Abstract
We studied the role of γ-aminobutyric acid (GABA)ergic septohippocampal projections in medial septum (MS) self-stimulation of behaving mice. Self-stimulation was evoked in wild-type (WT) mice using instrumental conditioning procedures and in J20 mutant mice, a type of mouse with a significant deficit in GABAergic septohippocampal projections. J20 mice showed a significant modification in hippocampal activities, including a different response for input/output curves and the paired-pulse test, a larger long-term potentiation (LTP), and a delayed acquisition and lower performance in the MS self-stimulation task. LTP evoked at the CA3–CA1 synapse further decreased self-stimulation performance in J20, but not in WT, mice. MS self-stimulation evoked a decrease in the amplitude of field excitatory postsynaptic potentials (fEPSPs) at the CA3–CA1 synapse in WT, but not in J20, mice. This self-stimulation-dependent decrease in the amplitude of fEPSPs was also observed in the presence of another positive reinforcer (food collected during an operant task) and was canceled by the local administration of an antibody-inhibiting glutamate decarboxylase 65 (GAD65). LTP evoked in the GAD65Ab-treated group was also larger than in controls. The hippocampus has a different susceptibility to septal GABAergic inputs depending on ongoing cognitive processes, and the GABAergic septohippocampal pathway is involved in consummatory processes related to operant rewards.
Keywords: GABA, glutamate decarboxylase, hippocampus, mice, self-stimulation
Introduction
In a seminal study, Olds and Milner (1954) reported that rats located in a Skinner box can learn to do a simple manipulation (i.e., to press a lever) in order to obtain a train electrical stimulation of selected brain sites. Presently, intracranial self-stimulation is a standardized operant conditioning paradigm by which animals are capable of self-administering electrical stimulation through electrodes chronically implanted in the brain (Carlezon and Chartoff 2007). Although it is generally assumed that the reinforcing electrical stimulus activates brain centers and/or pathways related with the neural processes involved in the recognition of natural rewards as water or food (Olds 1958; Wise 1996), no clear link has been yet established between the subsequent neural effects of internal (brain stimulation) and external (food, water) rewards on consummatory behavior. For example, both mesolimbic dopaminergic (Wise 2002) and γ-aminobutyric acid (GABA)ergic (Lassen et al. 2007; Leppä et al. 2011) systems have been reported to be involved in these processes.
Here, we studied the effects of medial septum (MS) self-stimulation on the synaptic strength of hippocampal CA3–CA1 synapses and compared these effects with those evoked by a food-rewarded operant task. It is known that rodents will work actively to obtain train electrical stimulations of this septal area (Ball and Gray 1971; Cazala et al. 1988), which have a triple (cholinergic, glutamatergic, and GABAergic) projection system to the hippocampus (Gulyás et al. 2003; Sotty et al. 2003; Habib and Dringenberg 2009). In addition, it has been recently reported that dorsal hippocampal CA3 connections with the ventral tegmental area (across the lateral septum) close a circuit putatively involved in the internal reward system (Luo et al. 2011).
Experiments were carried out in both wild-type (WT) and J20 mice (Mucke et al. 2000), which have been reported to have a severe deficit of GABAergic septohippocampal projections (Rubio et al. 2012). We tested the hypothesis that the decrease in GABAergic septal projection onto hippocampal interneurons could modify the functional properties of hippocampal circuits, including changes in input/output curves, paired-pulse facilitation, and experimentally evoked long-term potentiation (LTP). LTP was evoked by high-frequency stimulation (HFS) of Schaffer collaterals and recorded at the ipsilateral hippocampal CA1 area in the behaving animal. These functional changes could also have some putative consequences on MS self-stimulation. To this aim, and following procedures described elsewhere (Gruart et al. 2006), WT and J20 mice were prepared for the chronic recording of field excitatory postsynaptic potentials (fEPSPs) evoked at the CA3–CA1 synapse during the acquisition and performance of an operant self-stimulation task. Animals were rewarded either with a train of stimuli presented to the MS or with food pellets. In this regard, it has been already shown that the hippocampus is involved in the acquisition of food-rewarded operant tasks (Madroñal et al. 2010, Jurado-Parras et al. 2013). To determine further the role of GABAergic neurons in the decrease of fEPSPs evoked at the CA3–CA1 synapse by MS self-stimulation, we carried out local injections of the GAD65-specific monoclonal antibody b78, which specifically inhibit GAD65 enzyme activity. GAD65 is an isoform of GAD particularly prominent in axon terminals (Raju et al. 2005; Manto et al. 2011).
Results indicate that the GABAergic septohippocampal projection plays an important inhibitory role in the functional properties of hippocampal circuits, particularly on intrinsic neural mechanisms involved in consummatory behaviors of internal (MS self-stimulation) and external (food) rewards. In addition, the hippocampus seems to have a different susceptibility (i.e., synaptic potentiation) to ongoing cognitive processes, such as the acquisition or the performance of an already learned task, or during the display of specific behaviors, such as eating or resting (Jurado-Parras et al. 2013).
Materials and Methods
Animals
Histological and self-stimulation experiments (Figs 1–7) were carried out in mature (6/8-month old, 25–35 g) hemizygous transgenic male mice-expressing human amyloid precursor protein carrying both the Swedish and Indiana familial Alzheimer disease mutations (i.e., the J20 line; Mucke et al. 2000; Palop et al. 2003). These mice and their corresponding WT littermates were provided by the University of Barcelona Animal House (Barcelona, Spain) or by Drs Joaquín del Rio and Alberto Pérez-Mediavilla (CIMA Animal House, University of Navarra, Pamplona, Spain). Additional mature (6/7-month old, 24–35 g) WT male mice (C57BL/6J strain) obtained from an official supplier (University of Granada Animal House, Granada, Spain) were used in the operant conditioning test (Fig. 6G) and in the GAD65Ab-treatment study (Figs 8 and 9). Upon arrival to the Pablo de Olavide Animal House (Seville, Spain), animals were housed in shared cages (5 per cage), but they were switched to individual cages after surgery. Mice were kept on a 12-h light/dark cycle with constant ambient temperature (21.5 ± 1°C) and humidity (55 ± 8%), with food and water available ad libitum.
Figure 1.
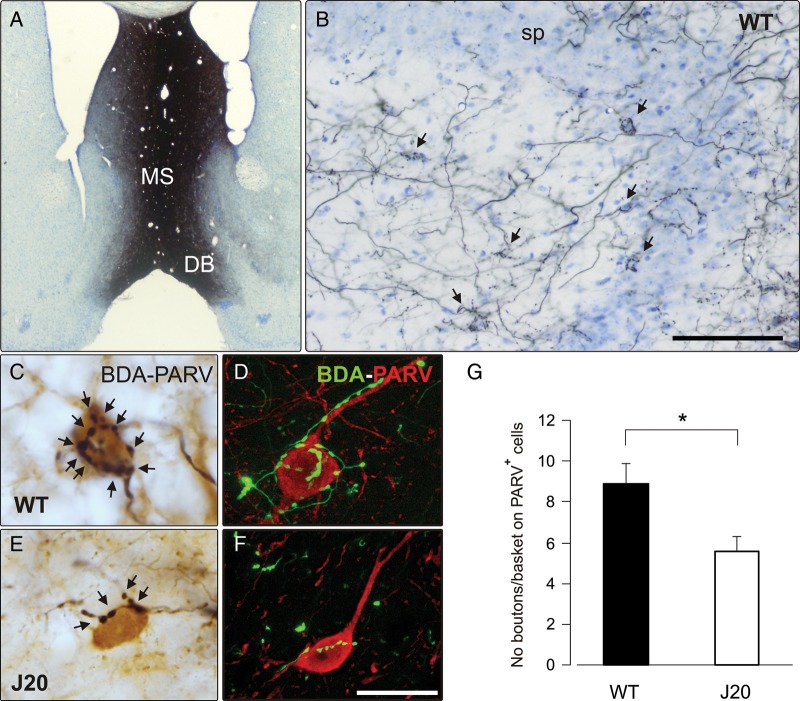
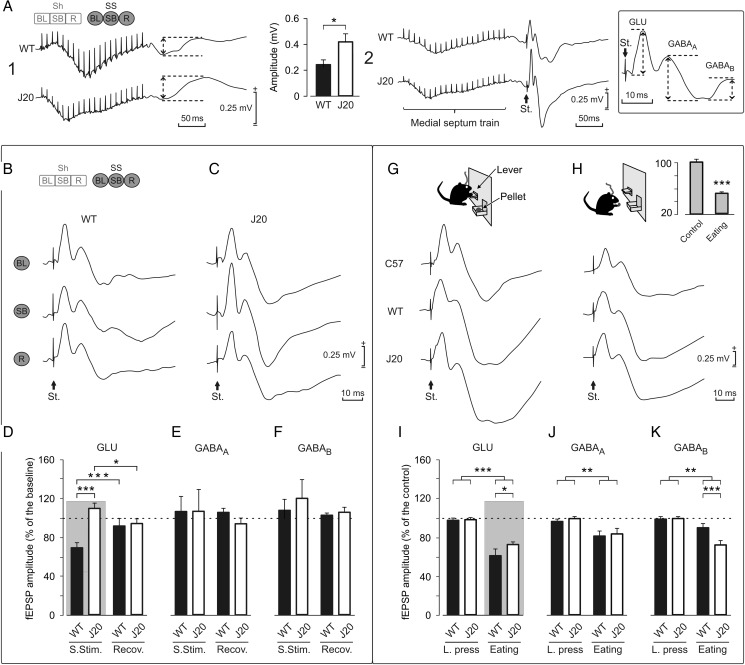
Histological analysis of the GABAergic septohippocampal connection in WT and J20 mice. (A) A photomicrograph of a Nissl-stained section showing the site of injection of BDA tracer (black staining) in the MS/DB complex. (B) BDA injection in the MS/DB produced an intense labeling of septohippocampal fibers in the hippocampus. The photomicrograph shows septal fibers throughout all hippocampal layers forming basket-shaped contacts (arrows) around the somata of neurons located mainly in, or close to, the stratum pyramidale (sp) in the hippocampal CA3 area of the WT animal. (C and E) The complexity of GABAergic septohippocampal baskets was assessed by combining BDA detection (black) with PARV immunostaining (brown). Complex baskets with numerous synaptic boutons (arrows) were abundant in the hippocampus of WT mice. (C) In contrast, GABAergic septal baskets were formed by a few boutons (arrows) in J20 transgenic mice (E). (D and F) Confocal photomicrographs of double-immunofluorescent detection of the BDA tracer (green) and PARV (red) showing an important reduction in the complexity of GABAergic septohippocampal contacts in J20 mice (F) compared with WT age-matched controls (D). (G) A quantitative analysis showed a significant reduction (*P < 0.05, Student's t-test) in the complexity of these baskets in J20 compared with WT mice, expressed by the number of boutons on PARV-positive neurons. Scale bars: (in B) 100 μm applies to B and 1000 μm applies to A and (in F) 20 μm applies to C–F.
Figure 2.
