Abstract
There has been much recent debate regarding the neural basis of motor response inhibition. An influential hypothesis from the last decade proposes that a module within the right inferior frontal cortex (RIFC) of the human brain is dedicated to supporting response inhibition. However, there is growing evidence to support the alternative view that response inhibition is just one prominent example of the many cognitive control processes that are supported by the same set of ‘domain general’ functional networks. Here, I test directly between the modular and network accounts of motor response inhibition by applying a combination of data-driven, event-related and functional connectivity analyses to fMRI data from a variety of attention and inhibition tasks. The results demonstrate that there is no inhibitory module within the RIFC. Instead, response inhibition recruits a functionally heterogeneous ensemble of RIFC networks, which can be dissociated from each other in the context of other task demands.
Keywords: Right inferior frontal cortex, Functional networks, Response inhibition, Cognitive control, Attention, Stop signal task, Go/no go, Target detection, Domain general cortex, Independent component analysis, Phase synchrony, FMRI
Highlights
-
•
ICA renders a consistent functional parcellation of the inferior frontal cortex (RIFC).
-
•
There is no evidence for a motor response inhibition module within the RIFC.
-
•
All RIFC sub-regions respond to motor inhibition and attentional control conditions.
-
•
RIFC sub-regions show heterogeneous responses to attentional task demands.
-
•
Inhibition increases connectivity throughout the entire ensemble of RIFC networks.
Classic approaches to functional neuroimaging have produced a plethora of models ascribing highly specific cognitive processes to dedicated modules within the frontal lobes. However, there is growing evidence to support the view that the human frontal lobes house sub-regions of ‘domain general’ networks, each of which makes a broader contribution to cognition. Consequently, many models from the neuroimaging literature are likely to be both functionally and anatomically over-specified because they do not account for the general involvement in cognition of the brain regions that they pertain to or for the co-recruitment of those brain regions with distributed functional networks. A prominent example of this issue is the controversy regarding whether there is a module within the right inferior frontal cortices (RIFC) that is uniquely and specifically dedicated to the process of motor response inhibition.
Motor response inhibition refers to the process by which routine, initialised or otherwise, pre-potent motor responses are effortfully withheld or cancelled. This particular aspect of top-down control has been a major focus of research because a lack of inhibitory control is characteristic of a range of important clinical populations. Consequently, paradigms that are designed to measure motor response inhibition are becoming increasingly popular in clinical research and assessment. Most prominent amongst these is the Stop Signal Task (SST), in which the participant makes button presses in response to a frequent go stimuli (Logan and Cowan, 1984) but must cancel the previously initiated response when an infrequent stop signal is presented at a brief offset after the go stimulus. Another prominent inhibition paradigm is the go/no go (GNG) task, in which the participant executes a button press in response to a frequent ‘go’ stimuli but must try to withhold that response when an infrequent ‘no go’ stimulus is displayed. Although the value of these paradigms as markers of cognitive impairment is well established, the neural mechanisms that support response inhibition remain the topic of much debate.
One prominent hypothesis states that a dedicated neural module within the RIFC is dedicated to supporting motor response inhibition (Aron, 2011; Aron et al., 2004). When environmental cues signal the requirement for inhibition, the RIFC module is proposed to down-regulate processes within the motor control areas of the brain via interactions with subcortical areas (Aron and Poldrack, 2006); in this manner, the RIFC module is proposed to work as a top-down braking system that rapidly halts all ongoing motor responses.
In support of the modular view, RIFC sub-regions reliably activate during GNG and SST tasks in healthy controls (Rubia et al., 2001a) but to a lesser extent in patients who suffer from impulsivity disorders, for example, attentional deficit hyperactivity disorder (Rubia et al., 1999, 2001b). Moreover, drugs that are used to treat patients with impulsivity disorders improve performance of and increase RIFC activation during the SST (Aron et al., 2003a; Chamberlain et al., 2008; Rubia et al., 2011). Finally, lesions to the RIFC are associated with impulsive behaviour and poor SST performance (Aron et al., 2003b), completing the active during and necessary for relationship that is a central tenet of cognitive neuroscience. Therefore, there is strong evidence to support the view that the RIFC is critically involved in motor response inhibition.
Nonetheless, it does not logically follow that the RIFC houses a dedicated response inhibition module, nor is it necessarily the case that an RIFC–subcortical pathway exists with the sole purpose of down regulating motor responses. Indeed, sub-regions of the human RIFC have been reported to be involved in a particularly broad range of cognitive tasks that require the top-down control of thoughts and actions (Duncan, 2001; Duncan and Owen, 2000). Representative examples include working memory maintenance (Hampshire et al., 2012; Owen, 1997; Owen and Hampshire, 2009), updating (Levy and Wagner, 2011; Verbruggen et al., 2010), attentional switching (Cools et al., 2002; Hampshire and Owen, 2006; Shallice et al., 2008), context monitoring (Chatham et al., 2012) and target detection (Hampshire et al., 2007, 2008; Linden et al., 1999). The latter example, target detection, is perhaps the most relevant because this paradigm requires motor responses to be initiated as opposed to inhibited when infrequent target cues are presented amongst sequences of more frequent distractor stimuli. Therefore, the design is similar to that of SST and GNG tasks with respect to the stimulus processing demands but differs in terms of the requirement for motor response inhibition (Erika-Florence et al., 2014; Hampshire et al., 2010). Given the results of the broader literature, it is likely that the RIFC regions observed during response inhibition are involved in a wider range of cognitive processes.
To complicate matters further, data-driven analyses have demonstrated that the RIFC contains multiple functionally distinct sub-regions and moreover, each sub-region activates in close association with an intrinsic network, the other components of which are distributed throughout the brain (Beckmann and Smith, 2004; Damoiseaux et al., 2006; Dosenbach et al., 2008; Erika-Florence et al., 2014; Rosazza and Minati, 2011; Smith et al., 2009a; Zhang and Li, 2012). In a recent series of studies, it has been demonstrated that components throughout RIFC–whole brain networks activate to a similar level during the SST and across a broad range of other task conditions that do not involve the effortful cancellation of motor responses (Erika-Florence et al., 2014; Hampshire et al., 2010; Sharp et al., 2010; Swick and Chatham, 2014). Of particular relevance to the current study, it has recently been reported that RIFC sub-regions show an increase in functional connectivity during motor response inhibition and other attentionally demanding task conditions, that is, they co-activate in a synchronous manner as an ensemble (Erika-Florence et al., 2014). The strength of this change in network connectivity correlates with individual differences in Stop Signal Reaction Times (SSRT), the most commonly applied measure of response inhibition ability. Based on this observation it has been proposed that motor response inhibition is just one specific example of a broader class of top-down control processes that are supported by dynamic interactions that occur throughout ‘domain general’ networks.
However, the original proponents of the modular hypothesis have questioned this alternative network perspective. More specifically, it has been suggested that the studies providing evidence counter to the modular hypothesis or RIFC function did not examine the precise location of the RI module and moreover, that some of the attentional control conditions that were applied may have had hidden inhibitory demands (Aron et al., 2014a,b). Here, I address these arguments with further analyses of two previously published SST studies and a new target detection/GNG task, which are designed to differentiate between the cognitive processes that are typically confounded in SST and GNG paradigms.
First, independent components analyses (ICA) are applied separately to data from each of the three studies to test whether there is a consistent data-driven functional parcellation of the RIFC across task contexts and whether there is any evidence within that parcellation of a distinct functional sub-region at the proposed coordinates of the response inhibition module. Then further analyses are undertaken of previously reported data to determine whether the exact proposed coordinates of the inhibition module, or any other sub-regions of the RIFC, activate either specifically or particularly strongly during motor response inhibition relative to a wide range of other task conditions. Data from the new study are then examined in greater depth in order to probe the conditions under which RIFC sub-regions can be dissociated from each other. Finally, functional connectivity analyses are conducted on data from the new study to determine whether the widespread increases in network functional connectivities that were previously reported during the SST may be replicated in the context of target detection and GNG paradigms.
Materials and methods
Participants
Fourteen participants undertook study 1, 16 participants undertook study 2 and 15 participants undertook study 3. All participants were right handed, aged between 18 and 40, had normal hearing and normal or corrected to normal vision. Exclusion criteria included a history of neurological or psychiatric illness and the taking of psychoactive medications. Participants gave informed consent prior to commencing the studies.
Task designs
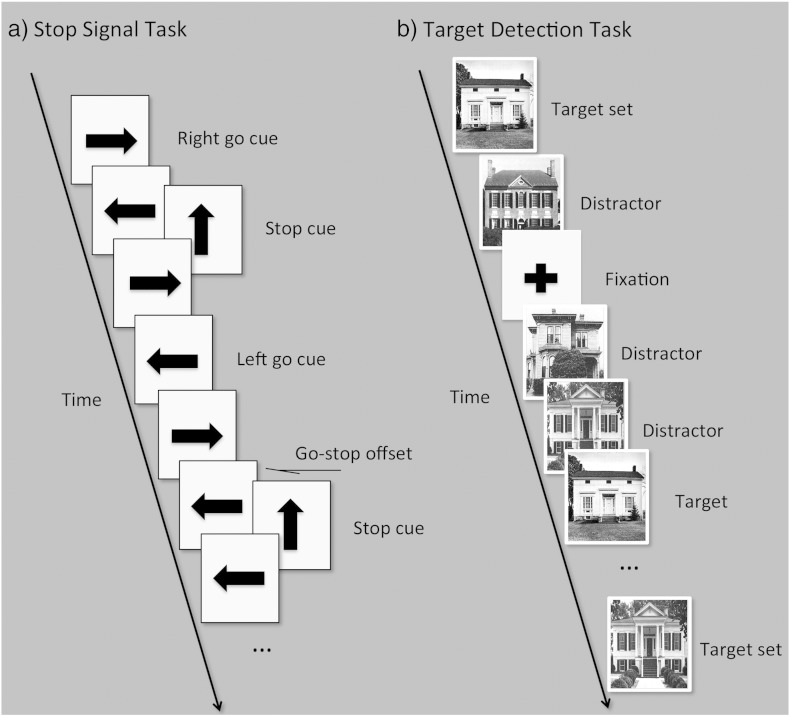
The design of study 1 has been reported in detail in a previous article in this journal (Hampshire et al., 2010); in brief, there were three blocks of scanning acquisition during which participants undertook a classic SST paradigm and two attentional control variants of the task. In all three of the acquisition blocks, participants viewed a series of left and right arrows that appeared on the screen in rapid succession. Less frequently, an up arrow appeared a short variable delay after the onset of the left or right arrow (Fig. 1a), and this formed the cue for an additional behaviour that varied across the three blocks. During the first block, participants were instructed to silently count the total number of up-arrow cues that were presented without making any motor responses (‘COUNT’). At the end of the block, participants were asked to report the total number of up arrows that they had counted. In the second block (RESPOND), participants responded to the up-arrow cue with a left or right button press the direction of which was defined by the immediately preceding lateral arrow. In the third block, participants were instructed to make left or right button presses as soon as possible after the appearance of the left and right arrows, but to try and cancel that response whenever an up arrow was presented (‘INHIBIT’). This latter condition was equivalent to the response inhibition manipulation employed in classical SST tasks. Participants viewed a total of 131 left and 131 right arrows per 9-min acquisition block, 68 of which were followed by up arrows. Left and right arrows were displayed on the screen for 300 ms with a predefined pseudo-randomised ISI such that arrows occurred at either 1600, 1700, 1800, 1900 or 2000 ms intervals. Up arrows were displayed unpredictably after the left and right arrows with a predefined and pseudo-randomised offset from the start of the left or right signal of between 300 and 900 ms.
