Comparing Sodium Intake Strategies in Heart Failure: Rationale and Design of the PROHIBIT Sodium (PRevent adverse Outcomes in Heart faIlure By limITing Sodium) Study
In the last two decades, heart failure (HF) research has focused primarily on drugs and devices. In contrast, evidence remains scarce and mostly observational for dietary sodium restriction,1–3 arguably the most widely recommended self-care measure for patients with HF. In recent studies, patients with HF consumed an average of 3600 to 4200mg sodium daily by 24-h urinary sodium excretion,4 with 65% consuming >3000mg.5 Although the evidence suggests that high sodium intake worsens outcomes, the level of sodium intake that achieves optimal outcomes for patients with HF is unknown.6–12 All current guidelines emphasize sodium intake restriction; however, there is no consensus on the actual level. Recommendations are either nonspecific or ranging between 2000–3000mg/d,9 largely based on opinions or observational studies. In explicit acknowledgement of the evidence gap, the recent European Society of Cardiology guidelines for HF12 have not assigned a level of evidence to sodium intake recommendations. The recent American College of Cardiology Foundation (ACCF) and American Heart Association (AHA) guidelines recommend (Class IIa) that sodium restriction is reasonable for HF patients with congestive symptoms but do not recommend a specific target level.13 The inconsistency of guidelines underlines the weak database that supports this “cornerstone” treatment.
Current Patterns of Sodium Intake Among Patients with Heart Failure
Current data indicate limited adherence with recommended sodium restriction among HF patients. In a recent interventional study, when instructed to limit sodium intake to 2500 mg/day, HF patients averaged a daily intake of 2700 to 3900 mg/day by 24-h urinary sodium, depending on the assigned arm, after 8 months of intervention.4 Sodium intake reduction is difficult to adhere to even among patients with symptomatic HF, with less than one-third of patients reporting sodium intake ≤2500 mg/day by 3-day food diaries, which underestimate actual sodium intake.4 Congruent with this observation, a recent study reported that only 34% of patients consume <3000 mg and only 15% consume <2000 mg sodium daily based on their 24-h urinary sodium excretion.5 Sodium consumption below 2000 mg/day is difficult to achieve even with dietitian education,14 and studies have demonstrated that gender15 and race16 affect dietary preferences and adherence to sodium restriction recommendations in patients with HF.
The Challenge of Sodium Restriction in Heart Failure: Need for a Phase III Clinical Trial
Heart failure may be associated with changes in cardiac output, systemic venous pressures, or shunting of blood away from the kidneys, leading to diminished renal perfusion and in turn activating the sympathetic17 and the renin angiotensin aldosterone system (RAAS)18 creating a vicious cycle of sodium and water retention despite fluid overload (Figure 1).18, 19 Moreover, inappropriate vasopressin levels are seen in HF. There is evidence that the natriuretic system is impaired early in the course of HF,20, 21 causing sodium and water retention, which in turn provides the physiologic basis for the low-sodium diet recommendation for patients with HF regardless of stage.
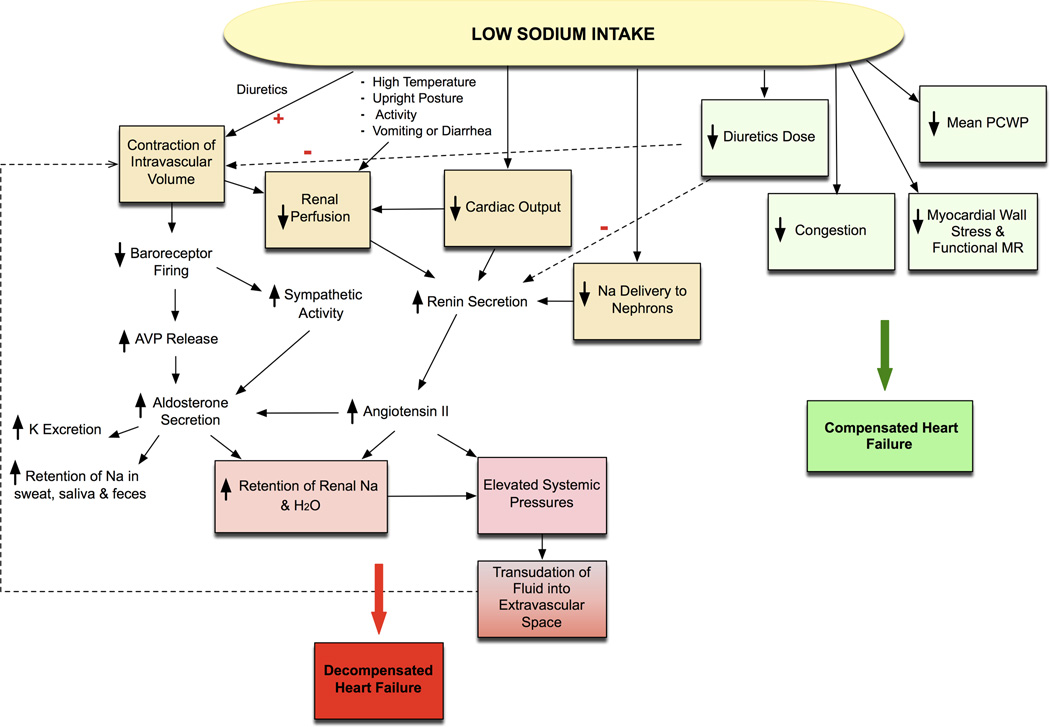
Figure 1. Effects of Sodium Intake in Heart Failure.
Low sodium intake may have varied effect on heart failure. Intravascular volume contraction improves hemodynamics and reduces diuretic requirement, congestion, and myocardial wall stress, leading to compensated heart failure. Intravascular volume contraction however may also lead to a vicious cycle of increased sodium and water retention through neurohormonal activation predisposing to decompensated heart failure. (AVP: arginine vasopressin; H2O: water; Na: sodium; K: potassium; MR: mitral regurgitation; PWCP: pulmonary wedge capillary pressure; red plus: diuretic action enhances contraction of intravascular volume; red minus: low diuretic doses reduce hormonal activation and contraction of intravascular volume). Reproduced with permission from Circulation. 2012;126:479–485.19
Although high sodium intake can cause fluid retention and stimulate sympathoexcitation and inflammation, neurohormonal activation induced by low sodium intake could potentially harm the failing heart also.22 In animal studies, a sodium-restricted diet leads to RAAS activation,23 and data suggest that dietary sodium restriction is associated with further neurohormonal activation in patients with HF also.24–29 It might be argued that further sympathetic and RAAS activation is less clinically relevant in the presence of RAAS-blocking agents and beta-blockers. However, higher plasma renin activity was an independent predictor of mortality in the Valsartan in Heart Failure Trial (Val-HeFT) regardless of angiotensin-converting enzyme inhibitor or beta-blocker treatment.22 In the Heart Outcomes Prevention Evaluation (HOPE) trial, high plasma renin activity was also an independent predictor of mortality in patients at high cardiovascular risk regardless of allocation to ramipril or placebo.30 These data suggest that neurohormonal activation may nevertheless be important regardless of drug treatments that modulate neurohormonal activation.
