Abstract
Respiratory Syncytial Virus (RSV) is a leading cause of severe respiratory disease in infants and the elderly. No vaccine is presently available to address this major unmet medical need. We generated a new genetic vaccine based on chimpanzee Adenovirus (PanAd3-RSV) and Modified Vaccinia Ankara RSV (MVA-RSV) encoding the F, N, and M2-1 proteins of RSV, for the induction of neutralizing antibodies and broad cellular immunity. Because RSV infection is restricted to the respiratory tract, we compared intranasal (IN) and intramuscular (M) administration for safety, immunogenicity, and efficacy in different species. A single IN or IM vaccination completely protected BALB/c mice and cotton rats against RSV replication in the lungs. However, only IN administration could prevent infection in the upper respiratory tract. IM vaccination with MVA-RSV also protected cotton rats from lower respiratory tract infection in the absence of detectable neutralizing antibodies. Heterologous prime boost with PanAd3-RSV and MVA-RSV elicited high neutralizing antibody titers and broad T-cell responses in nonhuman primates. In addition, animals primed in the nose developed mucosal IgA against the F protein. In conclusion, we have shown that our vectored RSV vaccine induces potent cellular and humoral responses in a primate model, providing strong support for clinical testing.
Introduction
Human respiratory syncytial virus (HRSV) is a highly infectious member of the Paramyxoviridae family causing upper and lower respiratory tract infections in humans. Respiratory syncytial virus (RSV) infection in children causes ~12 million severe and 3 million very severe cases of lower respiratory tract infection (LRTI) worldwide.1 RSV infection is also recognized as a significant problem in older adults. Epidemiological evidence indicates that the impact of RSV in the elderly may be similar to nonpandemic influenza.2 No effective treatment is available and the only preventative measure is a humanized monoclonal antibody specific to the RSV fusion (F) protein (Palivizumab) administered as monthly injections during the RSV season to prevent lower respiratory infections and severe disease in high-risk infants. However, it does not prevent infection of the upper respiratory tract and is not recommended for use in healthy infants or the elderly.3 In addition, because of the high costs, Palivizumab is not extensively used worldwide. A major barrier to pediatric vaccine development has been the occurrence of enhanced respiratory disease (ERD) seen following natural RSV infection of naive infants that had been vaccinated earlier with a poorly protective formalin-inactivated RSV (FI-RSV).4,5 Although the mechanisms responsible for FI-RSV induced ERD are not clear, the most prevalent hypotheses, based mainly on preclinical data, are that FI-RSV induced antibodies with poor functional activity resulting in immune complex deposition and complement activation in the lungs, and/or induced Th2-biased T-cells.6,7 Based on the current knowledge, an RSV vaccine for infants would ideally induce: (i) neutralizing antibodies against the F protein for protection against lung infection8; (ii) a Th1-biased cellular immunity to contribute to virus clearance and to prevent ERD9; and (iii) mucosal immunity (IgA, IgG, and T-cells) to protect at the portal of virus entry.10
Clinical manifestation of RSV disease and the immune response to infection differ in infants and the elderly, suggesting that vaccines designed to protect these populations may require different attributes. Low levels of RSV-specific nasal IgA against F and G proteins were found to be a significant risk factor for RSV infection in adults10 and increasing evidence suggests that deficient RSV-specific T-cell responses contribute to susceptibility to severe RSV disease in older adults.11,12 Therefore, a protective vaccine for the elderly should primarily aim at increasing mucosal IgA and cellular immune responses.
Genetic vaccine approaches and, in particular, those based on replication deficient Adenovirus vectors can address all of these needs.13 So far, Adeno-vectored vaccines against HRSV have been mainly investigated in mice or cotton rats. Replication defective Adenovirus serotype 5 (Ad5) expressing the RSV F protein administered intramuscularly (IM) or intranasally (IN) or by a mixed modality of IM prime/ IN boost has provided protection from RSV challenge in mice14,15 and cotton rats.16 Similarly, a gorilla-derived Adeno vector expressing the RSV F protein has been recently reported to be protective in mice and cotton rats after IM vaccination.17
We have generated a new RSV vaccine candidate consisting of a synthetic, consensus-based sequence encoding a soluble F protein for the induction of neutralizing antibodies and the conserved N and M2-1 internal proteins, for the induction of a broader T-cell repertoire.18 This antigen was inserted in a replication incompetent chimpanzee Adenovirus (PanAd3), which is insensitive to pre-existing antiadenovirus antibodies present in the human population,19 and in Modified Vaccinia Ankara (MVA). We show here that a single IN or IM administration of PanAd3-RSV completely protected mice against RSV replication in the lungs and a single IN administration in cotton rats resulted in complete protective efficacy in the upper and in the lower respiratory tract for at least 3 months after vaccination without signs of enhanced pulmonary pathology. We also show that IM administration of a MVA vector encoding the same RSV antigen protects cotton rats from LRTI in the absence of neutralizing antibodies and without potentiation of pulmonary pathology.
An improved regimen based on heterologous prime/boost with PanAd3 administered IN or IM and MVA injected IM was also tested in nonhuman primates (NHP). Vaccinated macaques exhibited strong and Th1 biased T-cell responses which were directed against all the vaccine antigens, and neutralizing antibody titers greater than the previously reported threshold for protection of infants.20 Moreover, IN vaccination elicited mucosal IgA.
These preclinical data strongly support the clinical development of the vectored vaccine and suggest that different combinations of routes and regimens can be exploited for the development of RSV vaccines for the pediatric and the elderly populations.
Results
Vaccine antigen structure, expression and vectored delivery
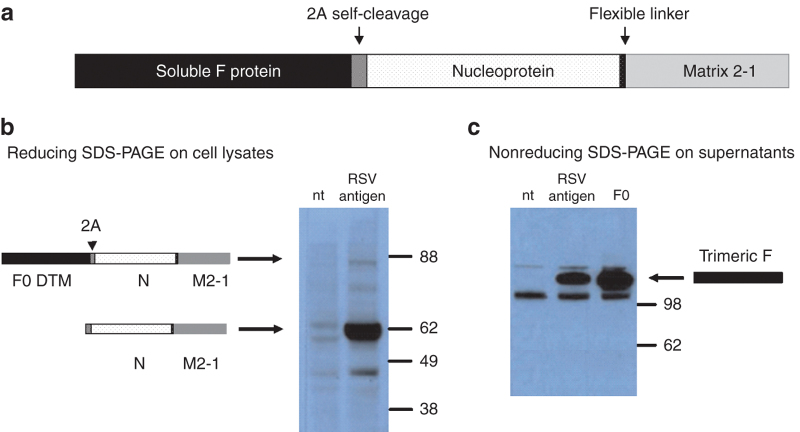
We designed a synthetic HRSV antigen composed of three viral proteins: F, N, and M2-1 (F0ΔTM–N–M2-1), which were encoded by a single open reading frame with a self-cleaving Foot and Mouth Disease virus 2A sequence21 between F and N sequences. A short sequence encoding a flexible linker was inserted between the N and the M2-1 sequences to facilitate folding (Figure 1a). The transmembrane and cytoplasmic regions of the F protein were deleted for efficient secretion of a soluble form of the F protein. The synthetic gene was codon-optimized for expression in human cells. Mature N–M2-1 fusion protein was detected by Western blot (WB) in cell lysates of DNA transfected HeLa cells (Figure 1b). The high molecular weight precursor F–N–M2-1 was barely detectable indicating efficient 2A-driven self-processing. Consistent with this observation, the soluble F protein was found in the medium of transfected cells with an apparent molecular weight of ~170 kDa on a nonreducing SDS–PAGE gel, compatible with F protein trimers, and was recognized by the conformation sensitive neutralizing antibody mAb 13 (ref. 22) (Figure 1c). When the soluble F protein was run on a reducing SDS–PAGE, two fragments were revealed consistent with the size of F1 and F2, indicating that the protein was correctly processed at the furin-cleavage sites (see Supplementary Figure S1).
Figure 1.
The RSV vaccine antigen. (a) Schematic diagram of the synthetic DNA fragment used to express the RSV antigens by PanAd3 and MVA vectors. 2A self-cleavage site derives from Foot and Mouth Disease virus sequence; (b) WB analysis of total cell lysates from HeLa cells not transfected (nt) or transfected with an expression plasmid bearing the RSV antigen (RSV) and revealed using a monoclonal antibody against M2-1 (mAb 8). The arrows indicate the bands corresponding to the MW of the unprocessed precursor or to the fused internal proteins N and M2-1; (c) WB analysis of supernatants of Hela cells transfected with an expression plasmid encoding the RSV antigen (RSV) or the F0ΔTM protein (F0), after migration on nonreducing SDS–PAGE. A band of ~170 kDa indicated by the arrow was revealed by the monoclonal antibody mAb 13 raised against the F protein which is consistent with a soluble F trimer.
