In a double-blind sham-controlled study in stroke patients, Lefebvre et al. show that transcranial direct current stimulation improves learning and one-week retention of a motor skill performed with a paretic upper limb. They also reveal a trend towards normalisation of brain activation patterns during execution of the learned motor skill.
Keywords: motor skill learning, stroke, fMRI, neurorehabilitation, tDCS
Abstract
Motor skill learning is one of the key components of motor function recovery after stroke, especially recovery driven by neurorehabilitation. Transcranial direct current stimulation can enhance neurorehabilitation and motor skill learning in stroke patients. However, the neural mechanisms underlying the retention of stimulation-enhanced motor skill learning involving a paretic upper limb have not been resolved. These neural substrates were explored by means of functional magnetic resonance imaging. Nineteen chronic hemiparetic stroke patients participated in a double-blind, cross-over randomized, sham-controlled experiment with two series. Each series consisted of two sessions: (i) an intervention session during which dual transcranial direct current stimulation or sham was applied during motor skill learning with the paretic upper limb; and (ii) an imaging session 1 week later, during which the patients performed the learned motor skill. The motor skill learning task, called the ‘circuit game’, involves a speed/accuracy trade-off and consists of moving a pointer controlled by a computer mouse along a complex circuit as quickly and accurately as possible. Relative to the sham series, dual transcranial direct current stimulation applied bilaterally over the primary motor cortex during motor skill learning with the paretic upper limb resulted in (i) enhanced online motor skill learning; (ii) enhanced 1-week retention; and (iii) superior transfer of performance improvement to an untrained task. The 1-week retention’s enhancement driven by the intervention was associated with a trend towards normalization of the brain activation pattern during performance of the learned motor skill relative to the sham series. A similar trend towards normalization relative to sham was observed during performance of a simple, untrained task without a speed/accuracy constraint, despite a lack of behavioural difference between the dual transcranial direct current stimulation and sham series. Finally, dual transcranial direct current stimulation applied during the first session enhanced continued learning with the paretic limb 1 week later, relative to the sham series. This lasting behavioural enhancement was associated with more efficient recruitment of the motor skill learning network, that is, focused activation on the motor-premotor areas in the damaged hemisphere, especially on the dorsal premotor cortex. Dual transcranial direct current stimulation applied during motor skill learning with a paretic upper limb resulted in prolonged shaping of brain activation, which supported behavioural enhancements in stroke patients.
Introduction
Stroke is a devastating pathology that causes restrictions in daily life activities, such as motor limitations due to upper limb hemiparesis in a majority of patients (Lai et al., 2002; Kwakkel et al., 2003). Neurorehabilitation aims to improve residual motor function and restore independence, but its impact is still limited. Hence, innovative strategies for enhancing neurorehabilitation have been developed, including non-invasive brain stimulation (NIBS) techniques such as transcranial direct current stimulation (DCS) (Hummel and Cohen, 2006; Madhavan and Shah, 2012). NIBS can modulate transiently brain excitability and behaviour in healthy individuals, as well as in stroke patients (Hummel and Cohen, 2006; Hummel et al., 2006; Vines et al., 2008; Madhavan and Shah, 2012). Pilot experiments have shown that NIBS has the potential to enhance neurorehabilitation (Boggio et al., 2007; Lindenberg et al., 2010; Bolognini et al., 2011). Upon which neural substrates NIBS acts in the brains of stroke patients and how exactly NIBS modulates brain activity is still poorly understood.
Researchers started to explore the neural substrates associated with the enhancement of neurorehabilitation interventions by NIBS with functional MRI (Lindenberg et al., 2010; Nair et al., 2011; Yamada et al., 2013). Typically, over a period of several days, hemiparetic stroke patients received occupational therapy coupled with real or sham NIBS; functional MRI data were acquired before and after the therapy programme. Motor performance enhancement of the paretic upper limb after neurorehabilitation with NIBS was associated with a reorganization of the functional MRI pattern, namely a transfer of brain activation from a bilateral network towards the ipsilesional sensori-motor-premotor areas (Lindenberg et al., 2010; Nair et al., 2011; Yamada et al., 2013). Recently, Stagg and collaborators (2012) explored the brain activation associated with the after-effects of transcranial DCS in chronic stroke patients performing a simple response time task. Immediately after applying anodal transcranial DCS over the primary motor cortex in the damaged hemisphere (M1damH), improved performance was associated with increased activation in M1damH and the supplementary motor area (SMAdamH), as well as increased activation bilaterally in the dorsal premotor cortex (PMd). Furthermore, after applying cathodal transcranial DCS over M1 in the undamaged hemisphere (M1undamH), activation was increased contralaterally in PMddamH and SMAdamH as well as bilaterally in M1 (Stagg et al., 2012).
In these pioneer studies, the tasks used to elicit brain activation were, for obvious practical reasons, relatively simple (e.g. repetitive fingers/elbow/wrist flexion/extension, simple reaction time task). These tasks are distinct from the scales commonly used to quantify motor impairment and recovery [i.e. the modified Rankin Scale, National Institutes of Health Stroke Score (NIHSS), Fugl-Meyer assessment, Action Research Arm Test, Barthel index, etc. (Kasner, 2006)]. Furthermore, during neurorehabilitation interventions, the stroke patients were not trained specifically to perform the functional MRI tasks. Therefore, these functional MRI tasks represent a generic exemplification of the overall improvement of paretic upper limb function. From a clinical point of view, such a transfer from neurorehabilitation interventions towards generic enhancement of motor control in the paretic upper limb is both highly satisfactory and promising. However, the specific components underlying neurorehabilitation-induced enhancements are still poorly understood.
Overall, post-stroke motor recovery driven by neurorehabilitation relies most obviously on restored muscle strength, reduced spasticity, increased endurance, resolution of metabolic events in the (sub)acute stroke phase, and neural plasticity (i.e. reorganization of the spared neuronal networks and connections). However, beyond these crucial components, any lasting improvement gained through training and experience (e.g. through neurorehabilitation) depends necessarily upon long-term motor memory retention. To some extent, recovering from hemiparesis can be conceptualized as a particular form of motor learning; in other words, learning to use the reconfigured motor network to optimize planning, execution, and control of movement with the affected limb. The idea that motor skill learning plays a central role in post-stroke motor recovery is becoming a focus of interest in neurorehabilitation (Matthews et al., 2004; Krakauer, 2006; Dipietro et al., 2012; Kitago and Krakauer, 2013). Motor skill learning is defined as a practice-dependent motor performance improvement that persists over time; it is characterized by a shift in the speed/accuracy trade-off (SAT), some degree of automatization, and a reduction in variability (Reis et al., 2009; Dayan and Cohen, 2011; Krakauer and Mazzoni, 2011).
It was demonstrated recently that NIBS can enhance online motor skill learning and overnight retention in stroke patients (Meehan et al., 2011a; Zimerman et al., 2012) and, more importantly, long-term retention of a motor skill involving a SAT (Lefebvre et al., 2013a). The demonstration that NIBS enhances motor skill learning and its long-term retention in stroke patients establishes a crucial link between bench observations [transient enhancement of motor function (Hummel et al., 2006; Lefebvre et al., 2013b)] and clinical implementation [enhanced neurorehabilitation (Lindenberg et al., 2010; Nair et al., 2011; Yamada et al., 2013)]. The science of neurorehabilitation will be advanced by elucidation of how NIBS boosts the effects of neurorehabilitation (i.e. through enhanced motor skill learning), and which neural substrates underlie (i) specific motor skill learning; and (ii) generic improvement.
Studies of the neural substrates underlying motor skill learning in healthy subjects have demonstrated activations in a network encompassing M1, SMA, premotor cortex, the dorsolateral prefrontal cortex (PFC), the cerebellum, and the basal ganglia (Karni et al., 1995; Halsband and Lange, 2006; Lefebvre et al., 2012; Hardwick et al., 2013). Based on recent concepts in motor learning, we designed a motor skill learning paradigm involving a SAT and demonstrated that efficient motor skill learning, characterized by a shift of this SAT, depends upon recruitment of the SMA in healthy individuals (Lefebvre et al., 2012).
The few studies that have explored the neural substrates of motor skill learning after stroke have shown a decreased activation in the undamaged hemisphere and an increased specific activity in the damaged hemisphere compared to pre-training activation (Carey et al., 2002; Boyd et al., 2010; Bosnell et al., 2011; Meehan et al., 2011b). Also, motor skill learning in chronic stroke patients induced a recruitment of additional areas compared to healthy individuals, such as dorsolateral PFC (Carey et al., 2002; Boyd et al., 2010; Bosnell et al., 2011; Meehan et al., 2011b). The network activated while learning with the paretic upper limb a motor skill involving a SAT has been described only recently (Lefebvre et al., personal communication); it seems that efficient motor skill learning relied upon the recruitment of the PMddamH in chronic stroke patients (S. Lefebvre, personal communication).
