Abstract
Fatty acid synthase (FASN) is the enzyme that synthesizes fatty acids de novo in human cells. Although FASN is generally expressed at low levels in most normal tissues, its expression is highly upregulated in many cancers. Consistent with this notion, inhibition of FASN activity has demonstrated potential to halt proliferation and induce cell death in vitro and to block tumor growth in vivo. Consequently, FASN is widely recognized as a valuable therapeutic target. In this report, we describe a variety of 1,4-quinones and 9,10-anthraquinones, including several natural compounds and some newly synthesized compounds, that potently inhibit the thioesterase (TE) domain of FASN. Inhibition of recombinant TE activity, inhibition of cellular FASN, and cytotoxicity in human prostate cancer cell lines and normal fibroblasts, is shown for the most potent inhibitors. Collectively, the data illustrate the novel inhibitory capacity of the 1,4-quinone and 9,10-anthraquinone pharmacophores against FASN.
Keywords: fatty acid synthase, thioesterase, chemotherapy
Fatty acid synthase (FASN) is the enzyme that synthesizes fatty acids in mammalian cells. FASN is a multifunctional protein comprised of seven functional domains. Over expression of FASN is a common characteristic of most tumors, and FASN expression levels correlate with disease progression.1) Accordingly, FASN activity is essential for the survival of cancer cells and tumor growth. Because this enzyme is absent in most normal adult tissues, there may be an opportunity to achieve a high therapeutic index with FASN-directed therapeutics that may be devoid of side effects typically associated with conventional anti-cancer agents. The FDA-approved drug Orlistat inhibits the thioesterase (TE) domain of FASN and can kill cancer cells in cell culture and xenograft models.2) However, Orlistat suffers from poor solubility and systemic bioavailability, and thus is not suitable as a cancer therapeutic in the current formulation.
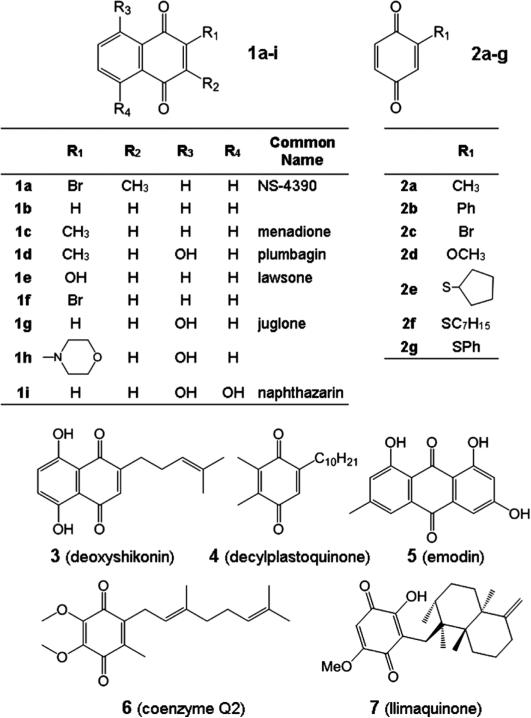
In an effort to identify novel discovery leads, a library of 8800 compound library (Nanosyn, Inc., U.S.A.) was screened using the recombinant FASN-TE domain in an assay containing 10 μm of each compound and 4-methylumbelliferyl heptanoate as the substrate.3,4) Among the compounds that inhibited FASN-TE was 2-bromo-3-methyl-1,4-benzoquinone (NS-4390, 1a) (Fig. 1). Interestingly, the 1,4-dione moiety is found in a wide range of bioactive molecules in the animal, fungal and plant kingdoms—the vitamin K family being a notable example.5,6) These compounds can modulate cellular redox pathways and exhibit a broad range of biological activities.7) Salient to this manuscript are recent investigations into the cytotoxic and anti-proliferative properties of compounds containing 1,4-benzoquinone (common name) moiety.8–13) To the best of our knowledge, inhibition of the TE domain of FASN has not been postulated as a mechanism for the ability of this class of compounds to inhibit tumor growth.
Fig. 1.