Few studies, and only one in US, have tested the impact of different sodium intake on clinical outcomes in HF.5, 26–28, 31–33 Observational and randomized studies have yielded contradicting results (Table 1). A number of single-center randomized studies26–28, 34–36 have suggested worse outcomes with strict sodium restriction in HF. However, these trials were conducted by the same investigators in a restricted geographic area, enrolled only post-discharge HF patients, and in the largest of these studies there were multiple treatment arms, increasing thus the potential for type I error.19 Although a significant proportion of patients in these studies were on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, few were on β-blockers or aldosterone antagonists. These shortcomings limit the generalizability of the findings.
Table 1.
Studies Investigating the Impact of Sodium Intake on Outcomes in Heart Failure
| Source | Design | Intervention | Clinical Impact |
|---|---|---|---|
| Paterna 200826 |
Randomized N 232; NYHA II post discharge; EF: <35% |
Group 1: 2760 mg/d Na diet Group 2: 1840 mg/d Na diet Fluid intake: 1 L/d |
6 months (death, death + readmission): Group 1: 7.6%, 12.7% Group 2: 26.3%, 39.5% |
| Paterna 200927 |
Randomized N 410; NYHA II post discharge; EF: <35% |
Group A & B: 2760 mg Na + 500 / 250 mg F Group C & D: 1840 mg Na + 500 / 250 mg F Fluid Intake: 1 L/d Group E & F: 2760 mg Na + 500 / 250 mg F Group G & H: 1840 mg Na + 500 / 250 mg F Fluid Intake: 2 L/d |
6 months (death, death + HF readmission): A: 1.9%, 7.7% B: 3.9%, 29.4% C: 9.8%, 49.0% D: 13.7%, 54.9% E: 9.6%, 51.9% F: 12.0%, 58.0% G: 11.5%, 71.1% H: 15.7%, 78.4% |
| Parrinello 200928 |
Randomized N 173; NYHA II post discharge; EF: <35% |
Group 1: 2760 mg/d Na + (250–500) mg F Group 2: 1840 mg/d Na + (250–500) mg F Fluid Intake: 1 L/d |
12 months (readmission, death+ readmission): Group 1: 12%, 16% Group 2: 44%, 64% |
| Arcand 201131 |
Observational N 123 NYHA I–IV EF: <35% |
Group 1: ≤1900 mg/d Na; Group 2: 2000–2700 mg/d Na; Group 3: ≥2800 mg/d Na Fluid intake: not mentioned |
12 months HF readmission: 5±3% (1), 5±3% (2), 17±6% (3) 36 months HF readmission: 12±6% (1), 15±7% (2), 46±11% (3) |
| Lennie 20115 |
Observational N 302 NYHA I–IV EF: either < or ≥40% |
Group 1: <3000 mg/d Na Group 2: ≥3000 mg/d Na Fluid intake: not mentioned |
12 months (death + admission + ED visits): NYHA I–II 1 vs. 2: higher event-rate; NYHA III–IV 1 vs. 2: lower event rate |
| Son 201132 |
Observational N 232 NYHA I–IV EF: <40% |
Group 1: <3000 mg/d Na Group 2: ≥3000 mg/d Na Fluid intake: not mentioned |
12 months (death + cardiovascular admission + cardiovascular ED visits): Group 1 vs. 2: lower event rate |
| Song 201433 |
Observational N 244 NYHA I–IV EF: either < or ≥40% |
Group 1: <2000 mg/d Na Group 2: 2000–3000 mg/d Na Group 3: >3000 mg/d Na Fluid intake: not mentioned |
12 months (death + all-cause admissions): NYHA I–II: <2 g/d higher risk vs. 2–3 g/d, >3 g/d lower risk vs. 2–3 g/d NYHA III–IV: >3 g/d highest risk, no difference between <2 g/d and 2–3 g/d groups |
HF: heart failure; ED: emergency department; EF: ejection fraction; F: furosemide; NYHA: New York Heart Association
Thus, although it seems reasonable to restrict sodium below <3000 mg/d in HF, it is currently unknown how “low” is appropriate for patients with HF. The net impact of sodium restriction on outcomes in HF patients can only be addressed through a well-designed trial testing different levels of sodium restriction. However, critical knowledge gaps exist in order to develop a Phase III trial of sodium restriction in HF.
KNOWLEDGE GAPS TO DESIGN A PHASE III CLINICAL TRIAL OF SODIUM RESTRICTION IN HEART FAILURE: RATIONALE FOR A CLINICAL TRIAL PILOT STUDY
Target Population and Estimating Event Rates
Although the evidence base to support sodium restriction in HF and preserved EF (HFpEF) is inadequate,37 the actual concerns with sodium restriction in HF have been raised for patients with HF and reduced EF (HFrEF) in the previous literature due to the neurohormonal activation and fluid retention with diuretic resistance in these patients.38 Enrolling chronic stable HFrEF patients would require a large sample size due to the lower event rates in this population.39, 40 Patients with acute HF have mortality and readmission rates of up to 15% and 30% respectively within 90 days post-discharge, necessitating further research.41 These event rates increase power to detect an effect on outcomes within a feasible active feeding period (e.g. 12 weeks). However, patients admitted for acute HF are given low-sodium diets different than their free living state, undergo adjustments in diuretics and other medications, and are given self-care education, rendering assessment of usual sodium intake pattern unreliable at discharge. Enrolling patients at the 2-week follow-up visit would be more conducive to assessment of patients’ usual dietary pattern and optimizing medications while the patient is still within the post-discharge vulnerable phase. Waiting longer may lead to inclusion of lower risk patients.
Most acute HF studies reported cumulative 90-to-180 day outcomes.41 Thus, event rates among HFrEF patients who are not readmitted 2 weeks after discharge can only be indirectly deducted from these data. Outcomes data specifically among HFrEF patients who are stable 2 weeks post discharge consuming >3000-mg/d sodium are unavailable. These estimates are needed to power a full-scale trial.
Proportion of Eligible and Willing Participants
Participating in a feeding trial for 12 weeks requires commitment. Although currently we do not know the optimal “low” sodium intake below 3000 mg/day for HF, it is unlikely that sodium intake >3000 mg/d would be beneficial. Thus, out of ethical considerations, we will include patients who continue to consume ≥3000mg/d sodium 2 weeks post-discharge, despite education and instructions at discharge. The proportion of HFrEF patients who are willing to participate, meet the trial eligibility criteria, and are eating ≥3000mg sodium daily, is not known. This knowledge is essential to project enrollment rate in a full-scale trial.