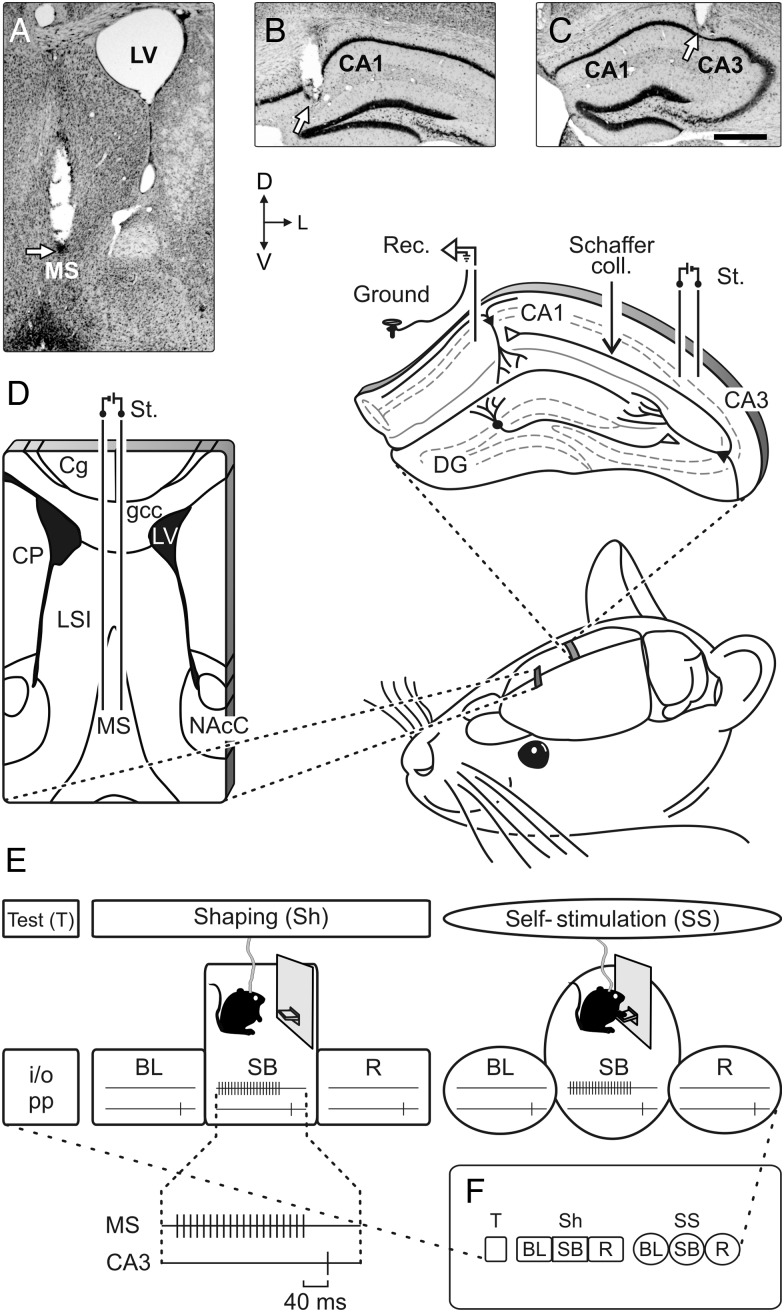
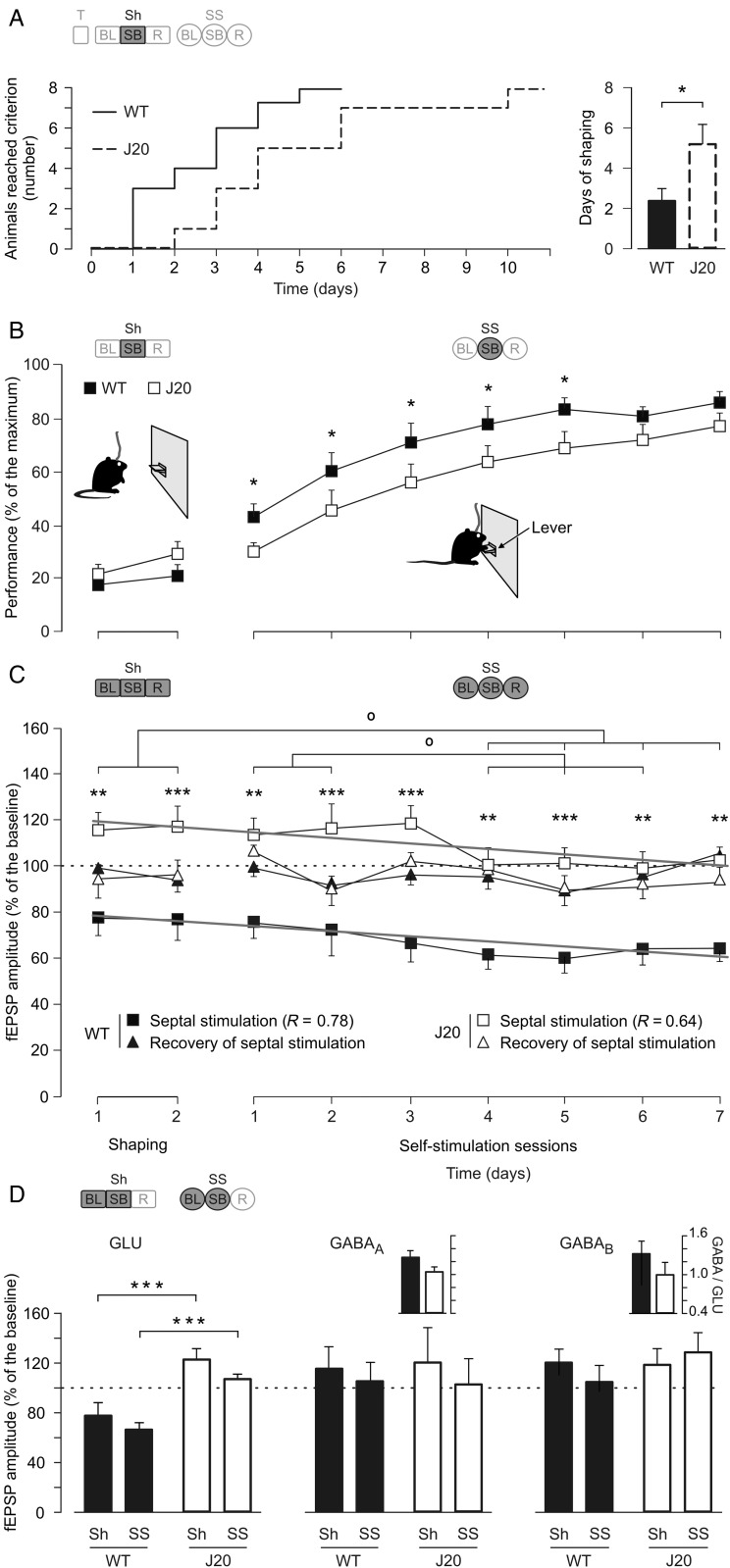
Experimental design. (A–C) Photomicrographs illustrating the location (arrows) of stimulating (A and C) and recording (B) sites. Calibration bar is 0.5 mm. (D) Bipolar stimulating (St.) electrodes were chronically implanted in the medial MS (left schematic drawing). As shown at the top-right diagram, animals were also implanted with stimulating and recording (Rec.) electrodes aimed to activate the CA3–CA1 synapses of the right hippocampus. Abbreviations: Cg: cingulate cortex; coll.: collaterals; CP: caudate-putamen; DG: dentate gyrus; gcc: genu of the corpus callosum; LSI: lateral septal nucleus, intermediate part; LV: lateral ventricle; NAcC: core of the accumbens nucleus; D, L, V: dorsal, lateral, and ventral. (E) In a first experimental step (test, T), we recorded the electrical activity of the hippocampal CA1 area, and input/output (i/o) curves and paired-pulse (pp) facilitation at the CA3–CA1 synapse in all of the animals. Animal's shaping session (shaping, Sh) consisted of: 1) a BL period 5 min long for recordings of fEPSPs evoked at the CA3–CA1 synapse with the animal located in a small box; 2) up to 10 shaping sessions (20 min each) in a Skinner box (SB), during which animals were presented with a train of stimuli to the MS followed 40 ms later by a single pulse applied to Schaffer collaterals every time the animals were located nearby the lever; and 3) a recovery (R) recording period 5 min long in the small box. Finally, the animals were allowed to self-stimulate (self-stimulation session, SS) when pressing the lever. For this, we used the same protocol as for animals' shaping, with a total of 7 self-stimulation sessions. Only 1 session (Sh or SS) per day was carried out. (F) A diagram summarizing all the experimental protocols.
Figure 3.
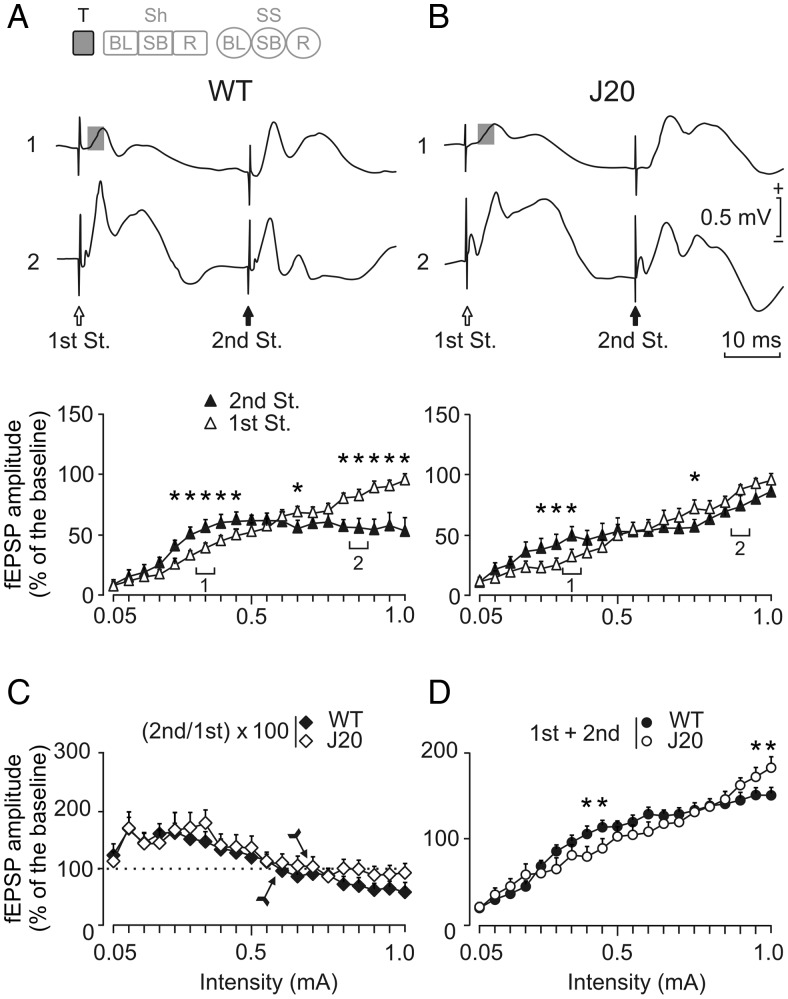
Input/output curves of the CA3–CA1 synapse in WT and J20 mice. Input/output curves were carried out with 2 pulses of increasing intensity. The code bar at the top left is defined in Figure 2E. (A and B, top panel) Representative averages (5 sweeps) of fEPSPs recorded in the CA1 area of representative WT (left) and J20 (right) animals following paired-pulse stimulation (first St. and second St., at 40 ms interstimulus interval) of the ipsilateral Schaffer collaterals at 2 increasing (1: 0.35 mA; and 2: 0.90–0.85 mA) intensities. Gray squares indicate the fEPSP that was further analyzed. (A and B, bottom panel) Relationships between the intensity of the paired pulses presented to Schaffer collaterals and amplitudes of fEPSPs evoked at the CA1 area, corresponding to the first (white triangles) and the second (black triangles) pulses. Facilitation and depression was observed in WT and J20 (P < 0.001 and P = 0.008 respectively, indicated by asterisks) animals. (C) Evolution of the paired-pulse ratio [(second/first) × 100] with increasing stimulus intensity for the data illustrated in A and B. The arrow indicates the intensity at which the relationship between fEPSPs turns from facilitation into depression. (D) Evolution of the total fEPSP response (first + second) to the pair of pulses with increasing stimulus intensity for the data illustrated in A and B. Note that J20 animals show less facilitation and depression (*P < 0.001) than WT mice at the indicated intensities. These data (illustrated as mean ± SEM) were collected and averaged from 10 animals per group.
Figure 4.
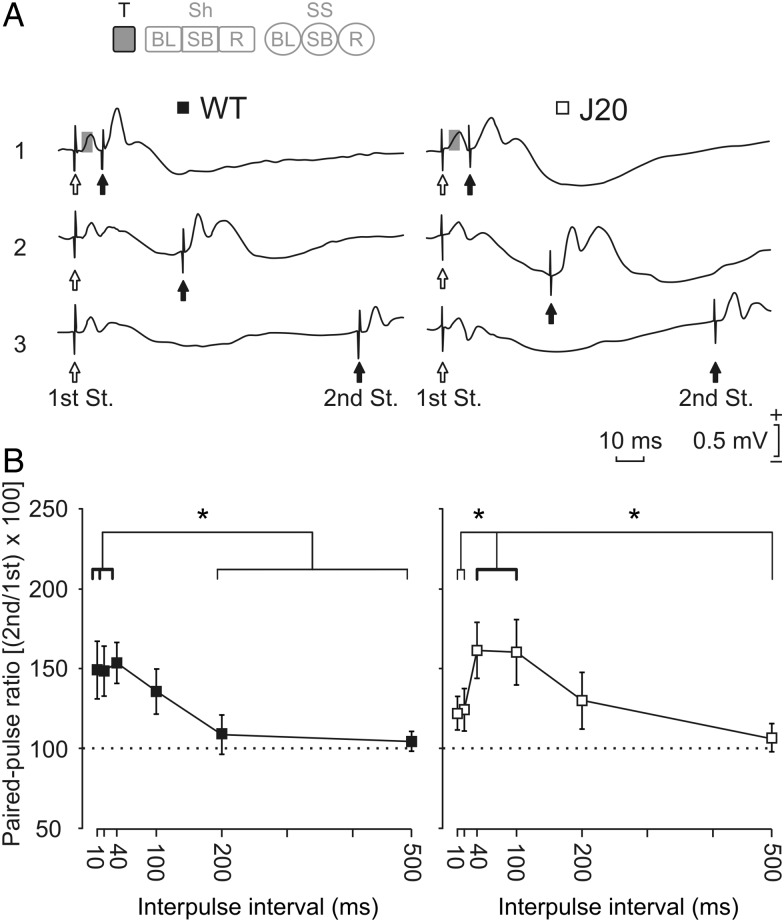
Effects of paired-pulse stimulation of the hippocampal CA3–CA1 synapse in WT and J20 mice. (A) Representative averaged (5 sweeps) records of fEPSPs evoked by paired-pulse stimulation at 3 different (1: 10 ms; 2: 40 ms, and 3: 100 ms) time intervals, and using intensities (mA) 30–40% of the maximum response value for WT (left set of records) and J20 (right set of records) animals. Gray squares indicate the component of the fEPSP that was considered for analysis. (B) Paired-pulse facilitation of fEPSPs recorded in the CA1 area following stimulation of the ipsilateral Schaffer collaterals. Paired-pulse facilitation was evoked by stimulating Schaffer collaterals with a fixed current (in accordance with animal's threshold) within 30–40% of the intensity necessary for evoking a maximum fEPSP response. fEPSPs paired traces were collected at interpulse intervals of 10, 20, 40, 100, 200, and 500 ms. Data shown are mean ± SEM amplitudes of the second fEPSP expressed as a percentage of the first fEPSP [(second/first) × 100] for each paired pulse averaged along the 6 interstimulus intervals used in this test. A delayed facilitation effect was found in J20 mice. The facilitation started at 40 ms (P = 0.034) was maintained at 100 ms (P = 0.040) with some like-remaining effects at 200 ms (P = 0.1). In contrast, WT mice presented paired-pulse facilitation from 10 ms (P = 0.01) until 40 ms (P = 0.007), but not at 100 ms (P = 0.299). The thicker line indicates statistically significant (*P < 0.05) intervals. No significant differences between groups were found (P = 0.196, 2-way ANOVA). These data (illustrated as mean ± SEM) were collected and averaged from 10 animals per group. The code bar at the top left is defined in Figure 2E.