The F0ΔTM–N–M2-1 RSV vaccine antigen was inserted into two different recombinant viral vectors: a replication-defective chimpanzee Adenovirus, PanAd3 (PanAd3-RSV) and a replication-defective poxvirus, Modified Vaccinia Ankara RSV (MVA-RSV).
PanAd3-RSV is immunogenic and protects BALB/c mice against HRSV infection
To test the immunological potency of the novel RSV vectored vaccines a dose–response immunization study with PanAd3-RSV was conducted in BALB/c mice (Figure 2a). T-cell responses were measured by IFN-γ ELISpot using three mapped CD8+ epitopes (F51-66, F85-93 and M2-182–90). As expected, given the presence of a strong Kd-restricted immunodominant epitope in the M2-1 protein, the T-cell response was mainly directed to the peptide M2-182–90 (see Supplementary Figure S2). The PanAd3-RSV vector showed strong T-cell responses over a wide range of doses, with 5 out 14 mice vaccinated with 106 viral particles (vp) still having RSV epitope-specific responses above the background.
Figure 2.
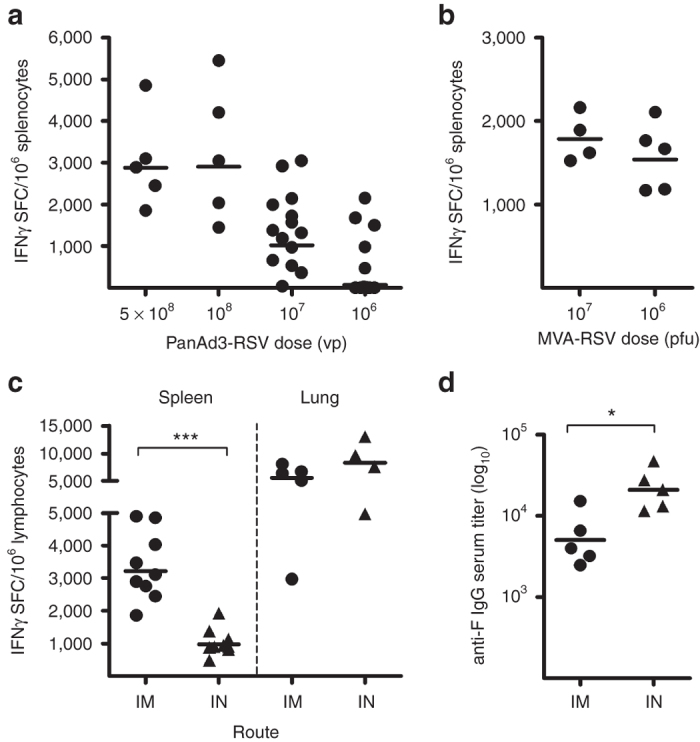
Immunological potency of PanAd3-RSV and MVA-RSV in BALB/c mice. (a) T-cell responses in spleens of animals vaccinated IM with different doses (vp) of PanAd3-RSV measured by IFNγ ELISpot. Symbols show individual mouse data, expressed as IFNγ spot forming cells (SFC)/million splenocytes, calculated as the sum of responses to the three immunodominant epitopes (F51-66, F85-93, and M2-182–90) corrected for background. Horizontal lines represent geometric mean; (b) T-cell responses in spleens of animals vaccinated IM with different doses (pfu) of MVA-RSV. (c) T-cell responses in spleen (left) or lung (right) of mice vaccinated with 5 × 108 vp of PanAd3-RSV IM or IN. Data are expressed as IFNγ SFC/million splenocytes or pulmonary lymphocytes; (d) Anti-F protein IgG endpoint titers in sera from IM and IN vaccinated animals. Responses in a, b, c, and d were measured 4 weeks after vaccination.
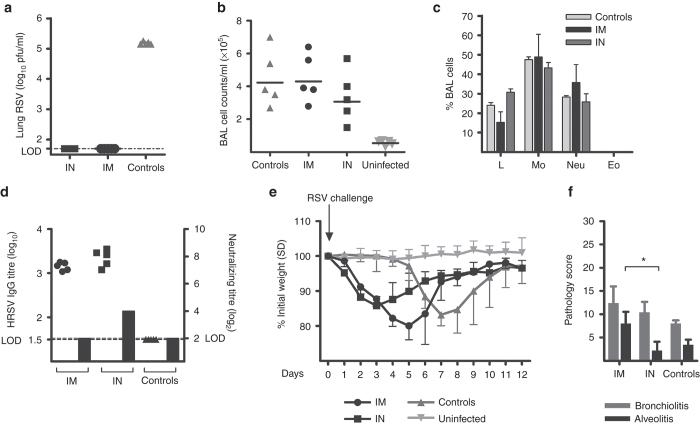
Because IN delivery can induce local immunity in the respiratory tract at the portal of virus entry,23 we compared T-cell and antibody responses in animals immunized by IM or IN delivery of 5 × 108 vp of PanAd3-RSV. IM immunization with PanAd3-RSV elicited stronger T-cell responses in the spleen compared with IN immunization (geometric mean = 3,224 versus 976 SFC/106 splenocytes), whereas comparable T-cell responses were observed in the lungs (8,300 versus 5,600 SFC/106) (Figure 2c). Low levels of serum antibodies to the F protein were induced 4 weeks after vaccination and unexpectedly, antibody titers were higher following IN vector delivery (Figure 2d). We next challenged BALB/c mice with HRSV, 4 weeks after IM or IN vaccination with 5 × 108 vp of PanAd3-RSV, to explore the effect of the strong Adeno-induced T-cell component in the absence of protective levels of neutralizing antibodies (Figure 3d). Following a high dose challenge with 4 × 106 plaque forming units (pfu) of HRSV, strain A2, vaccinated mice were fully protected against virus replication in the lung (Figure 3a). Importantly, none of the vaccinated animals showed eosinophils (Figure 3c) or increased number of leukocytes in bronchoalveolar lavages (BAL) compared with HRSV-infected, unvaccinated controls (Figure 3b). As expected, low levels of RSV-specific serum IgG were detected in both vaccinated groups at the day of the challenge, and levels of neutralizing antibodies were below the limit of detection in IM vaccinated mice and were log2 4 in IN vaccinated mice (Figure 3d). Following the high dose challenge, all mice lost weight following RSV infection; however, the onset of weight loss was more rapid in vaccinated mice than in controls and less pronounced in IN than in IM vaccinated mice (Figure 3e). IN delivery also showed a better efficacy profile also in terms of lung pathology relative to IM vaccinated mice. In fact, IM delivery induced higher scores of alveolitis (A) compared to either IN vaccinated mice or control animals (Figure 3f).
Figure 3.
RSV challenge in BALB/c mice. Vaccination with PanAd3-RSV at 5 × 108 vp dosage was performed IM or IN, and 4 weeks later the animals were challenged with 4 × 106 pfu of HRSV, strain A2. (a) RSV titer in lung homogenates 5 days postchallenge. Total (b) and differential (c) inflammatory cell counts in BAL from vaccinated and unvaccinated mice, either uninfected or RSV infected (controls) 6 days after RSV challenge. Single animal’s data are shown in (b), while mean + SD is shown in (c); Eo, Eosinophils; L, lymphocytes; Mo, monocytes; Neu, neutrophils; (d) RSV-specific IgG endpoint titer (filled symbols plotted on left y axis, individual mice) and RSV neutralization antibody titer (gray bar plotted on right y axis, pooled sera) in vaccinated and control animals. Limit of detection (LOD) for both assays is indicated by dashed lines; (e) Groups of five mice were vaccinated and challenged as described earlier and their body weight monitored for 12 days after challenge. Data are expressed as the percentage of weight loss compared to initial weight, and are presented as mean and SD for each group; (f) Mean + SD of pathology score recorded in each experimental group for bronchiolitis (gray bars) and A (black bars). Bronchiolitis (thickness of cells surrounding the bronchiole or blood vessel) and A (number of inflammatory cells in the air spaces) were scored on a scale from 0 to 3, and multiplied by the proportion of lung section affected on a scale from 0 to 4, giving a maximum score of 36 per mouse lung for each of the two parameters. Mean + SD of pathology score recorded in each experimental group for bronchiolitis (gray bars) and A (black bars) are shown.