Level of Sodium Intake and Relative Risk Between Trial Arms
A wide separation in sodium intake between trial arms, e.g. more than the average American diet vs. 1000 mg/d, would increase the probability to detect a difference in event rates. However, both very high and very low sodium intake would raise ethical and logistic concerns.24–29, 34–36 Americans consume ~3700 mg sodium daily42; whereas the US Department of Agriculture (USDA) and Department of Health and Human Services recommend 2300 mg/day in general and 1500 mg/day for African Americans, those over age 50, or those with hypertension, diabetes, or kidney disease.43 In a recent report, the Institute of Medicine concluded that there is inadequate evidence to suggest dietary sodium <1500 mg/day in any population and that, specifically for HF, more data are needed to establish appropriate targets.44 Therefore, testing the recommended level for at-risk populations (1500 mg/d) vs. (3000 mg/d) would achieve a reasonable balance between ethical and trial concerns. No data exist on the effect of 1500- vs. 3000-mg/d sodium diets on HFrEF outcomes to inform sample size for a full-scale clinical trial.
Long-term Adherence, Safety, and Follow Up
To assess efficacy of sodium restriction, the trial cannot rely on patients trying to reduce salt intake, as these attempts are likely going to be inconsistent at best or not effective at worst. Provision of prepared food is preferable in a phase III efficacy trial. A longer trial would increase power to detect a treatment effect, but adherence with provided food would likely decrease over time. The adherence of the target population with the provided meals over prolonged periods (e.g. 12 weeks) is unknown. There are concerns about the effects of low dietary sodium on renal function and blood pressure in HF patients taking diuretics,19 especially older adults, who constitute the majority of HF patients. Currently, there are limited data to support the safety of strict sodium restriction in HF over a longer-term (12-week) intervention.
A full-scale trial will need to include a number of follow-up visits to assess adherence with provided food and safety. However, to maintain logistic and fiscal feasibility of the trial, data on the minimum acceptable number and spacing of visits is required.
Fluid Intake
Data on the effects of fluid intake on outcomes and neurohormonal activation in HF are limited. One study suggested that fluid intake ≤1 L/day with a sodium intake of ~2760 mg/d is associated with better outcomes and neurohormonal profile.27 However, other studies suggest no difference in symptoms, weight, functional capacity, quality of life (QoL),45 or time to achieve clinical stability.46 To isolate the effect of sodium intake, we will advise participants to consume ≤2L of fluids daily as this level is recommended by most HF guidelines. However, we recognize that the evidence behind this recommendation is weak and more definitive data are needed.
CLINICAL TRIAL PILOT STUDY DESIGN
Registry Component
We will approach consecutive HFrEF patients with EF ≤40% during admission for a primary diagnosis of acute HF (Figure 2). We will ask patients who do not meet any exclusion criteria (Table 2) to participate in a 12-week feeding trial followed by a 12-week follow-up period. We will instruct willing patients to complete and bring back a 3-day food record (3DFR) at the 2-week, standard-of-care post-discharge visit. The study dietitian will analyze food records by using the Nutritionist Pro Diet Analysis software (Axxya Systems LLC, Redmond, WA) that allows for analysis of daily intake for 90 nutrients. The database of food and ingredients includes 52,000 foods, including 500 brands from over 7 manufacturers (www.nutritionistpro.com).
Figure 2. Design of the Registry Component.
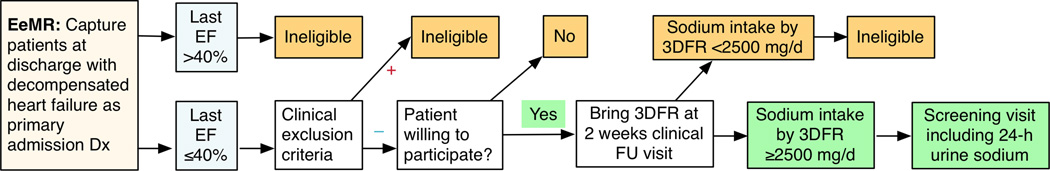
3DFR: 3-day food record; EeMR: Emory electronic medical record system; EF: ejection fraction; FU: follow-up
Table 2.
Eligibility Criteria for Entry in the Randomized Pilot Trial Component
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; EF: ejection fraction; HF: heart failure
This component will estimate the proportion of (1) discharged HFrEF patients who are both eligible and willing to participate, and (2) among these patients, the proportion consuming ≥3000 mg/d 2 weeks post discharge despite instructions. Our experience with previous acute and post-discharge HF trials has been that >50% of eligible patients will be willing to participate. Sodium intake data on HF patients in US are limited. In the NIH-funded Education and Supportive Partners Improving Self-Care (ENSPIRE) trial, patients with HF consumed an average of 3600 to 4200mg sodium daily by 24-h urinary sodium excretion at baseline.4 However, these data are from chronic HF patients. Patients admitted for HF receive dietary instructions. Therefore, the proportion of patients consuming >3000mg/d sodium 2 weeks post discharge is unknown.
Because large-scale screening with 24-urine sodium would be impractical for a full-scale trial, we will pre-screen patients with 3DFR.47 This validated tool will provide an estimate of sodium consumption to select patients for 24h urine sodium screening to determine eligibility for the randomized pilot trial component. The rationale for a lower sodium eligibility threshold (≥2500 mg/d) in the 3DFR is that food records systematically underestimate sodium intake compared to 24-hour urine collection,47 especially in HF patients taking loop diuretics.48 Average daily sodium excretion by 24-hour urine was >750mg higher than reported intake among 62 HF patients receiving loop diuretics.48 Therefore, we expect that most participants exceeding the 2500-mg/d sodium threshold by 3DFR will have ≥3000 mg/d sodium excretion by 24-h urine collection. This approach will reduce the number of urine collections and improve feasibility of screening and at the same time confirm sodium intake by more objective testing.
Randomized Pilot Trial Component and Follow-Up Surveillance
Eligible patients will enter the randomized, double blind pilot trial. We plan to randomize 50 patients to receive food with either 1500- or 3000-mg/d sodium for 12 weeks, followed by an additional 12 weeks of surveillance (Figure 3). Meals will be prepared under the nutritional and sodium-content surveillance of PurFoods, LLC (Ankeny, IA, www.purfoods.com), in a USDA certified kitchen. PurFoods will ship all prepared meals to participants. Meals will be stored under temperature-controlled conditions at all times during shipment and storage, until delivered to the subject. Patients will be given food diaries in order to record any additional food and/or drink as well as the portion of the prepared meal that they have consumed and will be instructed to a <2L/d fluid restriction. The purpose of this component is to estimate:
Overall retention of patients on study and adherence with prepared food;
Trends and between-arms differences in all-cause mortality, readmissions, and emergency department (ED) visits; NT-pro-B-type natriuretic peptide (NT-proBNP) levels; quality of life (QoL) and satisfaction with food; and
Safety of 1500- and 3000 mg/d sodium diets, including adverse events, vital signs, and biochemistry panels at 4, 8, and 12 weeks
Figure 3. Design of the Randomized Pilot Trial Component.