Figure 5.
Acquisition of the self-stimulation protocol and changes evoked at the CA3–CA1 synapse in WT and J20 mice. (A) The graph at the left illustrates the accumulative days needed to reach the selected criterion by each animal (to press the lever by itself a minimum of 20 times during a 10-min period), taken by the 2 groups of mice. The graph at the right illustrates the mean time (days) spent by each group to reach criterion (*P = 0.028, Mann–Whitney rank-sum test). (B) Group performances during shaping (Sh) and self-stimulation (SS) protocols. WT animals (black squares) reached larger values (*P = 0.011, 2-way ANOVA) than the J20 group (white squares). (C) Amplitude of fEPSPs evoked at the CA3–CA1 synapse 40 ms after each self-stimulation (black squares, WT; white squares, J20) and during recovery (black triangles, WT; white triangles, J20) across the successive sessions. Control values (100%, dashed line) were collected from the BL of the last 2 shaping sessions for each mouse. Significances between groups (***P < 0.001; **P < 0.01, 2-way ANOVA) and throughout sessions (° P ≤ 0.06, 2-way ANOVA) are indicated. Tendency lines in gray color show the progressive decrease in fEPSP amplitude across the sessions. (D) Comparative effects on the different components (glutamatergic, GLU; and GABAergic, GABAA and GABAB; see inset in Fig. 6A) of the fEPSP evoked in the pyramidal CA1 area by the electrical stimulation of Schaffer collaterals at 2 different times of the experiment: (1) prior to any MS stimulation or behavioral test, that is, at the beginning of the experiment: the 2 upper insets show the comparative ratio (GABAA or B/GLU amplitude) for late GABAergic components evoked, neither of them (GABAA and GABAB) were significantly modified (GABAA, P ≥ 0.138 and GABAB, P ≥ 0.194). (2) fEPSP analysis during shaping and self-stimulation: Bottom histograms illustrate the effect of MS stimulation in J20 mice, results show a significant (***P < 0.001, 2-way ANOVA) decrease in fEPSP amplitude only in the GLU component during both shaping and self-stimulation days. Code bars at the top in A–D are defined in Figure 2E.
Figure 6.
Comparison of the effects evoked in the CA3–CA1 synapse in WT and J20 mice by self-stimulation (SS) of the MS and during food-reward in an operant conditioning task using a fixed-interval (FI1) schedule. The code bar at the top left is defined in Figure 2E. (A) Illustrative recordings (averaged 10 times) at the pyramidal CA1 area of a self-stimulation train in the MS in the absence (1) or followed 40 ms later (2) by a single pulse (St.) presented to Schaffer collaterals. Histograms at the top center represent the amplitude (in mV) of the fEPSPs evoked at the CA1 area of hippocampus by MS stimulation in WT and J20 mice. The bottom right square shows how the field potential components were measured to obtain amplitude peak to peak (dashed arrows). The approximate start latency for the 3 different components measured in fEPSPs was as follow: GLU, 2–5 ms; GABAA, 12–15 ms; and GABAB, 26–32 ms. Calibrations as indicated. (B) Representative recordings (averaged 5 times) of fEPSP evoked at the CA1 area by Schaffer collateral stimulation during BL recordings, immediately following a self-stimulation (SB), and during the recovery period (R) collected from a representative WT animal. (C) As in B, but collected from a representative J20 mouse. (D, E, and F) A quantitative analysis of data shown in B and C. (D) Note that MS self-stimulation produced a significant decrease (***P < 0.001) in the amplitude of fEPSPs evoked at the CA3–CA1 synapse in WT mice, but an increase (*P < 0.05, 2-way ANOVA) in J20 mice. (E and F) No significant changes were observed in the later components of the fEPSP evoked in CA1 by CA3 stimulation following MS self-stimulation or during the recovery period (P = 0.608 for E and P = 0.306 for F, 2-way ANOVA). (G and H) fEPSPs evoked at the CA3–CA1 synapse in C57Bl/6 (n = 12), WT littermates (n = 10), and J20 (n = 10) mice. Representative fEPSPs recorded from C57Bl/6, WT, and J20 mice during lever presses (G) or during eating (H). The histogram at the top right is a comparison of the GLU component of the fEPSP evoked during control and eating behaviors for the C57Bl/6 group (***P < 0.001, 1-way ANOVA). (I–K) A quantitative analysis of data illustrated in G and H. (I) Note that food-reward is related with a decreased fEPSP amplitude during consummatory behavior (eating) in all components of the fEPSP (GLU, P < 0.001; GABAA, P = 0.004; and GABAB, P < 0.002) for both WT and J20 mice. In addition, the decreased inhibition in J20 mice is reflected in the relative GLU increase (*P = 0.019), and GABAB decrease (***P < 0.001) in the amplitude of fEPSPs evoked at the CA3–CA1 synapse in comparison with their WT littermates. Gray panels in D and I show the same relationship of decreased inhibition in 2 different types of consummatory behaviors in WT and J20 mice.
Figure 7.
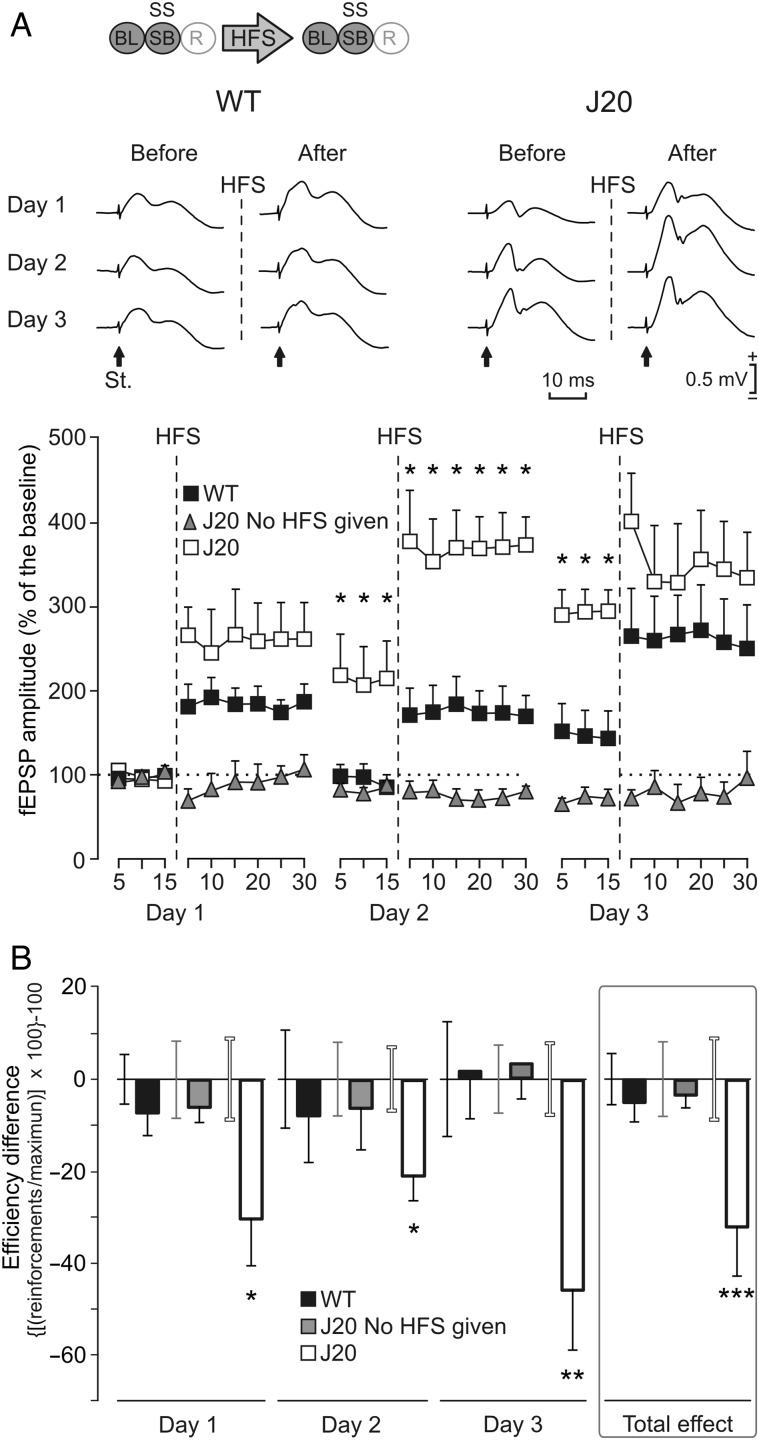
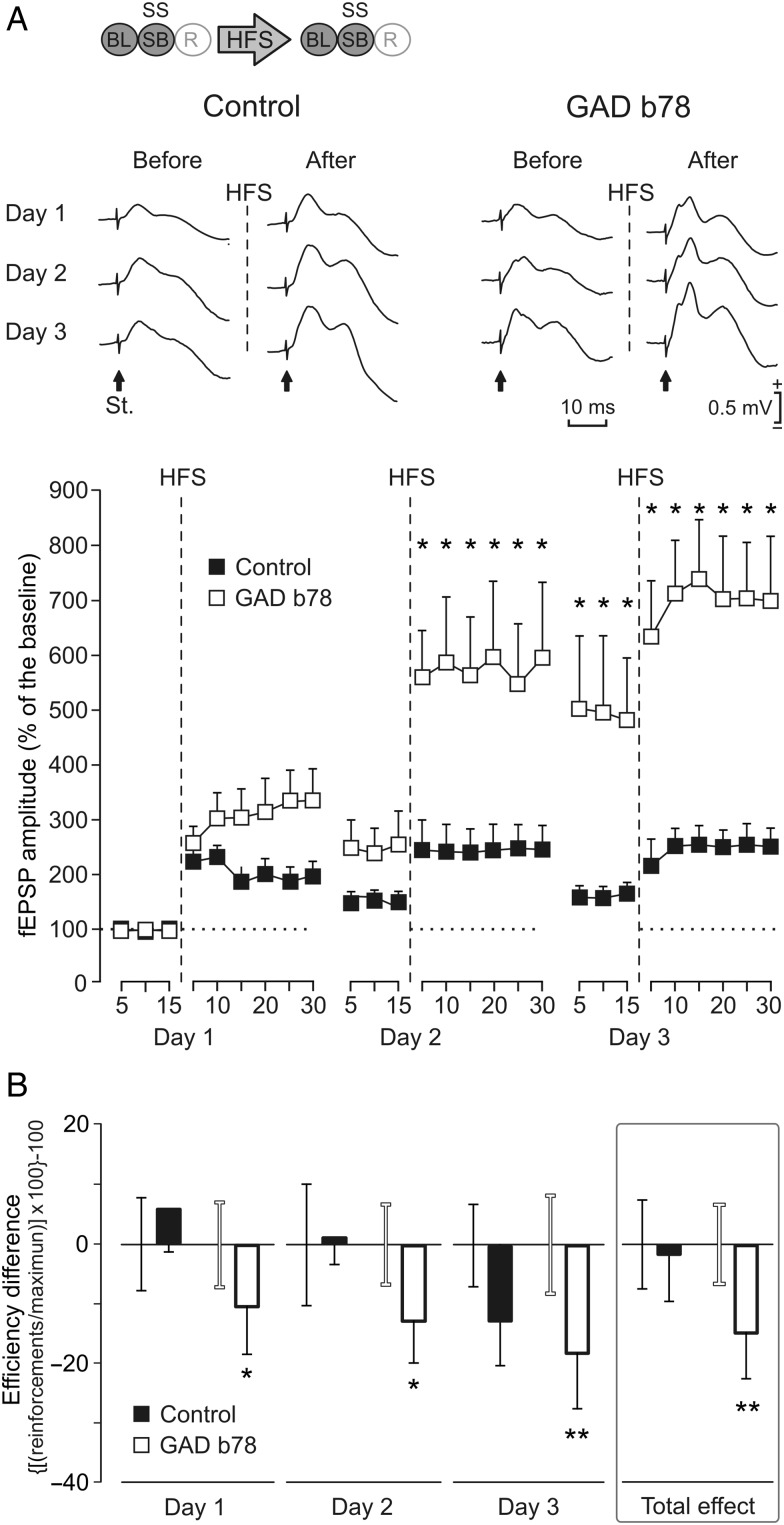
Different effects of LTP evoked at the CA3–CA1 synapse on MS self-stimulation (SS) carried out by WT and J20 mice. (A) Representative examples (averaged 5 times) of fEPSPs collected from WT and J20 animals, before (baseline recordings, BL) and after (days 1–3) 3 successive HFS sessions of the Schaffer collaterals. Arrows indicate the stimulus artifact (St.). The bottom graphs show the time course of LTP evoked in the CA1 area (fEPSP mean ± SEM) following the 3 HFS sessions for WT and J20 mice. The HFS was presented for 3 days after 15 min of BL recordings, at the time marked by the dashed line. The fEPSPs are given as a percentage of the BL (100%) amplitude. Although the 2 groups presented a significant increase (2-way ANOVA) in fEPSP amplitude following HFS when compared with BL records, values collected from the J20 group were significantly (*P = 0.023, 2-way ANOVA) larger than those collected from WT mice at the indicated times. The code bar at the top left is defined in Figure 2E. To prove that basal synaptic transmission was stable across time, a third group of J20 mice that did not receive the HFS protocol (gray triangles) is also illustrated. (B) The graphs illustrate the effects of LTP on self-stimulation for both WT and J20 mice. This effect was determined with the help of the efficiency coefficient per day and group: ([(actual number of self-stimulation reinforcements/maximum number of reinforcements obtained during BL recordings) × 100] − 100). HFS results are presented day by day. As BL we used the last 3 days before HFS with a stable execution level. The error bar of the BL (before HFS) is close to its respective data bar of the histogram (after HFS) with the matching corresponding color code (black, WT; gray, J20 no HFS; and white, J20 HFS). LTP significantly reduced self-stimulation of J20 mice every day (day 1, P = 0.038; day 2, P = 0.039; day 3, P = 0.004; and total effect, P < 0.001), but not (P = 0.234) in WT mice. In addition, no change (P = 0.166) was observed in J20 mice without LTP (*P < 0.05; **P < 0.01; ***P < 0.001).