A finer knowledge of the neural substrates underlying motor learning in stroke patients and the neural substrates upon which NIBS acts to enhance neurorehabilitation and motor learning after stroke is of key importance for the implementation of NIBS in routine clinical practice. The aim of the present study was to explore by means of functional MRI the neural substrates underlying the long-term retention of specific motor skill enhancement driven by motor skill learning with a paretic upper limb under dual-transcranial DCS versus sham (intervention), generic enhancement of untrained movements performed with the paretic limb, and continued motor skill learning 1 week post-intervention.
Materials and methods
Population
Nineteen chronic stroke patients provided written informed consent and were included in this study, which was conducted according to the recommendations of the Declaration of Helsinki after being approved by the local Ethical Committee (Comité d’éthique médicale, CHU Dinant Godinne UcL Namur). The inclusion criteria were: (i) being a chronic (>6 months) stroke patient aged 18–80 years; (ii) presenting with a chronic motor deficit in an upper limb; and (iii) having a vascular brain lesion demonstrated by cerebral imaging (Supplementary Fig. 1). The exclusion criteria were: (i) having a contraindication to MRI or to transcranial DCS; (ii) being unable to perform the task or to understand instructions; (iii) suffering from epilepsy, alcoholism, cognitive impairment, or a psychiatric disorder; and (iv) being pregnant. Each patient’s impairment was evaluated upon enrolment by means of the Purdue Pegboard Test (Tiffin and Asher, 1948), a maximal hand force (MaxHF) assessment with a whole-hand Jamar dynamometer, manual ability with the ABILHAND scale (Penta et al., 2001), and the NIHSS (Kasner et al., 1999). Overall degree of disability was determined with the modified Rankin Scale (Bonita and Beaglehole, 1988) (Supplementary Table 1). With the exception of Patient 8, all of the patients participated in a motor skill learning study within a single functional MRI session (without transcranial DCS), at least 1 week before the intervention (S. Lefebvre, personal communication). Four patients (Patients 2, 3, 4 and 8) participated in a study exploring the impact of a single session of dual-transcranial DCS on precision grip and dexterity, a year or more prior (Lefebvre et al., 2013b).
Study design
The stroke patients participated in a crossover experiment with two series. Each series consisted of two sessions: (i) an intervention session during which dual-transcranial DCS or sham was applied during motor skill learning of the paretic upper limb (using a double-blind, crossover randomized method); and (ii) an imaging session 1 week later (retention session), during which the patients performed the learned motor skill. The retention session permitted the exploration of the mean overall level of motor performance retention. Because, during the retention session, the stroke patients performed eight blocks of the circuit learned 1 week before, the performance evolution during this session was also analysed as continued learning. The general design was similar to our previous study exploring the impact of dual-transcranial DCS on motor skill learning in chronic hemiparetic stroke patients (Lefebvre et al., 2013a), except that in the current study the intervention sessions were performed in the supine position with the circuit projected on the ceiling, to accommodate the patient’s position in the MRI scanner 1 week later during the retention session (Fig. 1).
Figure 1.
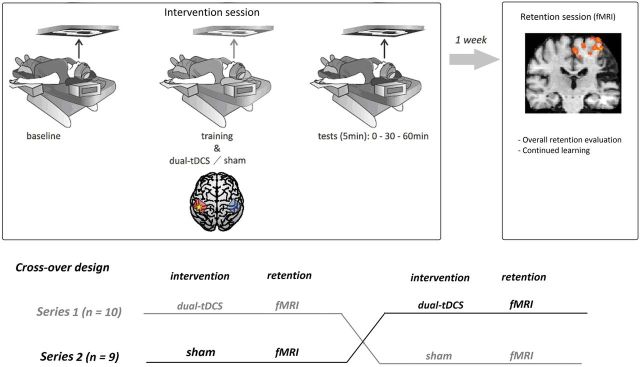
Study design. During the intervention, each stroke patient trained in the supine position, matching their position during the MRI retention session 1 week later. They participated in two separate series of two sessions each in a double-blind, cross-over randomized fashion. Each series contained one intervention session (one with dual-transcranial DCS, the other with sham) and a retention session 1 week after. Ten patients were enrolled in the first series (dual-transcranial DCS as the first intervention) and nine patients were enrolled in the second series (sham transcranial DCS as the first intervention). fMRI = functional MRI; tDCS = transcranial DCS.
Motor skill learning intervention with dual-transcranial DCS and sham
An Eldith DC-Stimulator® (NeuroConn) was used to deliver dual-transcranial DCS using two soaked (NaCl 0.9%) electrodes (35 cm2). The anode electrode was positioned over the ipsilesional M1 and the cathode electrode over the contralesional M1. The localization of both M1s was determined using a Magstim 2002 (Magstim Company) with a figure-of-8 coil. Dual-transcranial DCS at 1 mA was applied over 30 min. For sham, a short current up-ramp (8 s fade-in) was followed by 30 s of direct current to induce similar scalp sensations, then by 8 s of current fade-out. This technique of stimulation was used in our previous study (Lefebvre et al., 2013a).
The motor skill learning paradigm (circuit game and its analysis) has been described in detail elsewhere (Lefebvre et al., 2012, 2013a). Briefly, the circuit game consisted of moving a cursor with magnetic resonance compatible mouse held by the paretic hand along a complex path as quickly and accurately as possible with visual feedback. Velocity and error (the surface area between the actual trajectory and the ideal trajectory) were averaged across 3-s bins, resulting in 10 values for each 30-s training block. Movement and speed are reported in arbitrary grid unit (u) as u/s for velocity and u2 for error. Normalized mean error (Pe = constant error/subject mean error) and normalized mean velocity (Pv = subject mean velocity/constant velocity) were used to compute performance index (PI) values, which increase when the error is reduced and/or when the velocity is increased (PI = Pv × Pe). The evolution of motor skill learning (i.e. of the SAT) was quantified by a learning index {LI = [(PI − PIBaseline)/PIBaseline] × 100}, with baseline being the first block of training (Supplementary material).
During functional MRI scanning, the stroke patients performed two runs of the circuit game learned 1 week before. First, the amount of motor skill retention 1 week after the intervention (overall mean learning index of each retention session = Run 1 + 2) was compared between dual-transcranial DCS and sham series with paired sample t-tests. Second, continued learning (i.e. retraining on the circuit learned 1 week before during the intervention) was evaluated in terms of performance evolution during the functional MRI sessions. To quantify continued learning, the learning index was recalculated using the first circuit block of Run 1 as the new baseline, separately for each functional MRI session.
In addition, during functional MRI acquisition the patients also performed an untrained Easy condition (see below) during which they moved the cursor back and forth at a comfortable speed between two horizontal or vertical targets, without speed or accuracy constraints. During Easy, the number of movements (total distance), their speed and normalized jerk [with the formula: (Contreras-Vidal and Buch, 2003; Caimmi et al., 2008)] were compared between the two sessions using paired Student’s t-tests.
To explore further a potential transfer towards generic, untrained motor performance improvement, a repeated measures ANOVA was performed on the Purdue Pegboard Test and MaxHF scores recorded during the intervention sessions with Bonferroni t-test post hoc analysis; a paired t-test compared the Purdue Pegboard Test and MaxHF values recorded after functional MRI scanning.
Functional MRI design, acquisition and preprocessing
The functional MRI sessions consisted of one habituation run (2 min 40 s; four blocks of practice on a simple square circuit alternating with four blocks of rest) and two runs of the circuit learned the previous week (i.e. during the intervention sessions) (Fig. 1) (8 min 41 s, 172 scans). Each run contained three conditions which occurred four times each: (i) Learning (performing the learned circuit as quickly and accurately as possible); (ii) Easy (simple motor condition without speed or accuracy constraints); and (iii) Replay (visual-visuomotor condition: with a video clip of the last Learning performance displayed, patients were instructed to follow the cursor’s displacement with their eyes while keeping their hands motionless). The practice blocks were separated by rest blocks during which a fixation cross was visible.
The images were acquired with a 3-T scanner attached to a 32-channel head coil (Siemens Verio). Functional MRI scans were acquired by repeated single-shot echo-planar imaging with the following parameters: repetition time = 3000 ms, echo time = 23 ms, flip angle = 90°, matrix size = 64 × 64, field of view = 220 × 220 mm2, slice order descending and interleaved, slice thickness = 2 mm (no gap) and number of slices = 59 (whole-brain). A 3D T1-weighted data set covering the whole brain was acquired (1 mm3, repetition time = 1600 ms, echo time = 2.39 ms, flip angle = 9°, matrix size = 512 × 512, field of view = 256 × 256 mm3, 176 slices, slice thickness = 1 mm, no gap).