A dose–response immunization was also conducted in BALB/c mice to characterize the immunogenicity of the MVA-RSV vector after a single IM injection. The results showed that this vector was also able to induce potent cellular immunity (Figure 2b). However, neutralizing antibodies were not detected in sera from vaccinated mice (data not shown).
PanAd3-RSV induces safe and durable protective efficacy in cotton rats
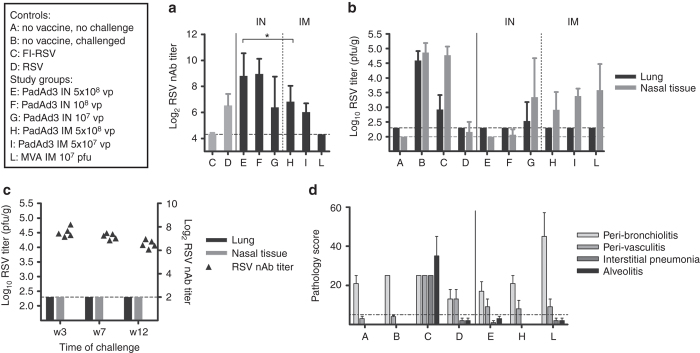
The cotton rat model is considered to be informative for the safety and efficacy profile of HRSV vaccines because (i) the animals can be infected with clinical isolates of HRSV; (ii) the virus replicates in both the upper and lower respiratory tract; and (iii) vaccination with FI-RSV vaccine primes for ERD-like hallmarks, such as alveolitis, following RSV challenge.24 Groups of cotton rats were immunized with single IN administrations of 107, 108 and 5 × 108 vp or with single IM administrations of 5 × 107 and 5 × 108 vp of PanAd3-RSV. Another group received 107 pfu MVA-RSV IM. All groups were challenged IN with RSV, 7 weeks after vaccination. Control groups included unvaccinated animals, a group earlier infected with RSV and a group vaccinated with FI-RSV as an internal control for enhanced pulmonary pathology. All animals vaccinated IM with PanAd3-RSV developed good levels of neutralizing antibodies (nAbs = Log2 6–7), comparable to those induced by an earlier RSV infection (Figure 4a). As observed in BALB/c mice, IN vaccination elicited higher titers of nAbs (Log2 8–9) respect to IM vaccination at the same dose. In contrast, none of the animals vaccinated with MVA-RSV showed detectable levels of neutralizing antibodies 7 weeks after vaccination. As shown in Figure 4b, all vaccinated animals except one outlier in the group receiving the lowest dose IN were fully protected against viral replication in the lung. In addition, mucosal vaccination with PanAd3-RSV at doses of 108 and 5 × 108 effectively blocked RSV replication in the upper respiratory tract (Figure 4b), In contrast, although systemic vaccination strongly reduced viral replication in the nose, it did not achieve complete protection even at high doses. These data support the importance of mucosal immunity in preventing RSV infection. Interestingly, a single IM dose of MVA-RSV was also fully protective in the lung, despite the lack of induction of circulating neutralizing antibodies, suggesting a role for other immune effector mechanisms in MVA-mediated protection. We further explored the durability of the protective efficacy given by the single IN dose of 5 × 108 vp PanAd3-RSV. Three groups of cotton rats were vaccinated IN with PanAd3 and challenged 3, 7, or 12 weeks after vaccination. All animals were fully protected from infection in the upper and lower respiratory tract and showed quite stable neutralizing antibody titers over the whole observation period (Figure 4c). As expected, RSV infection in FI-RSV vaccinated cotton rats was associated with increased interstitial pneumonia (IP) and alveolitis (A) scores (Figure 4d). In contrast, animals vaccinated with PanAd3-RSV IN or IM or MVA-RSV IM showed no differences in A or IP scores when compared with controls, supporting a favorable safety profile of genetic vaccine regimes.
Figure 4.
Vaccine immunogenicity, efficacy, safety, and durability in cotton rats. Controls and vaccine study groups are described in the text box. Animals were vaccinated with different doses of PanAd3-RSV (IN or IM as indicated), or MVA-RSV (IM). Seven weeks later they were challenged IN with 1 × 105 pfu of RSV/A/long strain. FI-RSV: formalin-inactivated RSV (Lot 100), two doses IM. RSV: previous infection IN with 1 × 105 pfu of RSV/A/long strain (a) RSV neutralizing antibody titers in vaccinated and control animals expressed as the serum dilution (log2) reducing plaques by 60% compared to controls. Sera were collected at study week 7 at the time of challenge. Bars represent group mean + SD. (b) RSV titers in lung homogenates and nasal tissue collected 5 days after RSV challenge. Data are expressed as RSV plaque forming units per gram of tissue (pfu/g). Bars represent group mean + SD. (c) RSV neutralizing titers (filled symbols plotted on right y axis, individual animals) and virus titers in lung and nasal tissue (black and gray bars plotted on left y axis, group mean + SD) at week 3, 7, and 12 after IM vaccination with 5 × 108 vp PanAd3-RSV. (d) Histological analysis of lung sections 5 days after RSV challenge. Formalin-fixed, paraffin-embedded lung sections were stained with hematoxylin and eosin. Four parameters of pulmonary inflammation were evaluated: peribronchiolitis (PB), perivasculitis (PV), interstitial pneumonia (IP), and alveolitis (A). Slides are scored blind on a 0–4 severity scale, and values are then converted to a 0–100% histopathology score. The dashed line (set at 5%) represents a threshold of IP and A pathology score considered not compatible with ERD. Bars show group mean + SEM.
PanAd3-RSV prime/MVA boost induces strong B- and T-cell responses in CD1 mice
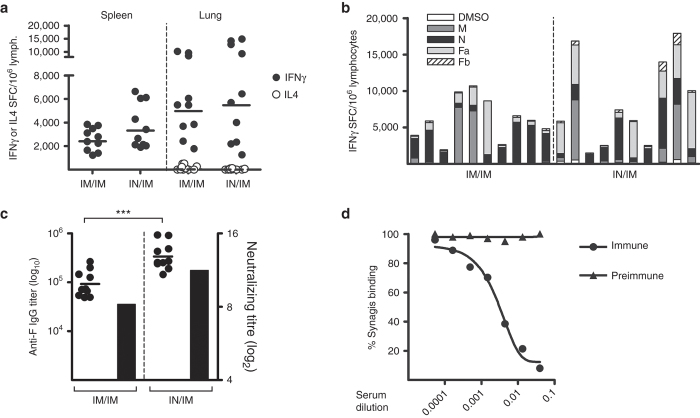
Outbred CD1 mice were used to test the immunological potency of various combinations of PanAd3 prime/MVA boost regimens as they are genetically heterogeneous and therefore represent a better model than inbred mice for human MHC heterogeneity. CD1 mice were administered IN or IM with 108 vp of PanAd3-RSV, followed by IM boosting with 107 pfu of MVA-RSV, 4 weeks later. Spleen and lung RSV-specific T lymphocytes were analyzed 3 weeks after the boost by IFN-γ ELISpot. Both regimens induced broad and potent IFN-γ T-cell responses which recognized all three RSV vaccine antigens to varying extents (Figure 5a,b). Pulmonary RSV-specific T-cells producing IL-4, which have been correlated with enhanced disease in animal models and in humans,6 were not detected in the lung of PanAd3-RSV/MVA-RSV immunized animals, indicating that the response was skewed toward a Th1 phenotype (Figure 5a).
Figure 5.
Cellular and humoral response to PanAd3-RSV/MVA-RSV vaccination in CD1 mice. (a) T-cell responses, measured by ELISpot in spleen (left) or lung (right) of mice vaccinated with 108 vp of PanAd3-RSV IM or IN, and boosted IM 4 weeks later with MVA-RSV at 107 pfu. For each mouse, the sum of responses to four overlapping peptide pools spanning the complete RSV vaccine antigen subtracted of DMSO background was calculated. Data are expressed as IFN-γ (closed symbols) or IL4 (open symbols) SFC/million splenocytes or pulmonary lymphocytes; (b) magnitude and breadth of IFN-γ lung lymphocyte response to individual peptide pools covering RSV transgene (Fa, Fb, M, and N) and to DMSO, the peptide diluent, in individual mice within each immunization group (indicated at the bottom of the graph); (c) Anti-F protein IgG endpoint titer (filled symbols plotted on left y axis, individual mice) and RSV neutralization titer (black bar plotted on right y axis, pooled sera) in vaccinated animals. Horizontal lines in a and c represent geometric mean values. All responses shown in a, b, and c were measured 3 weeks postboost; (d) Inhibition of Synagis (Palivizumab) binding to F protein by pooled sera from mice immunized with PanAd3-RSV and control preimmune sera. Synagis binding (percentage of ELISA signal without competing sera) is plotted as a function of the dilution of competing pooled sera.