Figure 8.
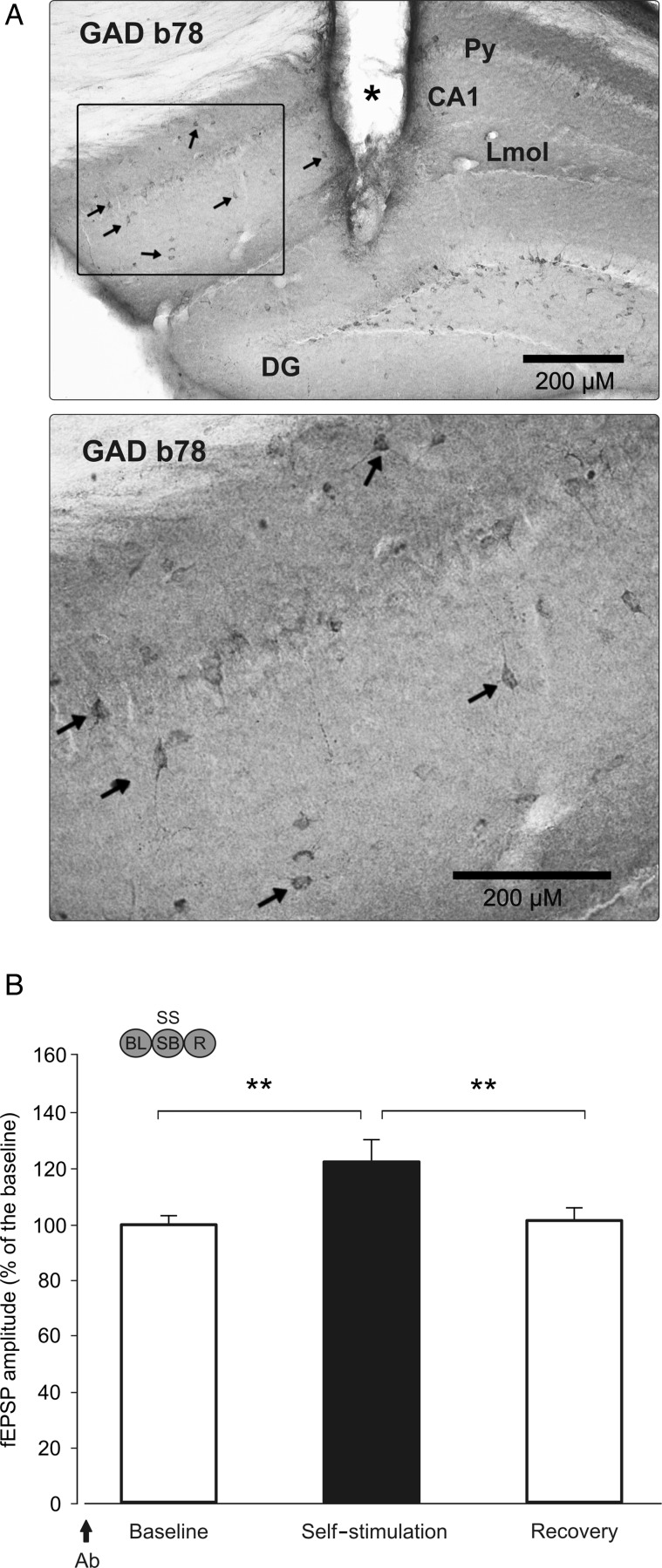
Effects of local injection of GAD65-specific antibody b78 on the amplitude of fEPSPs evoked at the CA3–CA1 synapse before, during, and following MS self-stimulation of WT mice. (A) At the top is illustrated a photomicrograph of an immunostaining indicating the presence of the injected b78 antibody in CA1 interneurons (arrows). The marked area is shown enlarged in the bottom photomicrograph. Calibration bar: 200 μM. The asterisk in the top photomicrograph indicates the location of the cannula. Abbreviations: DG: dentate gyrus; LMol: lacunosum moleculare layer; Py: pyramidal cell layer. (B) Amplitude of fEPSPs evoked at the CA3–CA1 synapse (fEPSP mean ± SEM) during BL records (left), 40 ms after self-stimulation (middle), and during the recovery period (right). Note that in the presence of GAD65-specific antibody b78, fEPSPs presented larger amplitudes following self-stimulation (SS) than during BL (BL, **P = 0.005) and recovery (R, **P = 0.006) periods (1-way ANOVA). The antibody (Ab) injection was carried out 30 min prior to the BL recording (arrow). The code bar at the top left is defined in Figure 2E.
Figure 9.
Different effects of LTP evoked at the CA3–CA1 synapse on MS self-stimulation (SS) carried out by b78-injected and control WT mice. (A) Representative examples (average of 5 sweeps) of fEPSPs collected from b78-injected and control animals, before (BL) and after (days 1–3) 3 successive HFS sessions of Schaffer collaterals. The arrows indicate the stimulus artifact (St.). The bottom graphs show the time course of LTP evoked in the CA1 area (fEPSP mean ± SEM) following the 3 HFS sessions for b78-injected and control mice. The HFS was presented for 3 days after 15 min of BL recordings, at the time marked by the dashed line. The fEPSPs are given as a percentage of the BL (100%) amplitude. Although the 2 groups presented a significant increase (2-way ANOVA) in fEPSP amplitude, those for the b78-injected group were significantly (*P = 0.028) larger than those collected from noninjected control mice at the indicated times. The code bar at the top left is defined in Figure 2E. (B) The graphs illustrate the effects of LTP on self-stimulation for both control and b78-injected mice. This effect was determined with the help of the efficiency coefficient per day and group: ([(actual number of self-stimulation reinforcements/maximum number of reinforcements obtained during BL recordings) × 100] − 100). HFS results are presented day by day. As BL we used the last 3 days before HFS with a stable execution level. The error bar of the BL (before HFS) is close to its respective data bar of the histogram (after HFS) with the matching corresponding color code (black, WT; gray, J20 no HFS; and white, J20 HFS). LTP significantly reduced self-stimulation of b78-injected mice every day (day 1, P = 0.05; day 2, P = 0.047; day 3, P = 0.006; and total effect, P < 0.010), but not of control (P = 0.847) mice (*P ≤ 0.05; **P ≤ 0.01).
All of the experiments were performed in accordance with the guidelines of the European Union Council (2003/65/EU) and Spanish regulations (BOE 252/34367-91, 2005) for the use of laboratory animals in chronic studies, and approved by the local Ethics Committee of the Pablo de Olavide University. Unless otherwise indicated, a total of 10 successful animals were used per experimental group. We considered as successful those animals that finished experimental protocols presenting fEPSPs that did not deteriorate over time.
Detection of GABAergic Septohippocampal Projections
For the detection of GABAergic septohippocampal fibers (Fig. 1A,B), mice were deeply anesthetized with a mixture (10/1; 0.003 mL/g, intraperitoneally) of ketamine (Ketolar, Parke-Davis, Madrid, Spain) and xylazine (Rompun, Bayer UK Ltd., Suffolk, UK), and stereotaxically injected with 10% biotinylated dextran amine (BDA; 10 000 MW, Molecular Probes, Eugene, OR, USA) in the MS/diagonal band (MS/DB) complex. Each animal received 2 iontophoretic (7-µA positive direct current and 7-s on–off cycle) injections of the tracer into the MS/DB (0.0 mm lateral, 0.7 mm anterior, and 3.0 and 3.7 ventral to bregma; Paxinos and Franklin 2001). This protocol results in intense BDA labeling in the MS/DB, which contains the highest proportion of GABAergic septohippocampal neurons (Pascual et al. 2004). Five or 6 days later, animals were deeply reanesthetized (sodium pentobarbital, 50 mg/kg) and perfused with 4% paraformaldehyde in 0.12 M phosphate buffer. Brains were removed and frozen, and 30-µm sections were obtained. Coronal sections were stored in a cryoprotectant solution (30% glycerin, 30% ethyleneglycol, and 40% 0.1 M phosphate buffer) at −20°C until use. After blocking, BDA was visualized by incubating the sections overnight at 4°C with the ABC complex (Vectastain ABC Kit; Vector Labs, Burlingame, CA, USA) diluted 1/100. Peroxidase activity was developed with diaminobenzidine intensified with nickel ammonium sulfate and cobalt chloride (DAB/Ni–Co), and H2O2 yielding a black end-product in septohippocampal fibers. Thereafter, sections were mounted onto gelatinized slides, Nissl-stained, and coverslipped with Eukitt.
Double Immunodetection of BDA Tracer and Parvalbumin
Hippocampal sections from iontophoretically injected animals were processed for the double detection of BDA and parvalbumin (PARV; Fig. 1C–G). After blocking, free-floating sections were incubated overnight at 4°C with the ABC complex simultaneously with the rabbit polyclonal antibody against PARV (1/3000, Swant Antibodies, Fribourg, Switzerland). Next, BDA was revealed using DAB/Ni–Co producing a black end-product. Primary antibody was then visualized by sequential incubation with biotinylated secondary antibodies and the ABC complex (2 h each, Vector Labs). The peroxidase reaction was developed with DAB to produce a brown end-product. The sections were mounted onto gelatinized slides, dehydrated, and coverslipped with Eukitt. These double immunolabeled sections were used to perform subsequent quantifications.
