Abstract
The aim of this study was to test whether an omental pouch can be used as an alternative site for islet implantation in diabetic monkeys. Here we report the successful engraftment of islets in diabetic cynomolgus monkeys when loaded on a synthetic biodegradable scaffold and placed in an omental pouch. One autologous and 5 allogeneic diabetic monkey transplants under the cover of steroid-free immune suppression were undertaken. Fasting blood glucose (FBG) and c-peptide (CP), exogenous insulin requirements (EIR), intravenous glucose tolerance test (IVGTT), A1C and histopathology were used to assess islet engraftment and survival. All animals achieved CP levels > 1.0 ng/ml following transplant, a 66 – 92% post-transplant decrease in EIR and reduced A1C. Following graft removal, CP became negative and histopathological analysis of the explanted grafts demonstrated well granulated and well vascularized, insulin positive islets, surrounded by T cell subsets and macrophages. Compared to intrahepatic allogeneic islet transplants (n=20), there was a delayed engraftment for omental pouch recipients but similar levels of CP production were ultimately achieved, with a broad range of IEQ/kg transplanted in both sites. Our results suggest this extrahepatic transplantation site has potential as an alternative site for clinical islet cell transplantation.
Keywords: Islet transplantation, alternative sites, graft survival, islet engraftment, scaffold
Introduction
Insulin independence has been achieved via clinical islet transplantation worldwide (1–9). The liver is currently the site of choice for clinical islet transplantation, chosen based on portal insulin delivery, avoidance of systemic hyperinsulinemia, and relative ease of transplant with a minimally invasive procedure. The liver also possesses distinct disadvantages, primarily in the early post transplant period, including activation of hemostasis and thrombotic mechanisms that contribute to early islet loss post transplant (10–13), macrophage and Kuppfer cell mediated inflammation (14, 15), and the potential for hemorrhagic and thrombotic events (16), as well as higher levels of immune suppression in the portal circulation that are directly toxic to or impair engraftment of islets (17). In addition, there is development of periportal steatosis (18) and an inability to routinely monitor the site with biopsies. A more favorable site for islet engraftment would: 1) provide portal venous drainage to permit normalization of blood glucose levels; 2) provide a rich arterial supply to the islets, thereby increasing oxygen tension; 3) allow for minimally invasive clinical islet infusion; 4) allow easy access for functional and morphological follow-up of the islets post-transplant; and 5) provide a microenvironment that prevents early islet loss, thereby enhancing islet engraftment. The greater omentum fits requirements 1–4, particularly if mobilized to a subcutaneous or intramuscular portion of the abdominal wall. The omentum has good arterial pedicles, numerous lymphatic vessels and exclusive portal drainage (19). The tissue has a high vascular density and allows for good neo-angiogenesis. It is commonly used in surgery to cover aseptic or septic areas. Although the volume of the greater omentum varies with nutritional status in humans, an omental pouch can be constructed even in cases of malnutrition. Its pedicles are long enough to place the pouch in a superficial, subcutaneous area, thereby enabling monitoring of implanted islets via Doppler-Ultrasonography and thermography, and graft biopsies. The omental pouch of diabetic dogs has been successfully used as a site for autologous islet implant (20–22). In a study by Ao, et. al., a few dogs were also transplanted with allogeneic islets; interestingly, an increased islet mass was required to achieve insulin independence in the omental pouch as compared to the spleen, and lower levels of insulin were observed in the omental pouch animals. Despite these earlier studies, the omentum as an alternative site for clinical transplantation has not gained acceptance. It is generally accepted that the nonhuman primate is a better model for humans than the dog. Reasons include the cross-reactivity of various biological agents with NHP cells, similarity of islet architecture and function (23) and the fact that nonhuman primates are upright, thereby imposing different physical constraints on alternative graft sites such as the omentum. We have shown that the omental anatomy of adult monkeys is very similar to that of humans (24). The aim of this study was to test an omental pouch as an alternative site for islet implantation in streptozotocin-induced diabetic cynomolgus monkeys.
Methods
Animals
Donor and recipient cynomolgus monkeys were obtained from Charles River BRF, Inc. (Houston, TX) and were negative for TB, Herpes B, SRV, SIV and STLV-1. Animals >4 and >2 years of age were used as donors and recipients, respectively. Pair-housed monkeys were supplied with water ad libitum and fed twice daily. The University of Miami complies with the Animal Welfare Act of 1966 (PL89-544) as amended by the Welfare Act of 1970 (PL91-279), adheres to the principals stated in the guide for the care and use of laboratory animals – NIH publication #85-23 (revised) and is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Donor-recipient pairs were ABO compatible (25).