1,4- and 9,10-Diones Tested against Recombinant FASN-TE and FASN
An analysis of naturally occurring, commercially available, and new synthetic 1,4-benzoquinones, 1,4-naphthoquinones, 9,10-anthraquinones (Fig. 1, Chart 1) was performed to develop structure–activity relationship (SAR) information for FASN-TE at an initial concentration of 10 μm. Included are the naturally occurring compounds menadione (1c), plumbagin (1d), lawsone (1e), juglone (1g), and naphthazarin (1i). The 1,4-naphthoquinones (1a–i) and 1,4-benzoquinones (2a–g) (Table 1) display potent inhibition of FASN-TE, except for compound 1e. In contrast, the more complex naturally occurring compounds (3–7) inhibit with less potency (Table 1). The 1,4-quinones with known anti-cancer activity like mitomycin C, doxorubicin, geldanamycin, streptonigrin and mitoxantone did not inhibit; lapachol and β-lapachone exhibited weak inhibition potency (data not shown). All of the 1,4-benzoquinones (2a–c, 2e–g) with the exception of 2d show complete inhibition (Table 1) of FASN-TE at 10 μm, but because of their high level of chemical reactivity were not assessed further with TE or in cellular assays.
Chart 1.
Table 1.
Effect of 1,4- and 9,10-Diones on FASN-TE, Fatty Acid Synthesis and Cell Killing
| Cmpd | rTEa)) % Inhibition at 10 μm | rTE IC50 (μM) | Inhibition of FA synthesis IC50 (μM) |
Cell killing EC50 (μM) |
||
|---|---|---|---|---|---|---|
| PC-3 | FS4 | PC-3 | DU145 | |||
| 1a | 78 | 12.5 | 10 | 9.4 | 11.8 | 19.2 |
| 1b | 100 | 0.9 | 2.8 | 10.6 | 12.8 | 6.8 |
| 1c | 54 | 21.9 | — b)) | 16.7 | 48.4 | — b)) |
| 1d | 59 | 10.9 | 2.4 | 2.3 | 4.1 | 5.3 |
| 1e | 10 | >100 | — b)) | >50 | >100 | — b)) |
| 1f | 100 | 0.3 | 11.5 | 21 | 23.3 | 35.4 |
| 1g | 100 | 0.1 | 6.8 | 6.3 | 5.8 | 10.6 |
| 1h | 26 | 26.4 | — | — b)) | — b)) | — b)) |
| 1i | 98 | 3.5 | 2.6 | 2.4 | 2.7 | 4.8 |
| 2a | 100 | — b)) | — b)) | — b)) | — b)) | — b)) |
| 2b | 100 | — b)) | — b)) | — b)) | — b)) | — b)) |
| 2c | 100 | — b)) | — b)) | — b)) | — b)) | — b)) |
| 2d | 66 | — b)) | — b)) | — b)) | — b)) | — b)) |
| 2e | 99 | 6.4 | >25 μM | — b)) | — b)) | — b)) |
| 2f | 96 | 6.2 | >25 μM | — b)) | — b)) | — b)) |
| 2g | 100 | 0.2 | >25 μM | — b)) | — b)) | — b)) |
| 3 | 4 | 52.4 | >25 μM | 31.1 | 8.3 | — b)) |
| 4 | 39 | — b)) | — b)) | — b)) | — b)) | — b)) |
| 5 | 35 | — b)) | — b)) | — b)) | — b)) | — b)) |
| 6 | 30 | — b)) | — b)) | — b)) | — b)) | — b)) |
| 7 | 31 | — b)) | — b)) | — b)) | — b)) | — b)) |
Recombinant TE domain of human FASN.
Not determined.
The IC50 values against recombinant TE were determined for compounds 1a–i, 2e–g, and 3. The 1,4-naphthylquinones (1a–i) show a much wider SAR profile with the IC50 values ranging from 0.1 μm to greater than 100 μm. As expected, compounds 1b, 1f, and 1g had the lowest IC50 values of 0.9 μm, 0.3 μm, and 0.1 μm, respectively, and compound 1e had the highest IC50 value of over 100 μm. Based on this dataset, we reasoned that the structure–activity is driven principally by substituent effects of moieties directly attached to the quinone ring. Overall activating groups (Electron Donating Groups, EDGs) diminish inhibition, while deactivating groups (Electron Withdrawing Groups, EWGs) have nominal to a positive effect on inhibition. Compound 1e containing a strong EDG consistent with the SAR has low inhibition capacity; likewise compound 4 with three weak EDGs is a nominal inhibitor. The natural products 6 and 7 that contain strong EDGs (methoxy and hydroxy) also show diminished ability to inhibit TE. Importantly, the best compounds are comparable in potency to Orlistat and its congener Ebelactone B, with IC50 values of 0.3 and 0.8 μm, respectively.