For double-immunofluorescent detection of BDA and PARV, the sections were first blocked and then incubated overnight with anti-PARV antibody. The primary antibody was detected by simultaneous incubation with goat anti-rabbit IgG Alexa Fluor 568 (Invitrogen, Carlsbad, CA, USA) and streptavidin-fluorescein (1/200, Amersham Biosciences, Buckinghamshire, UK), that later of which allows the detection of BDA tracer. The sections were then mounted onto slides with Mowiol 4-88 (Merck Chemicals, Darmstadt, Germany) and viewed under a Leica SPE confocal microscope (Leica, Inc., Heidelberg, Germany). Images were then processed with ImageJ software (Rasband, W.S., ImageJ, NIH, Bethesda, MD, USA), and brightness and contrast were adjusted using Adobe Photoshop™ (Adobe, Systems, San Jose, CA, USA).
To assess the complexity of the baskets, the number of boutons per basket around the somata of parvoalbumin-positive interneurons was hand-counted in the same regions. The sections used for quantifications were collected from equivalent hippocampal levels (sections between 1.60 and 2.30 mm posterior to bregma; Paxinos and Franklin 2001). Histological data were processed for statistical analysis with Statgraphics Plus 5.1 (Statistical Graphics, Rockville, MD, USA). Two-tailed Student's t-test was used to examine differences between the experimental groups. The significance was set at α ≤ 0.05, except for some intragroup tests, where α ≤ 0.06 was accepted as significant due to the general data context.
Animal Preparation for Self-Stimulation and Electrophysiological Recordings
Animals were anesthetized with 0.8–1.5% isoflurane delivered by a mouse anesthesia mask (David Kopf Instruments, Tujunga, CA, USA). The anesthetic gas was supplied from a calibrated Fluotec 5 (Fluotec-Ohmeda, Tewksbury, MA, USA) vaporizer, at a flow rate of 1–2 L/min oxygen (AstraZeneca, Madrid, Spain). Animals were implanted with bipolar stimulating electrodes on the right MS (0.1 mm lateral and 0.6 mm anterior to bregma, and 3.8 mm from the brain surface; Paxinos and Franklin 2001) and in the ipsilateral Schaffer collateral/commissural pathway of the dorsal hippocampus (2 mm lateral and 1.5 mm posterior to bregma, and 1–1.5 mm from the brain surface; Paxinos and Franklin 2001). A recording electrode was aimed to the CA1 stratum radiatum (1.2 mm lateral and 2.2 mm posterior to bregma, and 1–1.5 mm from the brain surface; Paxinos and Franklin 2001). Electrodes were made from 50 μm, Teflon-coated, tungsten wire (Advent Research, Eynsham, UK). A bare silver wire was affixed to the bone as ground. All the implanted wires were soldered to one 6-pin socket (RS Amidata, Madrid, Spain) and fixed to the skull with dental cement (Fig. 2A–D; see Gruart et al. 2006 for details).
For the administration of the GAD65-specific monoclonal antibody b78, selected animals were also implanted chronically with a blunted, stainless steel, 26-G cannula (Plastic One, Reanoke, VA, USA) in the CA3–CA1 areas, nearby hippocampal stimulating and recording electrodes (1.6 mm lateral and 1.8 mm posterior to bregma, and 1 mm from the brain surface; Paxinos and Franklin 2001). The tip of the cannula was aimed to be located 0.25 mm above the infusion target.
Electrocortical Field Recordings, Input/Output Curves, and Paired-Pulse Facilitation
Recording sessions started 1 week after surgery. To determine the functional capabilities of hippocampal circuits in the 2 groups of mice, we performed input/output and a paired-pulse stimulation tests at the CA3–CA1 synapse. For this, the animal was placed in a small (5 × 5 × 10 cm) plastic chamber located inside a larger Faraday box (30 × 30 × 20 cm). fEPSP recordings were made with Grass P511 differential amplifiers through a high-impedance probe (2 × 1012 Ω, 10 pF).
For input/output curves, animals were stimulated at the CA3 area with 2 pulses (40 ms of interstimulus interval) of increasing intensity (∼0.05–1.0 mA) until reaching a maximum fEPSP response (see below). Data were normalized using as 100% the highest amplitude (average of 5 selected sweeps with same stimulation intensity) of the first fEPSP of each mouse as their own baseline (BL). Additionally, the ratio “2nd fEPSP/1st fEPSP × 100” and the total response “1st fEPSP + 2nd fEPSP” were evaluated (Fig. 3).
For the paired-pulse facilitation test, the intensity was fixed in accordance with the threshold of each mouse, within 30–40% of the intensity necessary for evoking a maximum fEPSP response. The effects of paired pulses of different (10, 20, 40, 100, 200, and 500 ms) interstimulus intervals were measured. Data are presented (the average of 5 selected sweeps with the same interval) using the same ratio as for the input/output test [(2nd/1st) × 100], but for every interstimulus interval (Fig. 4). The stimuli of these 2 tests (input/output and paired-pulse) were repeated ≥5 times with time intervals of 10 s, to avoid as much as possible interferences with slow short-term potentiation (Zucker and Regehr 2002). In all cases, we computed fEPSP amplitudes in a normalized way (Fig. 6A, inset), taking each mouse as its own BL in accordance with every experimental stage (Fig. 2E). We restricted the quantitative analysis to fEPSPs free of population spikes, noisy components, as well as of saturation signals, artifacts, or instability signs.
Self-Stimulation Procedures
Training took place in a Skinner box module measuring 12.5 × 13.5 × 18.5 cm (MED Associates, St. Albans, VT, USA) equipped with a lever. The shaping (Sh, Fig. 2E) protocol was carried out as follows: 1) The animal was placed for 5 min in a small box (5 × 5 × 10 cm) located besides the Skinner box. In this situation, the animal was stimulated at the CA3–CA1 synapse at a rate of 6 stimuli/min as BL (Fig. 2E) recordings; 2) afterwards, the animal was carefully placed for 20 min in the Skinner box where it was shaped applying the successive approximation method with the lever as a target until pressing it. The lever was programmed as a trigger to deliver a train of pulses (bipolar, 100 μs pulses at 100 Hz for 200 ms, with intensity ≤2 mA) in the MS using a fixed time interval of 5 s (FI5). This train was followed 40 ms after its end by a single pulse presented at the CA3–CA1 synapse (SB, Fig. 2E); and 3) finally, the animal was returned to the small box for a recovery (5 min) period, in which it was stimulated at the CA3–CA1 synapse at the initial rate of 6 stimuli/min (R, Fig. 2E).
The shaping protocol was applied for a maximum of 10 sessions, but when the animal reached criterion the shaping was completely stopped and switched to self-stimulation at the next day's session. The criterion was that the animal performed by itself a minimum of 20 lever presses during a 10-min period (Fig. 5A). In accordance with previous reports (Hodos and Valenstein 1962; Miliaressis and Rompre 1987), during the first 2 shaping sessions, the reward intensity threshold was adjusted for each animal using an additional behavioral criterion; this is, to reach the minimum current to get constant bar pressing (at least 1 lever press per min) in the absence of any observable arrest, general body reaction, or overt movements associated with the reward presentation. After this adjustment, the reward intensity remains without any change along all the experiment. Animals that did not reach the selected criterion during a maximum of 10 shaping sessions were discarded from the study. In this way, we could guarantee that all MS stimulation data included in the analysis came from rewarding phenomena.
Shaping sessions were followed by 7 self-stimulation sessions (Fig. 5B–D). Self-stimulation sessions were organized as described for shaping ones, but in this case, train stimulation of the MS (reward) was only carried out when the animal pressed the lever by itself. In all cases, self-stimulation rewards could be received along 20 min recording at a maximum rate of 1/5 s, that is, with the same fixed time interval (FI5) schedule. To evaluate self-stimulation performance in the different groups of animals (control, J20, and GAD65 mAb b78), we analyzed different behavioral parameters including the time expended in lever presses, the number of nonrewarded lever presses, and the latency to start pressing the lever. However, significant differences between groups were better represented by the number of reinforced obtained/the number of maximum available reinforcements (Fig. 5B), mostly when evaluating LTP effects on MS self-stimulation (Figs 7B and 9B).
Instrumental Conditioning for Food Reward
For this, an additional group of WT (C57BL/6J) mice was prepared with an identical surgical protocol (see above) but without stimulated electrodes in the MS. These mice were trained and tested in the already-mentioned Skinner box module. In this case, the Skinner box was equipped with a food dispenser from which pellets (MLabRodent Tablet, 20 mg; Test Diet, Richmond, IN, USA) could be delivered by pressing a lever. Before training, mice were handled daily for 7 days and food deprived to 85–80% of their free-feeding weight. Training took place for 20 min during successive days, in which mice were allowed to press the lever to receive pellets from the food tray using a fixed-interval 1-s (FI1) schedule. Animals were maintained on this FI1 schedule until they reached the selected criterion—namely, until they were able to obtain ≥20 pellets for 3 successive sessions. During this test, we recorded fEPSPs evoked in the CA3–CA1 synapse across the session in relation with selected ongoing behaviors. For this, single pulses were applied at the CA3–CA1 synapse, similar to those used during the self-stimulation protocol. All operant sessions were recorded with a synchronized video capture system (Sony HDR-SR12E, Tokyo, Japan).
Long-Term Potentiation
For LTP induction in behaving mice, we followed procedures described previously (Gruart et al. 2006). BL values for the amplitude of fEPSPs evoked at the CA3–CA1 synapse were collected 15 min prior to LTP induction using single 100 μs, square, biphasic pulses every 20 s. Pulse intensity was the same as during behavioral tests carried out with each mouse. BL values collected from the first day were selected as the normalization value (100%) for the next 2 days (illustrated in Figs 7A and 9A as a dotted horizontal line). For LTP induction, animals were presented with a HFS session consisting of five 200 Hz, 100 ms trains of pulses at a rate of 1/s repeated 6 times, at intervals of 1 min, that is, a total of 600 pulses were presented during the HFS session. To avoid evoking large population spikes and/or the appearance of cortical seizures, the stimulus intensity during HFS was set at the same intensity as that used for generating BL recordings. After the HFS session, exactly the same single-stimuli parameters as for BL recordings were presented for the following 30 min. On days 2 and 3, the same HFS session was repeated following a BL recording session lasting for 15 min and followed by a 30-min recording session. All HFS data were normalized using as 100% BL fEPSP values collected at the first day; in this way, we could evaluate early and long LTP (Figs 7 and 9).
Histological Procedures for Electrode Location
Mice were perfused transcardially under deep pentobarbital anesthesia, with saline and then 4% paraformaldehyde in phosphate-buffered saline (PBS, 0.1 M, pH 7.4). Brains were cryoprotected with 30% sucrose in PB, and coronal sections (50 μm) were obtained with a sliding freezing microtome (Leica SM2000R, Nussloch, Germany) and stored at −20°C in 30% glycerol and 30% ethylene glycol in PB until used. Selected sections including the implanted (MS and hippocampus) sites were mounted on gelatinized glass slides and stained using the Nissl technique with 0.1% toluidine blue to determine the location of stimulating and recording electrodes (Fig. 2A–C).
Administration of Monoclonal Antibody b78
The human monoclonal antibody b78 specific to the 65kDa isoform of glutamate decarboxylase 65 (GAD65; Raju et al. 2005) was used in this experiment. Prior (30 min) to the sixth self-stimulation session, the b78 antibody was diluted in saline (1 μg/μL) and administered through the injection cannula. The injection cannula was 250 µm longer than the implanted cannula, which was used as a guide. The injection (1 µL) was administered with the help of a SP100i pump (WPI, Sarasota, FL, USA) at a rate of 0.2 µL/min.
Immunohistochemistry for b78 Detection
Selected brain slices, including the dorsal hippocampus, were processed “free-floating” for immunohistochemistry, and all of the sections studied passed through all procedures simultaneously to minimize any difference from immunohistochemical staining itself. After cooling down the sections to room temperature, they were incubated with 10% methanol and 0.003% H2O2 in PBS for 10 min to block endogenous peroxidase activity. After this, sections were treated for 1 h with 5% normal donkey serum (NDS; AbD Serotec, MorphoSys, Kidlington, UK) in PBS with 0.2% Triton X-100 (Sigma-Aldrich, Madrid, Spain), and then incubated overnight at room temperature with polyclonal rabbit antihuman lambda light chain (1:20; AbD Serotec) with PBS containing 0.2% Triton X-100 and 3% NDS. The following day, sections were incubated for 1 h with donkey anti-rabbit IgG conjugated to biotin (1:200; AbD Serotec) in PBS with 0.2% Triton-X-100 and 3% NDS. Then, sections were incubated with the avidin–biotin–peroxidase complex (Vector Labs) for 30 min in PBS. Color development was achieved by incubating with 3,3-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St. Louis, MO, USA) and 0.033% hydrogen peroxide in PB for 4 min. Finally, sections were mounted on slides, dried for 1 day at room temperature, dehydrated with ascending alcohols and rinsed in xylol. After this, sections were coverslipped using DPX mounting medium and viewed under a Leica light microscope (Leica, Madrid, Spain) for analysis.