Diabetes induction, metabolic monitoring, and insulin administration
The monkey was NPO the night before diabetes induction; streptozotocin (STZ, 1,250 mg/m2 i.v.) was infused over an eight minute period (13, 26). Diabetes in this model was defined as fasting CP levels <0.2 ng/ml, and a negative CP response (stimulated c-peptide <0.3 ng/ml) to a glucagon challenge undertaken four weeks after STZ treatment (13). For the autologous implant, diabetes was induced via pancreatectomy, as previously described (27); pancreatic exocrine insufficiency was compensated with Viokase-V (Fort Dodge Animal Health, Fort Dodge, IA). After diabetes induction, as well as following islet cell transplant, blood glucose levels were monitored 2–3 times daily via heel stick using a OneTouch® Ultra® Glucometer (LifeScan, Inc., Milpitas, CA). Subcutaneous insulin was administered (Humulin® N, Elli Lilly, Indianapolis, IN or Humulin® N + Lantus®, Sanofi Aventis, Bridgewater, NJ) as needed and based on an individualized sliding scale, aiming for fasting and post-prandial plasma glucose levels of 150–250 mg/dL post-STZ and prior to transplantation.
A double-antibody RIA method (Diagnostics Products, Los Angeles, CA) was used to assess plasma insulin and c-peptide levels. For IVGTT results, the incremental area under the curve (AUC) was calculated using the trapezium rule (28) and adjusted for the fasting values. CP values are represented in ng/ml.
Donor or recipient pancreatectomy, islet preparation, culture and islet quality control
The donor pancreas was recovered as previously described (13, 27, 29). In the one recipient of autologous islets, ligatures were placed around all vessels and then tied immediately before pancreas removal. NHP islet isolation was performed using modifications (29) of the automated method for human islet isolation (30) and purified in discontinuous Euroficoll gradients (13, 27, 29). After culture for 16–42 hours, islets were collected, washed and counted to determine IEQ (13, 29). Islets from one (animals 1 and 2) or two donors (animals 3–6) were seeded on the porous side of a synthetic absorbable scaffold composed of VICRYL (Polyglactin 910) and PDS (polyP-Dioxanon) (Codman Ethisorb™ Dura Patch, Raynham, MA) (size 40 × 60 mm). The scaffold had been pre-wet with culture medium containing serum autologous to the islets from POD −1 to POD 0.
Islet purity was estimated based on the percentage of dithizone positive particles present in the preparation (31, 32), and viability was estimated based on fluorescein diacetate/propidium iodide staining (33). In vitro functional capacity was determined via assessment of dynamic glucose-stimulated insulin secretion using a column-perifusion assay (34). The stimulation index (SI) was calculated as the ratio of insulin released under high glucose (11 mM) divided by the insulin released under low (3 mM) glucose concentrations.
Intrahepatic islet transplantation
Under general anesthesia, the recipients underwent a mini-laparotomy in order to access a mesenteric tributary of the portal vein. A small, supraumbilical central midline incision was made, and the islets were infused via gravity through a 24-gauge intravenous catheter over a period of 5 minutes (13).
Islet transplantation in the omental pouch
Under general anesthesia, the recipients underwent a mini-laparotomy and the greater omentum was mobilized and exposed. The pre-wetted scaffold was trimmed by approximately 20% and placed on the omentum with the porous side facing upwards. The islets were allowed to settle and then gently drawn into a 1 ml Hamilton syrynge and delivered to the porous side of the scaffold material. After placing the islets on the scaffold, it was wrapped 3 times with omentum, and the margins were sutured together with 2.0 silk. This composite omental pouch was then inserted into an intramuscular pocket created through the internal oblique and between the transverse abdominal muscles. The mobilized pouch was then fixed in placed with 2.0 silk and the muscle wall closed with 3.0 vycril.