Functional MRI data were preprocessed and analysed with BrainVoyager QX (Version 2.4.2.2070) software; the data were processed as described previously (Lefebvre et al., 2012; S. Lefebvre, personal communication), except that for patients with stroke lesions on the right side of the brain, the 3D-T1 and functional data were flipped: the 3D-T1 by flipping the x-axis and the functional data by flipping the data horizontally. Briefly, the preprocessing of the functional data consisted of a slice-time scan correction, temporal high-pass filtering, and 3D motion correction. A general linear model was used to analyse the functional MRI data. Co-registrations between functional runs and 3D-T1 weighted scans of each patient were performed automatically, and corrected manually when careful visual inspection identified imperfect co-registration. All anatomical and functional volumes were normalized in talairach space (Talairach, 1988) to allow group analysis. Functional runs were smoothed in the spatial domain with a 5-mm Gaussian filter.
Functional MRI processing
Whole-brain ANOVA
A whole-brain two-factors ANOVA using condition estimates (beta values) from a first-level random effect general linear model analysis constructed with 76 runs (19 stroke patients, two retention sessions with two runs each) was performed to directly compare the activation associated with motor skill retention between each intervention [first factor: functional MRI conditions (Learning, Easy and Replay), second factor: Intervention (1 week after dual-transcranial DCS or sham)].
Whole-brain random effect
Three whole-brain random effects were constructed and included the 19 stroke patients. The first random effect involved the two retention sessions and was used in the ANOVA and for the regions of interest analyses. The two others random effects were computed for each session separately and also used for regions of interest analyses. The brain activation associated with each condition was explored at the whole-brain level using the following contrasts for the three random effects: (i) [LEARNING] reflecting all the activation while performing the retained motor skill; (ii) [EASY] reflecting the neural substrates underlying the performance of simple, untrained movements performed with the paretic upper limb without SAT constraint; (iii) [REPLAY] reflecting visual and oculomotor activation elicited by looking at the video sequence of the last LEARNING block, while keeping the hand still; and (iv) [LEARNING – REPLAY] reflecting the activation of motor skill performance minus the activation relative to visual-oculomotor activity; highlighting the brain activation specifically dedicated to motor skill performance during retention
Region of interest analyses
Effect of intervention
To better understand the effect of intervention, for each region of interest found activated in the contrasts obtained with the random effect involving both sessions, the mean beta weights were extracted (one averaged beta weigh per condition and per retention session for each patient) and directly compared between the two retention sessions by means of paired Student t-tests.
Correlations analysis with motor skill retention
In the regions of interest with significant activation found in the two separate random effects (one for each intervention), Pearson correlation analyses were performed to identify the area(s) whose activation correlated most strongly with retained motor skill performance. For this analysis, the learning indices of each patient were averaged across the two runs (overall mean learning index, reflecting the general level of performance of the retained motor skill) and correlated with the mean beta weights of the condition of interest for each stroke patient across the two runs. The [LEARNING – REPLAY] was the contrast of interest for this analysis comparing the two retention sessions; a complementary region of interest analysis was performed on [REPLAY].
Individual contribution of each patient to the main (whole-group) pattern
The following predefined regions of interest were drew bilaterally in Talairach space: using both the Talairach Daemon [http://www.talairach.org (Talairach, 1988)] and the third edition of the ‘Atlas Of the Human Brain’ (Mai et al., 2007): M1, PMd, primary somatosensory cortex (S1), posterior parietal cortex (PPC), dorsolateral PFC, visual areas; based on Picard and Strick (2001) for SMA proper and pre-SMA; and cerebellum. These regions of interest were used to explore the individual contribution of each patient to the main (whole-group) pattern: the numbers of activated voxels with an uncorrected P-value of 0.05 (in order to reveal all areas involved) were counted inside each region of interest for [EASY], [REPLAY] and [LEARNING − REPLAY]. Each stroke patient was compared between the retention sessions for each predefined region of interest with paired Student t-tests.
Continued learning
Finally, the brain activations associated with continued learning were explored in three steps using the two separate whole-brain random effect analyses, including only the patients who achieved learning. For each session, a conjunction analysis ([LEARNING – REPLAY] ∩ [LEARNING – EASY]) was performed to explore the activation common to both contrasts. Then, Pearson correlation analyses were performed between the evolution of the beta weights and that of the learning index values and performance index values in the regions of interest obtained with the conjunction analysis at each functional MRI session.
Results
Behaviour
Compared to the sham procedure, dual-transcranial DCS improved both the magnitude (Fig. 2, Supplementary material and Supplementary Fig. 2) and the quality of motor skill learning with the paretic arm (Supplementary material and Supplementary Fig. 3). The overall mean learning index 1 week after dual-transcranial DCS [52% ± 29, mean ± standard deviation (SD)] was statistically superior to that observed after the sham procedure [12% ± 20, t(18) = 3.57; P = 0.002; Cohen’s d effect size (d) = 1.61] (Fig. 2). During the performance of simple, untrained movements (EASY condition), the two functional MRI sessions did not differ in speed [17 ± 3 u/s after sham versus 18 ± 4 u/s after dual-transcranial DCS, P = 0.28], total amount of movement [479 ± 92 u versus 503 ± 112 u, P = 0.50], or normalized jerk [353 070 ± 201 347 versus 513 756 ± 513 766, P = 0.18].
Figure 2.
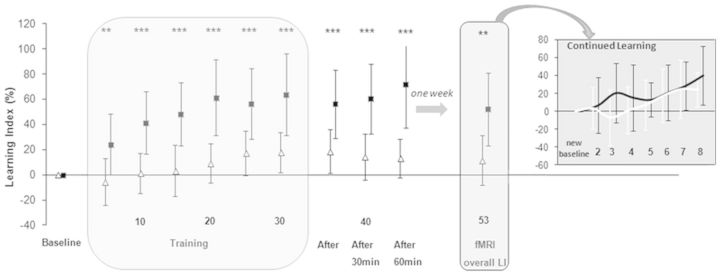
Differential evolution of motor skill learning under sham and dual-transcranial DCS. Evolution of learning index, expressed as a %, change from baseline during the intervention session (baseline, training, after 0 min, 30 min and 60 min) and 1 week later (overall learning index during the functional MRI session). The learning index is plotted as means ± SDs of five consecutive blocks of the circuit game, except for the functional MRI retention session for which the overall learning index is plot as means ± SD of the eight blocks. Insert: Continued learning is plotted as the learning index evolution compared the first block of the functional MRI session (‘new baseline’). Numbers on the x-axis refer these blocks. Open triangles = sham; filled squares = dual-transcranial DCS. *P < 0.05, **P < 0.01, ***P < 0.001. See Supplementary Fig. 2 for more details. LI = learning index; fMRI = functional MRI.
Dual-transcranial DCS also resulted in a transfer of motor performance enhancement in the paretic hand’s dexterity outside the magnetic resonance environment. One week after dual-transcranial DCS, Purdue Pegboard Test scores remained significantly improved [+0.88 pegs in 30 s, +12%, t(18) = 3.94; P = 0.001; d = 0.23] compared to baseline. There was a slight but significant deterioration after the sham procedure [−0.58 pegs, −8%, t(18) = 2.84; P = 0.01; d = 0.17], and a significant difference between the sham and dual-transcranial DCS series [t(18) = 3.81; P = 0.001; d = 0.23]. MaxHF remained unchanged in both series (Supplementary material).
Whole-brain ANOVA
Main effects
The whole-brain ANOVA presented significant effects of the two factors and mainly their interaction [at q(False Discovery Rate) = 0.05: functional MRI conditions: F(2.72) = 3.63; interventions: F(1.36) = 6; interaction: F(2.72) = 3.63].
Post hoc analyses
The post hoc analyses contrasted interventions for each functional MRI condition. There was no significant difference between the two interventions for [REPLAY] [t(74) = 2.30; q(FDR) = 0.05]. With [LEARNING] and [EASY], several areas were more activated during the retention session 1 week after sham compared to dual-transcranial DCS [t(74) = 2.30, Table 1]. By contrast, no area was more activated during the retention session 1 week after dual-transcranial DCS compared to sham at the same t.
Table 1.