Data Collection and Analysis
fEPSP and 5-V rectangular pulses corresponding to lever presses and brain stimulations were stored digitally on a computer through an analog/digital converter (CED 1401 mk II, Cambridge, UK). Filmed operant conditioning sessions were also stored with the help of the CED 1401 mk II. Extracellular recordings were analyzed using a home-modified MATLAB software (MathWorks, Madrid, Spain). Self-stimulation data were analyzed off-line for the quantification of each animal's performance in the Skinner box and fEPSP with the Spike 2 (CED) program. Five successive fEPSPs were averaged, and the mean value of the amplitude during the rise-time period (i.e., the period between the initial 10% and the final 10% of the fEPSP) was determined. Computed results were processed for statistical analysis using the Sigma Plot 11.0 package (SigmaPlot, San Jose, CA, USA). Data are always represented as mean ± SEM. Acquired data were analyzed with 2-tailed Student's t test or the 1-way or 2-way analysis of variance (ANOVA), with days as repeated measure and with a contrast analysis for a further study of significant differences.
Results
Density of GABAergic Septohippocampal Contacts Is Decreased in J20 Mice
Following Rubio et al. (2012), in a first series of experiments, we checked whether J20 mice presented a lower density of GABAergic septohippocampal contacts and therefore, could be used as a suitable model to determine the role of the GABAergic septohippocampal pathway on the effects of MS self-stimulation. For this, we performed iontophoretic injections of the anterograde tracer BDA in the MS/DB of J20 (Mucke et al. 2000) and WT mice. This protocol results in intense BDA labeling in the MS/DB (Fig. 1A). As shown previously in WT mice (Freund and Antal 1988; Gulyás et al. 1990; Pascual et al. 2004), septohippocampal axons innervated all layers of the hippocampus and dentate gyrus (Fig. 1B). Two types of fibers were easily recognizable: Thin axons with “en passant” synaptic boutons, corresponding to cholinergic fibers, and abundant thick GABAergic axons forming complex baskets with large synaptic boutons around the perisomatic region of hippocampal interneurons (Fig. 1B, arrows). The GABAergic septohippocampal fibers were also recognizable in J20 transgenic mice, in which septal axons formed baskets on the perisomatic region of hippocampal interneurons.
To determine the density of GABAergic septohippocampal contacts in J20 mice, we performed double immunostaining of BDA tracer and PARV, which specifically label basket and axo-axonic interneurons largely contacted by GABAergic septohippocampal axons (Freund and Buzsaki 1996; Matyas et al. 2004). In agreement with previous results from our group (Rubio et al. 2012), our data showed that the complexity (i.e., the number of boutons per target neuron) of GABAergic septohippocampal contacts on PARV-positive interneurons was diminished in J20, compared with WT, mice (Fig. 1C–F). By confocal microscopy, we confirmed a substantial decrease in the complexity of GABAergic septohippocampal contacts on PARV-immunolabeled neurons in J20 mice (Fig. 1D,F). Quantitatively, the number of boutons per basket on PARV-positive cells was significantly reduced (38%) in transgenic mice compared with age-matched controls (P = 0.024, Student's t-test; Fig. 1G). In accordance with a recently published study (Rubio et al. 2012), the present data demonstrate a substantial reduction in the GABAergic septohippocampal connection affecting all interneuron types (immunodetected by GAD65/67, PARV, and calretinin) in J20 mice. The reduction affected mainly PARV-positive basket and axo-axonic hippocampal interneurons.
Differences in the Functional Properties of Hippocampal Circuits Between WT and J20 Mice
In the next experimental step, we tested the functional properties of hippocampal circuits in behaving WT and J20 mice (see Fig. 2 for details). During the input–output test, both groups separately [WT, F19,171 = 12.128, P < 0.001; J20, F19,171 = 2.050, P = 0.008] showed similar increases in the amplitude of the second fEPSP evoked at the CA1 area by the second of 2 pulses (40 ms of interstimulus interval) of increasing intensity presented to ipsilateral Schaffer collaterals (Fig. 3A,B). Interestingly, and as already described in behaving WT mice (Madroñal et al. 2009), the input–output facilitation in the second fEPSP evoked in both groups of mice at low intensities was reversed into a depression at higher intensities, but the J20 group showed a delay to switch the relationship between fEPSPs (Fig. 3C). Additionally, the total response obtained in this test (first + second fEPSPs) showed that J20 mice have significantly lower facilitation and depression (F19,171 = 4.731, P < 0.001), compared with WT mice (Fig. 3D).
Using the paired-pulse test, we evaluated the synaptic facilitation evoked by the contiguity of a pair of pulses. This is a typical presynaptic short-term plastic property of the hippocampal CA3–CA1 synapse at short (<60 ms) intervals. This phenomenon has been related to the process of neurotransmitter release (Zucker and Regehr 2002; Madroñal et al. 2009). As illustrated in Figure 4, both groups of mice showed a normal-like paired-pulse facilitation profile without significant differences between them (F5,45 = 1.542, P = 0.196). However, J20 animals showed (i.e., within their own group) a delayed facilitation, that is, the increase of the second fEPSP was only significant for 40 (P = 0.034) and 100 ms (P = 0.040) intervals, while for WT mice the paired-pulse facilitation was restricted to 10 (P = 0.01) and 40 ms (P = 0.007) interpulse intervals (Fig. 4B). These results suggest that the short-term facilitation process was delayed to longer intervals in J20 mice when compared with WT animals.
WT Mice Present Better Self-Stimulation Performance and Activity-Dependent Hippocampal Synaptic Depotentiations Than J20 Mice
As detailed in the Materials and Methods section and following a selection of individual stimulus intensities, animals were firstly shaped to associate lever presses with train stimulation of the MS. A maximum of ten 20-min sessions (1 per day) were allowed for each animal to reach criterion. For criterion, the animal was required to press the lever by itself a minimum of 20 times during a 10-min period, with pauses between self-stimulus <60 s. WT mice reached the selected criterion significantly sooner (P = 0.028, Mann–Whitney rank-sum test) than J20 animals (Fig. 5A). In addition, WT mice reached significantly (F9,72 = 2.637, P = 0.011) larger self-stimulation scores than J20 animals during most (5 of 7) self-stimulation sessions (Fig. 5B).
We checked the effects of manual train stimulation of the MS on field responses evoked in the hippocampal CA1 area. In WT mice, train stimulation of the MS evoked a positive–negative (0.24 ± 0.05 mV, peak-to-peak) extracellular field potential with a latency of 20 ± 5 ms, that lasted for 100 ± 12 ms (Fig. 6A1). The negative component was smaller and the positive component larger (0.41 ± 0.09 mV) in J20 mice than in the WT group (Fig. 6A1). This significant (P = 0.028; Student's t-test; Fig. 6A1) difference in the amplitude of field potentials evoked in CA1 by train stimulation of the MS was probably the result of the imbalance in the inhibitory direction between GABAergic and cholinergic septohippocampal projections observed in J20 mice (Palop et al. 2007; Rubio et al. 2012).
In a next step, we studied the effects of train stimulation of the MS on fEPSPs evoked at the hippocampal CA1 area by single pulses presented to the ipsilateral Schaffer collaterals. Figure 6 illustrates several profiles of fEPSPs evoked at the CA3–CA1 synapse collected immediately (40 ms) after (Fig. 6A2, B, SB), or in the absence (Fig. 6B, BL and R) of a self-stimulation of the MS. As known, the first component (≍4 ms of latency) of fEPSPs evoked at the CA3–CA1 synapse (see inset at the right of Fig. 6A) corresponds to the activation of glutamate receptors (Collingridge et al. 1983a, 1983b; Bliss and Collingridge 1993). Similar fEPSPs have already been recorded in alert behaving mice (Gruart et al. 2006).
fEPSPs evoked at the CA3–CA1 synapse, recorded after MS stimulation (shaping or self-stimulation), presented a decreased amplitude during the shaping session stage, when compared with fEPSPs recorded without MS stimulation (BL) in WT mice (Figs 5C,D and 6A,B) with a decreasing tendency (y = −1.99x + 80.4, R2 = 0.78, Fig. 5C). This decrease disappeared during the recovery period (black triangles, Fig. 5C), that is, in the 5 min after the end of the MS stimulation session. In contrast, MS stimulation in J20 mice did not evoke a similar decrease in the amplitude of fEPSPs. In fact, after the first shaping session, the fEPSPs observed in J20 animals presented an increase in amplitude when compared with BL fEPSPs values with a decreasing tendency (y = −2.16x + 121.6, R2 = 0.64, Figs 5C,D and 6A,C). Interestingly, this increase returned to BL values, starting from the fourth self-stimulation session (white squares in Fig. 5C). The amplitude of fEPSPs in J20 mice during MS stimulation (shaping or self-stimulation) sessions returned to BL values during the recovery period (white triangles in Figs 5C and 6D). As a consequence of the different effects on synaptic strength evoked by MS self-stimulation in WT and J20 mice, fEPSPs evoked in WT mice during the successive self-stimulation sessions were significantly (F8,56 = 32.294, P < 0.001) smaller in amplitude than those evoked in J20 mice (asterisks in Figs 5C and 6D). Additionally, the data show for both groups a significant decrease throughout sessions, so that the last sessions are significantly (P ≤ 0.06) different from the first ones (circles in Fig. 5C).
The late negative components presented in the fEPSPs (Fig. 6A–C) correspond to the activation of GABAA and GABAB receptors (Schwartzkroin 1986). We also checked the effects of MS self-stimulation on these late components of fEPSP evoked at the CA3–CA1 synapse (see inset at the right of Fig. 6A) at 2 different times: First, at the beginning of the experiment, while placed in the small box (see Materials and Methods) and prior to any MS stimulation or behavioral test (insets in Fig. 5D) and secondly, during MS self-stimulation (Figs 5D and 6E,F). At the beginning of the experiment, a lower GABAA or B/glutamate (GLU) amplitude ratio was observed for J20 mice, but this difference was not significant (GABAA, P ≥ 0.138 and GABAB, P ≥ 0.194). Similar results were obtained during MS self-stimulation, whereas no significant differences (P ≥ 0.306) could be observed between the 2 groups of mice for GABAA and GABAB components of the field potential. These negative results indicate that (polysynaptic) hippocampal GABAergic circuits activated by the electrical stimulation of Schaffer collaterals were not differentially affected in the 2 groups of mice (WT and J20) by the preceding train of pulses applied to the MS nucleus.
The Amplitude of fEPSPs Evoked at the CA3–CA1 Was Decreased During Consummatory Behaviors in an Operant Conditioning Task
The fact that the amplitude of fEPSPs evoked at the CA3–CA1 synapse of WT animals was decreased by MS self-stimulation prompted us to determine whether this change in the strength of hippocampal synapses could be related to internal changes in the intrinsic value of the reward in the self-stimulated animal. As an indirect way of checking the intrinsic emotional state of the animal, we designed an instrumental test that allowed us to check the synaptic strength of the same hippocampal synapse during a positive reinforcement in an operant conditioning task. In a pilot study, we apply similar procedures reported elsewhere (Madroñal et al. 2010; Jurado-Parras et al. 2012), where C57Bl/6 (n = 12) mice were trained to press a lever to obtain a small pellet of food, using a fixed-interval 1-s (FI1) schedule. Once the animals reached criterion (i.e., pressing the lever ≥20 times/20 min session), we recorded for 3 additional (20 min) sessions fEPSPs evoked at the CA3–CA1 synapse during control behavior (i.e., when animals were resting in the Skinner box) and while the animals were eating the collected pellet. As illustrated in the top panel of Figure 6G,H, the amplitude of the GLU component of the fEPSPs evoked during the consummatory (eating) behavior was significantly (P = 0.001) reduced when compared with values collected during the control situation. No significant (P > 0.70) differences could be observed for the late GABAergic components of the evoked synaptic field potentials (data not shown). This negative result could be related to the high variability found in late (GABAergic) of the fEPSPs recorded in vivo.
To verify the above point, we decided to apply the same protocol in WT littermates and J20 mice (n = 10 per group). In this experiment, and following current reports (Jurado-Parras et al. 2013), the behavior “pressing the lever” (labeled as “L. press” in Fig. 6I–K) was used as a normalization value, in order to evaluate a pure appetitive behavior versus a consummatory one. For this experiment, the lever was programmed as a trigger to deliver a single-pulse stimulation of the CA3–CA1 synapse. Collected results shown a significant decrease during eating behavior in all of the components (GLU, F1,7 = 155.777, P < 0.001; GABAA, F1,7 = 18.572, P = 0.004; GABAB, F1,7 = 21.146, P < 0.002) of the evoked fEPSP in both WT and J20 mice (Fig. 6I–K). Importantly enough, we found in J20 mice that the GLU component was increased (P = 0.019) and the GABAB component decreased (P < 0.001) in relation to the amplitude values collected from their littermate WT mice (Fig. 6I,K).
In conclusion, both consummatory behaviors, MS self-stimulation and pellet-reward produced a similar decrease in the amplitude of fEPSPs evoked at the hippocampal CA3–CA1 synapse.