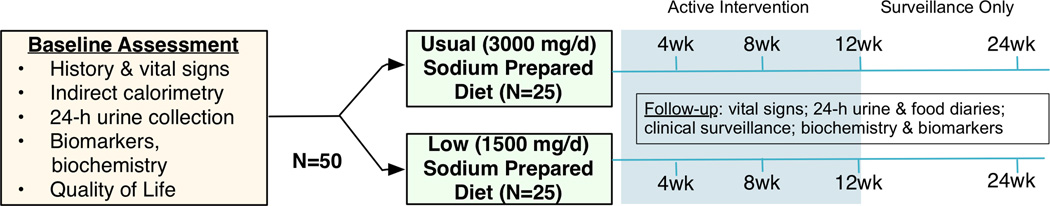
After the 12-week intervention, we plan 12 additional weeks of surveillance including 2 office visits at weeks 1–2 and 12 and a phone call at 3–4 weeks.
Study Procedures
Screening and Baseline Visits
The dietitian will review the 3DFR and interview participants about diet habits and preferences to customize meal plans. Research coordinators will provide education for 24-h urine collection. If the urine collection shows >3000 mg/d sodium excretion, the patient will be invited to come back for the baseline visit (vital signs, blood draws, QoL questionnaire) and start receiving individualized meal plans (either 1,500 mg or 3,000 mg/d).
Randomization
Participants will be randomly allocated to 3000- vs. 1500-mg/d sodium diets with a small-blocks (6 subjects per block) permuted block-randomization process to ensure balance between arms. The entire process will be managed by a study member without patient or clinical involvement and will be completely masked to investigators.
Masking procedures
Coordinators, investigators, and participants will be blinded to arm assignment (double-blind design), and only an administrative member of the study team and the dietitians (from Stony Brook and PurFoods) responsible for the preparation of the meals will be aware of this. Also, to ensure blinding and neutrality, follow up 24-hour urinary sodium values will not be disclosed to research personnel or participants until the end of study. Adherence will be reinforced through standardized scripts.
Dietary Intervention
Participants will be given instructions to complete the 3DFR, including details about food preparation, brands, and amounts, and any dietary supplements (nutrients and herbals). Visual guides corresponding to portion size will be provided including household measuring cups and spoons, rulers, etc., to help the recording process and quantitation. The dietitians will review the 3DFR for fluid consumption and participants will be able to continue to drink selected beverages (i.e. water, coffee, tea) but within the limit of 2 L/day total. Limits will be put on the type and amount of condiments to keep within study parameters. For caloric intake, basal metabolic rate will be calculated using indirect calorimetry. Protein intake will be adjusted to 0.8 g/kg of body weight.49 All other nutrients will be between 70–100% of reference intake.49 After randomization, patients will receive controlled diets that provide either 3000 or 1500 mg/d sodium for 12 weeks. All diets will have consistent macronutrients and caloric content throughout the feeding period to ensure weight maintenance. The menus will be planned with the collaboration of the two dietitians from Stony Brook and PurFoods. Food delivery will be conducted twice a week, with alternative arrangements In case of inadvertent circumstances. Participants will be instructed during all interactions to only eat what is provided to them. They will be also asked to keep a detailed diary of (1) any non-study food items consumed and (2) the proportion of the provided food consumed at each meal, with the option to provide reasons for deviations. To encourage adherence, the dietitians will keep in contact with the participants during phone or clinic visits with standard scripts for reinforcement. Select discretionary seasonings (without sodium), but not salt, will be allowed. The caloric, fat, protein, and carbohydrate value of the meals will stay consistent throughout the trial.
Assessment of Adherence
Participants will be instructed to record (1) every non-study item they have consumed; (2) the proportion of study food consumed per meal; and (3) fluid intake, on a daily food diary, which will be reviewed at the 4-weekly visits. This approach was successful in the Dietary Approaches to Stop Hypertension (DASH) trial.50 Between the clinic visits the dietitian will contact subjects by phone in order to assess the diet adherence and to resolve any meal related issues. Table 3 summarizes the schedule of visits and procedures.
Table 3.
Schedule of Study Visits and Procedures
| Procedure | Screening Day -2 |
Baseline (Day 0) |
Wk 2 (Phone) |
Wk 4 | Wk 6 (Phone) |
Wk 8 | Wk 10 (Phone) |
Wk 12 | Post Wk 2 |
Post Wk 2 (Phone) |
Post Wk 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline diet assessment (3-day food record) | X | X | |||||||||
| 24-h urine collection | X | X | X | X | X | X | |||||
| Indirect calorimetry | X | ||||||||||
| History, physical exam, vital signs, anthropometrics | X | X | X | X | X | X | |||||
| Biochemistry, NT-proBNP, and HF biomarkers | X | X | X | X | X | X | |||||
| Review of food diaries | X | X | X | ||||||||
| Adherence assessment | X | X | X | X | X | X | |||||
| Food palatability | X | X | X | ||||||||
| Quality of life (KCCQ) | X | X | |||||||||
| Adverse and clinical events | X | X | X | X | X | X | X | X | X |
KCCQ: Kansas City Cardiomyopathy Questionnaire; NT-proBNP: N-terminal-pro-B-type natriuretic peptide
Study Endpoints
Primary Endpoints - Patient On-Study Retention and Adherence
A longer (e.g. 6 months) trial would increase power to detect a treatment effect. However, retention and adherence with study food would likely decline over time, compromising intention-to-treat analyses. For example, in the Treatment of Mild Hypertension Study51, adherence with sodium intake declined over time as evident from the serial 24-h urinary sodium determinations. Currently, there are no data to inform the optimal duration of an outcome-driven feeding trial in HF. Previous studies either provided food for a short time or relied on educational interventions to modify sodium intake. In the proposed study, we will track (1) retention, defined as the proportion of patients remaining on the study in the absence of clinical or safety events, and (2) adherence, through patient diaries and 4-weekly 24-h urine collections. In a recent HF study,4 the correlation between 3DFR-derived and 24h-urine sodium was modest (r<0.5) despite statistical significance and 3DFR systematically underestimated sodium intake, supporting therefore the need for objective assessment of sodium intake adherence at least in the pilot phase. Our goal is to inform the optimal balance between trial duration and retention/adherence rates for a full-scale trial.