Postoperative monitoring and insulin administration
Clinical signs, fluid balance, blood glucose, body weight, and nutritional intake were monitored regularly, and weekly blood tests were done to monitor overall health. Blood samples for determination of Rapamycin and FK506 trough levels were obtained weekly. Following islet transplantation, insulin was administered as needed to maintain FBG in the 100–150 mg/dl range and post-prandial glucose in the 100–200 mg/dl range. Fasting c-peptide levels were monitored weekly and intravenous glucose tolerance tests (IVGTT) were undertaken every 6–8 weeks post-transplant to assess graft function (29).
Experimental design
Steroid-free immune suppression was initiated on POD −2 and consisted of FK506 (Astellas Pharma, Deerfield, IL) at 0.02 mg/kg, IM BID, adjusted to maintain trough levels of 4–6 ng/ml and Rapamycin (LC Laboratories, Woburn, MA) at 0.05 mg/kg, IM BID on POD −2 and −1 and SID thereafter, adjusted to maintain trough levels of 12–20 ng/ml; Zenapax induction was administered at 1 mg/kg, IV on POD −1 and repeated every other week for 5 doses. On POD 0, recipient animals were transplanted with islets placed intrahepatically (n = 20 allogeneic transplants), or on one (animals 2, 3, 5 and 6) or two scaffolds (animals 1 and 4) placed in an omental pouch. One autologous (animal 1) and 5 allogeneic (animals 2–6) implants were undertaken in an omental pouch.
Histopathological analysis
Explanted grafts were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned (5 μm) and stained with hematoxylin and eosin (H&E). Insulin expression in the islets was assessed by immunohistochemistry using a guinea pig anti-porcine insulin polyclonal antibody (Dako, Carpinteria, CA) and a biotinylated donkey anti-guinea pig immunoglobulin (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), followed by streptavidin-horseradish peroxidase and revealed by aminoethylcarbazole (Invitrogen, Carlsbad, CA). For immunofluorescence