Higher anti-F IgG titers were induced by IN PanAd3-RSV prime, but overall the levels of neutralizing antibodies were comparably high (up to log2 12) in both prime/boost regimens (Figure 5c). Furthermore, a competition assay showed that antibodies induced by vaccination with PanAd3-RSV IN/MVA-RSV IM were able to compete with Palivizumab, thus binding antigenic site II or overlapping epitopes (Figure 5d).
In conclusion, PanAd3/MVA induced potent humoral and cellular immunity in CD1 mice.
Heterologous prime/boost regimens are immunogenic in NHP
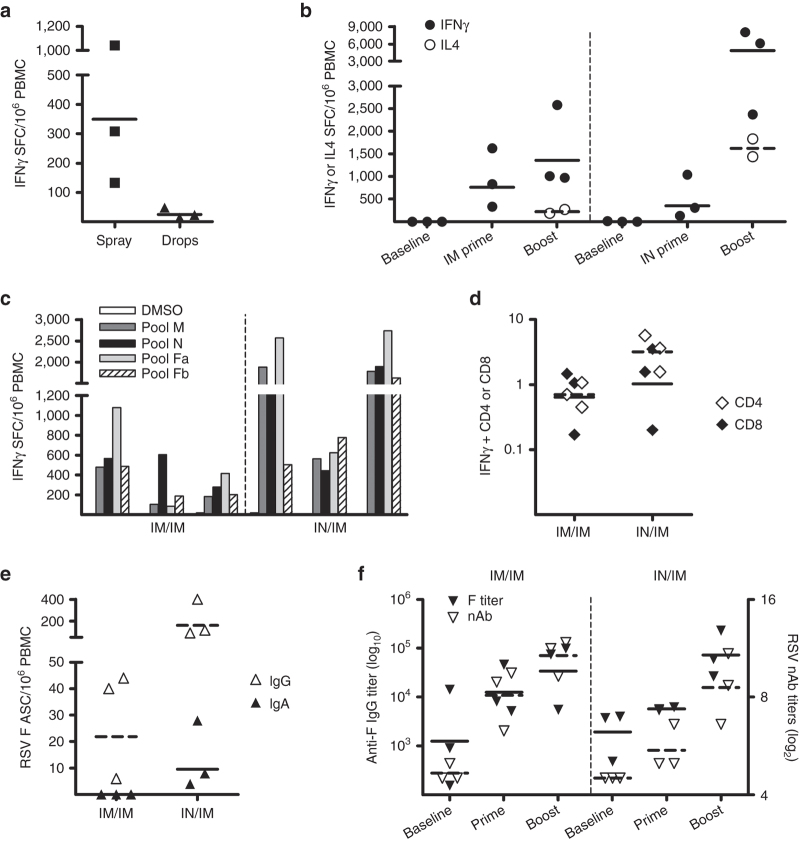
Data gathered in the different rodent models strongly supported the IN route as an immunogenic, protective, and safe route for the vectored RSV vaccine. However, it is generally recognized that genetic vaccines are often less effective in large animals than in rodents.25 Therefore, the immunogenicity of prime/boost vaccine regimens was explored in primates, which are a more relevant model for the development of a human vaccine. A crucial aspect of IN administration is the modality of vaccine delivery into the nasal cavity. Two different IN delivery systems were explored: instillation and IN spray. The spray device (Accuspray system, Becton Dickinson, Franklin Lakes, NJ) was chosen because it generates an aerosol of an average drop size of 30 µm, to limit penetration into the lower respiratory tract.26 Three groups of three Cynomolgus macaques (Macaca fascicularis) were immunized with 5 × 1010 vp PanAd3-RSV by: (i) IM injection, (ii) IN instillation of drops and (iii) IN spray. As shown in Figure 6a, T-cell responses in the blood were higher in animals inoculated with the spray device than those inoculated by IN instillation. Therefore, this group of animals and the group that received the prime IM were boosted 8 weeks later by IM injection of 1 × 108 pfu of MVA-RSV. T-cell responses after vaccination were measured by IFN-γ and IL4 T-cell ELISpot (Figure 6b). None of the animals had detectable RSV-specific T-cells at baseline, but all animals developed T-cell responses 2 weeks after prime and were effectively and potently boosted by MVA, with the IN/IM regimen showing higher peak levels of T-cell responses in peripheral blood mononuclear cells (PBMCs). IL4 secreting cells were detected after boost in both groups of vaccinated monkeys although they were more abundant in the IN/IM regimen. Overall, the RSV specific T-cell responses were skewed to a Th1 phenotype in both groups (Figure 6b). T-cell responses were broadly distributed over the three proteins encoded by the vaccine, confirming that they represented a good source of T-cell epitopes independently of individual MHC haplotype (Figure 6c). IFN-γ intracellular staining revealed that a large proportion of the RSV-specific T-cell response induced in the two groups of vaccinated animals consisted of CD4+ T-cells (Figure 6d).
Figure 6.
Vaccine immunogenicity in Cynomolgus macaques. (a) T-cell responses in PBMC of macaques 2 weeks after IN vaccination with 5 × 1010 vp PanAd3-RSV delivered by the spray device Accuspray (Becton Dickinson) or by instillation (drops). Data are expressed as IFN-γ SFC/million PBMC. For each animal, the sum of responses to four overlapping peptide pools spanning the complete RSV vaccine antigen subtracted of the background reactivity to the peptide diluent (DMSO) was calculated. (b) IFN-γ (filled symbols) and IL4- (open symbols) T-cell responses following PanAd3-RSV prime/MVA-RSV boost vaccination. Each dot represents the response in a single animal, each line corresponds to geometric mean in IM (left) or IN (right) primed animals. PBMC for IL4 testing after MVA boost was only available for two animals per group. (c) IFN-γ ELISpot responses to single RSV pools and DMSO is shown at week 9, 1 week after MVA boost, in each animal. (d) IFN-γ intracellular cytokine staining (ICS) and FACS analysis of IM (left) or IN (right) primed animals, 1 week after MVA IM boost. Data are expressed as the percentage of IFN-γ secreting CD4+ (open symbols) or CD8+ (filled symbols) T-cells on stimulation with RSV peptide pools. Each dot represents total RSV CD4+ or CD8+ response, obtained by summing reactivity to each peptide pool, corrected for DMSO background. (e) F-protein specific ASCs measured by B-cell ELISpot in PBMC of vaccinated macaques, 1 week after MVA boost. Data are expressed for each animal as number of IgG (open symbols) or IgA (filled symbols) ASC per million PBMC (f) Anti-F protein IgG titer (filled symbols plotted on left y axis), and anti-RSV neutralizing titer (open symbols plotted on right x axis in sera from individual vaccinated animals. IgG antibody titer calculated as the reciprocal of the minimal dilution giving a value three times higher than the SD of the preimmune serum. RSV neutralizing titers measured by a FACS-based RSV-EGFP infection assay are defined as the reciprocal of the dilution giving 50% of inhibition of infection (EC50) and expressed as log2. Horizontal lines in a, b, d, e, and f represent geometric mean values.
The number of circulating antibody secreting cells (ASCs) producing IgG or IgA specific for the F protein was measured by B cell ELISpot on freshly isolated PBMC 1 week after MVA boost (Figure 6e). Although detectable levels of F-specific IgG secreting B cells were measured in both groups, only IN primed animals had circulating IgA secreting B cells, showing that this route elicited mucosal immunity (Figure 6e). Both IN and IM PanAd3 prime induced serum anti-F and neutralizing antibodies, with the IM route being more effective (Figure 6f). IM boost with MVA increased the titers of anti-F IgG and neutralizing antibodies in both groups (GMT [geometric mean titer] = log2 9.8 in the IM/IM and GMT = log2 8.2 in the IN/IM) (Figure 6f).
Discussion
Our strategy to develop an effective and safe candidate RSV vaccine was based on: (i) the design of a novel immunogen including B- and T-cell antigens; (ii) the choice of a validated vector delivery platform capable of eliciting humoral and cellular immunity in humans; and (iii) the demonstration of consistent immunogenicity, safety, and efficacy of different mucosal and systemic regimens of administration in rodents and NHPs.