Whole-group ANOVA
| Contrast | Brain area/structure | BA | Mean x | Mean y | Mean z | mm3 |
|---|---|---|---|---|---|---|
| [LEARNING] | ||||||
| sham > dual tDCS | ||||||
| M1damH | 4 | −29 | −32 | 54 | 23 | |
| S1 damH | 3 | −39 | −35 | 54 | 36 | |
| PMd damH | 6 | −14 | −13 | 49 | 104 | |
| Frontal cortex damH | 10 | −6 | 59 | 12 | 185 | |
| DLPFC damH | 8 | −18 | 21 | 41 | 76 | |
| IPC damH | 40 | −38 | −37 | 51 | 86 | |
| Parietal cortex damH | 5 | −32 | −40 | 63 | 47 | |
| temporal cortex damH | 21 | −44 | −4 | −12 | 64 | |
| temporal cortex undamH | 21-38-39 | 42 | 1 | −25 | 6899 | |
| DLPFC undamH | 8-9 | 56 | 14 | 30 | 312 | |
| [LEARNING] | ø | |||||
| dual tDCS > sham | ||||||
| [EASY] | ||||||
| sham > dual tDCS | ||||||
| M1 damH | 4 | −26 | −35 | 47 | 1468 | |
| S1 damH | 2 | −40 | −26 | 41 | 260 | |
| PMd damH | 6 | −14 | −14 | 48 | 40 | |
| PMd undamH | 6 | 13 | −7 | 50 | 441 | |
| SMA undamH | 6 | 7 | −16 | 49 | 344 | |
| temporal cortex undamH | 20-21 | 48 | −3 | −20 | 1810 | |
| [EASY] | ø | |||||
| dual tDCS > sham | ||||||
| [REPLAY] | ø | |||||
| sham > dual tDCS | ||||||
| [REPLAY] | ø | |||||
| dual tDCS > sham | ||||||
t(74) = 2.30.
BA = Brodmann area; DLPFC = dorsolateral PFC; mm3 = number of activated voxels; tDCS = transcranial DCS.
Whole-brain random effect
The whole-brain activation patterns found with the three random effect analyses are shown in Fig. 3. As can be visually appreciated and as found with the ANOVA, the activation patterns were more widespread in both hemispheres 1 week after sham compared to dual-transcranial DCS.
Figure 3.
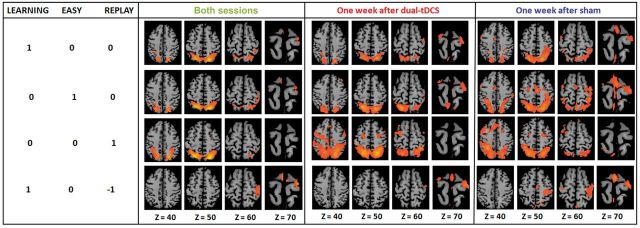
Activation patterns 1 week post-intervention. Whole-brain activation of the 19 stroke patients with the [LEARNING], [EASY], [REPLAY] and [LEARNING − REPLAY] contrasts, for both sessions [t(74) = 2.30] and for each session separately [i.e. 1 week after sham/dual-transcranial DCS; t(18) = 2.13]. The damaged hemisphere is on the right. Easy and Replay conditions evoked more consistent activation than the Learning condition. In fact, during Easy, the patients performed similar and consistent movements, associated with a larger amount of consistent brain activation. During Replay, as no movement was performed, the blood oxygen level-dependent signal observed was minimally contaminated by motion artefacts and resulted thus in higher levels of brain activation. tDCS = transcranial DCS.
With the first random effect (two sessions together), significant activation was found for [LEARNING] [at q(FDR) = 0.05, t(37) = 2.86] in SMAundamH, M1damH, bilateral PMd, S1 damH, dorsolateral PFCundamH, and bilaterally in the PPC, frontal cortex, putamen, visual areas and cerebellar hemispheres; for [EASY] [q(FDR) = 0.05, t(37) = 2.93], in M1damH and bilaterally in the SMA, PMd, S1, PPC, visual areas and cerebellar hemispheres; for [REPLAY] [at q(FDR) = 0.05, t(37) = 3.86] in dorsolateral PFCundamH and bilaterally in PMd, PPC, frontal cortex; putamen, visual areas in bilateral SMA, pre-SMAundamH, M1damH, PMd damH, S1 damH, and PPC damH; and finally for [LEARNING – REPLAY] [at q(FDR) = 0.05, t(37) = 2.922], in bilateral SMA, pre-SMAundamH, M1damH, PMd damH, S1 damH, and PPC damH.
The visual inspection of the separate random effect on Fig. 3 also confirmed that brain activation was both less bilateral and more restricted spatially in the motor/premotor network 1 week after dual-transcranial DCS compared to sham (Table 2). As demonstrated by the ANOVA, the activation pattern seemed to be similar between the two sessions for [REPLAY].
Table 2.
Activated areas with [LEARNING – REPLAY] and [EASY] for the two separate random effect analyses
| BA | Mean x | Mean y | Mean z | mm3 | Activation peak (t) | |
|---|---|---|---|---|---|---|
| Dual-transcranial DCS [EASY] | ||||||
| SMAdamH | 6 | −1 | −14 | 72 | 9 | 2.64 |
| SMAundamH | 6 | 2 | −16 | 72 | 42 | 2.77 |
| M1damH | 4 | −30 | −27 | 66 | 231 | 3.34 |
| PMddamH | 6 | −27 | −20 | 68 | 84 | 3.04 |
| PMdundamH | 6 | 29 | −11 | 65 | 14 | 2.53 |
| PPCdamH | 7 | −20 | −68 | 49 | 6990 | 5.45 |
| PPCundamH | 7 | 19 | −64 | 47 | 7027 | 4.84 |
| Cerebellum contralateral to paretic hand | −17 | −67 | −21 | 4719 | 3.83 | |
| Cerebellum ipsilateral to paretic hand | 17 | −65 | −21 | 9832 | 4.76 | |
| Visual areasdamH | 18–19 | −31 | −81 | −1 | 16 010 | 4.15 |
| Visual areasundamH | 18–19 | 27 | −79 | −7 | 19 969 | 5.25 |
| Lentiform nucleusdamH | −19 | −6 | 1 | 84 | 3.61 | |
| Sham [EASY] | ||||||
| SMAdamH | 6 | −3 | −13 | 38 | 339 | 3.42 |
| SMAundamH | 6 | 2 | −12 | 69 | 398 | 3.26 |
| pre-SMAundamH | 6 | 2 | −4 | 68 | 13 | 2.53 |
| M1damH | 4 | −32 | −32 | 55 | 2618 | 3.97 |
| M1undamH | 4 | 30 | −39 | 60 | 64 | 2.86 |
| PMddamH | 6 | −30 | −16 | 65 | 901 | 3.75 |
| PMdundamH | 6 | 37 | −6 | 56 | 540 | 3.00 |
| S1damH | 3 | −33 | −39 | 56 | 1755 | 4.03 |
| S1undamH | 3 | 32 | −40 | 60 | 199 | 2.86 |
| PPCdamH | 7 | −23 | −62 | 48 | 13 168 | 6.14 |
| PPCundamH | 7 | 20 | −62 | 47 | 9576 | 4.99 |
| Cerebellum contralateral to paretic hand | −20 | −67 | −22 | 5641 | 3.97 | |
| Cerebellum ipsilateral to paretic hand | 20 | −67 | −22 | 7640 | 4.74 | |
| Visual areasdamH | 18–19 | −33 | −79 | −1 | 18 669 | 5.28 |
| Visual areasundamH | 18–19 | 29 | −77 | −2 | 27 693 | 4.67 |
| Lentiform nucleusundamH | 16 | −5 | 3 | 130 | 3.07 | |
| Dual-transcranial DCS [LEARNING – REPLAY] | ||||||
| SMAdamH | 6 | −3 | −12 | 71 | 88 | 3.47 |
| SMAundamH | 6 | 1 | −14 | 71 | 97 | 3.32 |
| M1damH | 4 | −29 | −24 | 66 | 143 | 3.56 |
| PMddamH | 6 | −26 | −19 | 67 | 251 | 3.84 |
| Sham [LEARNING – REPLAY] | ||||||
| SMAdamH | 6 | −3 | −13 | 68 | 374 | 3.95 |
| SMAundamH | 6 | 2 | −11 | 69 | 257 | 4.01 |
| pre-SMAdamH | 6 | −2 | −4 | 69 | 6 | 2.51 |
| pre-SMAundamH | 6 | 1 | −4 | 69 | 9 | 2.65 |
| M1damH | 4 | −33 | −29 | 59 | 3471 | 4.37 |
| M1undamH | 4 | 32 | −32 | 59 | 71 | 2.45 |
| PMddamH | 6 | −31 | −19 | 63 | 989 | 4.71 |
| PPCdamH | 7 | −32 | −53 | 58 | 15 | 2.43 |
| S1damH | 3 | −37 | −37 | 58 | 952 | 3.69 |
t(18) = 2.13.
BA = Brodmann area; mm3 = number of activated voxels.