microscopy, sections were stained with anti-insulin (AbCam, Cambridge, MA), anti-glucagon (Sigma, St. Louis, MO), anti-somatostatin, anti-CD68 (DakoCytomation) for macrophages, anti-CD4 for helper T cells, anti-CD14 (BD) for monocytes, anti-CD8 for cytotoxic T cells, or anti-CD20 (Beckman Coulter, Fullerton, CA) for B cells, or anti-CD31 (AbCam) and anti-CD34 (BioGenex, San Ramon, CA) for blood vessels followed by Alexa Fluor conjugated secondary antibodies (Molecular Probes, Carlsbad, CA) and cell nuclei were stained with DAPI (Molecular Probes). As a negative control, the primary antibody was omitted, and immunofluorescence analysis was performed using a Zeiss LSM 510 confocal microscope.
Results
Islet transplantation in an omental pouch
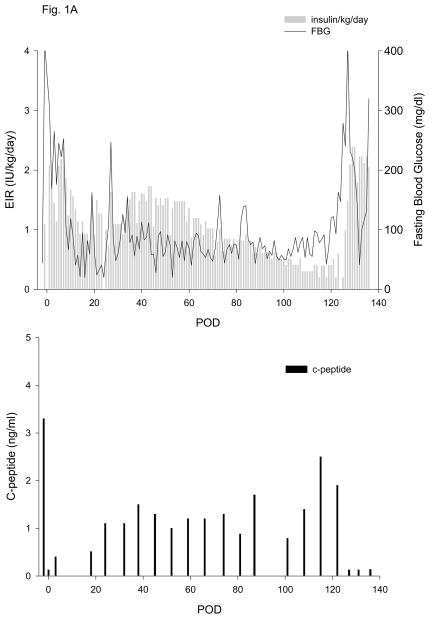
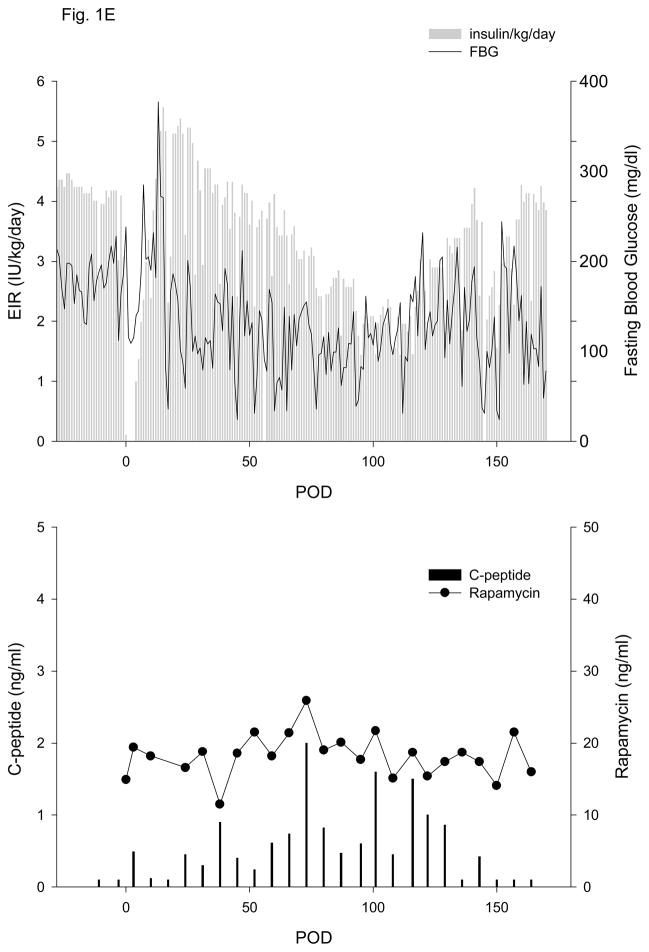
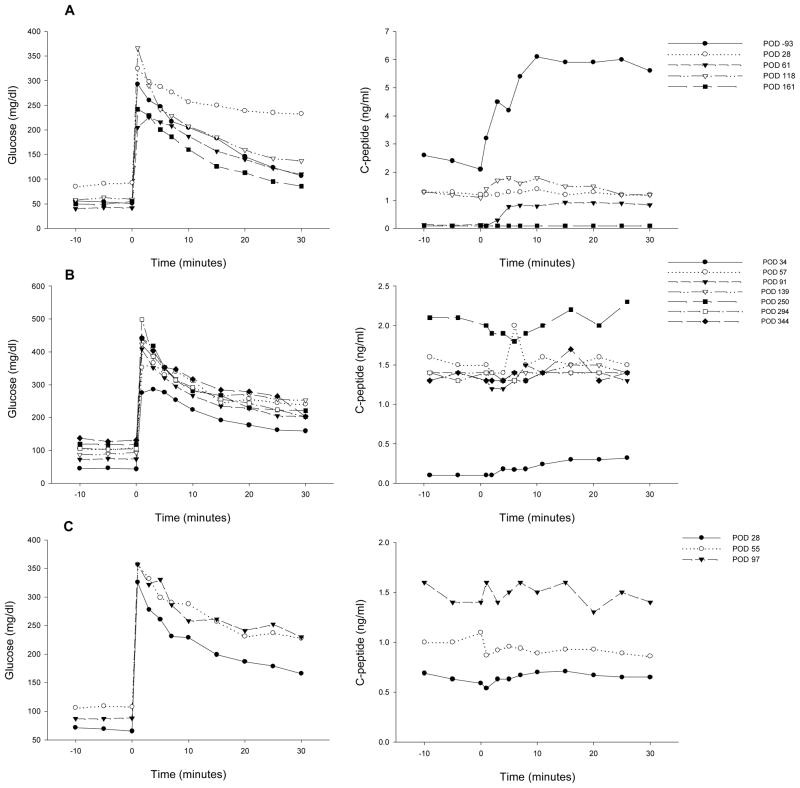
All islet grafts were >91% viable and were functional in a perifusion assay (data not shown). Duration of diabetes, number of IEQ/kg transplanted and data for exogenous insulin requirement (EIR) and fasting c-peptide (CP) are given in Table 1. One autologous islet transplant (5,093 IEQ/kg, Table 1, animal 1, Fig. 1A) was performed in order to test the efficacy of the new islet transplant technique in the absence of an immune response to the islets or the use of immunosuppressive drugs. Once the animal fully recovered from surgery, the average daily EIR gradually and progressively declined to a minimum of 0.3 to 0.4 IU/kg/day. CP levels above 0.2 and 1.0 ng/ml were detected on POD 3 and 24, respectively, and remained greater than 1.0 ng/ml (max of 2.5 ng/ml) until the scaffold was electively removed on POD 124. Omental graft removal resulted in a rapid increase in EIR, destabilization of metabolic control and loss of c-peptide (Fig. 1A). Analysis of c-peptide released during an IVGTT at different times post-transplant showed sustained release up until the time of explant (Fig. 2A).