The RSV F glycoprotein is a conserved target of nAbs, including Palivizumab (Synagis), which also contains human T-cell epitopes. The N and M2-1 internal proteins are highly conserved between RSV strains and are known to be a source of T-cell epitopes in humans.18 We therefore designed an artificial fusion protein including an N-terminal F protein followed by a picorna virus 2A consensus cleavage sequence and by the N and M2-1 coding sequences separated by a flexible gly-ser linker to allow for proper independent folding of the two polypeptides. The intervening 2A sequence led to efficient cotranslational cleavage of the polyprotein releasing the F protein from the rest of the N–M2-1 fusion protein. Although we did not perform structural analysis on this product, three lines of evidence suggest that the soluble F protein adopted a properly folded postfusion conformation. First, the design of our vector-encoded F is equivalent to that described by Chaiwatpongsakorn et al.27 who showed that a recombinant F, deleted of the transmembrane and cytoplasmic domains and produced in human HEK293 cells, self-assembles into soluble prefusion trimers that are metastable and can be easily triggered to a postfusion conformation. Second, on expression of the F–N–M2-1 polyprotein in human cells, the secreted F displays a migration pattern in PAGE electrophoresis consistent with the formation of homotrimers. Finally, conformation-sensitive antibodies specific for the native F protein (including Synagis) efficiently recognized the vector-expressed F. Based on this evidence, we concluded that the soluble F expressed by the vectored vaccine most probably contains antigenic sites I, II, and III, but we could not ascertain whether it contains the recently identified antigenic site Ø which is present only in the prefusion conformation.28
We showed that both IN and IM delivery of the F–N–M2-1 antigen by a single administration of the replication incompetent chimpanzee Adenovirus PanAd3 or by PanAd3 prime/MVA boost induced high levels of RSV nAbs in all the animal species tested, apart from BALB/c mice. Acknowledging the fact that comparison of antibody levels among different labs deserves some caution, the neutralizing antibody titers induced in CD1 mice and cotton rats by F–N–M2-1 vectored delivery were comparable to those previously reported to be induced by recombinant protein or VLP in adjuvant,29,30 and three- to fivefold higher than those required for protective immunity in the cotton rat model.31 In NHPs the genetic vaccine induced nAb titers of Log2 ≥ 8, which are above the threshold of RSV nAb titres of Log2 ≥ 6 that have been associated with a reduced risk of hospitalization in infants.20
In addition to neutralizing antibodies, RSV-specific CTLs may also play a crucial role in protection from RSV disease as suggested by studies in infants where decreased frequencies of CD8+ T-cells correlated with increased severity of RSV disease32 or by studies in which children with compromised cell-mediated immunity shed virus for a prolonged time and have increased disease.33,34 Furthermore, an age-related decline of RSV-specific cellular immunity has been described in older adults,11,12 whereas a similar decline was not observed for influenza-specific T-cell responses.35 These observations suggest that the elderly have a reduced capacity to generate or maintain RSV-specific T-cells and this defect may be correlated with their increased susceptibility to RSV disease. Therefore, boosting cellular immunity to RSV could be beneficial in reducing the burden of severe disease in the elderly.
We showed that the vectored RSV vaccine induced a broad, strong, and Th1-biased cellular immune response in all the animal species tested. A Th1-skewed T-cell response in BALB/c mice can prevent allergic inflammation and eosinophilia in the lung post-RSV challenge.36 On the other hand, RSV-specific CTLs are known to contribute to immune-mediated pathology. In this inbred model, the immunodominant Kd restricted M2-182–90 response has an extreme effector phenotype and can produce immunopathology when present at high frequencies.37 Furthermore, the strong CD8+ response induced in BALB/c mice may be responsible for the low levels of F-specific and neutralizing antibodies. The rapid, but transient weight loss seen in vaccinated BALB/c mice in our studies and the increased lung pathology seen in IM vaccinated BALB/c mice are most likely because of a high titer virus inoculum encountering the strong M2-182–90 CD8+ response in the absence of neutralizing antibodies. To provide experimental support to our hypothesis further experiments are needed, such as a lower titer RSV challenge, or the introduction of mutations in the CTL epitope M2-182–90 as described by Vallbracht et al.38 who showed significant reduction in weight loss after RSV infection without an impact on viral clearance.
In this study we demonstrated that vaccination of cotton rats with MVA-RSV by IM delivery was also completely protective in the lung, despite the absence of serum neutralizing antibodies; these animals had no signs of A or IP but showed higher scores of peri-bronchiolar infiltrates in the lung which could be interpreted as a consequence of the critical role played by cell mediated immunity in clearing the virus in the absence of nAbs.
Overall, the protective immunity given by the vectored vaccines in the absence of neutralizing antibodies in the two rodent models of HRSV infection was not associated to enhanced lung pathology: in BALB/c mice the vaccine did not induce pulmonary eosinophilia after challenge and in cotton rats it was protective without inducing pulmonary pathology.
In contrast to BALB/c mice, PanAd3-RSV induced good levels of neutralizing antibodies even at low doses in cotton rats. All the tested doses and routes conferred full protection in the lung, with no associated pathology. An important role for mucosal immunity in protective efficacy from RSV infection emerged from the cotton rat challenge studies where only the animals that received PanAd3-RSV IN were completely protected against RSV infection in both the upper and the lower respiratory tract. Further experiments are needed to find the dose of vaccine conferring partial protection in the lung and to assess any associated pulmonary pathology. Nevertheless, to confirm and expand our notions on safety, immunogenicity, and efficacy of the vectored vaccine, studies are ongoing in a model of natural infection, such as young sero-negative calves.
Evidence from human clinical trials has shown that heterologous chimpanzee Adeno prime/MVA boost vaccine regimen induces potent, broad, and durable T- and B-cell immunity.39 Here we demonstrate, consistently, that priming with PanAd3-RSV and boosting with MVA-RSV were very efficient at inducing high nAb titers as well as potent and broad RSV-specific T-cell responses in NHPs. T-cell responses were distributed over all vaccine antigens confirming that the strategy of including the conserved viral proteins N and M2-1 effectively augmented vaccine immunogenicity. Interestingly, the neutralizing antibody titers were similar between IM and IN vaccinated animals but only IN primed animals showed circulating RSV-specific IgA-secreting cells, suggesting that nasal delivery elicited mucosal immunity.
In conclusion, we have presented evidence that a new RSV antigen delivered by genetic vaccine vectors using a combination of routes and heterologous prime/boost regimen can induce a full array of immune responses that could address the different attributes required to protect infants and the elderly.
Materials and Methods
Vaccine antigen
The human RSV expression cassette contained consensus sequences of F, N, and M2-1. HRSV F, N, and M2-1 sequences were downloaded from the NCBI database. Protein sequences were chosen among RSV subgroup A for F, N, and M2-1 proteins, and consensus sequences were derived by alignment using MUSCLE version 3.6 (refs. 40,41) of all nonidentical sequences and applying the majority rule (see Supplementary Figure S3, S4, S5, and S6). The vaccine F protein lacked aa 525–574, which contains the transmembrane and intracellular regions. N and M2-1 sequences were spaced by a flexible linker (3GS3G). Consensus F0ΔTM, N, and M2-1 sequences were optimized for mammalian expression, including the addition of a Kozak sequence and codon usage optimization.
Adenovirus vector
Pan Adenovirus type 3 (PanAd3) was isolated from a stool specimen collected from a bonobo chimpanzee (Pan Paniscus). The PanAd3 isolate was amplified and the virus genome was then cloned in a plasmid vector and fully sequenced. The sequence analysis of the hexon region revealed a close relationship with species C human and chimpanzee adenovirus already used in preclinical and clinical trials (human Ad5, ChAd3). The E1 and E4 regions were deleted from the PanAd3 vector and the vector was propagated in PROCELL92 cells. An expression cassette based on human cytomegalovirus promoter with the TetO inserted downstream of the HCMV promoter TATA box, and bovine growth hormone polyadenylation signal was constructed to express RSV antigens and inserted into PanAd3 by replacing the E1 region. The expression cassette was first inserted into the mammalian expression vector pVJ generating the plasmid pVJ-RSV and then transferred by homologous recombination into the ΔE1ΔE4 preadeno plasmids.