Regions of interest analyses
Effect of intervention: regions of interest found with the first random effect
In each region of interest activated during [LEARNING], paired Student t-tests compared the blood oxygen level-dependent (BOLD) activation between the two sessions. Significant differences were observed in PMddamH [dual-transcranial DCS > sham: beta weights: 0.50 ± 0.47 versus 0.13 ± 0.58; t(18) = 3.21; P = 0.005], PMdundamH [sham > dual-transcranial DCS: 0.43 ± 0.34 versus 0.17 ± 0.37; t(18) = 2.29; P = 0.03] and in dorsolateral PFCundamH [sham > dual-transcranial DCS: 0.31 ± 0.52 versus 0.05 ± 0.39; t(18) = 2.95; P = 0.009] (Supplementary Table 2).
For [EASY], significant differences in activation were found in M1damH [sham > dual-transcranial DCS: 0.43 ± 0.31 versus 0.21 ± 0.44; t(18) = 2.29; P = 0.03] and PMddamH [sham > dual-transcranial DCS: 0.53 ± 0.50 versus 0.23 ± 0.54; t(18) = 2.56; P = 0.02].
For [REPLAY], there was no statistically significant difference in BOLD activation between the two sessions in any region of interest.
For [LEARNING-REPLAY], a significant difference in BOLD activation was found exclusively in PMd damH [dual-transcranial DCS > sham: 0.59 ± 0.44 versus 0.19 ± 0.59; t(18) = 3.14; P = 0.006].
Correlations with retention: regions of interest found with the two separate random effect
A week after sham, a significant correlation between the overall mean learning index and mean beta weights of [LEARNING – REPLAY] for each patient was observed exclusively in M1undamH (r = 0.61, P = 0.005). By contrast, 1 week after dual-transcranial DCS, there was a significant correlation only in PMddamH (r = 0.63, P = 0.004). There were no statistically significant correlations between overall mean learning index and mean beta weights of the regions of interest activated with [REPLAY].
Individual contribution of each patient to the main (whole-group) pattern
For each patient and each session separately, the numbers of activated voxels in [EASY], [REPLAY] and [LEARNING – REPLAY] were counted in all the brains, in the right and left hemispheres separately, and in each predefined region of interest with an uncorrected P-value of 0.05 (to reveal all the involved areas).
For [EASY], despite the fact that the kinematic parameters did not differ significantly between the sessions, at the whole-brain level, the number of activated voxels 1 week post-intervention was greater after sham [t(18) = 2.11; P = 0.049; d = 0.47 versus dual-transcranial DCS] (Supplementary Fig. 4). One-week after sham, functional MRI activation in the damaged hemisphere showed a trend of being more widespread than after dual-transcranial DCS [t(18) = 2.02; P = 0.059; d = 0.17], with significant differences between the series (sham > dual-transcranial DCS) in the SMAdamH proper [t(18) = 2.47; P = 0.023; d = 0.66], M1damH [t(18) = 2.56; P = 0.019; d = 0.65], PMddamH [t(18) = 2.38; P = 0.028; d = 0.70]. Meanwhile, functional MRI activation in the undamaged hemisphere was significantly more widespread after sham than after dual-transcranial DCS [t(18) = 2.11; P = 0.048; d = 0.13]; in the SMAundamH proper [t(18) = 2.58; P = 0.019; d = 0.73], M1undamH [t(18) = 2.55; P = 0.020; d = 0.44], and PMdundamH [t(18) = 2.12; P = 0.047; d = 0.52] (Supplementary Fig. 4). There were no significant differences in the other regions of interest.
With [REPLAY], the number of activated voxels did not significantly differ between the two functional MRI sessions.
As shown in Supplementary Fig. 5, with [LEARNING – REPLAY] the number of activated voxels at the whole-brain level was greater 1 week after the sham intervention than after dual-transcranial DCS [t(18) = 2.48; P = 0.02; d = 0.59]. In the damaged hemisphere, functional MRI activation was more widespread after the sham intervention than after dual-transcranial DCS [t(18) = 2.87; P = 0.01; d = 0.99], with the following specific differences (regions of interest comparison): SMAdamH proper [t(18) = 2.47; P = 0.02; d = 0.70], pre-SMAdamH [t(18) = 2.34;P = 0.03; d = 0.74], PMddamH [t(18) = 3.60; P = 0.002; d = 0.79], M1damH [t(18) = 3.22; P = 0.004; d = 0.88] and S1damH [t(18) = 2.76; P = 0.01; d = 0.73]. In the undamaged hemisphere, there was a non-significant trend for greater activation 1 week after the sham intervention than after dual-transcranial DCS [t(18) = 1.76; P = 0.09; d = 0.63], driven mainly by a significantly larger activation in the SMAundamH proper [t(18) = 2.17; P = 0.04; d = 0.68] and a trend in the pre-SMAundamH [t(18) = 2.02; P = 0.058; d = 0.61] (Supplementary Fig. 5). There were no significant differences in the other regions of interest between the two interventions.
Continued learning
Only the patients who achieved continued learning 1 week after intervention were included in this analysis separately for each session (dual-transcranial DCS n = 16, sham n = 13) (Supplementary material).
An ANOVA exploring the effects of stimulation and time (Blocks 1–8) on the learning index evolution during the continued learning period showed a significant effect of stimulation [F(1) = 6.47; P = 0.01; dual-transcranial DCS versus sham], demonstrating that dual-transcranial DCS enabled patients to achieve greater continued motor skill learning, compared to the sham intervention, and a significant effect of time [F(7) = 4.63; P < 0.001], showing that the cohort of stroke patients improved generally in both series. There was no Stimulation × Time interaction.
Conjunction analysis
The conjunction analysis ([LEARNING – REPLAY] ∩ [LEARNING – EASY]) demonstrated that 1 week after sham, a widespread network including the bilateral M1, S1 and the parietal cortex was implied in continued learning (Fig. 4 and Table 3). In contrast, 1 week after dual-transcranial DCS, significant brain activation was observed exclusively in PMddamH.
Figure 4.
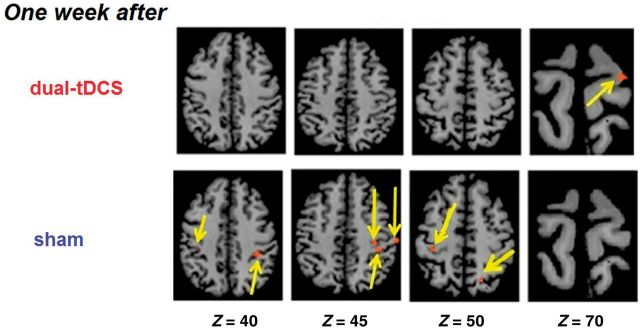
Continued motor skill learning. Group activation with the conjunction analysis [LEARNING − REPLAY] ∩ [LEARNING − EASY] for the patients who achieved successful continued motor skill learning 1 week after dual-transcranial DCS [n = 16, t(15) = 2.23] and after sham [n = 13, t(12) = 2.23), random effect; Puncorrected < 0.05. The damaged hemisphere is on the right. tDCS = transcranial DCS.
Table 3.
Continued learning: conjunction analysis
| BA | Mean x | Mean y | Mean z | mm3 | |
|---|---|---|---|---|---|
| Dual-transcranial DCS | |||||
| PMddamH | 6 | −22 | −18 | 70 | 19 |
| Sham | |||||
| M1damH | 4 | −33 | −33 | 44 | 34 |
| M1undamH | 4 | 32 | −28 | 38 | 23 |
| PPCdamH | 7 | −17 | −62 | 48 | 33 |
| PPCundamH | 7 | 14 | −68 | 36 | 5 |
| S1damH | 3 | −35 | −34 | 46 | 28 |
| S1undamH | 3 | 38 | −26 | 41 | 4 |
| IPCdamH | 40 | −44 | −30 | 26 | 292 |
t = 2.23.
BA = Brodmann area; IPC = inferior parietal cortex; mm3 = number of activated voxels.
Correlation analysis
Correlation analyses between the learning index or performance index after sham intervention and beta weights of the activated regions of interest showed a statistically significant positive correlation in the M1damH [learning index: r = 0.81, P = 0.01; performance index: r = 0.74, P = 0.04], M1undamH [learning index: r = 0.84, P < 0.01; performance index: r = 0.82, P = 0.01], PPC damH [learning index: r = 0.89, P < 0.01, performance index: r = 0.85, P < 0.01], PPCundamH [learning index: r = 0.75 P = 0.03, performance index: r = 0.64, P = 0.09], inferior parietal cortexdamH [learning index: r = 0.85, P < 0.01, performance index: r = 0.85, P < 0.01], S1damH [learning index: r = 0.73, P = 0.04, performance index: r = 0.69, P = 0.05]. By contrast, a week after dual-transcranial DCS, a statistically significant positive correlation between the learning index or performance index and the beta weights of the activated regions of interest was found exclusively for PMddamH [learning index: r = 0.72, P = 0.04, performance index: r = 0.81, P = 0.02].