Table 1.
Omental Pouch Transplant Data
| ID | Days Post STZ | IEQ/kg | EIR pre-tx | % Drop EIR on POD 14 | POD 50% Drop in EIR | Max % Drop EIR | POD c-peptide >0.2/value/FBG | POD c-peptide >1.0/value/FBG | Max C-peptide (ng/ml) | Day of elective scaffold explant∫ |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 * | N/A | 5,093 | N/A | N/A | N/A | N/A | 3/0.4/265 | 24/1.1/20 | 2.5 | 124 |
| 2 | 670 | 4,200 | 2.1 | −6.2 | 44 | 90 | 5/0.4/189 | 54/1.1/100 | 4.0 | 376 |
| 3 | 268 | 9,441 | 2.4 | −6.7 | 39 | 92 | 4/0.5/133 | 32/1.2/98 | 4.1 | † N/A |
| 4 * | 241 | 8,540 | 2.7 | 36.7 | 93 | 70 | **3/1.7/310 | **45/2.3/195 | 3.7 | 223 |
| 5 | 271 | 10,291 | 4.1 | −26.1 | 94 | 66 | 3/0.5/115 | 73/2.0/155 | 2.0 | 145 |
| 6 | 151 | 14,544 | 3.3 | †† N/A | †† N/A | †† N/A | 3/0.6/159 | †† N/A | †† N/A | †† N/A |
2 scaffolds;
animal had c-peptide >1.0 ng/ml on POD 3 but it decreased and did not return to >1.0 ng/ml until POD 45;
died during a technical procedure on POD 139;
died from infection on POD 16. Days post-STZ: duration of diabetes pre-transplant; EIR: exogenous insulin requirements; POD c-peptide >0.2 ng/ml/value/FBG: POD when the c-peptide values detected were >0.2 ng/ml/corresponding c-peptide value/corresponding fasting blood glucose value; POD c-peptide > 1.0 ng/ml/value/FBG: POD when the c-peptide values detected were >1.0 ng/ml/corresponding c-peptide value/corresponding fasting blood glucose value.
All animals were c-peptide positive at the time of scaffold explant.
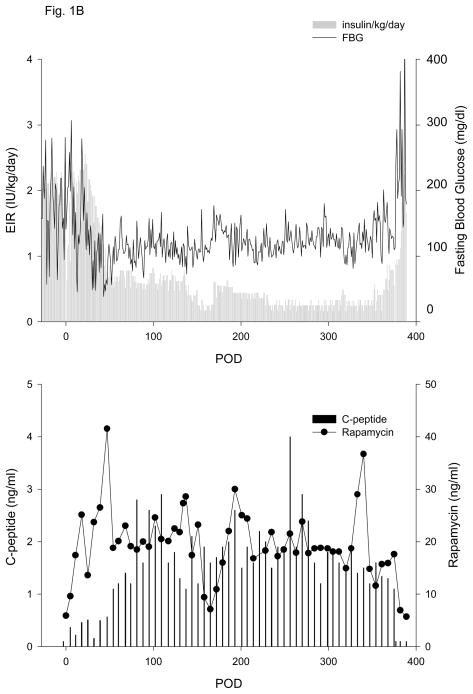