Adenovirus particle production
The PanAd3 vector was produced in PROCELL92 cells, a HEK293-based cell line suitable for manufacturing developed at Okairos, Rome, Italy. PROCELL92 cells were derived from the HEK 293 cell line originally banked at the University of Leiden in 1973 and obtained from Graham et al.42 at McMaster University (Hamilton, Canada), and further adapted at Okairos to be suitable for manufacturing by incorporation of a plasmid carrying a Tet repressor expression cassette and G418-resistance gene. The protocol for generating the PROCELL92 cell line followed essentially that published by Matthews et al.43 In brief, HEK 293 cells were transfected with an expression vector containing a cassette encoding the Tet repressor under control of the human phosphoglycerate kinase-1 promoter, and the G418-resistance gene. Single clones were selected by growing the transfected cells in the presence of 1 mg/ml G418 in culture medium. Single clones were amplified and tested for Tet repressor expression by WB analysis. The stability of Tet repressor expression in the selected clone was tested up to passage 63. PanAd3 vectors grown in these cells were purified by cesium chloride gradients and stored in buffer A195.44
MVA vector
MVA, attenuated by ~500 passages in primary chick embryo fibroblasts (CEFs),45 was used to create a recombinant MVA carrying the HRSV genes.
The HRSV genes were subcloned into the TPG shuttle vector.45 The so-generated transfer vector TPG-HRSV drives the HRSV antigen expression using the vaccinia P7.5 early/late promoter and enhanced green fluorescent protein (EGFP) expression using the synthetic promoter sP. The production of the recombinant virus was obtained by a previously described method46 based on in vivo recombination between homologous sequences (FlankIII-1 and -2 regions) present in both the acceptor virus MVA-RED virus46 and the plasmid transfer vector TPG-HRSV. Primary CEF cells were then infected/transfected with MVA-Red, a recombinant MVA carrying the HcRED1.1 gene and with the TPG-HRSV plasmid. The virus-containing cell lysate derived from infection/transfection was diluted 1:100 and used to infect fresh CEF in the presence of 1 μmol/l cytochalasin D (Sigma-Aldrich, St. Louis, MO). Infected cells, collected 24 hours p.i. by trypsinization, were washed, and kept on ice. Green cells were either bulk- or single cell-sorted by a Becton Dickinson FACSVantage SE flow cytometer (Becton Dickinson, San José, CA). EGFP fluorescence (excited at 488 nm) was detected using a 530/30 nm bandpass filter. HcRed1-1 fluorescence (excited at 633 nm) was detected using a 660/20-nm bandpass filter. Sorted cells were seeded onto CEF monolayers in microplate cultures to produce virus-containing cell lysate. Finally, markerless recombinant viruses (rMVA-HRSV) were cloned by terminal dilution, screened by whole plate fluoroimaging (Typhoon FLA 9000, GE Healthcare, Uppsala, Sweden), cloned again by terminal dilution and expanded in CEF by conventional methods.
In vitro expression and WB
HeLa cells were transfected with 4 µg of pVJ-RSV plasmid using Lypofectamine (Invitrogen, CA). Extracts were prepared 48 hours after transfection using TEN buffer (20 mmol/l Tris pH 7.5, 150 mmol/l NaCl, 1 mmol/l EDTA pH 8, 1% Triton X100 and protease inhibitors). Nuclei and cell debris were spun out by centrifugation at 7,500×g, 60 minutes at 4°C. Glycerol was added to supernatants to 10% and stored at −20 °C. Expression of the antigen proteins in the cell extracts was assessed by running the samples in reducing SDS–PAGE and WB with the monoclonal antibody mAb 8, specific for the M2-1 protein.22 Supernatants were collected 24 hours after infection. Expression of F protein was analyzed by running supernatant samples in nonreducing SDS–PAGE and WB with mAb 13, specific for the F protein.22
Peptides and proteins
CD8+ immunodominant peptides in BALB/c mice (F51-66, F85-93, and M2-182–90) were purchased from JPT Peptide Technologies GMBH. HRSV peptide pools covering the complete sequence of the vaccine antigens F, NP, and M2-1, consisting of 15-mer sequences with 11 amino acids overlap were purchased from JPT. The 269 peptides were dissolved in 100% DMSO and arranged in four pools as follows: pool M (46 peptides), pool N (95 peptides), pool Fa (N terminal half of F protein, 64 peptides), and pool Fb (C terminal half, 64 peptides). Recombinant (r)F protein from strain HRSV A2 was purchased from SinoBiologicals, Beijing, China.
Immunogenicity studies in mice and macaques
All experimental procedures were approved by institutional review boards and were performed in accordance with national and international laws and policies. The ethical committee of the Italian Ministry of Health approved this research. Animal handling procedures were performed under anesthesia and all efforts were made to reduce animal numbers and minimize suffering.
Six-week-old female BALB/c and CD1 mice were purchased from Charles River (Calco (Lecco), Italy). For IM immunizations, the intended dose of PanAd3-RSV or MVA-RVS vectors in a total volume of 100 µl was injected bilaterally in quadriceps muscles (50 µl/site). For IN administration, the intended dose of PanAd3-RSV in 20 µl volume was administered by delivering 10 µl in each nostril. Mice were euthanized after vaccination/challenge as specified in the figure legends to test immune responses. For experiments involving prime/boost, the two vaccinations were spaced 4 weeks apart, and mice were euthanized 3 weeks after boost. All manipulations were performed under isofluorane anesthesia. Splenocytes were isolated with standard techniques. To isolate infiltrating lymphocytes from the lungs of immunized mice, the organs were minced and incubated in DMEM/10% FBS with Collagenase Type I (210,000 U/mg, Gibco) for 60 minutes at 37 °C on an orbital shaker. All tissues were then homogenized, filtered through a sterile 70 µm Nylon cell strainer and lymphocytes were isolated by density gradient centrifugation (Ficoll-Paque). White cells were collected from the interface, and finally treated with ACK (#A10492, Gibco) to lyse red blood cells.
Female Cynomolgus macaques (Macaca fascicularis) from a purpose-bred colony housed at the IBCN primate facility (ENEA-Casaccia, Rome, Italy) were distributed in groups of three animals each, equivalent for mean body weight. During handling the animals were anesthetized by injection of 10 mg/kg ketamine hydrochloride. The injected dose was 5 × 1010 vp for adenoviral vectors, and 2 × 108 pfu for MVA. IM vaccination was performed in the deltoid muscle in 0.5 ml volume. For IN immunization, the same PanAd3-RSV dose was suspended in 0.2 ml total volume and 0.1 ml volume were administered per nostril as either drops or sprayed using the Accuspray (Becton Dickinson) device. To administer the vaccine with Accuspray, manufacturer recommendations for correct head position and device angle were followed. Sedated animals were kept with their head upright, and the tip of the device was inserted in the nostril keeping the syringe as horizontal as possible to orient the spray cloud correctly toward the nasal cavity. Active inspiration is not required for correct nasal deposition, thus sedation does not interfere with correct vaccination procedure. After Accuspray actuation, the animals were observed for a few minutes, and no vaccine leakage was seen from nostrils. The animals were closely monitored in the following days, and no signs of nasal irritation or discharge were observed in the IN vaccinated animals. PBMCs were isolated at selected time points with standard techniques.
IFNγ and IL4 ELISPOT
RSV-specific T-cells in mice splenocytes and pulmonary lymphocytes, or in macaque PBMC, were quantitated by a standard ELISpot assay exactly as described47 using antibody pairs obtained from U-CyTech (Utrecht, the Netherlands) and overnight stimulation with either CD8+ immunodominant peptides (BALB/c) at final concentration of 1 µg/ml, or with overlapping 15mer peptide pools at a final concentration of 3 µg/ml of each single peptide.
IFNγ intracellular cytokine staining and FACS analysis
The contribution of CD4 and CD8 subsets to the overall IFNγ T-cell response was analyzed in macaque PBMC by IFNγ intracellular staining and FACS analysis exactly as described earlier.47
Ex vivo F-specific IgG and IgA ASC ELISpot
Multiscreen HTS MSIP4510 plates (Millipore, MA, United States) were preactivated with 50 µl/well of 70% ethanol, washed with sterile water, and coated overnight with either 500 ng/well of recombinant F protein (Sinobiologicals, Beijing, China) diluted in 50 mmol/l NaHCO3 coating buffer, or with 150 ng/well of anti-IgG or anti-IgA capture mAbs diluted in PBS as a positive control. After washing, five times with PBS, plates were blocked with R10 (RPMI, 10% FBS, 200 mmol/l L-Glutamine, 50µg/ml Streptomycin, 50U Penicillin, 1M HEPES) culture medium for 30 minutes at room temperature. Freshly isolated PBMC were plated in R10 at 500,000 and 250,000 cells/well in duplicate (250,000/well and 125,000/well for total IgA and IgG, respectively) and left overnight at 37°C, 5% CO2. Plates are developed by subsequent incubation with biotinylated anti-IgG and anti-IgA detection mAbs, followed by streptavidin–alkaline phosphatase conjugate and finally with BCIP/NBT-plus till distinct spots emerged. All antibodies and ELISpot reagents were from Mabtech, Sweden. Plates were analyzed by an A.EL.VIS automated plate reader and responses were expressed as the number of antigen-specific IgG or IgA ASC per million PMBCs.