Fig. 1.
(a) In the stop signal task, participants are instructed to respond as quickly and accurately as possible to ‘go’ stimuli in the form of left and right arrows, which are presented in a rapid and pseudo-randomised sequence. During the inhibition trials, they must try to cancel the already initiated response when an infrequent stop signal cue is presented at a short offset after the go cue. The go–stop offset is typically set to produce a 50% failure rate on the inhibition trials. (b) In the target detection paradigm, participants are instructed to monitor sequences of distractors for an infrequent target stimulus. The stimulus that is the target is periodically changed. When motor responses are made to distractors but not to targets, this design is equivalent to a go–no go paradigm.
The design of study 2 was similar to that of study 1 and has been reported in detail elsewhere (Erika-Florence et al., 2014); in brief, there were four blocks of scanning acquisition during which participants undertook either a variant of the classic SST or one of three attentional control variants of the task. In all four blocks, frequent left and right arrows were displayed with a variable inter-stimulus interval (1600, 1700, 1800, 1900 or 2000 ms). In 91 trials per block an infrequent stimulus interrupted a frequent stimulus at an unpredictable offset (mean = 323 ms, SD = 122 ms). Unlike study 1, the infrequent stimulus could be either an up or a down arrow presented randomly and with equal frequency. Furthermore, each task consisted of 4 × 180 s periods of task interleaved with 5 × 40 second periods of rest. This design allowed sustained task vs. rest activations and transient activations during infrequent vs. frequent stimuli to be estimated separately using a mixed block/event-related model. In one block (MONITOR), participants were instructed to respond to frequent stimuli with a corresponding left or right button press and to simply monitor the infrequent stimuli with no change to the ongoing motor responses. In another block (INHIBIT), they were instructed to respond as fast as they could to the left and right arrows but to try and cancel that response if an up or down arrow was displayed, this being the classic SST design. In a third block (RESPOND), participants only responded when they were presented with an up or down arrow by indicating the direction of the previous left or right arrow. Therefore, they were planning but withholding a response on all trials but only made that response when infrequent up or down arrow cues were displayed. The fourth block (COMPLEX) was identical to the RESPOND condition, except that a dual button press was made in response to down arrows; therefore, this block involved a more complex set of stimulus–response mappings.
Study 3 consisted of six acquisition blocks during which participants undertook different variants of a target detection paradigm (Hampshire et al., 2007). These were designed to differentiate between cognitive control processes in a hierarchical manner whilst controlling for stimulus parameters. Specially, all six blocks were similar with respect to the rate and frequency of stimulus presentation; however, they differed according to the type of stimuli and the type of response required. In all six blocks, stimuli were presented in variable length sequences with targets first defined for 2.5 s subsequent to which distractor and target stimuli were presented at a rate of 1 stimulus displayed for 1 s every 1.5 s (Fig. 1b). In four of the blocks, stimuli were either images of faces or buildings. In one of these blocks (MONITOR), participants were instructed to simply look for the target on the screen but to make no overt motor response to either targets or distractors. In another (COUNT), they were instructed to silently count the total number of targets displayed throughout the block and there were no overt motor responses. In a third condition (PREPARE), participants were instructed to respond with a button press at the end of the block if the final stimulus was the target; therefore, because sequence length varied unpredictably, they always prepared a response when a target was displayed. In a fourth condition (RESPOND IMAGES), participants were instructed to respond as quickly as possible with a button press when the target was detected. These four conditions were designed to hold stimulus presentation parameters constant whilst varying the response requirements of the task. In the other two blocks, stimuli were either animal or object words presented in the centre of the screen. In one block (RESPOND WORDS), participants were instructed to respond with a button press as quickly as possible when the target was detected and to make no response to distractors. In the other condition (INHIBIT), participants were instructed to respond to all stimuli except the target with a button press; this condition was equivalent to a GNG task. These blocks were designed to test whether there were GNG-specific activations related to the requirement to withhold a routine response and to determine whether there was any sensitivity to stimulus type within the RIFC. Sequences varied in length from 1 to 11 stimuli (average length = 6) in a pseudo-randomised and unpredictable manner. One in every three stimuli was a fixation cross and one in six stimuli was a target. There were a total of 32 sequences per acquisition block with 32 targets and 96 distractors. This design enabled BOLD activation related to targets and distractors to be estimated relative to fixation. In a given block, there were four stimuli from each of two categories. During any given sequence, distractors were always drawn from the same category as the current target. Target and distractor stimuli were drawn from the same pool of stimuli and different stimuli were used in each acquisition block, thereby controlling for differences in stimulus salience. Unlike the classic SST, this design also controlled for the potentially confounding effects of stimulus familiarity when contrasting targets and distractors.
FMRI acquisition
Prior to being scanned, participants read an information sheet detailing the task requirements. Once in the scanner, they were reminded of the current instructions verbally before each acquisition block began. Responses were made using an MRI compatible button box. The tasks were displayed on a projector screen at the end of the scanner bore, visible via a mirror placed just in front of the eyes. Brain images were collected using a 3-T Siemens Scanner. T2-weighted echo-planar images depicting BOLD contrast were acquired throughout each block of scanning acquisition. The first 10 images were discarded to account for equilibrium effects. Each image consisted of 32 × 3 mm slices, each with a 64 × 64 matrix and a 192 × 192 mm field of view. Images were collected with a 2-s repetition time, a TE of 30 ms, a flip angle of 78°, echo spacing of 0.51 ms, and a bandwidth of 2232 Hz/Px. Prior to analysis, the data were pre-processed in SPM (Statistical Parametric Mapping, Welcome Department of Imaging Neuroscience, London, UK). Data were slice-timing and motion corrected, spatially normalised to the standard Montreal Neurological Institute template using SPM default parameters and spatially smoothed with an 8 mm full width at half maximum Gaussian kernel. The data were high-passed filtered (cutoff period = 180 s) to remove low frequency drifts in the MRI signal.
Event-related fMRI analyses
FMRI data were modelled at the individual participant level using general linear modelling (GLM) in SPM. Predictor functions were created for the psychological events of interest from each task by convolving the onsets and durations of the events with the canonical haemodynamic response function (HRF). Noise due to movement was accounted for by inclusion of six nuisance variables for each task acquisition, corresponding to translations and rotations in the x, y and z planes. In study 1, each acquisition block was modelled with one predictor function capturing the onsets and durations of the infrequent ‘stop cue’ trials, that is, trials where the frequent left and right ‘go’ arrows were interrupted by presentation of the infrequent up arrow. In the classic SST, the frequent trials are not reliably separable from the constant of the GLM once they have been convolved with the HRF as they are close together in time and the HRF is sustained. Consequently, they were not explicitly modelled with a predictor function.
The design of study 2 included interleaved periods of task and rest, which unlike the classic SST design, allowed the frequent events to be estimated relative to rest. Therefore, the acquisition blocks from study 2 were each modelled with two predictor functions. The first predictor function was designed to capture activations during routine responding to go stimuli and consisted of the onset and duration times for all trials convolved with the canonical haemodynamic response function (HRF). The second predictor function was designed to capture transient increases in activation in response to infrequent trials, that is, trials where the frequent left and right arrows were interrupted by presentation of the infrequent up or down arrows. This latter predictor function consisted of the onsets and durations for all infrequent trials convolved with the HRF, minus the onsets and durations for all frequent trials convolved with the HRF.
Study 3 included randomly interspersed fixation events, which also allowed both frequent targets and infrequent distractors to be estimated relative to fixation. Each acquisition block was modelled with six predictor functions, which captured the onsets and durations of targets, distractors and events where the target was set, separately for each of the two categories of stimuli (i.e., face targets, face distractors, face target set, building targets, building distractors and buildings target set).
Whole brain maps depicting parameter estimates for the experimental predictor functions were collated for group level random effects analyses. Focused regions of interest (ROI) analyses were carried out with the MarsBaR toolbox (Brett et al., 2002), which calculates the average value from all voxels within the ROI. Unless stated otherwise, voxelwise group level analyses were carried out in SPM8 with a false discovery rate (FDR) corrected threshold of p < 0.05 for multiple comparisons across the whole brain volume.
Functional connectivity analyses
Spatial independent components analyses (ICA) were carried out using the MELODIC command line function (Smith et al., 2009b) from the FSL toolbox. The ICA was restricted to an anatomical mask, including the right lateral frontal cortex and insula, which was defined by combining regions from the automatic anatomical labelling templates (Tzourio-Mazoyer et al., 2002). This mask was applied to try and achieve a complete and accurate decomposition of this region (Braga et al., 2013), the functional organisation of which is the focus of this article. The activation time courses for all voxels from within the RIFC mask were input to the ICAs using the concatenate option to combine across all individuals and all acquisition blocks for the study. The MELODIC tensor option was not used.
Seed analyses were conducted in SPM8. Specifically, time courses were extracted from 10 mm radius spheres placed at peak coordinates averaged across the three studies for each of the components identified in the ICA using the volume of interest (VOI) function, which extracts the first eigenvector across all voxels within the mask. These time courses were fitted together onto data for each voxel within the brain using GLMs with movement parameters included as nuisance variables. The resultant whole brain maps, depicting beta weights for each seed time course were examined at the group level using a one-way repeated ANOVA.
Psychophysiological interaction (PPI) analyses were carried out in SPM8 (Friston et al., 1997) in the following standard manner. BOLD activation time courses were extracted from 10 mm spheres within the seed ROIs using the VOI function. The neural signal underlying the BOLD response was estimated using the deconvolution function in SPM8 prior to being interacted with psychological time courses for the contrast of interest to produce the PPIs (e.g., targets = 1, distractors = − 1 and all other time points = 0). The physiological, psychological and psychophysiological time courses were convolved with the HRF to produce the predictor functions which were input together to GLMs in SPM8 for each of the seed regions and for each contrast of interest. Movement parameters were also included in the GLMs as nuisance variables. Mean beta weights for the PPI predictor functions were extracted for each task and from each ROI using the MarsBaR ROI toolbox and these data were exported for group level analyses in SPSS.