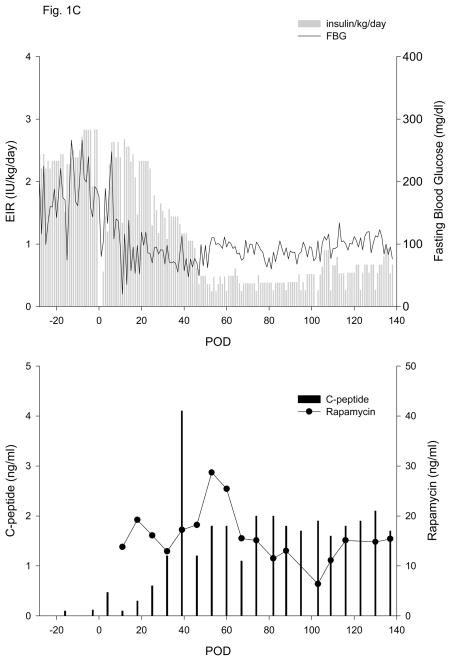
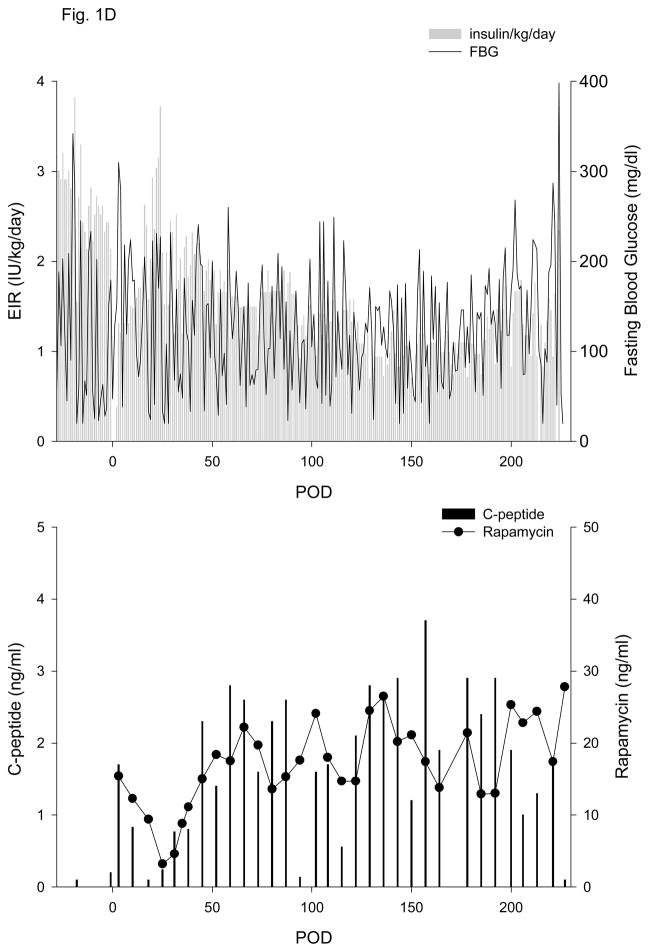
Figure 1. Exogenous insulin requirements (EIR), fasting blood glucose (FBG) (upper panel) and c-peptide and rapamycin levels (lower panel).
A. Animal 1 was pancreatectomized on POD −2 and received 5,093 autologous IEQ/kg on POD 0 onto 2 scaffolds, each one wrapped in an omental pouch. The bilateral omental pouches were explanted on POD 124. For recipients of allogeneic islets (animals 2–5), diabetes was induced with streptozotocin. With the exception of animal 4 (2 scaffolds), the other monkeys were implanted with one scaffold; each scaffold was wrapped in an omental pouch, which was explanted on the indicated day. B. Animal 2 received 4,200 IEQ/kg onto one scaffold, which was explanted on POD 376. C. Animal 3 received 9,441 IEQ/kg onto one scaffold; the animal died during a technical procedure on POD 139. D. Animal 4 received 8,540 IEQ/kg onto 2 scaffolds, which were explanted on POD 223. E. Animal 5 received 10,291 IEQ/kg onto one scaffold, which was explanted on POD 145. All animals gradually became c-peptide positive, with reduction of EIR and varying degrees of improvement in glycemic control (upper panels). With the exception of animal 3 (died with functioning islets), all recipients became c-peptide negative after explant (lower panels).
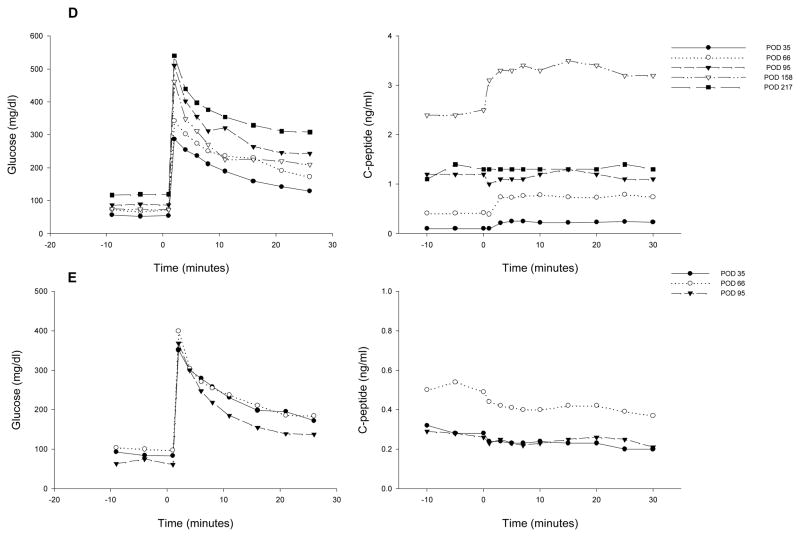
Figure 2. Plasma glucose (left panel) and c-peptide (right panel) during an intravenous glucose tolerance test (IVGTT) at different times post-transplant.