Humoral response analysis in mice and macaque sera
RSV-F protein-specific antibody levels were measured by enzyme-linked immunosorbent assay (ELISA). Nunc-MaxiSorp (SIGMA) 96-well plates were coated at 4°C overnight with 0.5 μg/100 μl of rF (Sinobiologicals) in 0.05 M NaHCO3 buffer. Data are expressed as endpoint titers calculated from 1 hour absorbance readings, defined as the highest dilution of sample giving an OD405 reading greater than three SD above the mean of preimmune samples. Levels of HRSV-specific IgG in mouse sera were determined using a lysate of HRSV A2 infected fetal calf kidney cells as described earlier.48 A lysate of mock-infected cells was used as a control antigen.
Neutralizing antibody titers were measured by a FACS-based assay which relies on a recombinant RSV expressing GFP,49 a generous gift from Prof. Mark E. Peeples, Nationwide Children’s Hospital, Columbus, OH, United States. The assay was performed essentially as described earlier.50 In brief, HEp-2 cells were plated (5 × 104 cells/100 μl each well) in 96-well plate(s) and incubated for 2 hours at 37 °C, 5% CO2. Serial dilutions of heat-inactivated sera were incubated with RSV expressing GFP (0.1 multiplicity of infection (MOI)) for 1 hour at 37 °C with 5% CO2 in 100 ml DMEM 7% FCS and then added to the cells and left for 20–22 hours at 37 °C in 5% CO2. Palivizumab (Synagis 100 mg, Abbott, IL, US Cat. 131-026-19) was used as a positive control. Cells were detached by trypsin, collected in PBS containing 1% FBS and 1% formaldehyde and then fluorescence was read on a FACS Canto cytometer, BD, using FACS DiVa Software (Becton Dickinson). The percentage of fluorescent cells for each serum dilution was calculated with respect to the negative control (100% of infection). The EC50 was calculated by curve fitting and nonlinear regression analysis (GraphPad Prism, GraphPad Software, San Diego, CA). In vaccination and challenge studies in BALB/c mice, neutralizing antibody titers in heat-inactivated serum were determined by a plaque reduction assay using the HRSV, A2 strain and calculated using a 50% reduction of the virus control.
BALB/c challenge study
Specific-pathogen-free, female BALB/c mice, weighing ~20 g were obtained from Charles River UK. Mice (N = 15/group) were vaccinated either IM with 5 × 108 vp of PanAd3-RSV in a volume of 50 μl, or PanAd3 expressing irrelevant antigens; or IN under isoflurane general anesthesia. Mice were bled from the tail vein, 2 and 4 weeks after vaccination and were challenged IN with 4 × 106 pfu of the A2 strain of HRSV, in a volume of 50 μl, under isoflurane general anesthesia, 4 weeks after vaccination. Groups of five mice were weighed daily for 12 days following HRSV challenge. Groups of five mice were sacrificed 5 days postchallenge, and HRSV titres in lung homogenates were determined by plaque assay on Vero cells. Further groups of five mice were sacrificed 6 days after challenge, and their lungs subjected to BAL with 1 ml of 12 mmol/l lidocaine in PBS, and then fixed in 10% buffered formalin. Cytocentrifuge preparations of BAL cells were stained with May-Grunwald Giemsa and differential cell counts of 300 cells per slide were made under oil immersion microscopy. Lung sections were stained with hematoxylin and eosin and histopathological lesions were scored as the sum of the scores for each of three different lung lobes/mouse. The extent of peribronchiolar and perivascular inflammation was scored on a scale from 0 to 3 depending on the thickness of cells surrounding the bronchiole or blood vessel multiplied by the proportion of lung section showing peribronchiolar inflammation on a scale from 0 to 4, giving a maximum bronchiolitis score of 36 for each mouse lung. The extent of A was scored on a scale of 0 to 3 depending on the number of inflammatory cells in the air spaces multiplied by the proportion of lung section with A on a scale from 0 to 4, giving a maximum A score of 36 for each mouse lung.
Cotton rat challenge study
Cotton rat studies were performed at Sigmovir Biosystems Inc (Rockville, MD). Groups of five or eight animals were vaccinated once either IN with 5 × 108 vp of PanAd3 (50 μl/nares) or IM with 100 μl PanAd3-RSV at different doses or MVA-RSV at 1 × 107 pfu or FI-RSV Lot#100 (1 : 125). Seven weeks after the vaccination, the animals were challenged IN with 100 μl HRSV/Long at 105 pfu per animal. Five days after challenge, the animals were euthanized, bled, and the nasal tissue and lung were harvested. Lungs were dissected and fixed with 10% neutral buffered formalin. Following fixation, the lungs were embedded in paraffin, sectioned and stained with hematoxylin and eosin. Four parameters of pulmonary inflammation were evaluated: peribronchiolitis (inflammatory cell infiltration around the bronchioles), perivasculitis (inflammatory cell infiltration around the small blood vessels), IP (inflammatory cell infiltration and thickening of alveolar walls), and A (cells within the alveolar spaces). Slides were scored blind on a 0–4 severity scale. The scores were subsequently converted to a histopathology scale 0–100%. Virus titers in lung and nose homogenates were determined by plaque assay on Hep-2 cells. RSV neutralizing antibodies were measured by plaque reduction assay on Hep-2 cells infected with RSV/Long and the reciprocal neutralizing antibody titers were determined at the 60% reduction end-point of the virus control using the statistics program “plqrd.manual.entry”.
Statistical analysis
Statistical analysis and graphs were made using GraphPad Prism version 5.02 for Windows (GraphPad Software, San Diego, CA, http://www.graphpad.com). As data were not always normally distributed, a two-tailed Mann–Whitney (nonparametric) test was used to compare two groups. Statistically significant differences are shown as follows: *P = 0.01–0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Statistical analysis was applied to immunological or pathology data when comparing groups vaccinated at the same dose via different routes.
Acknowledgments
The authors acknowledge Andrea Sbardellati (CRS4, Cagliari, Italy) for bioinformatic help in the design of the vaccine antigens, Sigmovir Biosystems Inc (Rockville, MD, United States) for expert assistance in the cotton rat studies, Mark Peeples (The Research Institute at Nationwide Children’s Hospital, Columbus, OH, United States) for kindly providing the recombinant RSV expressing GFP virus, Jean-François Toussaint (GSK Vaccines, Rixensart, Belgium, and Ann-Muriel Steff (GSK, Laval, Canada) for critical reading of the manuscript. BBSRC Institute Strategic Programme on Livestock Viral Diseases at The Pirbright Institute and MRC grant MR/J014648/1. G.T. is a Jenner Investigator. A.V., R.C., and A.N. are named inventors on patent applications covering RSV antigen expression system (WO 2012/089833). The other authors declare that they have no competing interests.
The first two authors equally contributed to this work.