Results
Independent components analyses of the right IFC
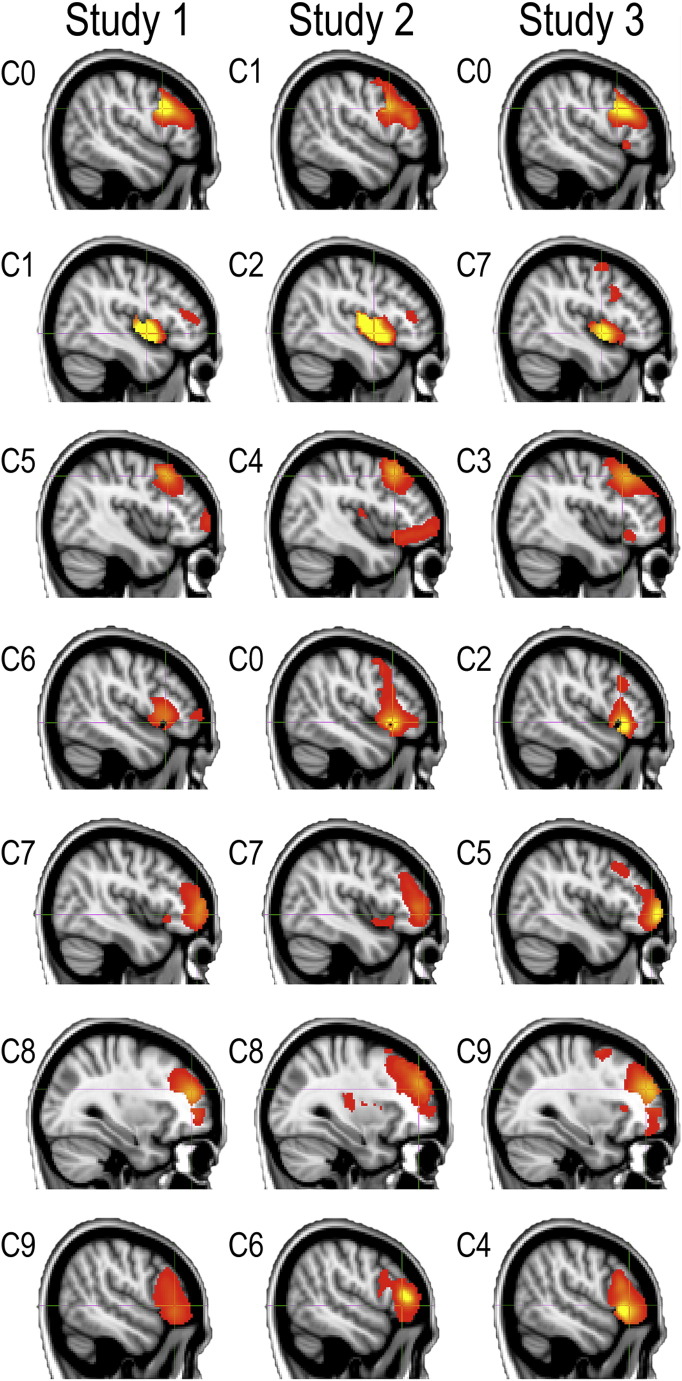
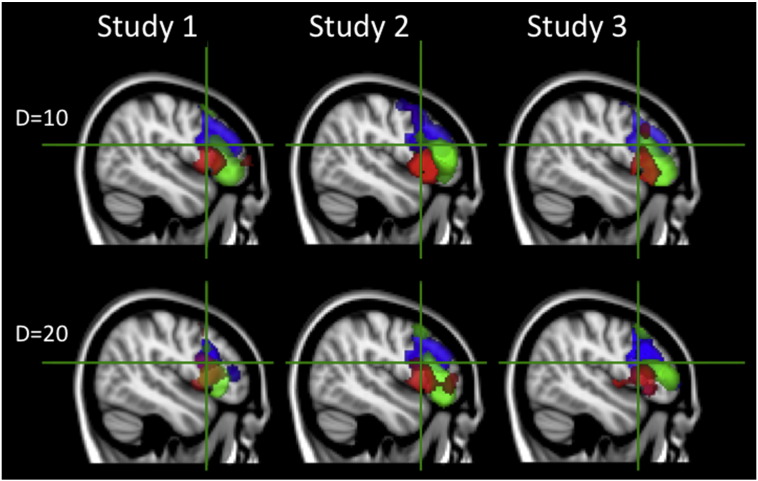
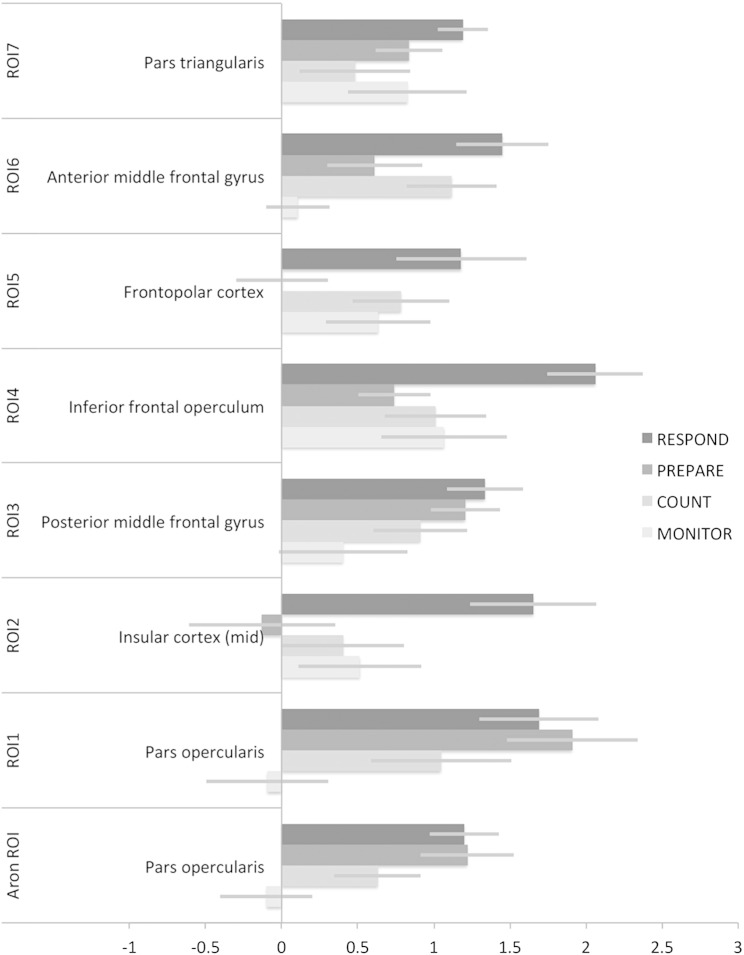
In a recent article, Aron and colleagues proposed that the coordinates of the inhibition module are at x = 48, y = 16, z = 18 in MNI space (Aron et al., 2014b); this was based on the results of a meta-analysis conducted by another group (Levy and Wagner, 2011). In order to determine whether there was any evidence for a functionally distinct region of the RIFC at these proposed coordinates, ICA decompositions were generated separately for each of the three studies. MELODIC was set to extract 10 components; this initial model order was selected based on previous studies that have indicated approximately 7 components within this volume when applying the Akaike information Criteria (Erika-Florence et al., 2014) and was intended to allow for the possibility of additional noise components, which are often extracted by this method, e.g., due to movement. Two of the components from study 1, three from study 2 and two from study 3, appeared to capture movement artefacts. There was a close conformity between seven of the remaining components across the three studies (Fig. 2). None of the components from any of the studies centred on the proposed coordinates of the inhibition module; however, 5 of them had mean peak values within 3 cm of those coordinates with the closest being dorsal by approximately 1 cm. Three of the components had clusters of peak activation that appeared to overlap with the coordinates of the inhibition module in all three studies (Fig. 3 upper and Table 1). Increasing the dimensionality of the ICA to 20 components also did not identify a component with closer peak coordinates in any of the three studies. However, in study 3, one of the proximal clusters was broken down into two further components (Fig. 3 lower). These results demonstrated that the data-driven parcellation of RIFC was robust across studies and at multiple levels of ICA dimensionality. 10 mm radius ROIs were defined for further analysis at the peak coordinates of the 7 components, averaged across studies. For completeness, an additional 10 mm radius ROI (Aron ROI) was defined at the exact proposed coordinates of the inhibition module.
Fig. 2.
Independent components analyses conducted separately on data from each of the three studies produced a highly consistent parcellation of the RIFC.
Fig. 3.
Rendering of the ICA components with peaks that were proximal to the putative inhibition module. Crosshairs are set to the proposed central coordinates of the inhibition module. Upper panel—none of the 10 components from the ICAs included a cluster that was centred on the proposed coordinates of the inhibition module; instead, those coordinates were at the overlap area of clusters from three distinct components. Lower panel—increasing the dimensionality of the ICA to 20 components still did not render a component with a cluster at the proposed coordinates of the inhibition module.
Table 1.
Peak coordinates from ICA of studies 1–3.