Discussion
The present study showed that, compared to the sham procedure, dual-transcranial DCS (i) enhanced online motor skill learning with the paretic upper limb in chronic hemiparetic stroke patients; (ii) induced a transfer of performance improvement to an untrained task; and (iii) translated online improvement more successfully into long-term retention of the motor skill.
In the dual-transcranial DCS series, the enhanced retention of the motor skill learned 1 week prior was associated with lesser activation in both hemispheres compared to the sham series, especially in the premotor/motor areas of the damaged hemisphere (e.g. in PMddamH).
The kinematic parameters of simple untrained movements with the paretic limb (without a SAT constraint) 1 week after the intervention did not differ between the sham and dual-transcranial DCS interventions. However, functional MRI activation was strikingly focused (i.e. less widespread) after dual-transcranial DCS, especially in the premotor/motor areas of both hemispheres.
Finally, compared to the sham intervention, dual-transcranial DCS applied during a first session of motor skill learning enhanced continued learning with the paretic upper limb 1 week later. This lasting behavioural improvement was associated with more efficient recruitment (see below) of the motor skill learning network after dual-transcranial DCS—that is, a focusing of activity within the motor-premotor areas of the damaged hemisphere, especially PMddamH.
Neural substrates underlying the retention of dual-transcranial DCS enhanced motor skill performance
This study confirms, in a new cohort of stroke patients, our previous observation that dual-transcranial DCS enhances not only online motor skill learning with the paretic upper limb, but also improves long-term retention of the learned motor skill (Lefebvre et al., 2013a). The retention level of the learned motor skill was strikingly comparable across the two studies: mean learning index values 1 week after the sham intervention were 12% in the present study (n = 19) and 6% in the previous study (n = 18), and the analogous mean learning index values after dual-transcranial DCS were 52% and 51%, respectively. Thus, behaviourally, there was no major impact of the experimental modifications specific to the current study (e.g. supine position without direct visual feedback of the paretic hand, in the scanner environment during the retention session, and the addition of the Replay and Easy conditions).
It is worth noting that the brain activation related to visual-visuomotor processes (Replay) 1 week after the interventions was similar in both series and did not correlate with skill performance. Thus, the Replay condition did not participate directly in motor skill retention (e.g. through rehearsal or by acting with additional feedback), which justifies its subtraction from the activation data during motor skill performance (Learning).
The less efficient 1-week retention of the learned motor skill after the sham intervention was associated with extensive recruitment of both hemispheres and prominent involvement of M1undamH; which might be interpreted as compensatory based on the positive correlation with motor skill retention. By contrast, the highly focused functional MRI pattern observed after dual-transcranial DCS tended towards normalization with lesser activation in both hemispheres. It is particularly interesting to note that for PMddamH, the ANOVA and the predefined regions of interest analysis showed more widespread activation 1 week after sham compared to dual-transcranial DCS whereas the other regions of interest analyses revealed another activation peak 23 mm away in PMddamH, which activation was more intense 1 week after dual-transcranial DCS and correlated with retention, suggesting a key role for PMddamH in the efficient long-term retention of motor skills learned with a paretic limb. This is the first study to unveil specific functional MRI activation supporting long-term retention of a motor skill learned with dual-transcranial DCS facilitation. As dual-transcranial DCS was applied 1 week before the functional MRI retention session, the brain activation patterns cannot be attributed to neuronal or vascular after-effects of transcranial DCS (Stagg et al., 2012). Several hypotheses may explain this overall lesser activation found 1 week after dual-transcranial DCS compared to sham such as diminished need for sensory feedback processing, online error correction and/or attentional resources once the skill is learned, or enhanced neural efficiency, but the present experiment was not designed to explore this issue. Whichever the precise mechanisms, the combination of motor skill learning and dual-transcranial DCS in the present study durably shaped the activation of the motor network for at least 1 week as this combination resulted in lesser bilateral functional MRI activation supporting superior motor skill retention compared to sham.
Regarding the possible mechanisms underlying this enhancement, it is important to consider that transcranial DCS can modulate neuronal membrane excitability and ongoing neuronal firing rate, alter glutamatergic and GABA concentrations, and finally lead to long-term modifications of synaptic strength in the motor cortex, which may be particularly important in the context of motor learning (Stagg and Nitsche, 2011). First, dual-transcranial DCS may have normalized deregulated interhemispheric interactions, which interfere with paretic hand performance and can be corrected by transcranial DCS (Murase et al., 2004; Bolognini et al., 2011). Second, it is unlikely that the effects of dual-transcranial DCS were focused under the electrodes. Rather, direct current had likely spread and could have activated a much larger network, resulting in changes beyond the effects of anodal transcranial DCS on M1damH and of cathodal transcranial DCS on M1undamH (Lindenberg et al., 2013; Sehm et al., 2013). Third, because the current’s direction is different in the dual M1-M1 montage compared with the ‘classical’ montage (one electrode over M1 and the other over the prefrontal cortex on the contralateral forehead), other cortical neuronal populations may have been modulated by dual-transcranial DCS, driving the observed effects. Recently, O’Shea et al. (2004) demonstrated that the classical montage was superior to dual-transcranial DCS for (i) modulating corticospinal excitability in healthy individuals; and (ii) enhancing simple reaction time in chronic stroke patients. However, applying dual-transcranial DCS in chronic stroke patients (Lindenberg et al., 2010; Bolognini et al., 2011) enhanced neurorehabilitation compared to sham. Similarly, we already demonstrated enhanced fine motor control of the paretic hand (Lefebvre et al., 2013b) as well as online motor skill learning and 1-week retention (Lefebvre et al., 2013a). From these findings, dual-transcranial DCS appears to be efficient in chronic stroke patients. Furthermore, Waters-Metenier et al. (2014) recently demonstrated that dual-transcranial DCS was also very efficient to enhance complex motor skill learning in healthy individuals, including generalization. The apparent discrepancy between these results and those by O’Shea et al. (2004) might be attributed to several factors: (i) difference in task complexity and motor engagement (simple reaction time cannot be compared with complex motor skill learning or neurorehabilitation training); (ii) difference in transcranial DCS parameters; (iii) overlooked effect of the direct current flowing in the prefrontal cortex in the classical montage; and (iv) potential—yet to be explored—mechanisms specific to dual-transcranial DCS. Future studies should compare the impact of dual and classical transcranial DCS montages on complex tasks and neurorehabilitation.
Behavioural and neurophysiological transfers 1 week post-intervention
We observed two types of transfer: (i) a behavioural improvement on an untrained dexterity task; and (ii) an improvement of the functional MRI pattern underlying simple movements even in the absence of a performance difference. First, the improvement of the learned motor skill facilitated by dual-transcranial DCS transferred to improvement of general paretic hand’s dexterity as evidence by performance in an untrained motor task (the Purdue Pegboard Test). The former type of behavioural transfer following NIBS has been associated with increased functional MRI activation in the damaged hemisphere during performance of a generic (untrained) motor task in previous studies (Lindenberg et al., 2010; Nair et al., 2011; Yamada et al., 2013). Such a transfer of behavioural enhancement to an untrained dexterity task 1 week after dual-transcranial DCS is very promising for neurorehabilitation. Motor skill learning boosted by dual-transcranial DCS could reshape activity in the motor system enduringly and lead to more efficient recruitment of neural resources.
In the latter form of transfer, a striking change of brain activation pattern was observed in the absence of behavioural difference. During the retention functional MRI sessions, the stroke patients performed simple untrained movements with the paretic upper limb, without a SAT constraint (Easy). One week after the intervention, the kinematic parameters of these simple back and forth movements did not differ between the sham and dual-transcranial DCS series. However, after the sham intervention, activation was more widespread (and thus likely less efficient), especially in the premotor-motor areas, compared to that observed in the dual-transcranial DCS series. As the Easy condition was interleaved with the performance of the learned motor skill, we cannot conclude whether this activation pattern change (i.e. a much focused activation pattern in the absence of a behavioural difference) was independent of the practice of the learned motor skill. Two different, but equally interesting, mechanisms could explain this observation. The most optimistic interpretation is that learning a complex visuomotor skill with concurrent dual-transcranial DCS shapes the motor system in such an efficient and lasting way that, subsequently, even simple and untrained movements are performed with a less widespread activation pattern, suggesting lesser neural activation. The most restrictive interpretation would be that reperforming the motor skill acquired with dual-transcranial DCS facilitation primes the current activity of the motor system and enhances its efficiency, even for untrained movements. Either mechanism is promising but entails different implications for implementation in neurorehabilitation. It has to be acknowledged that these interpretations are speculative and that more work is needed before implementing transcranial DCS in routine clinical practice, determining whether patients with a cortical or subcortical stroke would equally respond, etc.