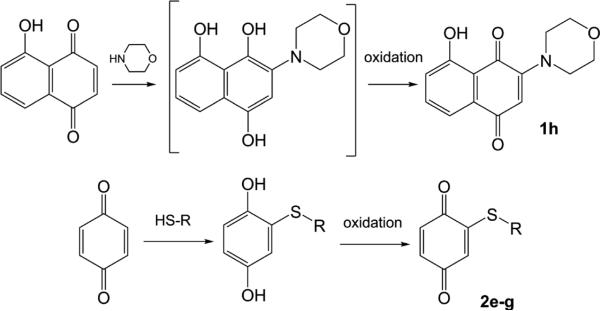
In an effort to test substituent effect hypotheses and to reduce overall chemical reactivity, several analogs were synthesized: one compound with a strong EDG (1h) resulting from the treatment of juglone with morpholine and some with weak to very weak electron donating groups (EDGs; 2e–g). As shown in Chart 1, these compounds were synthesized by reaction of the quinone precursor with a sulfur or nitrogen nucleophile leading to the substituted 1,4-dihydroxy compound (the reduced form of the quinone). Oxidation of the thiol intermediates was accomplished in high yield by dissolving the thiol substrates in methylene chloride and using an aqueous solution of sodium periodate and benzyltrimethylammonium bromide as a phase transfer catalyst13) to afford the desired product (2e–g). In contrast, the morpholino containing intermediate auto-oxidized to provide the desired compound (1h).
Supporting our hypothesis, the thio compounds (2e–g) are potent inhibitors; while the morpholino compound (1h) was a nominal inhibitor. The improved inhibition of the former compounds is most likely due to their lack of aromatic rings around the 1,4-benzoquinone core structure, as the naphthoquinone 1h or the anthraquinone 5 do, and the missing electron cloud of the phenyl rings decreases the electron donating capability of the 2-alkyl-substituted 1,4-benzoquinone derivatives. We surmise that Michael addition is one plausible mechanism for enzyme inhibition, though analogs with fully substituted quinone rings (1a, 5, 6, 7) still retain the ability to inhibit. Further work will be required to develop a mechanistic basis for the observed SAR; for example, affinity measurements (Ki) and determination of reversibility of inhibition will be critical in uncovering the physicochemical determinants of the observed inhibition data.
Inhibition of cellular FASN activity was performed for selected compounds in two prostate cancer cell lines (PC3 and DU145) and in normal fibroblast (FS4) using an assay that measures the incorporation of the 14C-acetate precursor into lipids.14) Not surprisingly, likely owing to low solubility in solution and cellular availability, there is little correlation between the ability of a compound to inhibit recombinant TE and inhibition of cellular FASN activity (Pearson's R=0.1). Interestingly, the compounds that best inhibited 14C-acetate incorporation are in the simple 1,4-naphthoquinone family (1a, 1b, 1d, 1f, 1g, 1i). The most potent inhibitors of recombinant TE that showed no inhibition of endogenous enzyme are the 2-thio-substituted 1,4-benzoquinones (2e–g). A high level of nonspecific chemical reactivity and/or a myriad other factors may be contributing to the observed contrast in SAR between these assays.