|
MNI coordinates |
|||||
|---|---|---|---|---|---|
|
Component |
x |
y |
z |
||
| Aron ROI | 48 | 16 | 18 | RIFC/pars opercularis | |
| Study 1 | |||||
| C0 | 48 | 16 | 26 | Pars opercularis | |
| C7 | 44 | 4 | − 4 | Insular cortex (mid) | |
| C3 | 46 | 24 | 42 | Posterior middle frontal gyrus | |
| C2 | 44 | 24 | − 8 | Anterior insula/inferior frontal operculum | |
| C5 | 44 | 54 | − 2 | Frontal polar cortex | |
| C9 | 34 | 48 | 24 | Anterior middle frontal gyrus | |
| C4 | 54 | 28 | − 4 | Pars triangularis | |
| C8 | 40 | − 10 | 6 | Insula cortex (posterior) | |
| Study 2 | |||||
| C1 | 50 | 14 | 30 | Pars opercularis | |
| C2 | 44 | 0 | − 2 | Insular cortex (mid) | |
| C4 | 46 | 20 | 48 | Posterior middle frontal gyrus | |
| C0 | 52 | 20 | − 4 | Anterior insula/inferior frontal operculum | |
| C7 | 40 | 46 | 4 | Frontal polar cortex | |
| C8 | 28 | 46 | 30 | Anterior middle frontal gyrus | |
| C6 | 48 | 38 | 8 | Pars triangularis | |
| Study 3 | |||||
| C0 | 50 | 16 | 28 | Pars opercularis | |
| C1 | 42 | − 2 | − 2 | Insular cortex (mid) | |
| C5 | 34 | 20 | 44 | Posterior middle frontal gyrus | |
| C6 | 44 | 20 | 0 | Anterior insula/inferior frontal operculum | |
| C7 | 44 | 50 | − 6 | Frontal polar cortex | |
| C8 | 34 | 46 | 22 | Anterior middle frontal gyrus | |
| C9 | 52 | 32 | − 4 | Pars triangularis | |
| C3 | 44 | 16 | − 10 | Insula cortex/OFC | |
| Average | |||||
| ROI1 | 49 | 15 | 28 | Pars opercularis | |
| ROI2 | 43 | 1 | − 3 | Insular cortex (mid) | |
| ROI3 | 42 | 21 | 45 | Posterior middle frontal gyrus | |
| ROI4 | 47 | 21 | − 4 | Anterior insula/inferior frontal operculum | |
| ROI5 | 43 | 50 | − 1 | Frontal polar cortex | |
| ROI6 | 32 | 47 | 25 | Anterior middle frontal gyrus | |
| ROI7 | 51 | 33 | 0 | Pars triangularis | |
Seed analyses with RIFC ROIs
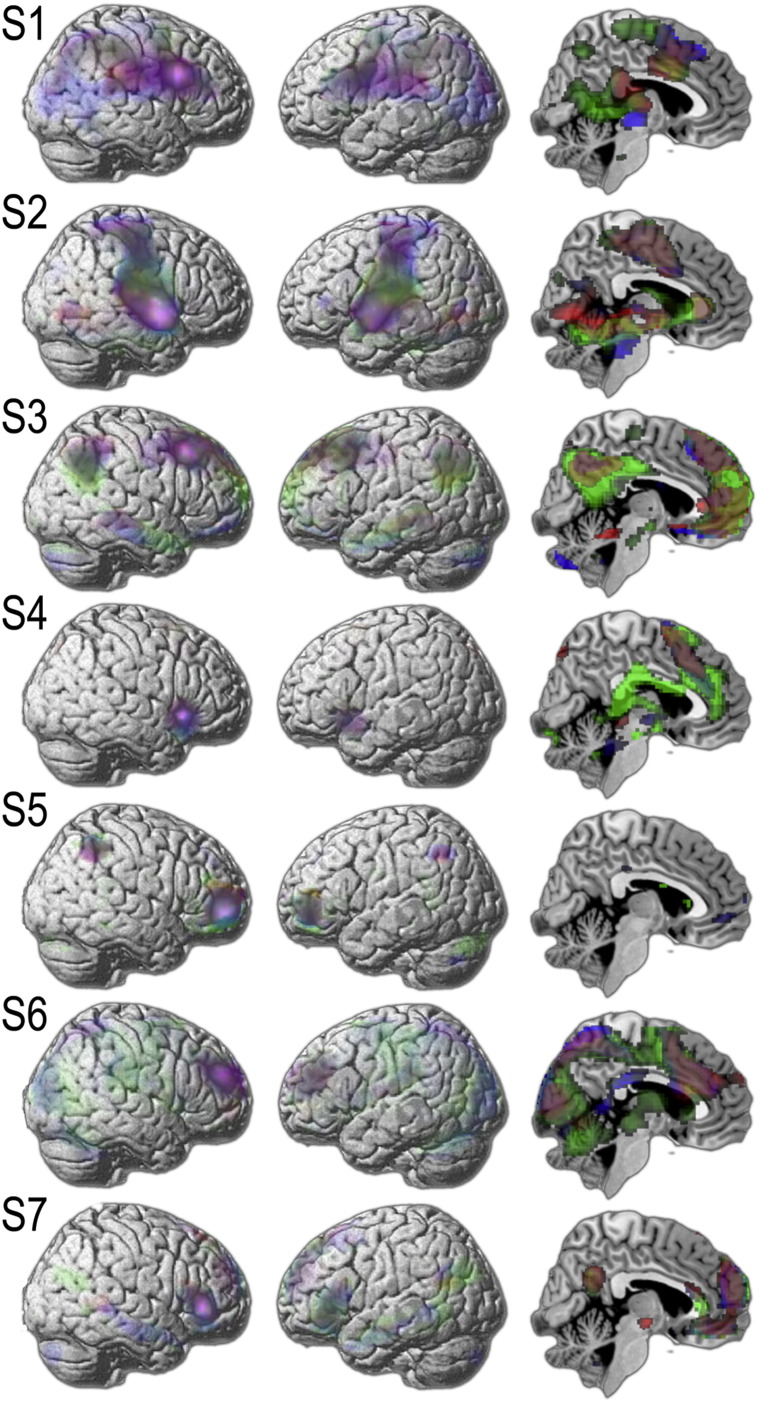
Seed analyses were conducted separately for the three studies using time courses extracted from the seven ICA ROIs to determine whether RIFC sub-regions consistently form parts of the same distributed functional networks. Contrasting the beta maps for each seed region relative to the others generated similar results across the three studies (Fig. 4). Seed region 1 was centred on the most posterior and dorsal extent of the right inferior frontal gyrus (pars opercularis). This region was most proximal to the proposed location of the inhibition module. It co-activated with the corresponding region of the left hemisphere, and the precentral gyri, supramarginal gyri, superior lateral occipital cortices, temporo-occipital cortices and thalamus bilaterally. Seed region 2 was centred on the middle section of the right insula. It co-activated with the corresponding region of the left hemisphere and the sensorimotor cortices, hippocampi and parahippocampal gyri bilaterally. Seed region 3 was centred on the most posterior extent of the right middle frontal gyrus. It co-activated with the corresponding left region and the parietal cortices, lateral orbitofrontal cortices and regions middle temporal gyri bilaterally. Seed region 4 was centred on the right anterior insula/inferior frontal operculum. It co-activated with the corresponding left region and the anterior cingulate cortex. Seed region 5 was centred on the right lateral frontopolar cortex. It co-activated with the corresponding left region and the superior parietal cortices. Seed region 6 was centred on the anterior portion of the right middle frontal gyrus. It co-activated with sub-regions of the supplementary motor cortex, caudate, precuneus, superior parietal cortices and occipital cortices bilaterally. Seed region 7 was centred on the right pars triangularis. It co-activated with the corresponding left region, and the medial frontal polar cortex, and anterior middle temporal gyri bilaterally.
Fig. 4.
Seed analyses conducted separately on data from each of the three studies rendered highly similar networks for each of the RIFC regions identified in the ICA (green = study 1, red = study 2 and blue = study 3).
Reanalysis of studies 1 and 2
It has been claimed that studies 1 and 2 focused on the wrong regions of the RIFC and, consequently, did not report results from an area that responds to response inhibition demands specifically (Aron et al., 2014b). Therefore, mean parameter estimates were first extracted from the Aron ROI for each of the three acquisition blocks in study 1 (Fig. 5a). These data were examined at the group level using a one-way repeated-measures analysis of variance (ANOVA) in which the condition was acquisition block (COUNT, RESPOND and INHIBIT). In accordance with a role for this region in the task, there was a significant positive effect of condition (i.e., T contrast collapsed across all three conditions t = 2.13, p < 0.02); however, there was no significant main effect of acquisition block (F = 0.51, p = 0.61). Contrasting INHIBITION minus the other two control blocks also showed no significant effect (t = 0.02, p = 0.49), a result that accords poorly with the notion of a dedicated motor response inhibition module. Furthermore, repeating the analysis with ROIs from the ICA showed similar and in some cases more pronounced positive effects of condition (Table 2), with the strongest effects being for the frontopolar cortex and the anterior insula/inferior frontal operculum. There were no significant main effects of acquisition block in any of the ICA ROIs.
Fig. 5.
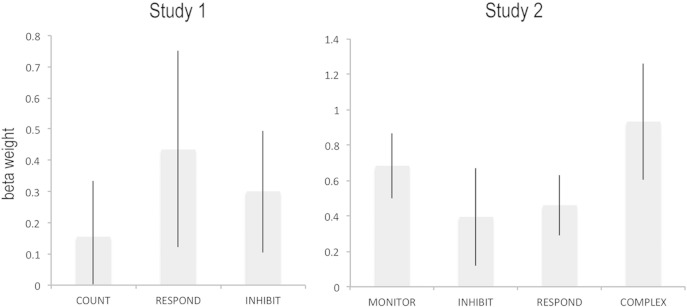
Data extracted from the proposed coordinates of the inhibition module for studies 1 and 2. There was significant activation across a range of target detection conditions regardless of the requirement to inhibit a motor response. Error bars report the standard error of the mean.
Table 2.
reanalysis of ROI activation magnitudes from study 1.
| Positive effect of condition | t | p (one-tailed) | |
| Aron ROI | Pars opercularis | 2.08 | 0.022 |
| ROI1 | Pars opercularis | 2.14 | 0.019 |
| ROI2 | Insular cortex (mid) | 1.61 | 0.057 |
| ROI3 | Posterior middle frontal gyrus | 1.94 | 0.029 |
| ROI4 | Anterior insula/inferior frontal operculum | 2.53 | 0.008 |
| ROI5 | Frontopolar cortex | 3.15 | 0.001 |
| ROI6 | Anterior middle frontal gyrus | 1.97 | 0.028 |
| ROI7 | Pars triangularis | 1.41 | 0.082 |
| Main effect of acquisition block | F | p | |
| Aron ROI | Pars opercularis | 0.53 | 0.594 |
| ROI1 | Pars opercularis | 1.05 | 0.359 |
| ROI2 | Insular cortex (mid) | 0.95 | 0.395 |
| ROI3 | Posterior middle frontal gyrus | 2.35 | 0.108 |
| ROI4 | Anterior insula/inferior frontal operculum | 0.22 | 0.803 |
| ROI5 | Frontopolar cortex | 1.96 | 0.154 |
| ROI6 | Anterior middle frontal gyrus | 2.42 | 0.101 |
| ROI7 | Pars triangularis | 0.23 | 0.798 |
| Inhibition—other blocks | t | p (one-tailed) | |
| Aron ROI | Pars opercularis | 0.02 | 0.493 |
| ROI1 | Pars opercularis | − 1.34 | 0.906 |
| ROI2 | Insular cortex (mid) | − 1.04 | 0.848 |
| ROI3 | Posterior middle frontal gyrus | − 2.17 | 0.982 |
| ROI4 | Anterior insula/inferior frontal operculum | 0.17 | 0.433 |
| ROI5 | Frontopolar cortex | − 0.19 | 0.574 |
| ROI6 | Anterior middle frontal gyrus | − 0.78 | 0.780 |
| ROI7 | Pars triangularis | − 0.07 | 0.528 |
Mean parameter estimates were then extracted from the Aron ROI and the ICA ROIs for the infrequent regressor from study 2 (Fig. 5b). These data were examined in a one-way repeated-measures ANOVA in which the condition was acquisition block (MONITOR, INHIBIT, RESPOND and COMPLEX). In close accordance with study 1, the Aron ROI showed a significant positive effect of condition (T contrast collapsed across all conditions t = 3.22, p = 0.001) but no significant main effect of acquisition block (F = 1.77, p = 0.16). Contrasting INHIBITION minus the other three conditions also showed no significant effect (t = − 0.88, p = 0.81), a result that again accords poorly with the notion of a dedicated motor response inhibition module. Furthermore, a similar and often more pronounced positive effect of condition was evident across all other ICA ROIs (Table 3) with no significant effects of acquisition block. Notably, the strongest response was within the anterior insula/inferior frontal operculum, with the scale of response being ~ 3 times that observed in the putative inhibitory module. These results demonstrate that in study 1 and study 2, although there was significant activation in response to stop signals at the proposed location of the inhibition module that activation was somewhat lesser than for other regions of the RIFC and was unrelated to the requirement for motor response inhibition.