Secondary Endpoint - Clinical Outcomes
The secondary endpoint will be the composite of all-cause mortality, hospitalization, or emergency department visits, whichever occurs first (time-to-event analysis), to generate the most clinically relevant evidence for the appropriate level of sodium intake in HF. We opted for all-cause hospitalizations and emergency department visits because reduction in HF-related events might be offset by non-HF related but still intervention-related events e.g. renal impairment or hypotension. In a recent study,31 HF patients consuming <2800 mg/d sodium were less likely to be admitted for HF compared with those consuming ≥2800 mg/d; however there was no difference in all-cause admissions. Patients and caregivers will be asked to report any interim event at any institution to the study team during the regular encounters. We will contact the patient or family in case of a patient no-show. Additional data for healthcare system encounters will be collected through electronic health records and contact with patients and caregivers. For encounters in outside hospitals, we will obtain information through patient inquiry and a copy of the medical record will be requested for adjudication.
Tertiary Endpoints – NT-proBNP levels and Patient-Oriented Outcomes (QoL and Food Palatability)
NT-proBNP levels are closely associated with prognosis in HF patients regardless of functional class.52 Therefore, we will measure NT-proBNP levels, a sensitive, responsive to treatment, and widely available HF prognostic biomarker, as a surrogate for efficacy. QoL is an important therapeutic goal in HF, especially for dietary interventions, as food palatability may affect QoL.53 Thirst and sodium appetite are physiologic sensations aroused by perceived lack of water and sodium. Sodium deprivation stimulates aldosterone production, which promotes renal sodium conservation54 and angiotensin II stimulates sodium appetite and thirst (but does not, on its own, selectively stimulate the ingestion of sodium relative to water).55, 56 Many HF patients experience increases in sodium appetite,57 further complicating any attempt to improve sodium restriction adherence. Few studies have investigated the effects of dietary sodium on QoL of HF patients. In one study of 12 weeks of sodium and fluid restriction, thirst, appetite, and QoL were not affected.58 However, 21% of patients complained about sodium restriction. Two studies reported better QoL among patients following the prescribed diet.29, 59 We plan to investigate the effects of the prescribed diets both on QoL and food palatability.
Safety and Post-intervention Surveillance
A number of studies with sodium restriction in HFrEF patients have reported a drop in blood pressure24, 28, 34 and worsening renal function25–29, 34, 60, 61 in the low sodium arm. We will collect data on blood pressure and renal function serially at 4, 8, and 12 weeks and withdraw those participants who meet prespecified safety criteria despite appropriate modification of HF therapy. We will keep tracking blood pressure and renal function in the two planned office visits (at 1–2 weeks and 12 weeks post-intervention) during the post-intervention surveillance period.
Safety endpoints will include (1) systolic blood pressure (SBP) drop >20mmHg for those with baseline SBP >120mmHg, >10mmHg for those with baseline SBP 100–120mmHg, and any SBP <100mmHg with symptoms, at any visit (planned or unplanned); (2) creatinine increase >0.5mg over baseline at any visit. For patients meeting these criteria (except SBP <90mmHg), medical therapy will be adjusted accordingly and patients will be re-evaluated after 1 week; if these effects persist, patients will be withdrawn. Patients with SBP <90mmHg will be withdrawn immediately. Allergic responses or food poisoning events will be considered safety events.
Because we cannot exclude the possibility of delayed or prolonged effects of dietary sodium on clinical and safety events, we will follow up all participants for an additional 12 weeks, with two clinic visits and an interim phone, after the end of the intervention.
Analytic Plan
On-study retention (primary endpoint) will be calculated according to the Kaplan-Meier principle, i.e. patients meeting a clinical event (death, admission, emergency department visit) will be censored as on-study at the time of the event. For retention calculation purposes, safety endpoints will be considered as withdrawals. We will calculate adherence (co-primary endpoint) on the basis of “adherent” days (days during which all study food was consumed and no non-study items were consumed) divided by the total number of days in the trial. A ≥ 90% adherence will be considered adequate. In studies with fixed sodium intake,62, 63 90% of ingested sodium was excreted in the urine across a wide range of sodium intake (1500 to 4600 mg/d). Therefore, we expect average 24-h urinary sodium to be 1350 mg in the 1500-mg/d group and 2700 mg in the 3000-mg/d group. The random variation of 24-h urine excretion had a coefficient of variation (CV) of ~15% in these studies, estimated from the published data.62, 63 We will therefore consider values outside the ±15% limits, i.e. outside 1150–1550 mg for the 1500-mg/d sodium arm and 2300–3100 mg for the 3000-mg/d sodium arm, as evidence of non-adherence. We will provide average values per-person and per-arm over time and the proportion of values outside the prespecified range.
DISCUSSION
The results of the pilot study will provide necessary information to assess the feasibility and design of a efficacy trial of dietary sodium intake in HFrEF, including information on (1) expected patient willingness and eligibility rates; (2) patient retention and adherence with prepared food; (3) expected event rates in the target population and between the trial arms; (4) safety; and (5) appropriate follow-up scheduling to balance scientific rigor and feasibility. If the proposed pilot study suggests key impediments to a Phase III trial, this will (1) prevent a costly, problematic full-scale trial; (2) provide the basis for alternative trial designs.
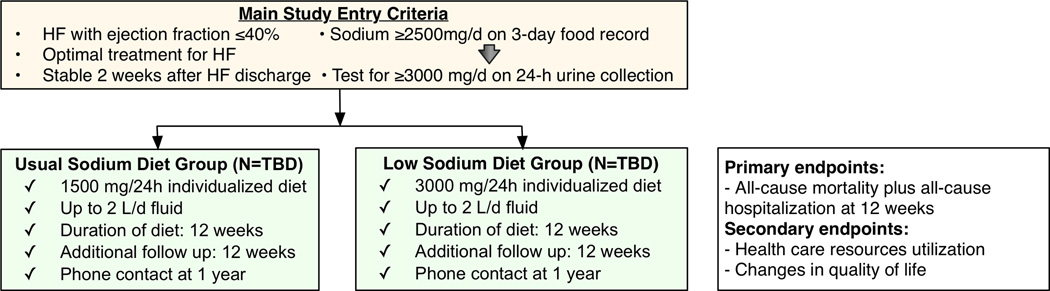
If the pilot results are encouraging, this will lead to an outcome-driven clinical trial to assess the efficacy of two different levels of sodium intake (3000 vs. 1500 mg/day) in HF patients with EF <40% recently discharged after an acute HF episode, with clinical events, health care resource utilization, and QoL as the endpoints of interest (Figure 4). Our hypothesis is that in recently hospitalized HFrEF patients, a sodium intake of 1500 mg/day as compared to 3000 mg/day for 12 weeks will result in: (1) reduction in the composite of death and all-cause hospitalization; (2) reduction in health care resources utilization; and (3) significant improvement in QoL. We expect the results of this study to inform HF guidelines and similar studies in HFpEF. If the full-scale trial proved efficacy of the low-sodium diet, then the low-sodium DASH diet would be a reasonable recommendation for HF, backed by advocacy efforts.