Neural substrates underlying the enhancement of continued motor skill learning after intervention
This study is the first to explore continued motor skill learning 1 week after NIBS. One week after the sham intervention, six of the hemiparetic stroke patients did not achieve continued learning (non-learners). In striking contrast, 1 week after dual-transcranial DCS, there were only three non-learners. The amount of continued motor skill learning was superior 1 week after dual-transcranial DCS compared to that of the sham intervention, although the rate of continued motor skill learning did not differ. Hence, the advantage yielded by dual-transcranial DCS since the first block of motor skill learning persisted 1 week later, which is obviously appealing for neurorehabilitation. For example, if applying dual-transcranial DCS during motor skill learning on Monday could enhance neurorehabilitation and continued skill learning for the rest of the week, then weekly transcranial DCS treatments would be easier to organize than daily sessions. However, it would first need to be demonstrated that such a ‘dual-transcranial DCS priming’ regimen is as effective as daily sessions during motor skill learning/neurorehabilitation.
This study is also the first to explore the neural substrates supporting continued motor skill learning after NIBS in stroke patients. In sharp contrast to the widely distributed activation observed after the sham intervention, 1 week after dual-transcranial DCS, efficient continued motor skill learning was supported by a less widespread network focused on the damaged hemisphere, which resembled the activation pattern observed in healthy individuals (i.e. M1damH, SMAdamH, PMddamH, and the contralesional cerebellum). Moreover, a significant correlation between activation and performance was found exclusively in PMddamH 1 week after dual-transcranial DCS, compared to the numerous significant correlations observed after sham (M1damH, M1undamH, PPC damH, PPCundamH, IPCdamH, and S1damH). These differences suggest that the less efficient continued motor skill learning after the sham intervention required a larger amount of bilateral neural resources. As the after-effects of dual-transcranial DCS are unlikely to persist for an entire week, such long-lasting enhancements suggest that a (durable) modification of synaptic and neural activity had consolidated in the motor network after dual-transcranial DCS. One can thus safely infer that it is precisely this lasting enhancement of brain activation (i.e. the trend towards normalization of the functional MRI pattern and recruitment of PMddamH), which supported more efficient continued learning 1 week after dual-transcranial DCS.
Limitations
The current experiment has several limitations. First, the sample of patients with hemiparetic chronic stroke was relatively heterogeneous, as in several other recent studies (O’Shea et al., 2004; Lindenberg et al., 2010; Bradnam et al., 2012; Stagg et al., 2012). However, we think this apparent weakness might be considered as strength (generalization) when considering the implementation of transcranial DCS in clinical neurorehabilitation settings with a diversity of stroke patients.
Second, before implementing NIBS in standard neurorehabilitation, larger multi-centre trials should be performed. The number of stroke patients recruited in the current experiment compares fairly with previous studies.
Third, it has to be acknowledged that the subgroups of patients who achieved continued learning 1 week post-intervention were not identical between the dual-transcranial DCS and sham series. Indeed, some patients could not achieve continued motor skill learning and were thus excluded from the analysis as non-learners (three after dual-transcranial DCS, six after the sham intervention). Despite this limitation, the striking focusing of functional MRI activation during continued motor skill learning after dual-transcranial DCS suggests that the more efficient recruitment of neural resources lasted at least 1 week.
Fourth, a previous study reported that transcranial DCS targeting M1 exclusively in the damaged (anodal) or undamaged (cathodal) hemisphere improved simple reaction time task in chronic stroke patients whereas dual-transcranial DCS failed to do so (O’Shea et al. 2004). Furthermore, cathodal transcranial DCS over the undamaged hemisphere can worsen residual function of the paretic upper limb in severely impaired stroke patients (Bradnam et al., 2012). In contrast to these experiments that used relatively simple motor tasks, in the current experiment and in previous ones (Lefebvre et al., 2013a, b), we used challenging motor tasks and found consistent enhancement of digital dexterity and motor skill learning with dual-transcranial DCS in a large number of chronic stroke patients with mild to moderate hemiparesis (modified Rankin Scale 0–4, NIHSS 0–7), with worsening of neither the paretic nor non-paretic upper limb. Future studies shall aim to compare different transcranial DCS protocols in stroke patients formally using challenging motor tasks and to identify surrogate markers of responsiveness, such as markers based on the lesion burden of the corticospinal tract (Zhu et al., 2010; Rosso et al., 2013) or magnetic resonance spectroscopy (O’Shea et al. 2004).
Fifth, this study has several statistical limitations. The thresholds used for the functional MRI analyses were voluntarily liberal (P uncorrected < 0.05). This is because of several factors intrinsic to this type of study in stroke patients. High variability derives from the recruitment of a heterogeneous patient’s cohort with different lesions along the corticospinal tract and a residual motor function ranging from good to moderate. The high variability due to the lesions location and extend also induced variability in the blood oxygen level-dependent response explaining the choice of the liberal threshold to explore the data. Nevertheless, we performed a random-effect group analysis at the whole-brain level to isolate all the areas we described. This allows a generalization of our observations to the majority of chronic hemiparetic stroke patients. In addition, the whole-brain ANOVA unambiguously demonstrated that there were differences between the retention sessions after dual-transcranial DCS compared to sham for the Easy and Learning conditions. Finally, we used the regions of interest analyses to explore the individual contribution of each patient to the whole-group pattern and to perform correlation analyses with behaviour, showing highly consistent data across all types of analyses.
General conclusion
The combination of motor skill learning and dual-transcranial DCS resulted in lasting enhancements of paretic upper limb function in chronic stroke patients, both in the form of improvement specific to the learned motor skill benefit and of generic enhancement (transfer). The enhancement specific to motor skill learning was supported by a (relative) normalization of the brain activation 1 week after dual-transcranial DCS (i.e. compared to sham, less activation in the undamaged hemisphere and a focusing in the damaged hemisphere, especially in PMddamH). Thus, dual-transcranial DCS combined with motor skill learning gave rise to a durable modification of brain activation pattern in stroke patients, which resulted in enhanced retention and continued motor skill learning.
The generic enhancement driven by dual-transcranial DCS benefitted both dexterity of the paretic hand on an untrained task (behavioural transfer) and less widespread brain activation pattern when performing simple, untrained movements with the paretic limb. It remains an open question as to whether these generic enhancements resulted from a lasting shaping of brain activation or from a priming of the motor system after performing the motor skill learned 1 week before during dual-transcranial DCS. Both interpretations are promising for neurorehabilitation but imply different approaches. Overall, the functional MRI patterns observed 1 week after the intervention tended towards a normalization of brain activation and an apparently adaptive recruitment of PMddamH, suggesting that dual-transcranial DCS combined with motor skill learning induced a prolonged shaping of brain activation.
Acknowledgements
We thank the stroke patients who participated in this experiment and their families as well, the collaborators from Siemens (Roger Demeure, Dimitri Haye, Jean-Pierre Bastin and Patrick Caluwaerts), and the radiology team from the CHU Dinant-Godinne UcL Namur for flexibility and assistance. We are grateful to Dr J-J. Orban de Xivry and Pr Ph. Lefèvre for helpful discussion and comments about the study design. We thank G. Tincani (Hopital Saint Joseph, CHC, Liège) and A. Findik (Cliniques Universitaires Saint-Luc UcL, Brussels) for their help with patients screening and recruitment. The authors are grateful to Professor Martin G. Edwards (Psychological Sciences Research Institute (IPSY)/IoNS, UcL) for careful re-reading of the revised manuscript.
Glossary
Abbreviations
- damH
damaged hemisphere
- DCS
direct current stimulation
- MaxHF
maximal hand force
- NIBS
non-invasive brain stimulation
- PMd
dorsal premotor cortex
- PFC
prefrontal cortex
- PPC
posterior parietal cortex
- SAT
speed/accuracy trade-off
- SMA
supplementary motor area
- undamH
undamaged hemisphere
Funding
The work of Y.V. was supported by the following grants: Fonds de la Recherche Scientifique Médicale (FRSM) 3.4.525.08.F in 2008, 2010 & 2012, Fonds Spécial de Recherche (FSR) grant from the Université Catholique de Louvain (UcL) in 2008 and 2010, and Fondation Van Goethem-Brichant. The work of S.L. was supported by UcL FSR grants in 2008 and 2010, and by a grant from the Fondation Mont-Godinne 2012. The work of L.D. was supported by a grant from the Fondation LOUVAIN, UcL.