LTP Evoked at the Hippocampal CA3–CA1 Synapse in J20 Mice Presented Larger Values and Produced a Larger Depressing Effect on MS Self-Stimulation Than in WT Animals
We expected that the decrease in GABAergic septohippocampal projections observed in J20 mice could modify the excitability of hippocampal circuits. To check this possibility, we decided to evoke LTP at the hippocampal CA3–CA1 synapse and to check its effects on MS self-stimulation and food-reward in both J20 and WT mice.
For the initial 3 days, each animal underwent a set of BL recordings (see Materials and Methods). Afterwards, the HFS protocol was applied followed by 30 min of post-HFS recordings at the same stimulation rate and intensity as for BL records (Fig. 7A). The same recording session with same stimulation parameters was repeated 24 h later. BL recordings of the second were used to determine the remaining LTP. After this, a second HFS session was carried out, and a 30-min session of additional recordings was repeated as well. Finally, 48 h after the first HFS, we carried out a third BL recording that was followed by a third HFS session. As before, the third HFS session was followed by a post-HFS recording session. The BL of the first day was used as a normalization value (see dotted lines in Fig. 7A). With this experimental protocol, both WT and J20 mice presented a significant LTP for the 3 recording days (P ≤ 0.05). Notably, the LTP response presented by J20 mice was significantly (F26,104 = 1.765, P = 0.023) larger and longer-lasting than that presented by WT animals (Fig. 7A). The long-lasting effect is easily observable by the progressive increase in the second and third BL values collected from J20 animals, while BL values in WT mice remained without significant changes across the 3 recording sessions.
To check the effects of LTP on MS self-stimulation and food-reward, we calculated several indexes of performance, but food-reward did not show any significant difference (P = 0.89). In contrast, a daily efficiency coefficient ([(actual number of self-stimulation reinforcements/maximum number of reinforcements obtained during BL recordings) × 100]−100) showed differences in the MS self-stimulation performance, as illustrated in Figure 7B. Following the experimental induction of LTP, J20 mice were more affected in their performance of MS self-stimulation than WT animals. Indeed, the scores reached along 3 days, by J20 for the efficiency coefficient, were significantly (F1,5 = 129.792; P < 0.001) decreased, with respect to their control values following each of the 3 HFS sessions (see Fig. 7B for P-values quantified day by day). In contrast, values collected for WT mice indicated that self-stimulation performance was not significantly modified (F1,6 = 1.751; P = 0.234) by the LTP evoked at the hippocampal CA3–CA1 synapse. In addition, self-stimulation was not significantly modified (F1,4 = 2.683; P = 0.166) in those J20 mice that did not receive a HFS session. In conclusion, the larger LTP evoked in J20 mice seems to have a more deleterious effect on MS self-stimulation than the smaller LTP evoked in WT animals.
Hippocampal GABAergic Neurons Are Involved in the Decrease of fEPSPs Evoked at the CA3–CA1 by MS Self-Stimulation
Using the same criterion for selecting stimulus intensity (see Materials and Methods), and to determine the effect of reduced GABA levels in the hippocampus on the reported decrease in the amplitude of CA3–CA1 fEPSPs during the same MS self-stimulation protocol, we employed GAD65-specific monoclonal antibody b78. This antibody inhibits the conversion of glutamate into GABA catalyzed by the GAD65 enzyme, an isoform of GAD which is particularly prominent in many axon terminals (Esclapez et al. 1994; Ishida et al. 1999; Mitoma et al. 2003).
The b78 antibody was injected 30 min before the sixth self-stimulation session (n = 8) WT mice. Figure 8A illustrates the presence of the b78 antibody in hippocampal interneurons, including some interneurons in, or close to, the pyramidal cell layer, which correspond to the PARV-positive interneurons population (Freund and Buzsaki 1996; Matyas et al. 2004). Since b78 diffusion and effects were restricted to a rather small area of the dorsal hippocampus, its injection did not have any significant (P = 0.87) effect on MS self-stimulation performance of injected animals. Nevertheless, the local injection of b78 in the close proximity of the CA3–CA1 stimulating and recording electrodes produced a significant (P = 0.006) increase in the amplitude of evoked fEPSPs during the self-stimulation session when compared with fEPSPs collected during BL and recovery period (Fig. 8B). The collected data indicate that, after acute reduction in GABA levels, the expected decrease in fEPSP amplitude during self-stimulation was reversed into an increase, similar to what was observed in J20 mice.
Since the presence of experimentally evoked LTP in J20 mice significantly decreased MS self-stimulation (i.e., the efficiency coefficient; see Fig. 7B), we decided to repeat a similar study evoking LTP in GAD b78-injected mice. As shown in Figure 9A, the animals injected with the GAD65 antibody b78 presented a significantly (F26,130 = 7.180; P = 0.028) larger LTP than the controls. The efficiency coefficient calculated for both groups before and following the experimentally evoked LTP showed no significant differences (F1,4 = 0.0426; P = 0.847) for MS self-stimulation in the control group. In contrast, the GAD b78 group presented daily a significant (F1,5 = 16.550; P = 0.010) decrease in the performance of MS self-stimulation (Fig. 9B).
Discussion
A Shared Neural Mechanism for Internal (Brain) and External (Natural) Rewards
We have shown here that train electrical stimulation of the MS can be rewarding for alert behaving WT mice, and that it can serve as an operant reinforcer—namely, the experimental animal will generate specific behaviors (lever presses) to obtain this internal reward (Olds and Milner 1954; Mora and Cobo 1990; Wise 1996). Indeed, the MS has been recognized for years as an important neural center involved in self-stimulation reward (Ball and Gray 1971; Buño and Velluti 1977; Grauer and Thomas 1982; Cazala et al. 1988). The present results support the notion that this internal reward system shares similar neural mechanisms with those activated by a natural reinforcer (e.g., food), that is, both of them produced a significant decrease in the amplitude of hippocampal fEPSPs evoked at the very moment of the reinforcement. The involvement of the hippocampus in these processes is probably related to the cognitive aspects, like attention and learning, of motivational processes. Further support of this model comes from recent reports of the presence of a hippocampal–septal–ventral tegmental area circuit involved in the relationships between reward-motivated behaviors and the learned value of contextual stimuli (Luo et al. 2011). The present results suggest that there are also septohippocampal feedback projections relating internal motivational states with their cognitive counterparts.
MS self-stimulation appears to be highly dependent on the proper functioning of the GABAergic septohippocampal pathway, since J20 mice characterized by decreased density of GABAergic terminals on hippocampal baskets and axo-axonic interneurons, showed a delayed acquisition and a lower performance of MS self-stimulation than their littermate controls. A further confirmation that hippocampal GABAergic circuits are involved in the decreased amplitude of hippocampal fEPSPs during self-stimulation reward was that local inhibition of the GABA-producing GAD65 enzyme (Ishida et al. 1999; Mitoma et al. 2003) also prevented these changes.
Functional Consequences of an Increased LTP in J20 Mice and Local Inhibition of GAD65
As reported here, J20 mice presented changes in hippocampal functional properties, including a larger hippocampal LTP. Similar findings were obtained following the local inhibition of GAD65 by monoclonal antibody b78 in the dorsal hippocampus, always without any motor disturbance. These effects could be ascribed to the imbalance between septohippocampal excitatory cholinergic (Krnjević and Ropert 1982; Markram and Segal 1990) and glutamatergic (Sotty et al. 2003; Habib and Dringenberg 2009) projections versus inhibitory GABAergic (Krnjević et al. 1988) projections. In particular, it has been described that septal cholinergic projections to the hippocampus increase the excitability and LTP of hippocampal circuits (Ovsepian et al. 2004; Palop et al. 2007; Dringenberg et al. 2008). As indicated below, this increased excitability and longer-lasting effects of HFS of the CA3–CA1 synapse have some important consequences on the reinforcing value of MS self-stimulation of J20 mice.
It has already been reported in a caloric restriction program that nonsaturating LTP evoked in hippocampal synapses (CA3–CA1) has no effect on the normal acquisition and/or performance of appetitive (lever press) and consummatory (food intake) behaviors evoked during operant conditioning tasks (Jurado-Parras et al. 2012). Here, nonsaturating LTP evoked at the hippocampal CA3–CA1 synapse did not have any noticeable effect on MS self-stimulation performance in WT mice. Nevertheless, when the evoked LTP reached high and long-lasting levels as those reported here in J20 mice —due probably to the specific decrease in GABAergic septohippocampal projections (present data and Rubio et al. 2012) or to an increased effect of CA3 feedback signals onto MS neurons (Gulyás et al. 2003; Colom 2006)—MS self-stimulation performance was significantly decreased. A putative reason for the above results is that the large facilitation of fEPSPs evoked during LTP in J20 and in mice injected with GAD65-inhibition antibody b78 undoes the internal rewarding effects of MS self-stimulation. These results further support our proposal that internal and/or natural rewards are related to a decrease in the amplitude of hippocampal fEPSP responses.
Role of GABAergic Septohippocampal Pathways in Self-Stimulation and Related Processes
It has been recently proposed that a complex network of electrically coupled GABAergic neurons widely distributed in the midbrain, hypothalamus, and thalamus could contribute to the brain stimulation reward system (Lassen et al. 2007). Indeed, there is additional evidence of the involvement of GABAergic pathways and receptors in diverse behavioral modalities, including emotional displays and motivational states (Macey et al. 2001; Leppä et al. 2011). Thus, GABAergic pathways could play a complementary role versus the widely accepted dopaminergic modulation of rewarding mechanisms (Liebman 1983; Gerhardt and Liebman 1985; Wise 2002). Indeed, it has been reported that septal self-stimulation is independent from the activation of dopaminergic terminals (Prado-Alcalá et al. 1984). Thus, and as shown here, septal GABAergic circuits play an important role in internal and external rewarding processes.
GABAergic septohippocampal projections terminate on hippocampal basket and axo-axonic interneurons (Freund and Buzsaki 1996; Matyas et al. 2004) and play an important regulatory role on the intrinsic excitability and rhythmic activities of hippocampal circuits (Buzsaki 2002; Ovsepian 2006). The deficit of the GABAergic septohippocampal innervation in J20 mice may result in the significant functional alterations affecting the potential role of MS self-stimulation as an internal rewarding agent. These results point to a particular role of MS-hippocampal circuits in the integration between internal motivational states, typical of the mesolimbic dopaminergic system (Wise 1996, 2002), and cognitive, learning, and memory processes characteristically ascribed to hippocampal circuits (Bliss and Collingridge 1993; Gruart et al. 2006). Early studies proposed that septal nuclei could play a definitive role in the integration between internal drives and learning and memory processes (Cazala et al. 1988), and that septal networks represent a nodal point for processing of information from brainstem and hypothalamic centers and archicortical and neocortical structures (Colom 2006). In addition, it has been already reported that MS self-stimulation is phase-locked to hippocampal theta rhythms, a fact that further supports the functional integration between motivational and cognitive activities taking place in septohippocampal circuits (Buño and Velluti 1977). Moreover, activation of the MS enhances the synchronized firing of hippocampal pyramidal cells and contributes to the fine tuning of hippocampal rhythmic activities (Ovsepian 2006).
The decreased fEPSP amplitudes observed in WT mice appears to contradict previous reports (Tóth et al. 1997). A putative explanation could be the different frequency of septal stimulation used as reward. This train used by us (100 Hz, 200 ms, 20 pulses) could excite in a recurrent manner: the MS-hippocampus-lateral septum-ventral tegmental area circuit, and the hippocamposeptal pathway, modifying the final effect on inhibitory hippocampal neurons (Risold and Swanson 1997; Rokers et al. 2002; Manseau et al. 2008). This is supported by a late inhibitory response recorded after every train of septal-stimulation (Fig. 6A1, WT mice). In fact, the local field potential recorded in the CA1 area of J20 mice after MS train stimulation was increased (Fig. 6A1) in comparison with values collected from WT mice. In addition, the GLU component of the fEPSP evoked at the CA3–CA1 synapse was also increased in J20 mice (Fig. 6A2,B–D). These 2 results clearly indicate a disturbance in the septohippocampal inhibitory mechanism present in J20 mice. Additionally, we decided to present the CA3 single-pulse stimulation at 40 ms after the end of the MS train stimulation, that is, at the moment during which the local field potential evoked by MS train stimulation was noticed (Fig. 6A).
Although the expected response on a GABAergic septohippocampal dysfunction would be a decreased GLU component of the CA3–CA1 response, some functional alterations cannot be discarded in the local interneuronal population of J20 mice that would try to counteract the GABA septohippocampal deficit. These compensatory mechanisms at the interneuronal level would lead to an increased response of the principal cell population, as described previously (Palop et al. 2007).