Figure 4. Outline of the Proposed Full-Scale Clinical Trial.
Adherence is an important component of any dietary intervention. Consistent results in terms of adherence to specified diet are difficult to produce even with coordinated efforts.4 In our pilot study, we will provide prepared meals to reduce the uncertainty associated with educational and socio-behavioral components related to preparation of prescribed diets. However, for practical implementation of any level of sodium restriction, the effectiveness of behavior modification interventions and adherence to sodium restriction over time has to be explicitly tested.
This study will not include HFpEF patients. There are no data on the appropriate level of sodium intake in these patients either, and sodium restriction is recommended on the basis of consensus. However, the underlying pathophysiology of HFpEF, especially in older adults who constitute the majority of HFpEF patients, is different than HFrEF. For example, NT-proBNP levels are less elevated in HFpEF patients, trials with neurohormonal blockade have not shown to improve outcomes, and unlike HFrEF patients who have mostly cardiovascular adverse outcomes events, outcomes related to comorbidity play a more significant role in HFpEF patients. Therefore, the appropriate level of sodium intake in this group of patients should be investigated in dedicated, well-designed studies.
If no separation trends in the efficacy endpoint are observed (mortality, readmission, emergency department visits) in our study, this might signify the need to design a non-inferiority trial. Finally, if safety concerns arise, we will propose a dose-finding study with multiple arms; these arms will be narrowly spaced in terms of dietary sodium intake spread to establish the safest level.
In trials of sodium intake in HFrEF, a lower-sodium diet (1800 mg/d) was associated with increased all-cause mortality and HF readmission rates risk compared to a higher-sodium diet (2800 mg/d). Although a single group has conducted all these trials and the results have not been independently validated, the potential impact of sodium intake recommendations on HF outcomes cannot be overemphasized. With over a million HF hospitalizations annually in US, even a fraction of the treatment effect observed in previous studies, e.g. a 20% relative risk between sodium arms, could lead to dramatic reductions in the absolute number of deaths and hospitalizations from HF and substantial savings for the healthcare system.
Acknowledgments
Sources of Funding
This work is supported by a National Heart, Lung, and Blood Institute grant (R34 HL119773).
Footnotes
Disclosures
None.
References
- 1.Ghali JK, Kadakia S, Cooper R, Ferlinz J. Precipitating factors leading to decompensation of heart failure. Traits among urban blacks. Arch Intern Med. 1988;148:2013–2016. [PubMed] [Google Scholar]
- 2.Michalsen A, König G, Thimme W. Preventable causative factors leading to hospital admission with decompensated heart failure. Heart. 1998;80:437–441. doi: 10.1136/hrt.80.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsuyuki RT, McKelvie RS, Arnold JM, Avezum A, Barretto AC, Carvalho AC, Isaac DL, Kitching AD, Piegas LS, Teo KK, Yusuf S. Acute precipitants of congestive heart failure exacerbations. Arch Intern Med. 2001;161:2337–2342. doi: 10.1001/archinte.161.19.2337. [DOI] [PubMed] [Google Scholar]
- 4.Dunbar SB, Clark PC, Reilly CM, Gary RA, Smith A, McCarty F, Higgins M, Grossniklaus D, Kaslow N, Frediani J, Dashiff C, Ryan R. A trial of family partnership and education interventions in heart failure. J Card Fail. 2013;19:829–841. doi: 10.1016/j.cardfail.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lennie TA, Song EK, Wu J-R, Chung ML, Dunbar SB, Pressler SJ, Moser DK. Three Gram Sodium Intake is Associated With Longer Event-Free Survival Only in Patients With Advanced Heart Failure. J Card Fail. 2011;17:325–330. doi: 10.1016/j.cardfail.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malcom J, Arnold O, Howlett JG, Ducharme A, Ezekowitz JA, Gardner MJ, Giannetti N, Haddad H, Heckman GA, Isaac D, Jong P, Liu P, Mann E, McKelvie RS, Moe GW, Svendsen AM, Tsuyuki RT, O'Halloran K, Ross HJ, Sequeira EJ, White M Canadian Cardiovascular Society. Canadian Cardiovascular Society Consensus Conference guidelines on heart failure--2008 update: best practices for the transition of care of heart failure patients, and the recognition, investigation and treatment of cardiomyopathies. Can J Cardiol. 2008;24:21–40. doi: 10.1016/s0828-282x(08)70545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 8.Royal College of Physicians. Chronic Heart Failure: National clinical guideline for diagnosis and management in primary and secondary care. 2010 [PubMed] [Google Scholar]
- 9.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WHW, Teerlink JR, Walsh MN. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 10.McKelvie RS, Moe GW, Cheung A, Costigan J, Ducharme A, Estrella-Holder E, Ezekowitz JA, Floras J, Giannetti N, Grzeslo A, Harkness K, Heckman GA, Howlett JG, Kouz S, Leblanc K, Mann E, O'Meara E, Rajda M, Rao V, Simon J, Swiggum E, Zieroth S, Arnold JMO, Ashton T, D'Astous M, Dorian P, Haddad H, Isaac DL, Leblanc M-H, Liu P, Sussex B, Ross HJ. The 2011 Canadian Cardiovascular Society heart failure management guidelines update: focus on sleep apnea, renal dysfunction, mechanical circulatory support, and palliative care. Can J Cardiol. 2011;27:319–338. doi: 10.1016/j.cjca.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Krum H, Jelinek MV, Stewart S, Sindone A, Atherton JJ Cardiac Society of Australia and New Zealand. 2011 update to National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand Guidelines for the prevention, detection and management of chronic heart failure in Australia, 2006. Med J Aust. 2011;194:405–409. doi: 10.5694/j.1326-5377.2011.tb03031.x. [DOI] [PubMed] [Google Scholar]
