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458:1180–1184. doi: 10.1038/nature07774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vangasseri DP, et al. Lipid-protamine-DNA-mediated antigen delivery. Curr Drug Deliv. 2005;2:401–406. doi: 10.2174/156720105774370168. [DOI] [PubMed] [Google Scholar]
- 68.Song E, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 69.Peer D, et al. Selective gene silencing in activated leukocytes by targeting siRNAs to the integrin lymphocyte function-associated antigen-1. Proc Natl Acad Sci U S A. 2007;104:4095–4100. doi: 10.1073/pnas.0608491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwarze SR, et al. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 71.Torchilin VP, et al. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc Natl Acad Sci U S A. 2001;98:8786–8791. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim WJ, et al. Cholesteryl oligoarginine delivering vascular endothelial growth factor siRNA effectively inhibits tumor growth in colon adenocarcinoma. Mol Ther. 2006;14:343–350. doi: 10.1016/j.ymthe.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 73.McNamara JO, 2nd, et al. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol. 2006;24:1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 74.Chu TC, et al. Aptamer mediated siRNA delivery. Nucleic Acids Res. 2006;34:e73. doi: 10.1093/nar/gkl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou J, et al. Novel dual inhibitory function aptamer-siRNA delivery system for HIV-1 therapy. Mol Ther. 2008;16:1481–1489. doi: 10.1038/mt.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou J, et al. Selection, characterization and application of new RNA HIV gp 120 aptamers for facile delivery of Dicer substrate siRNAs into HIV infected cells. Nucleic Acids Res. 2009;37:3094–3109. doi: 10.1093/nar/gkp185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marshall E. Gene therapy. Second child in French trial is found to have leukemia. Science. 2003;299:320. doi: 10.1126/science.299.5605.320. [DOI] [PubMed] [Google Scholar]
- 78.Hacein-Bey-Abina S, et al. Gene therapy of X-linked severe combined immunodeficiency. Int J Hematol. 2002;76:295–298. doi: 10.1007/BF02982686. [DOI] [PubMed] [Google Scholar]
- 79.Song E, et al. Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J Virol. 2003;77:7174–7181. doi: 10.1128/JVI.77.13.7174-7181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Omi K, et al. Long-lasting RNAi activity in mammalian neurons. FEBS Lett. 2004;558:89–95. doi: 10.1016/S0014-5793(04)00017-1. [DOI] [PubMed] [Google Scholar]
- 81.Cardoso AL, et al. Tf-lipoplexes for neuronal siRNA delivery: a promising system to mediate gene silencing in the CNS. J Control Release. 2008;132:113–123. doi: 10.1016/j.jconrel.2008.08.014. [DOI] [PubMed] [Google Scholar]