References
- Nair H, Simões EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JS, Severe Acute Lower Respiratory Infections Working Group Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381:1380–1390. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey AR. Editorial commentary: respiratory syncytial virus: a global pathogen in an aging world. Clin Infect Dis. 2013;57:1078–1080. doi: 10.1093/cid/cit473. [DOI] [PubMed] [Google Scholar]
- The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89:435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC, 3rd, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack FP, Teng MN, Collins PL, Prince GA, Exner M, Regele H. A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med. 2002;196:859–865. doi: 10.1084/jem.20020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis JR, Simoes EA, Levin MJ, Hall CB, Long CE, Rodriguez WJ. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- Hussell T, Baldwin CJ, O’Garra A, Openshaw PJ. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur J Immunol. 1997;27:3341–3349. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190:373–378. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- Cherukuri A, Patton K, Gasser RA, Jr, Zuo F, Woo J, Esser MT. Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin Vaccine Immunol. 2013;20:239–247. doi: 10.1128/CVI.00580-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bree GJ, Heidema J, van Leeuwen EM, van Bleek GM, Jonkers RE, Jansen HM. Respiratory syncytial virus-specific CD8+ memory T cell responses in elderly persons. J Infect Dis. 2005;191:1710–1718. doi: 10.1086/429695. [DOI] [PubMed] [Google Scholar]
- Liniger M, Zuniga A, Naim HY. Use of viral vectors for the development of vaccines. Expert Rev Vaccines. 2007;6:255–266. doi: 10.1586/14760584.6.2.255. [DOI] [PubMed] [Google Scholar]
- Fu Y, He J, Zheng X, Wu Q, Zhang M, Wang X. Intranasal immunization with a replication-deficient adenoviral vector expressing the fusion glycoprotein of respiratory syncytial virus elicits protective immunity in BALB/c mice. Biochem Biophys Res Commun. 2009;381:528–532. doi: 10.1016/j.bbrc.2009.02.075. [DOI] [PubMed] [Google Scholar]
- Kohlmann R, Schwannecke S, Tippler B, Ternette N, Temchura VV, Tenbusch M. Protective efficacy and immunogenicity of an adenoviral vector vaccine encoding the codon-optimized F protein of respiratory syncytial virus. J Virol. 2009;83:12601–12610. doi: 10.1128/JVI.01036-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Okada K, Beeler JA, Crim RL, Piedra PA, Gilbert BE. Development of an adenovirus-based respiratory syncytial virus vaccine: preclinical evaluation of efficacy, immunogenicity, and enhanced disease in a cotton rat model. J Virol. 2014;88:5100–5108. doi: 10.1128/JVI.03194-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TR, Rangel D, Graham BS, Brough DE, Gall JG. Genetic vaccine for respiratory syncytial virus provides protection without disease potentiation. Mol Ther. 2014;22:196–205. doi: 10.1038/mt.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Huang Y, Langley JM. Prospects for defined epitope vaccines for respiratory syncytial virus. Future Microbiol. 2010;5:585–602. doi: 10.2217/fmb.10.22. [DOI] [PubMed] [Google Scholar]
- Vitelli A, Quirion MR, Lo CY, Misplon JA, Grabowska AK, Pierantoni A. Vaccination to conserved influenza antigens in mice using a novel Simian adenovirus vector, PanAd3, derived from the bonobo Pan paniscus. PLoS One. 2013;8:e55435. doi: 10.1371/journal.pone.0055435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedra PA, Jewell AM, Cron SG, Atmar RL, Glezen WP. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine. 2003;21:3479–3482. doi: 10.1016/s0264-410x(03)00355-4. [DOI] [PubMed] [Google Scholar]
- Fang J, Qian JJ, Yi S, Harding TC, Tu GH, VanRoey M. Stable antibody expression at therapeutic levels using the 2A peptide. Nat Biotechnol. 2005;23:584–590. doi: 10.1038/nbt1087. [DOI] [PubMed] [Google Scholar]
- Taylor G, Stott EJ, Furze J, Ford J, Sopp P. Protective epitopes on the fusion protein of respiratory syncytial virus recognized by murine and bovine monoclonal antibodies. J Gen Virol. 1992;73(Pt 9):2217–2223. doi: 10.1099/0022-1317-73-9-2217. [DOI] [PubMed] [Google Scholar]
- Woodland DL. Intranasal vaccination. Viral Immunol. 2013;26:125. doi: 10.1089/vim.2013.ed.26.2. [DOI] [PubMed] [Google Scholar]
- Boukhvalova MS, Blanco JC. The cotton rat Sigmodon hispidus model of respiratory syncytial virus infection. Curr Top Microbiol Immunol. 2013;372:347–358. doi: 10.1007/978-3-642-38919-1_17. [DOI] [PubMed] [Google Scholar]
- Gerdts V, Littel-van den Hurk Sv, Griebel PJ, Babiuk LA. Use of animal models in the development of human vaccines. Future Microbiol. 2007;2:667–675. doi: 10.2217/17460913.2.6.667. [DOI] [PubMed] [Google Scholar]
- Brand P, Häussinger K, Meyer T, Scheuch G, Schulz H, Selzer T. Intrapulmonary distribution of deposited particles. J Aerosol Med. 1999;12:275–284. doi: 10.1089/jam.1999.12.275. [DOI] [PubMed] [Google Scholar]
- Chaiwatpongsakorn S, Epand RF, Collins PL, Epand RM, Peeples ME. Soluble respiratory syncytial virus fusion protein in the fully cleaved, pretriggered state is triggered by exposure to low-molarity buffer. J Virol. 2011;85:3968–3977. doi: 10.1128/JVI.01813-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G, Raghunandan R, Wu Y, Liu Y, Massare M, Nathan M. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS One. 2012;7:e50852. doi: 10.1371/journal.pone.0050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci U S A. 2011;108:9619–9624. doi: 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince GA, Horswood RL, Chanock RM. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985;55:517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrañaga CL, Ampuero SL, Luchsinger VF, Carrión FA, Aguilar NV, Morales PR. Impaired immune response in severe human lower tract respiratory infection by respiratory syncytial virus. Pediatr Infect Dis J. 2009;28:867–873. doi: 10.1097/INF.0b013e3181a3ea71. [DOI] [PubMed] [Google Scholar]
- Fishaut M, Tubergen D, McIntosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr. 1980;96:179–186. doi: 10.1016/s0022-3476(80)80799-2. [DOI] [PubMed] [Google Scholar]
- Hall CB, Powell KR, MacDonald NE, Gala CL, Menegus ME, Suffin SC. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315:77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- Lee FE, Walsh EE, Falsey AR, Liu N, Liu D, Divekar A. The balance between influenza- and RSV-specific CD4 T cells secreting IL-10 or IFNgamma in young and healthy-elderly subjects. Mech Ageing Dev. 2005;126:1223–1229. doi: 10.1016/j.mad.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Olson MR, Varga SM. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J Immunol. 2007;179:5415–5424. doi: 10.4049/jimmunol.179.8.5415. [DOI] [PubMed] [Google Scholar]
- Cannon MJ, Openshaw PJ, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbracht S, Jessen B, Mrusek S, Enders A, Collins PL, Ehl S. Influence of a single viral epitope on T cell response and disease after infection of mice with respiratory syncytial virus. J Immunol. 2007;179:8264–8273. doi: 10.4049/jimmunol.179.12.8264. [DOI] [PubMed] [Google Scholar]
- Capone S, D’Alise AM, Ammendola V, Colloca S, Cortese R, Nicosia A. Development of chimpanzee adenoviruses as vaccine vectors: challenges and successes emerging from clinical trials. Expert Rev Vaccines. 2013;12:379–393. doi: 10.1586/erv.13.15. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Matthews DA, Cummings D, Evelegh C, Graham FL, Prevec L. Development and use of a 293 cell line expressing lac repressor for the rescue of recombinant adenoviruses expressing high levels of rabies virus glycoprotein. J Gen Virol. 1999;80 (Pt 2):345–353. doi: 10.1099/0022-1317-80-2-345. [DOI] [PubMed] [Google Scholar]
- Evans RK, Nawrocki DK, Isopi LA, Williams DM, Casimiro DR, Chin S. Development of stable liquid formulations for adenovirus-based vaccines. J Pharm Sci. 2004;93:2458–2475. doi: 10.1002/jps.20157. [DOI] [PubMed] [Google Scholar]
- Di Lullo G, Soprana E, Panigada M, Palini A, Agresti A, Comunian C. The combination of marker gene swapping and fluorescence-activated cell sorting improves the efficiency of recombinant modified vaccinia virus Ankara vaccine production for human use. J Virol Methods. 2010;163:195–204. doi: 10.1016/j.jviromet.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Di Lullo G, Soprana E, Panigada M, Palini A, Erfle V, Staib C. Marker gene swapping facilitates recombinant Modified Vaccinia Virus Ankara production by host-range selection. J Virol Methods. 2009;156:37–43. doi: 10.1016/j.jviromet.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Capone S, Naddeo M, D’Alise AM, Abbate A, Grazioli F, Del Gaudio A. Fusion of HCV nonstructural antigen to MHC class II-associated invariant chain enhances T-cell responses induced by vectored vaccines in nonhuman primates. Mol Ther. 2014;22:1039–1047. doi: 10.1038/mt.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G, Stott EJ, Hughes M, Collins AP. Respiratory syncytial virus infection in mice. Infect Immun. 1984;43:649–655. doi: 10.1128/iai.43.2.649-655.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak LK, Spillmann D, Collins PL, Peeples ME. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol. 2000;74:10508–10513. doi: 10.1128/jvi.74.22.10508-10513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Chang JS, Nason M, Rangel D, Gall JG, Graham BS. A flow cytometry-based assay to assess RSV-specific neutralizing antibody is reproducible, efficient and accurate. J Immunol Methods. 2010;362:180–184. doi: 10.1016/j.jim.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.