There is also a correlation between the ability of a quinone analog to inhibit cellular FASN and the potency (EC50) at killing tumor cells. The inhibition of 14C-acetate uptake and EC50 for inhibition of PC3 cell growth is correlated with a Pearson's r=0.85. Prostate cancer cells expressing the highest level of FASN (DU145) used in this study show an even higher correlation, r=0.92. Despite this strong relationship, only a few of the compounds demonstrated a marginal therapeutic index which was calculated as the cell killing ratio of cancer and FS4 fibroblasts EC50 values (data not shown). Based on the 8 μm EC50 value of Orlistat for PC-3 cells and negligible cytotoxicity to fibroblasts and normal epithelial cells; the compounds studied herein clearly need significant optimization to move toward therapeutic use.2)
In summary, we have demonstrated that 1,4-benzoquinones and 1,4-naphthoquinones, as well as a number of natural products containing the 1,4-benzoquinone and the 9,10-anthraquinone moieties, inhibit the TE domain of FASN as well as cellular FASN activity. These compounds are not based on Orlistat structure, thus are expected to have different pharmacological properties than Orlistat. Nonetheless, the ability of these compounds to inhibit human pancreatic lipase will need to be performed in the future. We surmise that the bioactivity of these compounds may in some cases contribute to the widely observed anti-cancer properties of 1,4-quinones.7–12) Additionally, the SAR shown herein is a starting-point for the rational design of therapeutics that target the TE domain of FASN that are distinct from Orlistat.
Experimental
General
All reagents and solvents were used as purchased without further purification. 1H-NMR spectra were recorded in the indicated solvent on a Bruker 300 MHz with tetramethylsilane (TMS) as internal standard (chemical shift in δ ppm). Mass [ES-MS (70 eV)] spectra were recorded on Agilent 1100 LC/MSD spectrophotometer. Analytical TLC was performed on Silica Gel GHLF250 plates (Analtech) with visualization by UV (254 nm) chamber with protective filters. All compounds were purified by precipitation or by column chromatography performed on silica gel (230–400 mesh, grade 60, Fisher).
Synthesis of 8-Hydroxy-2-morpholinonaphthalene-1,4-dione (1h)
To a solution of juglone (30 mg, 0.172 mmol) in ethanol (1.4 mL) was added morpholine (17 μL, 0.172 mmol) at room temperature. A dark precipitate was observed. The sample was stirred at room temperature for 20 h, and then it was filtered (paper) and rinsed with cold ethanol (ca. 2 mL) and dried to give 34 mg (76% isolated yield) of 8-hydroxy-2-morpholinonaphthalene-1,4-dione as a purple-brown solid. 1H-NMR (DMSO-d6) δ: 7.655 (1H, t, J=0.028, 0.025 Hz), 7.408 (1H, d, J=0.024 Hz), 7.199 (1H, d, J=0.028 Hz), 5.993 (1H, s), 3.700–3.696 (4H, br s), 3.450–3.299 (4H, br s), 2.049 (1H, s). LCMS m/z: 260 [{M+1}+].
General Procedure for the Synthesis of 2-Alkylthio-1,4-benzoquinones (2e–g)
To a solution of 1.0 eq (2.11 mmol) of 1,4-benzoquinone in ethanol (9.2 mL) was added 1.0 eq (2.11 mmol) of thio-derivative at room temperature. The sample was stirred at room temperature for 1 h and monitored by thin layer chromatography until it was complete. Ethanol was removed by reduced pressure to obtain a solid (usually brown to orange in color) that was collected by filtration or purified by flash column chromatography with a hexane–ethyl acetate gradient. The resulting 2-alkylthiohydroxyquinone was dissolved in methylene chloride, placed in a separatory funnel and treated with an aqueous 0.467 m solution of 1.0 eq (2.11 mmol) of sodium periodate and a 0.022 eq (0.046 mmol) of benzyltrimethylammonium bromide (phase transfer re-agent) and was shaken for 20 min in a Glas-Col Bench Top Shaker at room temperature. Reaction completion was identified by TLC. Upon completion, the heterogeneous mixture was washed with water (2×10 mL and brine (10 mL). The aqueous layers were back-extracted with methylene chloride (10 mL). The organic layers were combined, dried (Na2SO4), filtered (paper) and concentrated to dryness in vacuo to afford the desired compounds as a solid. The resulting solid was purified by column chromatography, using hexanes and ethyl acetate gradient as mobile phase or by precipitation/recrystallization from methylene chloride.
2-(Cyclopentylthio)cyclohexa-2,5-diene-1,4-dione (2e)
Orange solid, yield 34%, 1H-NMR (CDCl3) δ: 6.832–6.714 (2H, m), 6.491 (1H, d, J=0.008 Hz), 3.461–3.445 (1H, m), 2.232–2.182 (2H, m), 1.860–1.714 (6H, m). LCMS m/z: 208 [M+].