Supplementary material
Supplementary material is available at Brain online.
References
- Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25:123–9. [PubMed] [Google Scholar]
- Bolognini N, Vallar G, Casati C, Latif LA, El-Nazer R, Williams J, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair. 2011;25:819–29. doi: 10.1177/1545968311411056. [DOI] [PubMed] [Google Scholar]
- Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke. 1988;19:1497–500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]
- Bosnell RA, Kincses T, Stagg CJ, Tomassini V, Kischka U, Jbabdi S, et al. Motor practice promotes increased activity in brain regions structurally disconnected after subcortical stroke. Neurorehabil Neural Repair. 2011;25:607–16. doi: 10.1177/1545968311405675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd LA, Vidoni ED, Wessel BD. Motor learning after stroke: is skill acquisition a prerequisite for contralesional neuroplastic change? Neurosci Lett. 2010;482:21–5. doi: 10.1016/j.neulet.2010.06.082. [DOI] [PubMed] [Google Scholar]
- Bradnam LV, Stinear CM, Barber PA, Byblow WD. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb Cortex. 2012;22:2662–71. doi: 10.1093/cercor/bhr344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimmi M, Carda S, Giovanzana C, Maini ES, Sabatini AM, Smania N, et al. Using kinematic analysis to evaluate constraint-induced movement therapy in chronic stroke patients. Neurorehabil Neural Repair. 2008;22:31–9. doi: 10.1177/1545968307302923. [DOI] [PubMed] [Google Scholar]
- Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, et al. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–88. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Buch ER. Effects of Parkinson's disease on visuomotor adaptation. Exp Brain Res. 2003;150:25–32. doi: 10.1007/s00221-003-1403-y. [DOI] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–54. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipietro L, Krebs HI, Volpe BT, Stein J, Bever C, Mernoff ST, et al. Learning, not adaptation, characterizes stroke motor recovery: evidence from kinematic changes induced by robot-assisted therapy in trained and untrained task in the same workspace. IEEE Trans Neural Syst Rehabil Eng. 2012;20:48–57. doi: 10.1109/TNSRE.2011.2175008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsband U, Lange RK. Motor learning in man: a review of functional and clinical studies. J Physiol Paris. 2006;99:414–24. doi: 10.1016/j.jphysparis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage. 2013;67:283–97. doi: 10.1016/j.neuroimage.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708–12. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Voller B, Celnik P, Floel A, Giraux P, Gerloff C, et al. Effects of brain polarization on reaction times and pinch force in chronic stroke. BMC Neurosci. 2006;7:73. doi: 10.1186/1471-2202-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–8. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5:603–12. doi: 10.1016/S1474-4422(06)70495-1. [DOI] [PubMed] [Google Scholar]
- Kasner SE, Chalela JA, Luciano JM, Cucchiara BL, Raps EC, Mcgarvey ML, et al. Reliability and validity of estimating the NIH stroke scale score from medical records. Stroke. 1999;30:1534–7. doi: 10.1161/01.str.30.8.1534. [DOI] [PubMed] [Google Scholar]
- Kitago T, Krakauer JW. Motor learning principles for neurorehabilitation. Handb Clin Neurol. 2013;110:93–103. doi: 10.1016/B978-0-444-52901-5.00008-3. [DOI] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Mazzoni P. Human sensorimotor learning: adaptation, skill, and beyond. Curr Opin Neurobiol. 2011;21:636–44. doi: 10.1016/j.conb.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Kollen BJ, Van Der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–6. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- Lai SM, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the stroke impact scale. Stroke. 2002;33:1840–4. doi: 10.1161/01.str.0000019289.15440.f2. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Dricot L, Gradkowski W, Laloux P, Vandermeeren Y. Brain activations underlying different patterns of performance improvement during early motor skill learning. Neuroimage. 2012;62:290–9. doi: 10.1016/j.neuroimage.2012.04.052. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Laloux P, Peeters A, Desfontaines P, Jamart J, Vandermeeren Y. Dual-tDCS enhances online motor skill learning and long-term retention in chronic stroke patients. Front Human Neurosci. 2013a;6:343. doi: 10.3389/fnhum.2012.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Thonnard JL, Laloux P, Peeters A, Jamart J, Vandermeeren Y. Single session of dual-tDCS transiently improves precision grip and dexterity of the paretic hand after stroke. Neurorehabil Neural Repair. 2013b;28:100–10. doi: 10.1177/1545968313478485. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Nachtigall L, Meinzer M, Sieg MM, Floel A. Differential effects of dual and unihemispheric motor cortex stimulation in older adults. J Neurosci. 2013;33:9176–83. doi: 10.1523/JNEUROSCI.0055-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75:2176–84. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan S, Shah B. Enhancing motor skill learning with transcranial direct current stimulation - a concise review with applications to stroke. Front Psychiatry. 2012;3:66. doi: 10.3389/fpsyt.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J, Paxinos G, Voss T. Atlas of the Human Brain. 3rd edn 2007. [Google Scholar]
- Matthews PM, Johansen-Berg H, Reddy H. Non-invasive mapping of brain functions and brain recovery: applying lessons from cognitive neuroscience to neurorehabilitation. Restor Neurol Neurosci. 2004;22:245–60. [PubMed] [Google Scholar]
- Meehan SK, Dao E, Linsdell MA, Boyd LA. Continuous theta burst stimulation over the contralesional sensory and motor cortex enhances motor learning post-stroke. Neurosci Lett. 2011a;500:26–30. doi: 10.1016/j.neulet.2011.05.237. [DOI] [PubMed] [Google Scholar]
- Meehan SK, Randhawa B, Wessel B, Boyd LA. Implicit sequence-specific motor learning after subcortical stroke is associated with increased prefrontal brain activations: an fMRI study. Hum Brain Mapp. 2011b;32:290–303. doi: 10.1002/hbm.21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–9. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nair DG, Renga V, Lindenberg R, Zhu L, Schlaug G. Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tDCS. Restor Neurol Neurosci. 2011;29:411–20. doi: 10.3233/RNN-2011-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'shea J, Boudrias MH, Stagg CJ, Bachtiar V, Kischka U, Blicher JU, et al. Predicting behavioural response to TDCS in chronic motor stroke. Neuroimage. 2014;85(Pt 3):924–33. doi: 10.1016/j.neuroimage.2013.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penta M, Tesio L, Arnould C, Zancan A, Thonnard JL. The ABILHAND questionnaire as a measure of manual ability in chronic stroke patients: rasch-based validation and relationship to upper limb impairment. Stroke. 2001;32:1627–34. doi: 10.1161/01.str.32.7.1627. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–72. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106:1590–5. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso C, Valabregue R, Attal Y, Vargas P, Gaudron M, Baronnet F, et al. Contribution of corticospinal tract and functional connectivity in hand motor impairment after stroke. PLoS One. 2013;8:e73164. doi: 10.1371/journal.pone.0073164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehm B, Kipping J, Schafer A, Villringer A, Ragert PA. Comparison between Uni- and bilateral tDCS effects on functional connectivity of the human motor cortex. Front Hum Neurosci. 2013;7:183. doi: 10.3389/fnhum.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, O'shea J, Allman C, Bosnell RA, Kischka U, et al. Cortical activation changes underlying stimulation-induced behavioural gains in chronic stroke. Brain. 2012;135:276–84. doi: 10.1093/brain/awr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Talairach JTP. Co-planar Stereotaxic Atlas of the Human Brain. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Stuttgart: Thieme Verlag; 1988. [Google Scholar]
- Tiffin J, Asher EJ. The purdue pegboard; norms and studies of reliability and validity. J Appl Psychol. 1948;32:234–47. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects' non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008;9:103. doi: 10.1186/1471-2202-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters-Metenier S, Husain M, Wiestler T, Diedrichsen J. Bihemispheric transcranial direct current stimulation enhances effector-independent representations of motor synergy and sequence learning. J Neurosci. 2014;34:1037–50. doi: 10.1523/JNEUROSCI.2282-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Kakuda W, Kondo T, Shimizu M, Mitani S, Abo M. Bihemispheric repetitive transcranial magnetic stimulation combined with intensive occupational therapy for upper limb hemiparesis after stroke: a preliminary study. Int J Rehabil Res. 2013;36:323–9. doi: 10.1097/MRR.0b013e3283624907. [DOI] [PubMed] [Google Scholar]
- Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41:910–5. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimerman M, Heise KF, Hoppe J, Cohen LG, Gerloff C, Hummel FC. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke. 2012;43:2185–91. doi: 10.1161/STROKEAHA.111.645382. [DOI] [PMC free article] [PubMed] [Google Scholar]