Table 3.
reanalysis of ROI activation magnitudes from study 2.
| Positive effect of condition | t | p (one-tailed) | |
| Aron ROI | Pars opercularis | 3.22 | p = 0.001 |
| ROI1 | Pars opercularis | 3.21 | p = 0.001 |
| ROI2 | Insular cortex (mid) | 7.01 | p < 0.001 |
| ROI3 | Posterior middle frontal gyrus | 5.13 | p < 0.001 |
| ROI4 | Anterior insula/inferior frontal operculum | 7.13 | p < 0.001 |
| ROI5 | Frontopolar cortex | 4.84 | p < 0.001 |
| ROI6 | Anterior middle frontal gyrus | 5.28 | p < 0.001 |
| ROI7 | Pars triangularis | 5.22 | p < 0.001 |
| Main effect of acquisition block | F | p | |
| Aron ROI | Pars opercularis | 1.33 | 0.094 |
| ROI1 | Pars opercularis | 1.39 | 0.085 |
| ROI2 | Insular cortex (mid) | − 2.36 | 0.989 |
| ROI3 | Posterior middle frontal gyrus | − 0.84 | 0.798 |
| ROI4 | Anterior insula/inferior frontal operculum | 0.64 | 0.263 |
| ROI5 | Frontopolar cortex | − 0.59 | 0.720 |
| ROI6 | Anterior middle frontal gyrus | 0.29 | 0.388 |
| ROI7 | Pars triangularis | − 2.23 | 0.985 |
| Inhibition—other blocks | t | p (one-tailed) | |
| Aron ROI | Pars opercularis | − 0.88 | 0.808 |
| ROI1 | Pars opercularis | − 0.75 | 0.773 |
| ROI2 | Insular cortex (mid) | − 0.2 | 0.579 |
| ROI3 | Posterior middle frontal gyrus | − 0.47 | 0.678 |
| ROI4 | Anterior insula/inferior frontal operculum | − 0.21 | 0.583 |
| ROI5 | Frontopolar cortex | 0.02 | 0.492 |
| ROI6 | Anterior middle frontal gyrus | − 0.73 | 0.767 |
| ROI7 | Pars triangularis | 0.74 | 0.230 |
It has been suggested that one of the three control conditions in study 1 and study 2 (RESPOND) requires a motor response to be actively withheld during frequent trials and, consequently, that the task has a hidden inhibitory component that would be expected to tax the response inhibition module (Aron et al., 2014b). The mixed block-event-related design of study 2 allows activations during routine/frequent trials to be examined relative to a resting baseline. Therefore, to test this hypothesis, activations in response to frequent stimuli were compared across the four task blocks using repeated-measures ANOVA with acquisition block as the factor. The Aron ROI showed no positive effect of condition (t = 0.35, p = 0.79), no main effect of condition (F = 1.33, p = 0.09) and no greater activation when contrasting the RESPOND block minus the other three blocks (t = 0.19, p = 0.42). These results do not support the view that frequent trials in the RESPOND control task involved additional recruitment of an inhibition module within the RIFC.
Study 3—behavioural results
Performance was good for all variants of the task that involved overt responses. In the RESPOND OBJECTS block participants missed 11.3% (SD = 9.2) of infrequent targets and responded to 1.4% (SD = 2.0) of frequent distractors. In the RESPOND WORDS block participants missed 9.7% (SD = 11.5) of infrequent targets and responded to 0.7% (SD = 1.3%) of frequent distractors. In the INHIBIT WORDS block participants made erroneous responses on 9.5% (SD = 6.5) of infrequent no-go trials and missed 7.1% (SD = 4.8) of frequent go trials. The other three acquisition blocks did not record immediate responses to stimuli. Response times were not available for study 3.
Study 3—analysis of activation magnitudes
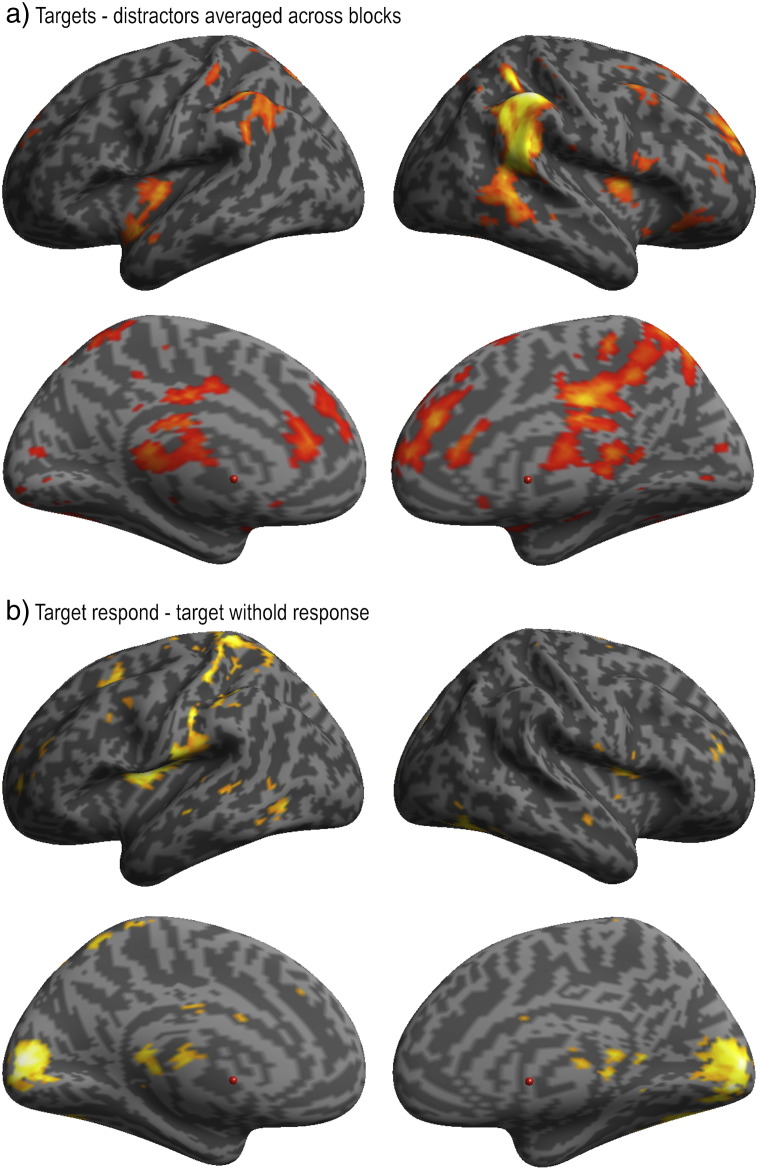
In the analysis of study 3, mean contrast values were first extracted from the Aron and ICA ROIs for targets minus distractors during each of the four acquisition blocks with object stimuli (faces and houses). Data were examined using a 4 × 8 repeated measure ANOVA in which the conditions were acquisition block and ROI. There was a significant positive effect of condition (t = 6.089, p < 0.001), a significant main effect of acquisition block (F(3,42) = 3.464, p = 0.025), a significant acquisition block × ROI interaction (F(21,294) = 2.912, p < 0.001) and no significant main effect of ROI (F(7,98) = 1.511, p = 0.173). In order to determine the basis of the main effects and interactions, the data from each ROI were examined separately using one-way repeated-measures ANOVAs with the condition acquisition block (MONITOR, COUNT, PREPARE and RESPOND). There was a positive effect of condition (T contrast averaged across all conditions) within all ROIs and a significant main effect of acquisition block in many ROIs, including the Aron ROI (Table 4). Plotting the target minus distractor contrasts for each ROI and for each acquisition block produced a highly variable pattern of results (Fig. 6). Specifically, although the ROIs generally activated more in the RESPOND block, some ROIs activated to targets under all conditions, including MONITOR, that is, regardless of whether an overt, covert or no response whatsoever was required. Prominent amongst these was the anterior insula/inferior frontal operculum. By contrast, the ROI placed within the mid insula activated selectively when a button press was made in response to the target. Several ROIs showed significant activation only for the three conditions in which targets triggered some further process, be that internal (i.e., counting or preparing to make a response) or overt (i.e., pressing a button). Prominent amongst these was the Aron ROI. These results demonstrate that although the sub-regions of the IFC that were delineated by the ICA all co-activate during motor response inhibition, they most likely sub-serve different cognitive functions as they are sensitive to different demands of the task. The results also demonstrate that the putative inhibition module responds to a broad range of conditions that do not require motor response inhibition, including when a stimulus is silently counted or a planned motor response is executed.
Table 4.
Analysis of ROI activation magnitudes for study 3 with picture stimuli.
| Positive effect of condition | t | p (one-tailed) | |
| Aron ROI | Pars opercularis | 4.91 | p < 0.001 |
| ROI1 | Pars opercularis | 5.19 | p < 0.001 |
| ROI2 | Insular cortex (mid) | 2.25 | 0.014 |
| ROI3 | Posterior middle frontal gyrus | 5.14 | p < 0.001 |
| ROI4 | Anterior insula/inferior frontal operculum | 5.95 | p < 0.001 |
| ROI5 | Frontopolar cortex | 2.77 | 0.003 |
| ROI6 | Anterior middle frontal gyrus | 4.46 | p < 0.001 |
| ROI7 | Pars triangularis | 4.63 | p < 0.001 |
| Main effect of acquisition block | F | p | |
| Aron ROI | Pars opercularis | 5.87 | p = 0.001 |
| ROI1 | Pars opercularis | 5.67 | p = 0.001 |
| ROI2 | Insular cortex (mid) | 3.61 | 0.017 |
| ROI3 | Posterior middle frontal gyrus | 1.89 | 0.137 |
| ROI4 | Anterior insula/inferior frontal operculum | 3.52 | 0.019 |
| ROI5 | Frontopolar cortex | 1.78 | 0.157 |
| ROI6 | Anterior middle frontal gyrus | 3.53 | 0.018 |
| ROI7 | Pars triangularis | 1.32 | 0.273 |
Fig. 6.
Analysis of ROI data for the four blocks of the target detection task with object images as stimuli showed a heterogeneous response profile across the RIFC. At one extreme, the mid insula was selectively activated when a motor response was executed in response to targets. At the other extreme, the pars triangularis and anterior insula/inferior frontal operculum responded to all target detection conditions, including when stimuli were passively monitored. Other ROIs, including the one centred on the proposed coordinates of the inhibition module, activated under all conditions in which target detection triggered a further process, be that the silent counting of targets, preparation to make a motor response, or the execution of a motor response. Error bars report the standard error of the mean.