Two additional experiments reported here reinforce this integrative role of septohippocampal circuits regarding motivation and learning and memory processes. First, both consummatory internal (self-stimulation) and external (food) rewards depressed in a similar way, fEPSPs evoked in hippocampal CA3–CA1 synapses and, secondly, that this functional depression of fEPSPs is prevented by the local inhibition of the GAD65 enzyme by monoclonal antibody b78 (Esclapez et al. 1994; Raju et al. 2005; Manto et al. 2011). Previous studies using slice-patch recordings have shown that b78 acts on the terminals of GABAergic neurons to suppress the release of GABA, thereby depressing the inhibitory transmission with a gradual and long-lasting time course. Administration of b78 antibody was also associated with an increase of glutamate concentrations after its administration (Mitoma et al. 2003; Manto et al. 2011). Both activity-dependent gradual functional loss of GABAergic neurons and the increase in glutamate concentrations seem to be confirmed here by the larger LTPs evoked in b78-injected mice.
In conclusion, the hippocampus can play a dual role in cognitive processes underlying internal and external rewards where septohippocampal pathways represent an important path of this intrinsic network. One of these roles could be during the acquisition process when the hippocampus is more active and, in addition, less susceptible to inhibitory effects. The other role could be during consummatory behaviors when the MS inhibitory inputs seem to be more effective.
Funding
This work was supported by grants from the Spanish Ministry of Science and Innovation (BFU2008-00899 and BFU2008-03390) and Junta de Andalucía (Spain, BIO-122, CVI-02487, and P07-CVI-02686 to J.M.D.G. and A.G. and BFU2008-3980 to E.S.), and Spanish Fondo de Investigaciones Sanitarias (PI11/00704) to M.P. The research leading to these results also received funding from the European Community's seventh Framework Program (FP7/2007-2013) under grant agreement n° 201714 (DEVANX). M.A.G.C. was supported by a JCI-2011-09013 post-doctoral fellowship from the Spanish Ministry of Science and Innovation.
Notes
The authors thank Dr R. Sánchez-Campusano for adapting MATLAB software, Mr. J.A. Santos for help in the experimental set-up, Natalia Ruiz for histological support, and Mr. R. Churchill for his help in manuscript editing. Conflict of Interest: None declared.
References
- Ball GG, Gray JA. Septal self-stimulation and hippocampal activity. Physiol Behav. 1971;6:547–549. doi: 10.1016/0031-9384(71)90203-4. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Buño W, Jr, Velluti JC. Relationships of hippocampal theta cycles with bar pressing during self-stimulation. Physiol Behav. 1977;19:615–621. doi: 10.1016/0031-9384(77)90035-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Cazala P, Galey D, Durkin T. Electrical self-stimulation in the medial and lateral septum as compared to the lateral hypothalamus: differential intervention of reward and learning processes? Physiol Behav. 1988;44:53–59. doi: 10.1016/0031-9384(88)90345-9. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. J Physiol (Lond) 1983a;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol (Lond) 1983b;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom LV. Septal networks: relevance to theta rhythm, epilepsy and Alzheimer's disease. J Neurochem. 2006;96:609–623. doi: 10.1111/j.1471-4159.2005.03630.x. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Oliveira D, Habib D. Predator (cat hair)-induced enhancement of hippocampal long-term potentiation in rats: involvement of acetylcholine. Learn Mem. 2008;15:112–116. doi: 10.1101/lm.778108. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gerhardt S, Liebman JM. Self-regulation of ICSS duration: effects of anxiogenic substances, benzodiazepine antagonists and antidepressants. Pharmacol Biochem Behav. 1985;22:71–76. doi: 10.1016/0091-3057(85)90488-5. [DOI] [PubMed] [Google Scholar]
- Grauer E, Thomas E. Conditioned suppression of medial forebrain bundle and septal intracranial self-stimulation in the rat: evidence for a fear-relief mechanism of the septum. J Comp Physiol Psychol. 1982;96:61–70. doi: 10.1037/h0077860. [DOI] [PubMed] [Google Scholar]
- Gruart A, Muñoz MD, Delgado-García JM. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci. 2006;26:1077–1087. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás AI, Gorcs TJ, Freund TF. Innervation of different peptide-containing neurons in the hippocampus by GABAergic septal afferents. Neuroscience. 1990;37:31–44. doi: 10.1016/0306-4522(90)90189-b. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Hájos N, Katona I, Freund TF. Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur J Neurosci. 2003;17:1861–1872. doi: 10.1046/j.1460-9568.2003.02630.x. [DOI] [PubMed] [Google Scholar]
- Habib D, Dringenberg HC. Alternating low frequency stimulation of medial septal and commissural fibers induces NMDA-dependent, long-lasting potentiation of hippocampal synapses in urethane-anesthetized rats. Hippocampus. 2009;19:299–307. doi: 10.1002/hipo.20507. [DOI] [PubMed] [Google Scholar]
- Hodos W, Valenstein ES. An evaluation of response rate as a measure of rewarding intracranial stimulation. J Comp Physiol Psychol. 1962;55:80–84. doi: 10.1037/h0047681. [DOI] [PubMed] [Google Scholar]
- Ishida K, Mitoma H, Song SY, Uchihara T, Inaba A, Eguchi S, Kobayashi T, Mizusawa H. Selective suppression of cerebellar GABAergic transmission by an autoantibody to glutamic acid decarboxylase. Ann Neurol. 1999;46:263–267. [PubMed] [Google Scholar]
- Jurado-Parras MT, Gruart A, Delgado-García JM. Observational learning in mice can be prevented by medial prefrontal cortex stimulation and enhanced by nucleus accumbens stimulation. Learn Mem. 2012;19:99–106. doi: 10.1101/lm.024760.111. [DOI] [PubMed] [Google Scholar]
- Jurado-Parras MT, Sánchez-Campusano R, Castellanos NP, del-Pozo F, Gruart A, Delgado-García JM. Differential contribution of hippocampal circuits to appetitive and consummatory behaviors during operant conditioning of behaving mice. J Neurosci. 2013;33:2293–2304. doi: 10.1523/JNEUROSCI.1013-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K, Ropert N. Electrophysiological and pharmacological characteristics of facilitation of hippocampal population spikes by stimulation of the medial septum. Neuroscience. 1982;7:2165–2183. doi: 10.1016/0306-4522(82)90128-2. [DOI] [PubMed] [Google Scholar]
- Krnjević K, Ropert N, Casullo J. Septohippocampal disinhibition. Brain Res. 1988;438:182–192. doi: 10.1016/0006-8993(88)91337-6. [DOI] [PubMed] [Google Scholar]
- Lassen MB, Brown JE, Stobbs SH, Gunderson SH, Maes L, Valenzuela CF, Ray AP, Henriksen SJ, Steffensen SC. Brain stimulation reward is integrated by a network of electrically coupled GABA neurons. Brain Res. 2007;1156:46–58. doi: 10.1016/j.brainres.2007.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppä E, Linden AM, Vekovischeva OY, Swinny JD, Rantanen V, Toppila E, Höger H, Sieghart W, Wulff P, Wisden W, et al. Removal of GABA(A) receptor γ2 subunits from parvalbumin neurons causes wide-ranging behavioral alterations. PLoS One. 2011;6(9):e24159. doi: 10.1371/journal.pone.0024159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman JM. Discriminating between reward and performance: a critical review of intracranial self-stimulation methodology. Neurosci Biobehav Rev. 1983;7:45–72. doi: 10.1016/0149-7634(83)90007-6. [DOI] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Froestl W, Koob GF, Markou A. Both GABA(B) receptor agonist and antagonists decreased brain stimulation reward in the rat. Neuropharmacology. 2001;40:676–685. doi: 10.1016/s0028-3908(00)00204-5. [DOI] [PubMed] [Google Scholar]
- Madroñal N, Gruart A, Delgado-García JM. Differing presynaptic contributions to LTP and associative learning in behaving mice. Front Behav Neurosci. 2009;3:7. doi: 10.3389/neuro.08.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madroñal N, López-Aracil C, Rangel A, del Río JA, Delgado-García JM, Gruart A. Effects of enriched physical and social environments on motor performance, associative learning, and hippocampal neurogenesis in mice. PLoS One. 2010;5(6):e11130. doi: 10.1371/journal.pone.0011130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manseau F, Goutagny R, Danik M, Williams S. The hippocamposeptal pathway generates rhythmic firing of GABAergic neurons in the medial septum and diagonal bands: an investigation using a complete septohippocampal preparation in vitro. J Neurosci. 2008;28:4096–4107. doi: 10.1523/JNEUROSCI.0247-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manto MU, Hampe CS, Rogemond V, Honorat J. Respective implications of glutamate decarbocylase antibodies in stiff person syndrome and cerebellar ataxia. Orphanet J Rare Dis. 2011;6:3. doi: 10.1186/1750-1172-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Segal M. Long-lasting facilitation of excitatory postsynaptic potentials in the rat hippocampus by acetylcholine. J Physiol (Lond) 1990;427:381–393. doi: 10.1113/jphysiol.1990.sp018177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas F, Freund TF, Gulyas AI. Immunocytochemically defined interneuron populations in the hippocampus of mouse strains used in transgenic technology. Hippocampus. 2004;14:460–481. doi: 10.1002/hipo.10191. [DOI] [PubMed] [Google Scholar]
- Miliaressis E, Rompre PP. Effects of concomitant motor reactions on the measurement of rewarding efficacy of brain stimulation. Behav Neurosci. 1987;101:827–831. doi: 10.1037//0735-7044.101.6.827. [DOI] [PubMed] [Google Scholar]
- Mitoma H, Ishida K, Shizuka-Ikeda M, Mizusawa H. Dual impairment of GABAA- and GABAB-receptor-mediated synaptic responses by autoantibodies to glutamic acid decarboxylase. J Neurol Sci. 2003;208:51–56. doi: 10.1016/s0022-510x(02)00423-9. [DOI] [PubMed] [Google Scholar]
- Mora F, Cobo M. The neurobiological basis of prefrontal cortex self-stimulation: a review and an integrative hypothesis. Prog Brain Res. 1990;85:419–431. doi: 10.1016/s0079-6123(08)62693-x. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of αβ1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J. Self-stimulation of the brain; its use to study local effects of hunger, sex, and drugs. Science. 1958;127:315–324. doi: 10.1126/science.127.3294.315. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Ovsepian SV. Enhancement of the synchronized firing of CA1 pyramidal cells by medial septum preconditioning: time-dependent involvement of muscarinic cholinoceptors and GABAB receptors. Neurosci Lett. 2006;393:1–6. doi: 10.1016/j.neulet.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Ovsepian SV, Anwyl R, Rowan MJ. Endogenous acetylcholine lowers the threshold for long-term potentiation induction in the CA1 area through muscarinic receptor activation: in vivo study. Eur J Neurosci. 2004;20:1267–1275. doi: 10.1111/j.1460-9568.2004.03582.x. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Jones B, Kekonius L, Chin J, Yu GQ, Raber J, Masliah E, Mucke L. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer's disease-related cognitive deficits. Proc Natl Acad Sci USA. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Perez-Sust P, Soriano E. The GABAergic septohippocampal pathway in control and reeler mice: target specificity and termination onto Reelin-expressing interneurons. Mol Cell Neurosci. 2004;25:679–691. doi: 10.1016/j.mcn.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego (CA); London: Academic Press; 2001. [Google Scholar]
- Prado-Alcalá R, Streather A, Wise RA. Brain stimulation areward and dopamine terminal fields. II. Septal and cortical projections. Brain Res. 1984;301:209–219. doi: 10.1016/0006-8993(84)91089-8. [DOI] [PubMed] [Google Scholar]
- Raju R, Foote J, Banga JP, Hall TR, Padoa CJ, Dalakas MC, Ortqvist E, Hampe CS. Analysis of GAD65 autoantibodies in Stiff-Person syndrome patients. J Immunol. 2005;175:7755–7762. doi: 10.4049/jimmunol.175.11.7755. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Rev. 1997;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Rokers B, Mercado E, Allen MT, Myers CE, Gluck MA. A connectionist model of septohippocampal dynamics during conditioning: closing the loop. Behav Neurosci. 2002;116:48–62. [PubMed] [Google Scholar]
- Rubio SE, Vega-Flores G, Martínez A, Bosch C, Pérez-Mediavilla A, del Río J, Gruart A, Delgado-García JM, Soriano E, Pascual M. Accelerated aging of the GABAergic septohippocampal pathway in a mouse model of Alzheimer's disease. FASEB J. 2012;26:4458–4467. doi: 10.1096/fj.12-208413. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA. Regulation of excitability in hippocampal neurons. In: Isacson RL, Pribram KH, editors. The hippocampus. New York (NY): Plenum Press; 1986. pp. 113–136. [Google Scholar]
- Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S. Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. J Physiol (Lond) 2003;551:927–943. doi: 10.1113/jphysiol.2003.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J Physiol. 1997;500(Pt 2):463–474. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Ann Rev. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Ann Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]