- 12.McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GYH, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 13.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL American College of Cardiology F, American Heart Association Task Force on Practice G. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Arcand JA, Brazel S, Joliffe C, Choleva M, Berkoff F, Allard JP, Newton GE. Education by a dietitian in patients with heart failure results in improved adherence with a sodium-restricted diet: a randomized trial. Am Heart J. 2005;150:716. doi: 10.1016/j.ahj.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Chung ML, Moser DK, Lennie TA, Worrall-Carter L, Bentley B, Trupp R, Armentano DS. Gender differences in adherence to the sodium-restricted diet in patients with heart failure. J Card Fail. 2006;12:628–634. doi: 10.1016/j.cardfail.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kollipara UK, Mo V, Toto KH, Nelson LL, Schneider RA, Neily JB, Drazner MH. High-sodium food choices by southern, urban African Americans with heart failure. J Card Fail. 2006;12:144–148. doi: 10.1016/j.cardfail.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Bashor TM, Granger CB, Hranitzky P, Patel MR. CURRENT Medical Diagnosis & Treatment. In: McPhee SJ, Papadakis MA, editors. Chapter 10. Heart Disease. The McGraw-Hill Companies, Inc.; 2011. [Google Scholar]
- 18.Skorecki KL, Brenner BM. Body fluid homeostasis in congestive heart failure and cirrhosis with ascites. Am J Med. 1982;72:323–338. doi: 10.1016/0002-9343(82)90824-5. [DOI] [PubMed] [Google Scholar]
- 19.Gupta D, Georgiopoulou VV, Kalogeropoulos AP, Dunbar SB, Reilly CM, Sands JM, Fonarow GC, Jessup M, Gheorghiade M, Yancy C, Butler J. Dietary sodium intake in heart failure. Circulation. 2012;126:479–485. doi: 10.1161/CIRCULATIONAHA.111.062430. [DOI] [PubMed] [Google Scholar]
- 20.Volpe M, Tritto C, DeLuca N, Rubattu S, Rao MA, Lamenza F, Mirante A, Enea I, Rendina V, Mele AF, Trimarco B, Condorelli M. Abnormalities of sodium handling and of cardiovascular adaptations during high salt diet in patients with mild heart failure. Circulation. 1993;88:1620–1627. doi: 10.1161/01.cir.88.4.1620. [DOI] [PubMed] [Google Scholar]
- 21.McKie PM, Schirger JA, Costello-Boerrigter LC, Benike SL, Harstad LK, Bailey KR, Hodge DO, Redfield MM, Simari RD, Burnett JC, Jr, Chen HH. Impaired natriuretic and renal endocrine response to acute volume expansion in pre-clinical systolic and diastolic dysfunction. J Am Coll Cardiol. 2011;58:2095–2103. doi: 10.1016/j.jacc.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masson S, Solomon S, Angelici L, Latini R, Anand IS, Prescott M, Maggioni AP, Tognoni G, Cohn JN. Elevated plasma renin activity predicts adverse outcome in chronic heart failure, independently of pharmacologic therapy: data from the Valsartan Heart Failure Trial (Val-HeFT) J Card Fail. 2010;16:964–970. doi: 10.1016/j.cardfail.2010.06.417. [DOI] [PubMed] [Google Scholar]
- 23.Mimran A, Guiod L, Hollenberg NK. The role of angiotensin in the cardiovascular and renal response to salt restriction. Kidney Int. 1974;5:348–355. doi: 10.1038/ki.1974.50. [DOI] [PubMed] [Google Scholar]
- 24.Cody RJ, Covit AB, Schaer GL, Laragh JH, Sealey JE, Feldschuh J. Sodium and water balance in chronic congestive heart failure. J Clin Invest. 1986;77:1441–1452. doi: 10.1172/JCI112456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damgaard M, Norsk P, Gustafsson F, Kanters JK, Christensen NJ, Bie P, Friberg L, Gadsbøll N. Hemodynamic and neuroendocrine responses to changes in sodium intake in compensated heart failure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1294–R1301. doi: 10.1152/ajpregu.00738.2005. [DOI] [PubMed] [Google Scholar]
- 26.Paterna S, Gaspare P, Fasullo S, Sarullo FM, Di Pasquale P. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clin Sci (Lond) 2008;114:221–230. doi: 10.1042/CS20070193. [DOI] [PubMed] [Google Scholar]
- 27.Paterna S, Parrinello G, Cannizzaro S, Fasullo S, Torres D, Sarullo FM, Di Pasquale P. Medium term effects of different dosage of diuretic, sodium, and fluid administration on neurohormonal and clinical outcome in patients with recently compensated heart failure. Am J Cardiol. 2009;103:93–102. doi: 10.1016/j.amjcard.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 28.Parrinello G, Di Pasquale P, Licata G, Torres D, Giammanco M, Fasullo S, Mezzero M, Paterna S. Long-term effects of dietary sodium intake on cytokines and neurohormonal activation in patients with recently compensated congestive heart failure. J Card Fail. 2009;15:864–873. doi: 10.1016/j.cardfail.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Nakasato M, Strunk CMC, Guimarães G, Rezende MVC, Bocchi EA. Is the low-sodium diet actually indicated for all patients with stable heart failure? Arq Bras Cardiol. 2010;94:92–101. doi: 10.1590/s0066-782x2010000100015. [DOI] [PubMed] [Google Scholar]
- 30.Verma S, Gupta M, Holmes DT, Xu L, Teoh H, Gupta S, Yusuf S, Lonn EM. Plasma renin activity predicts cardiovascular mortality in the Heart Outcomes Prevention Evaluation (HOPE) study. Eur Heart J. 2011;32:2135–2142. doi: 10.1093/eurheartj/ehr066. [DOI] [PubMed] [Google Scholar]
- 31.Arcand J, Ivanov J, Sasson A, Floras V, Al-Hesayen A, Azevedo ER, Mak S, Allard JP, Newton GE. A high-sodium diet is associated with acute decompensated heart failure in ambulatory heart failure patients: a prospective follow-up study. Am J Clin Nutr. 2011;93:332–337. doi: 10.3945/ajcn.110.000174. [DOI] [PubMed] [Google Scholar]
- 32.Son Y-J, Lee Y, Song EK. Adherence to a sodium-restricted diet is associated with lower symptom burden and longer cardiac event-free survival in patients with heart failure. J Clin Nurs. 2011;20:3029–3038. doi: 10.1111/j.1365-2702.2011.03755.x. [DOI] [PubMed] [Google Scholar]
- 33.Song EK, Moser DK, Dunbar SB, Pressler SJ, Lennie TA. Dietary sodium restriction below 2 g per day predicted shorter event-free survival in patients with mild heart failure. Eur J Cardiovasc Nurs. 2014;13:541–548. doi: 10.1177/1474515113517574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Licata G, Di Pasquale P, Parrinello G, Cardinale A, Scandurra A, Follone G, Argano C, Tuttolomondo A, Paterna S. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long-term effects. Am Heart J. 2003;145:459–466. doi: 10.1067/mhj.2003.166. [DOI] [PubMed] [Google Scholar]