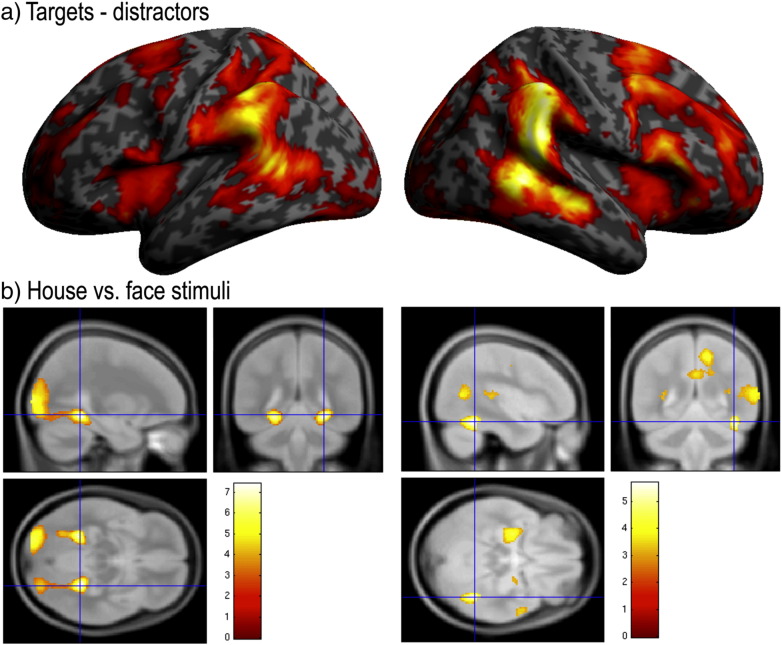
Supplemental voxelwise analyses rendered a broad set of frontal, parietal and sensorimotor areas for the positive effect of condition (Fig. 7a). Peak activation within the whole brain was in the right parietal cortex (PC right 54 − 38 50, t = 11.28 PC left x = − 56, y = − 38, z = 50, t = 7.29) and right temporal–parietal junction (TPJ right t x = 60, y = − 44, z = 10, t = 10.06, TPJ left x = − 56, y = − 52, z = 12, t = 5.77). Peak activation within the frontal lobes was in the right IFG pars opercularis (x = 60, y = 18, z = 8, t = 8.73). ROIs were defined at the peak PC and TPJ coordinates for further analysis. There was a significant main effect of Task within the right PC (t = 6.47, p < 0.001) but not within the right TPJ (t = 2.14, p = 0.069). These results were drive by greater activation in the PC when detecting targets with motor responses and lesser activation when simply monitoring for targets; however, there were significant responses to targets in the right PC in all six acquisition blocks (all p < 0.05).
Fig. 7.
(a) Voxelwise whole brain analysis contrasting targets minus distractors for the four acquisition blocks with object images as stimuli rendered activation across a set of brain regions, including the anterior insular/inferior frontal operculum bilaterally and the temporal–parietal junction bilaterally. (b) Contrasting the presentation of building minus face stimuli rendered activation within the ventral visual processing streams extending close to the expected coordinates of the parahippocampal place area. The reverse contrast rendered a distributed set of brain regions, including close to the expected coordinates of the fusiform face area (all rendered with FDR correction at p < 0.05 for the whole brain mass).
A supplemental analysis was conducted to identify category-specific regions of the visual processing streams (Fig. 7b). Contrasting blocks where targets and distractors were buildings minus blocks where they were faces rendered activation throughout a set of brain regions, including close to the expected coordinates of the parahippocampal place area (Epstein and Kanwisher, 1998) (right x = 28, y = − 44, z = − 10; left x = − 26, y = − 46, z = − 10). The opposite contrast rendered activation throughout a different set of brain regions, including close to the expected coordinates of the fusiform face area (Kanwisher et al., 1997; Sergent et al., 1992) (right x = 42, y = − 48, z = − 18), and a region between the lateral occipital cortex and middle temporal gyrus that is often observed during face processing (x = 60, y = − 58, z = 8).
Next, mean contrast estimates were extracted from the Aron ROI and the ICA ROIs for the two acquisition blocks with word stimuli. These data were examined in a 2 × 8 repeated-measures ANOVA in which the conditions were acquisition block (INHIBIT vs. RESPOND WORDS) and ROI. There was a significant positive effect of condition (t = 4.516, p < 0.001), a significant main effect of ROI (F(7,98) = 2.13, p = 0.047), no significant main effect of acquisition block (F(1,14) = 0.068, p = 0.798) and a sub-threshold ROI × acquisition block interaction (F(7,98) = 1.904, p = 0.077). Further analyses examined data individually for each ROI. Contrasting targets minus distractors showed significantly greater activation in the Aron ROI and the majority of the ICA ROIs (Table 5). There was no significant effect in any of the ROIs when comparing the target–distractor contrast across the INHIBIT minus the RESPOND WORDS blocks (Table 5). There were also no significant effects when contrasting between targets that triggered the withholding of a frequent motor response (INHIBIT block) vs. targets that triggered the execution of an infrequent motor response (RESPOND WORDS block) in any of the ROIs (all p > 0.4 one-tailed). Similarly, there were no significant effects when contrasting distractors that triggered a frequent response (INHIBIT block) vs. distractors that triggered no response (RESPOND WORDS block) in any of the ROIs (all p > 0.2 one-tailed).
Table 5.
Analysis of ROI activation magnitudes from study 3 with word stimuli.
| Targets minus distractor contrast | t | p (one-tailed) | |
| Aron ROI | Pars opercularis | 1.93 | 0.028 |
| ROI1 | Pars opercularis | 1.26 | 0.106 |
| ROI2 | Insular cortex (mid) | 2.88 | 0.003 |
| ROI3 | Posterior middle frontal gyrus | 2.42 | 0.009 |
| ROI4 | Anterior insula/inferior frontal operculum | 2.62 | 0.005 |
| ROI5 | Frontopolar cortex | 2.22 | 0.015 |
| ROI6 | Anterior middle frontal gyrus | 2.56 | 0.006 |
| ROI7 | Pars triangularis | 1.48 | 0.072 |
| Inhibition vs. respond | t | p (one-tailed) | |
| Aron ROI | Pars opercularis | − 0.259 | 0.600 |
| ROI1 | Pars opercularis | − 0.552 | 0.705 |
| ROI2 | Insular cortex (mid) | 1.662 | 0.060 |
| ROI3 | Posterior middle frontal gyrus | 0.116 | 0.455 |
| ROI4 | Anterior insula/inferior frontal operculum | 0.959 | 0.177 |
| ROI5 | Frontopolar cortex | 0.984 | 0.171 |
| ROI6 | Anterior middle frontal gyrus | 0.702 | 0.247 |
| ROI7 | Pars triangularis | 1.468 | 0.082 |
Supplemental voxelwise analyses were conducted to determine whether any voxel within the RIFC responded particularly strongly during the condition in which a routine response was withheld. Contrasting targets minus distractors collapsed across both acquisition blocks rendered activation within the anterior insula bilaterally and the RIFC at the whole brain corrected threshold (Fig. 8a). Contrasting targets where the response was withheld minus those where the response was made rendered no voxels within the inferior frontal cortices even at the very liberal uncorrected threshold of p < 0.05 one-tailed and uncorrected for multiple comparisons. However, the reverse contrast generated significant activation at a number of location within the brain at the whole brain corrected threshold, including right sensorimotor cortex; this activation most likely relates to the execution of a button press with the right hand in response to targets (Fig. 8b). Taken together, these results demonstrate that the putative inhibition module does not show particularly strong activation when motor responses are withheld relative to other regions of the RIFC or relative to other attentionally demanding task conditions in which there is no requirement for motor response inhibition.
Fig. 8.
(a) Voxelwise whole brain analysis contrasting targets minus distractors for the two acquisition blocks with object word stimuli rendered significant activation across a set of brain regions, including the anterior insula/inferior frontal operculum and the temporal–parietal junction bilaterally (FDR corrected at p < 0.05 for the whole brain mass). (b) Comparing the target–distractor contrast across the INHIBIT minus the RESPOND blocks did not render any significant voxels within the RIFC even at a liberal uncorrected threshold. The reverse contrast rendered activation across a set of brain regions, including the left sensorimotor cortices at the whole brain corrected threshold.
Study 3—psychophysiological interactions
In a previous article, we reported that connectivity across IFC sub-regions increases during the inhibition condition of the SST and other attentionally demanding conditions (Erika-Florence et al., 2014). In order to replicate this finding, psychophysiological interactions were generated for each reciprocal connection between the ROIs from the ICA. Specifically, data were extracted from one ROI for one acquisition block as the seed physiological time course, and this was interacted with a psychological time course consisting of all target events minus all distractor events. The resultant psychological, physiological and psychophysiological predictor functions were fitted to the time courses extracted from each of the other ICA ROIs. This process was repeated for each acquisition block and with each ROI as the seed. Parameter estimates for the PPI predictor function were averaged across target ROIs, and the resultant data were examined at the group level using a repeated-measures ANOVA in which the conditions were Seed (7) and Task (6). One participant's data set was excluded from this analysis due to multiple outlier values > 2.5 SDs from the mean. In accordance with the prediction of heightened inter-network connectivity during target detection, there was a significant positive effect of condition (T contrast averaged collapsed across all condition t = 2.31, p = 0.019). There was no significant main effect of Seed (F(6,78) = 1.105, p = 0.367), no significant main effect of Task (F(5,65) = 0.639, p = 0.67) and no significant Seed × Task interaction (F(30,390) = 0.818, p = 0.743). These results support the view that target detection involves an increase in the coupling between RIFC sub-regions across a range of stimulus and motor response conditions, including, but not limited to, motor response inhibition.
Discussion
Is there an inhibition module within the posterior inferior frontal gyrus?
Proponents of the response inhibition hypothesis have recently restated their modular perspective and have dismissed conflicting evidence from the broader neuroimaging literature (Aron et al., 2014a,b). Most notably, they have suggested that previous results indicating a more general role for sub-regions of the RIFC did not include any analysis of a region at the dorsal extent of the posterior inferior frontal gyrus (pIFG) and that it is this area that houses the response inhibition module (Levy and Wagner, 2011). This argument is somewhat misleading because those studies (Erika-Florence et al., 2014; Hampshire et al., 2010) included voxelwise whole brain analyses, which were reported at a very liberal uncorrected threshold. It was observed that no voxels within any part of the RIFC were activated during response inhibition either specifically or even particularly strongly relative to carefully designed control conditions.
Here, I have reinforced these findings by examining how activation levels within an ROI placed at the exact proposed pIFG coordinates (Aron et al., 2014b) varied across multiple studies and in response to a variety of task demands. The results clearly demonstrated that while this area of the RIFC activates during the classic SST it also activates to the same level during a wide variety of other conditions that have no obvious requirement for motor response inhibition. Furthermore, the pIFG region was less active during motor response inhibition conditions than some of the other RIFC ROIs, for example, the anterior insula/inferior frontal operculum.