2-(Heptylthio)cyclohexa-2,5-diene-1,4-dione (2f)
Black solid, yield 22%, 1H-NMR (CDCl3) δ: 6.844–6.720 (2H, m), 6.408 (1H, d, J=0.007 Hz), 2.800 (2H, t, J=0.024 Hz), 1.798–1.747 (2H, m), 1.499–1.472 (2H, m), 1.396–1.298 (6H, m), 0.932 (3H, t, J=0.022 Hz). LCMS m/z: 238 [M+].
2-(Phenylthio)cyclohexa-2,5-diene-1,4-dione (2g)
Orange solid, yield 96%, 1H-NMR (CDCl3) δ: 7.523 (5H, bs), 6.854 (1H, d, J=0.033 Hz), 6.706 (1H, dd, J=0.033 Hz, J=0.008 Hz), 5.918 (1H, d, J=0.008 Hz. LCMS m/z: 216 [M+].
Acknowledgments
The authors thank Lynnette Johnson and Frances Wheeler for their technical support. This work was supported by a Biotechnology Research Grant (BRG1216) from the North Carolina Biotechnology Center and the Department of Defense Prostate Cancer Research Program (PC08165).
Footnotes
The authors declare no conflict of interest.
References
- 1.Kridel SJ, Lowther WT, Pemble CW. Expert Opin. Investig. Drugs. 2007;16:1817–1829. doi: 10.1517/13543784.16.11.1817. [DOI] [PubMed] [Google Scholar]
- 2.Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Cancer Res. 2004;64:2070–2075. doi: 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]
- 3.Purohit VC, Richardson RD, Smith JW, Romo D. J. Org. Chem. 2006;71:4549–4558. doi: 10.1021/jo060392d. [DOI] [PubMed] [Google Scholar]
- 4.Pemble CW, 4th, Johnson LC, Kridel SJ, Lowther WT. Nat. Struct. Mol. Biol. 2007;14:704–709. doi: 10.1038/nsmb1265. [DOI] [PubMed] [Google Scholar]
- 5.Costa PRR. Rev. Virtual Quím. 2009;1:58–66. [Google Scholar]
- 6.Martínez MJA, Benito PB. Stud. Nat. Prod. Chem. 2005;30:303–366. [Google Scholar]
- 7.Brunmark A, Cadenas E. Free Radic. Biol. Med. 1989;7:435–477. doi: 10.1016/0891-5849(89)90126-3. [DOI] [PubMed] [Google Scholar]
- 8.Tandon VK, Maurya HK, Tripathi A, ShivaKeshava GB, Shukla PK, Srivastava P, Panda D. Eur. J. Med. Chem. 2009;44:1086–1092. doi: 10.1016/j.ejmech.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Zagotto G, Sissi C, Lucatello L, Pivetta C, Cadamuro SA, Fox KR, Neidle S, Palumbo M. J. Med. Chem. 2008;51:5566–5574. doi: 10.1021/jm800160v. [DOI] [PubMed] [Google Scholar]
- 10.Bernardo PH, Chai CL, Heath GA, Mahon PJ, Smith GD, Waring P, Wilkes BA. J. Med. Chem. 2004;47:4958–4963. doi: 10.1021/jm049625o. [DOI] [PubMed] [Google Scholar]
- 11.Kogan NM, Rabinowitz R, Levi P, Gibson D, Sandor P, Schlesinger M, Mechoulam R. J. Med. Chem. 2004;47:3800–3806. doi: 10.1021/jm040042o. [DOI] [PubMed] [Google Scholar]
- 12.Basarić N, Cindro N, Bobinac D, Mlinaric-Majerski K, Uzelac L, Kralj M, Wan P. Photochem. Photobiol. Sci. 2011;10:1910–1925. doi: 10.1039/c1pp05182b. [DOI] [PubMed] [Google Scholar]
- 13.Afrasiabi Z, Sinn E, Chen J, Ma Y, Rheingold AL, Zakharov LN, Rath N, Padhye S. Inorganica Chimica Acta. 2004;357:271–278. [Google Scholar]
- 14.Little JL, Wheeler FB, Fels DR, Koumenis C, Kridel SJ. Cancer Res. 2007;67:1262–1269. doi: 10.1158/0008-5472.CAN-06-1794. [DOI] [PubMed] [Google Scholar]