- 35.Paterna S, Di Pasquale P, Parrinello G, Fornaciari E, Di Gaudio F, Fasullo S, Giammanco M, Sarullo FM, Licata G. Changes in brain natriuretic peptide levels and bioelectrical impedance measurements after treatment with high-dose furosemide and hypertonic saline solution versus high-dose furosemide alone in refractory congestive heart failure: a double-blind study. J Am Coll Cardiol. 2005;45:1997–2003. doi: 10.1016/j.jacc.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 36.Paterna S, Fasullo S, Parrinello G, Cannizzaro S, Basile I, Vitrano G, Terrazzino G, Maringhini G, Ganci F, Scalzo S, Sarullo FM, Cice G, Di Pasquale P. Short-Term Effects of Hypertonic Saline Solution in Acute Heart Failure and Long-Term Effects of a Moderate Sodium Restriction in Patients With Compensated Heart Failure With New York Heart Association Class III (Class C) (SMAC-HF Study) Am J Med Sci. 2011;342:27–37. doi: 10.1097/MAJ.0b013e31820f10ad. [DOI] [PubMed] [Google Scholar]
- 37.Hummel SL, Seymour EM, Brook RD, Sheth SS, Ghosh E, Zhu S, Weder AB, Kovacs SJ, Kolias TJ. Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail. 2013;6:1165–1171. doi: 10.1161/CIRCHEARTFAILURE.113.000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann Intern Med. 1985;103:1–6. doi: 10.7326/0003-4819-103-1-1. [DOI] [PubMed] [Google Scholar]
- 39.Granger CB, McMurray JJV, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 40.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 41.Giamouzis G, Kalogeropoulos A, Georgiopoulou V, Laskar S, Smith AL, Dunbar S, Triposkiadis F, Butler J. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. J Card Fail. 2011;17:54–75. doi: 10.1016/j.cardfail.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Bernstein AM, Willett WC. Trends in 24-h urinary sodium excretion in the United States 1957–2003: a systematic review. Am J Clin Nutr. 2010;92:1172–1180. doi: 10.3945/ajcn.2010.29367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.United States Department of Agriculture. Dietary Guidelines for Americans. 2010 [Google Scholar]
- 44.Strom BL, Anderson CAM, Ix JH. Sodium reduction in populations: insights from the Institute of Medicine committee. JAMA. 2013;310:31–32. doi: 10.1001/jama.2013.7687. [DOI] [PubMed] [Google Scholar]
- 45.Holst M, Strömberg A, Lindholm M, Willenheimer R. Description of self-reported fluid intake and its effects on body weight, symptoms, quality of life and physical capacity in patients with stable chronic heart failure. J Clin Nurs. 2008;17:2318–2326. doi: 10.1111/j.1365-2702.2008.02295.x. [DOI] [PubMed] [Google Scholar]
- 46.Travers B, O'Loughlin C, Murphy NF, Ryder M, Conlon C, Ledwidge M, McDonald K. Fluid restriction in the management of decompensated heart failure: no impact on time to clinical stability. J Card Fail. 2007;13:128–132. doi: 10.1016/j.cardfail.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Bentley B. A review of methods to measure dietary sodium intake. J Cardiovasc Nurs. 2006;21:63–67. doi: 10.1097/00005082-200601000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Arcand J, Floras JS, Azevedo E, Mak S, Newton GE, Allard JP. Evaluation of 2 methods for sodium intake assessment in cardiac patients with and without heart failure: the confounding effect of loop diuretics. Am J Clin Nutr. 2011;93:535–541. doi: 10.3945/ajcn.110.004457. [DOI] [PubMed] [Google Scholar]
- 49.IOM. Dietary Reference Intakes (DRIs): Recommended Intakes for individuals. 2011 [Google Scholar]
- 50.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 51.Neaton JD, Grimm RH, Prineas RJ, Stamler J, Grandits GA, Elmer PJ, Cutler JA, Flack JM, Schoenberger JA, McDonald R Treatment of Mild Hypertension Study. Final results. Treatment of Mild Hypertension Study Research Group. JAMA. 1993;270:713–724. [PubMed] [Google Scholar]
- 52.Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ. 2005;330:625. doi: 10.1136/bmj.330.7492.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lerner A. New therapeutic strategies for celiac disease. Autoimmun Rev. 2010;9:144–147. doi: 10.1016/j.autrev.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 54.Makhanova N, Sequeira-Lopez MLS, Gomez RA, Kim H-S, Smithies O. Disturbed homeostasis in sodium-restricted mice heterozygous and homozygous for aldosterone synthase gene disruption. Hypertension. 2006;48:1151–1159. doi: 10.1161/01.HYP.0000249902.09036.e7. [DOI] [PubMed] [Google Scholar]
- 55.Prakash MR, Norgren R. Comparing salt appetites: induction with intracranial hormones or dietary sodium restriction. Brain Res Bull. 1991;27:397–401. doi: 10.1016/0361-9230(91)90132-4. [DOI] [PubMed] [Google Scholar]
- 56.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 57.Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, Felder RB. Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H2241–H2251. doi: 10.1152/ajpheart.2001.281.5.H2241. [DOI] [PubMed] [Google Scholar]
- 58.Philipson H, Ekman I, Swedberg K, Schaufelberger M. A pilot study of salt and water restriction in patients with chronic heart failure. Scand Cardiovasc J. 2010;44:209–214. doi: 10.3109/14017431003698523. [DOI] [PubMed] [Google Scholar]
- 59.Colín Ramírez E, Castillo Martínez L, Orea Tejeda A, Rebollar González V, Narváez David R, Asensio Lafuente E. Effects of a nutritional intervention on body composition, clinical status, and quality of life in patients with heart failure. Nutrition. 2004;20:890–895. doi: 10.1016/j.nut.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 60.Volpe M, Magri P, Rao MA, Cangianiello S, DeNicola L, Mele AF, Memoli B, Enea I, Rubattu S, Gigante B, Trimarco B, Epstein M, Condorelli M. Intrarenal determinants of sodium retention in mild heart failure: effects of angiotensin-converting enzyme inhibition. Hypertension. 1997;30:168–176. doi: 10.1161/01.hyp.30.2.168. [DOI] [PubMed] [Google Scholar]
- 61.Alvelos M, Ferreira A, Bettencourt P, Serrão P, Pestana M, Cerqueira-Gomes M, Soares-Da-Silva P. The effect of dietary sodium restriction on neurohumoral activity and renal dopaminergic response in patients with heart failure. Eur J Heart Fail. 2004;6:593–599. doi: 10.1016/j.ejheart.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 62.Luft FC, Sloan RS, Fineberg NS, Free AH. The utility of overnight urine collections in assessing compliance with a low sodium intake diet. JAMA. 1983;249:1764–1768. [PubMed] [Google Scholar]
- 63.Luft FC, Fineberg NS, Sloan RS. Estimating dietary sodium intake in individuals receiving a randomly fluctuating intake. Hypertension. 1982;4:805–808. doi: 10.1161/01.hyp.4.6.805. [DOI] [PubMed] [Google Scholar]