More broadly, it is questionable precisely where within the RIFC volume an inhibition module would be located. For example, although activation during SST inhibition is invariably widespread, the results from studies 1–3 demonstrate that it tends to be strongest within a ventral area that includes the anterior insula and inferior frontal operculum. A similar ventral area has been reported in a conjunction analysis of SST and GNG studies (Rubia et al., 2001a) and has been observed to hypo-activate in attentional deficit hyperactivity disorder patients (Rubia et al., 1999). Moreover, drugs that are used to treat such patients have been reported to modulate activity within more ventral areas, including the anterior insula/inferior frontal operculum with concomitant improvements in SST performance (Chamberlain et al., 2008; Cubillo et al., 2014). Therefore, the findings reported here and from the broader literature are at odds with the notion that it is the dorsal pIFG in particular that mediates motor response inhibition.
The incongruence between meta-analysis and ICA decompositions of the RIFC
The advantage of the ICA method is that it is data driven and model free; consequently, is not biased by prior assumptions regarding the functional organisation of the analysed brain volume. Here, the local ICA decompositions of the RIFC were relatively consistent across all three studies. However, none of the components centred on the putative pIFG inhibition module; instead, the pIFG coordinates corresponded with a point in the brain where clusters from at least three ICA components overlapped (Fig. 3). The most likely explanation for this incongruence is that the functional resolution of any meta-analysis contrast is limited by the accuracy of the assumptions that are made regarding exactly what the distinct components of human cognition are. For example, when contrasting inhibition minus attentional orienting tasks, if the inhibition construct was heterogeneous and captured several distinct processes that were less prominent within the attention category, then that contrast would have identified multiple functionally distinct brain regions. If some of those brain regions were in close proximity to each other, as appears to be the case within the RIFC, then the peak region from the meta-analysis would have been in between them.
One objection to this interpretation could be that the ICA decompositions reported here may have been incomplete; consequently, they might have missed a distinct inhibition module within the pIFG. This is highly unlikely to be the case for several reasons. First, the inhibition module should be strongly activated during inhibition tasks; therefore, one would expect that it would contributed significant variance to and be evident within the ICA decomposition. More importantly, doubling the dimensionality of the ICA decomposition still did not produce a component at the expected pIFG coordinates. Finally, as discussed above, even when an ROI was placed at the precise pIFG coordinates of the meta-analysis, it showed no particular sensitivity to the inhibition demands of the three studies.
The behavioural literature on response inhibition provides further support for this interpretation. There is little in the way of evidence to support the view that response inhibition is a unitary construct (Stuss and Alexander, 2007). For example, it has been observed that individual differences in performances tend not to correlate strongly across inhibition tasks (Rush et al., 2006; Shilling et al., 2002) and that individual difference in SST performance may be accounted for by other cognitive processes, including attention and motor speed (Salinas and Stanford, 2013). It has also been reported that stop signal reaction time, which is the main measure of motor inhibition performance from the SST, is sensitive to conditions that would not typically be considered part of an inhibition construct; a prominent example is motivational state when the reward parameters of the task are manipulated (Herrera et al., 2014). It is questionable whether it makes sense to map a complex and heterogeneous cognitive construct like motor response inhibition onto a unique and dedicated module within the brain.
The notion of hidden inhibitory processes
It has also been suggested that the observed lack of a significant difference between activations during the response inhibition and attentional control conditions of study 1 and study 2 could have been a consequence of there being hidden motor inhibition demands in some of the control blocks (Aron et al., 2014b). It was proposed that such hidden inhibitory processes were evident in the slowing of routine motor responses when attention was captured by infrequent, salient or otherwise surprising stimuli. This interpretation of the results has a number of notable shortcomings.
First, the notion of hidden inhibitory processes accounts poorly for those conditions in which there is no slowing of routine responses. For example, in study 2, a control condition was included in which participants were asked to respond to frequent go stimuli and to simply monitor the infrequent stimuli with no change to their routine motor responses. This manipulation activated all of the RIFC sub-regions to a similar level as the response inhibition condition, including the pIFG; however, there was no slowing of the routine response on trials in which the infrequent stimuli were attended. Furthermore, one of the control conditions from study 2 was singled out as involving a hidden inhibition process (Aron et al., 2014b), yet this interpretation cannot account for the observed results. Specifically, in the RESPOND condition individuals were instructed to make a button press only when infrequent cues were detected and to do so by indicating the direction of the most recent of the frequently presented left and right cues. It was suggested that the frequent cues would have triggered an immediate motor response that would then have to be inhibited until an infrequent cue was detected. Certainly, this manipulation was likely to involve some degree of preparatory processing, although the notion that this involved top-down inhibition as opposed to the planning of a response is debatable. Either way, this manipulation was designed to invert the relationship that frequent and infrequent stimuli have with going and stopping in the SST. More specifically, the infrequent cues were presented in an unpredictable pseudo-randomised sequence. Therefore, any preparatory processes would have been elicited on all trials regardless of whether there was an infrequent cue; consequently, contrasting the trials with infrequent cues minus those without would have isolated the execution of a response as opposed to the preparatory processes. Finally, it has been reported in a parallel line of research (Sharp et al., 2010) that when attentional orienting was associated with the slowing of routine responses, the scale of that slowing was independent from the level of activation observed within the RIFC. This result does not accord with the notion that an RIFC region mediates hidden inhibitory processes during attentional orienting.
On a theoretical level, any slowing of routine responses when attentionally salient stimuli are presented may equally be accounted for without recourse to a dedicated inhibition module or connection pathway. For example, cortical regions typically have many local inhibitory connections. Consequently, when two items of information form distinct distributed representations across the same population of neurons they compete with each other. It has long been established that focusing attention on one or other competing item will bias processing towards that items corresponding representation; a secondary consequence of this top-down potentiation is the suppression of competing representations via local lateral inhibition (Desimone and Duncan, 1995). It logically follows that when top-down resources are reoriented towards one stimulus or response then other stimulus–response processes will be impeded, that is, assuming there is local competition between them at some point in the stimulus–response processing stream. Consequently, the slowing of routine responses when infrequent stimuli are detected cannot be cited as evidence either for or against the existence of a motor inhibition module. Indeed, in this respect, all cognitive processes involve hidden inhibitory processes, but they occur ubiquitously within local neural populations.
Functional dissociations within the RIFC
SST and GNG tasks do not typically include adequate controls for non-inhibitory demands. For example, in the classic SST, the exact same stimuli are always used for go and stop trials; therefore, the stop-go contrast is confounded by differences in the visual properties and the familiarities of the stimuli. Moreover, both paradigms conflate the requirement to inhibit a response with the detection of a target and the execution of an effortful and infrequent task plan. Study 3 was designed to address these issues by controlling for stimulus conditions whilst varying the response required from simple target detection, through internal counting and preparation of a motor response, to the immediate execution of a motor response. Heightened activations were still evident throughout the RIFC for the contrast of targets minus distractors when the salience and familiarity of the individual stimuli were balanced. Furthermore, significant activations were evident throughout the RIFC regardless of whether stimuli were object words or images of faces and buildings.
An informative comparison can be made with the findings of a previous study that controlled for stimulus frequency in the context of a novel GNG paradigm. More specifically, it was observed that there was little difference in RIFC activation when frequent and infrequent Go stimuli were contrasted (Chikazoe et al., 2009). Conversely, contrasting the no-go minus go stimuli that were matched for frequency did activate the RIFC. Contrasting the infrequent no-go and go stimuli in this manner demonstrated that the level of stimulus infrequency employed in the GNG task was not sufficient to account for the observed RIFC activation. Notably, both the frequent and infrequent Go stimuli mapped to the same routine response; therefore they had the same relevance to the overarching task schema. By comparison, the studies presented here applied control conditions that were more analogous to the stop condition of the SST insofar as they explicitly assigned additional task-related processes to the infrequent stimuli. Thus, RIFC activation during target detection and response inhibition most likely relates to the allocation of cognitive resources as opposed to the relative frequencies of stimuli. Indeed, in a previous study, I reported that the RIFC activated in response to targets even when the overall frequencies of the targets and distractors were matched at a 1:1 ratio (Hampshire et al., 2009). When taken together, these results accord with the task-oriented nature of processing within multiple demand regions of the brain (Duncan, 2001, 2006; Hampshire et al., 2009; Chatham et al., 2012).
More interestingly, the results from the acquisition blocks with object pictures demonstrated that the RIFC ROIs were dissociable across the four response conditions. For example, the anterior insula/inferior frontal operculum ROI activated significantly to the presentation of targets in all acquisition blocks, which highlights a likely role at the very general level of attention to task-relevant stimuli. This result accords well with the meta-analysis of Levy and colleagues, which reported a similar broad role for this region in both inhibition and attentional orienting tasks (Levy and Wagner, 2011). It is notable that the associated regions in the seed analysis are similar to those that are often labelled the ‘salience network’ and have been reported for contrasts intended to isolate attentional demands (Hampshire and Owen, 2006; Seeley et al., 2007). However, it is unlikely that this region of the RIFC is involved only in attentional orienting because it was most strongly activated when a motor response was elicited in study 3. Furthermore, this region has previously been observed to activate under conditions in which attention is not focused on external stimuli, for example, working memory maintenance (Owen et al., 1996; Owen and Hampshire, 2009) and mental imagery (Hampshire et al., 2013).
At the other extreme, the mid insula ROI activated selectively when a motor response was executed, suggesting a role that is more closely related to either sensory feedback or motor processing. In this respect, it is notable that whole brain analysis with this regions as a seed rendered activation across the sensorimotor cortices.
By contrast, several of the more dorsal and anterior RIFC sub-regions showed little activation when targets were simply being monitored in study 3 but significant activation when the targets elicited some further task process, for example, the incrementing of an internal count and the preparation or immediate execution of a motor response. Notably, these regions included the putative coordinates of the inhibition module, which responded most strongly when a motor response was either prepared or executed. Again, this pattern of results accords well with the findings of Levy and colleagues (Levy and Wagner, 2011), who observed greater activation in this region during response inhibition relative to simple attentional orienting. However, the nature of that dissociation is more general than inhibition vs. attention. Thus, the observed heterogeneity of response demonstrates that the RIFC sub-regions identified in the data-driven analyses most likely support different aspects of the task program, varying from the detection of task-relevant stimuli, through the processing of internal task plans, to the overt execution of motor responses. In the context of classic response inhibition paradigms, these functionally distinct sub-regions are co-recruited and must interact closely.
Future challenges
In a previous article, it was reported that attentionally demanding conditions, including target detection and motor response inhibition, not only elicit heightened activation across RIFC sub-regions but also lead to an increase in the functional connectivity between those sub-regions (Erika-Florence et al., 2014). Here, these results were replicated in the PPI analysis of ROI data from study 3. Specifically, there was a general increase in cross correlation between RIFC sub-regions during target detection. The increase in inter-network connectivity did not vary significantly between seed regions or across acquisition blocks. Thus, the results accord particularly closely with the view that response inhibition is an emergent property of interactions that occur throughout a functionally heterogeneous ensemble of domain general networks. A major future challenge is to determine how this set of networks coordinate to support such diverse cognitive demands. For example, more research is required to determine the distinct contributions of each RIFC sub-region. Also, methods with finer temporal resolution than fMRI should be applied to determine how those sub-regions interact with each other; including whether there is a hierarchical organisation with a consistent direction to the flow of information between RIFC sub-regions or whether the nature of interactions vary dependent on the type of cognitive process that is being undertaken. A future question also regards whether the network interactions occur within specific frequency domains. From a clinical-translational perspective, there may be value in going beyond the simple analyses of regional activation magnitudes or individual connection pathways to determine how the dynamic interactions of RIFC networks mediate the relationship between pathologies, interventions and response inhibition impairments.
A number of other important cognitive processes have been related to RIFC function in carefully controlled studies, some of which have been explored using elegant quantitative models (Band et al., 2003; Verbruggen and Logan, 2008). Prominent examples include action updating (Verbruggen et al., 2010), expectancy violation (Zandbelt et al., 2013), attentional switching (Hampshire and Owen, 2006) and working memory maintenance (Owen et al., 1996; Petrides, 1994). For a model of RIFC function to be plausible, it must parsimoniously account for all of the process attributed to each sub-region whilst being specific enough to account for the robust dissociations that are evident between those regions. I have previously argued that a diverse range of putatively distinct cognitive tasks may be accounted for by the same basic process of top-down potentiation of task-relevant information in the context of local lateral inhibition. The dissociations between RIFC sub-regions observed here lead me to tentatively propose an extension to this view; specifically, these dissociations may reflect the distinct components of an intentional processing stream that maps task-relevant stimulus inputs to internal task plans and then onwards to the relevant responses. Such a pathway would complement more direct stimulus–response mappings for routine and habitual behaviours that require little top-down control. From a quantitative perspective, these alternative pathways could account for the ‘horse-race’ dynamics of response inhibition behaviour (Band et al., 2003) and the dissociation between stimulus processing and control (Verbruggen et al., 2010) in a model that generalises to a broader range of tasks, including target detection. An important future direction is to instantiate this multi-stage model as a working simulation in order to determine whether it is able account for the range of processes associated with the RIFC whilst conforming to the observed dynamic interactions of RIFC networks.
Acknowledgements
This work was supported by the Medical Research Council (grant no. U1055.01.002.00001.01) and the European Research (grant no. PCIG13-GA-2013-618351).
References
- Aron A.R. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol. Psychiatry. 2011;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Poldrack R.A. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Dowson J.H., Sahakian B.J., Robbins T.W. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Fletcher P.C., Bullmore E.T., Sahakian B.J., Robbins T.W. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans (vol 6, pg 115, 2003) Nat. Neurosci. 2003;6:1329. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. Right inferior frontal cortex: addressing the rebuttals. Front. Hum. Neurosci. 2014;8:905. doi: 10.3389/fnhum.2014.00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A., Robbins T., Poldrack R. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn. Sci. 2014;18(4):177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Band G.P.H., van der Molen M.W., Logan G.D. Horse-race model simulations of the stop-signal procedure. Acta Psychol. 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Braga R.M., Sharp D.J., Leeson C., Wise R.J., Leech R. Echoes of the brain within default mode, association, and heteromodal cortices. J. Neurosci. 2013;33:14031–14039. doi: 10.1523/JNEUROSCI.0570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J., Valabregue R., Poline J. 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan. 2002. Region of interest analysis using an SPM toolbox. [Google Scholar]
- Chamberlain S.R., Hampshire A., Muller U., Rubia K., Campo N.D., Craig K., Regenthal R., Suckling J., Roiser J.P., Grant J.E., Bullmore E.T., Robbins T.W., Sahakian B.J. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol. Psychiatry. 2008;65(7):550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Chatham C.H., Claus E.D., Kim A., Curran T., Banich M.T., Munakata Y. Cognitive Control Reflects Context Monitoring, Not Motoric Stopping, in Response Inhibition. PLoS ONE. 2012;7(2) doi: 10.1371/journal.pone.0031546. e31546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J., Jimura K., Asari T., Yamashita K., Morimoto H., Hirose S., Miyashita Y., Konishi S. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb. Cortex. 2009;19:146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- Cools R., Clark L., Owen A.M., Robbins T.W. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J. Neurosci. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A., Smith A.B., Barrett N., Giampietro V., Brammer M.J., Simmons A., Rubia K. Shared and drug-specific effects of atomoxetine and methylphenidate on inhibitory brain dysfunction in medication-naive ADHD boys. Cereb. Cortex. 2014;24:174–185. doi: 10.1093/cercor/bhs296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R., Duncan J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat. Rev. Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- Duncan J. EPS Mid-Career Award 2004: brain mechanisms of attention. Q. J. Exp. Psychol. (Colchester) 2006;59:2–27. doi: 10.1080/17470210500260674. [DOI] [PubMed] [Google Scholar]
- Duncan J., Owen A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Epstein R., Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Erika-Florence M., Leech R., Hampshire A. A functional network perspective on inhibition and attentional control. Nat. Commun. 2014;5 doi: 10.1038/ncomms5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Hampshire A., Owen A.M. Fractionating attentional control using event-related fMRI. Cereb. Cortex. 2006;16:1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Hampshire A., Duncan J., Owen A.M. Selective tuning of the blood oxygenation level-dependent response during simple target detection dissociates human frontoparietal subregions. J. Neurosci. 2007;27:6219–6223. doi: 10.1523/JNEUROSCI.0851-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Thompson R., Duncan J., Owen A.M. The target selective neural response--similarity, ambiguity, and learning effects. PLoS ONE. 2008;3(6) doi: 10.1371/journal.pone.0002520. e2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Thompson R., Duncan J., Owen A.M. Selective tuning of the right inferior frontal gyrus during target detection. Cogn. Affect. Behav. Neurosci. 2009;9:103–112. doi: 10.3758/CABN.9.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Chamberlain S.R., Monti M.M., Duncan J., Owen A.M. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Highfield R.R., Parkin B.L., Owen A.M. Fractionating human intelligence. Neuron. 2012;76:1225–1237. doi: 10.1016/j.neuron.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Hampshire A., Parkin B., Cusack R., Pickard J., Owen A.M. Assessing residual reasoning ability in overtly non-communicative patients using fMRI. Neuroimage. 2013;2:174–183. doi: 10.1016/j.nicl.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera P.M., Speranza M., Hampshire A., Bekinschtein T.A. Monetary rewards modulate inhibitory control. Front. Hum. Neurosci. 2014;8:257. doi: 10.3389/fnhum.2014.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N., McDermott J., Chun M.M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B.J., Wagner A.D. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann. N. Y. Acad. Sci. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D.E.J., Prvulovic D., Formisano E., Vollinger M., Zanella F.E., Goebel R., Dierks T. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cereb. Cortex. 1999;9:815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- Logan G.D., Cowan W.B. On the ability to inhibit thought and action—a theory of an act of control. Psychol. Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Owen A.M. The functional organization of working memory processes within human lateral frontal cortex: the contribution of functional neuroimaging. Eur. J. Neurosci. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Owen A.M., Hampshire A. The mid-ventrolateral frontal cortex and attentional control. In: Frank Rosler C.R., Roder Brigitte, Kluwe Rainer, editors. Neuroimaging in Human Memory. Oxford University Press; Oxford: 2009. pp. 195–212. [Google Scholar]
- Owen A.M., Evans A.C., Petrides M. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cereb. Cortex. 1996;6:31–38. doi: 10.1093/cercor/6.1.31. [DOI] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and working memory: evidence from investigations of the effects of cortical excisions in nonhuman primates. In: Boller F., Grafman J., editors. Handbook of Neuropsychology. Elsevier; Amsterdam: 1994. pp. 59–82. [Google Scholar]
- Rosazza C., Minati L. Resting-state brain networks: literature review and clinical applications. Neurol. Sci. 2011;32:773–785. doi: 10.1007/s10072-011-0636-y. [DOI] [PubMed] [Google Scholar]
- Rubia K., Overmeyer S., Taylor E., Brammer M., Williams S.C., Simmons A., Bullmore E.T. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am. J. Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubia K., Russell T., Overmeyer S., Brammer M.J., Bullmore E.T., Sharma T., Simmons A., Williams S.C., Giampietro V., Andrew C.M., Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A., Lidzba K., Toone B., Simmons A., Williams S.C.R., Taylor E. Neural substrates of successful versus unsuccessful stopping in a cognitively challenging event related stop task. Neuroimage. 2001;13:S351. [Google Scholar]
- Rubia K., Halari R., Mohammad A.M., Taylor E., Brammer M. Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2011;70:255–262. doi: 10.1016/j.biopsych.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush B.K., Barch D.M., Braver T.S. Accounting for cognitive aging: context processing, inhibition or processing speed? Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2006;13:588–610. doi: 10.1080/13825580600680703. [DOI] [PubMed] [Google Scholar]
- Salinas E., Stanford T.R. The countermanding task revisited: fast stimulus detection is a key determinant of psychophysical performance. J. Neurosci. 2013;33:5668–5685. doi: 10.1523/JNEUROSCI.3977-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent J., Ohta S., MacDonald B. Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain. 1992;115(Pt 1):15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- Shallice T., Stuss D.T., Picton T.W., Alexander M.P., Gillingham S. Mapping task switching in frontal cortex through neuropsychological group studies. Front. Neurosci. 2008;2:79–85. doi: 10.3389/neuro.01.013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D.J., Bonnelle V., De Boissezon X., Beckmann C.F., James S.G., Patel M.C., Mehta M.A. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilling V.M., Chetwynd A., Rabbitt P.M. Individual inconsistency across measures of inhibition: an investigation of the construct validity of inhibition in older adults. Neuropsychologia. 2002;40:605–619. doi: 10.1016/s0028-3932(01)00157-9. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss D.T., Alexander M.P. Is there a dysexecutive syndrome. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D., Chatham C.H. Ten years of inhibition revisited. Front. Hum. Neurosci. 2014;8:329. doi: 10.3389/fnhum.2014.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Verbruggen F., Logan G.D. Response inhibition in the stop-signal paradigm. Trends Cogn. Sci. 2008;12:418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F., Aron A.R., Stevens M.A., Chambers C.D. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13966–13971. doi: 10.1073/pnas.1001957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbelt B.B., Bloemendaal M., Hoogendam J.M., Kahn R.S., Vink M. Transcranial magnetic stimulation and functional MRI reveal cortical and subcortical interactions during stop-signal response inhibition. J. Cogn. Neurosci. 2013;25:157–174. doi: 10.1162/jocn_a_00309. [DOI] [PubMed] [Google Scholar]
- Zhang S., Li C.S. Functional networks for cognitive control in a stop signal task: independent component analysis. Hum. Brain Mapp. 2012;33:89–104. doi: 10.1002/hbm.21197. [DOI] [PMC free article] [PubMed] [Google Scholar]