A: animal 1; B: animal 2; C: animal 3; D: animal 4 and E: animal 5.
Four of five recipients of allogeneic islets experienced a gradual decline in EIR that was associated with increasing CP levels (animals 2–5, Table 1, Figs. 1 B–E); the fifth monkey died from infection on POD 16 (animal 6, Table 1). All recipients of allogeneic islets attained adequate levels of rapamycin by POD 11. The initial drop in EIR in the 1st week post-transplant was a reflection of reduced appetite subsequent to surgery; as the animals recovered and began to eat, EIR were similar to pre-transplant values, with any changes primarily reflective of blood glucose control pre- and post-transplant. EIR data in Table 1 is, therefore, reported from POD 14 onward. Positive CP values (i.e., >0.2 ng/ml) were observed by POD 3–5 and all 4 animals eventually achieved CP levels of >1.0 ng/ml between POD 32–73. For animal 2 (Figure 1B), a 50% reduction in EIR was achieved by POD 44, accompanied by stabilization of FBG and a substantial increase in CP levels, ultimately achieving a maximum level of 4.0 ng/ml. After omental pouch explant on POD 376, a rapid return to hyperglycemia and pre-transplant EIR was observed, with a concomitant loss of c-peptide. A similar post-transplant course was observed for animal 3, with a 50% reduction in EIR achieved by POD 39 and a continued reduction to a maximum of 92%. Corresponding increases in CP levels were observed; levels >1.0 ng/ml were achieved by POD 32 and a maximum of 4.1 ng/ml was attained (Fig. 1C). The animal died with excellent graft function during a technical procedure on POD 139.
Animals 4 and 5 experienced a more gradual reduction in EIR. As seen for animal 4 in Figure 1D, the EIR slowly decreased, reaching 50% by POD 93 and a maximal reduction of 70%. Fasting blood glucose control remained erratic post-transplant, despite the presence of significant levels of c-peptide (Fig. 1D), which were >1.0 ng/ml by POD 45 (Table 1, Fig. 1D) and reached a maximum of 3.7 ng/ml. This animal experienced sub-immunosuppressive levels of rapamycin from POD 18–38. EIR began to increase on POD 187, and the animal was explanted on POD 223 due to continued deterioration of metabolic control. The POD 227 c-peptide sample subsequently tested negative. Animal 5 also experienced a slow decrease in EIR, with a 50% reduction achieved by POD 94 and a maximum of 66%. Similar to animal 4, pronounced improvement in glycemic control was not achieved and it took 73 days for c-peptide levels to rise above 1.0 ng/ml (Table 1, Fig. 1E). The animal began to lose function after POD 117 and the omental pouch was explanted on POD 145, followed by a return to negative c-peptide.
The plasma glucose and c-peptide profiles during an IVGTT for 5 animals at different times post-transplant are shown in Fig. 2. C-peptide area under the curve (AUC) during an IVGTT in healthy cynomolgus monkeys has a wide distribution, with a mean ± SD of 167.5 ± 52.8 ng/ml x min (range: 79.4 – 334.7 ng/ml x min, n = 81). Prior to pancreatectomy and transplant (POD −93), the islet autograft recipient (animal 1) had a c-peptide AUC of 145.2 ng/ml x min. After pancreatectomy and islet transplant, c-peptide AUC was partially restored, with maximum values reaching 35.0 ng/ml x min (24.1 % of the pre-transplant levels) on POD 118 (Fig. 2A). These levels decreased to 1.4% after omental pouch removal. The data obtained for the 4 allogeneic recipients that were followed long-term (Figs. 2 B–E) revealed a pattern similar to what was observed for CP values; i.e., the animals with higher CP (2–4 in Table 1) had better c-peptide AUC in IVGTT (Figs. 2 B–D). The curves were relatively flat, except for the data obtained on POD 158 for animal 4 (Fig. 2D).
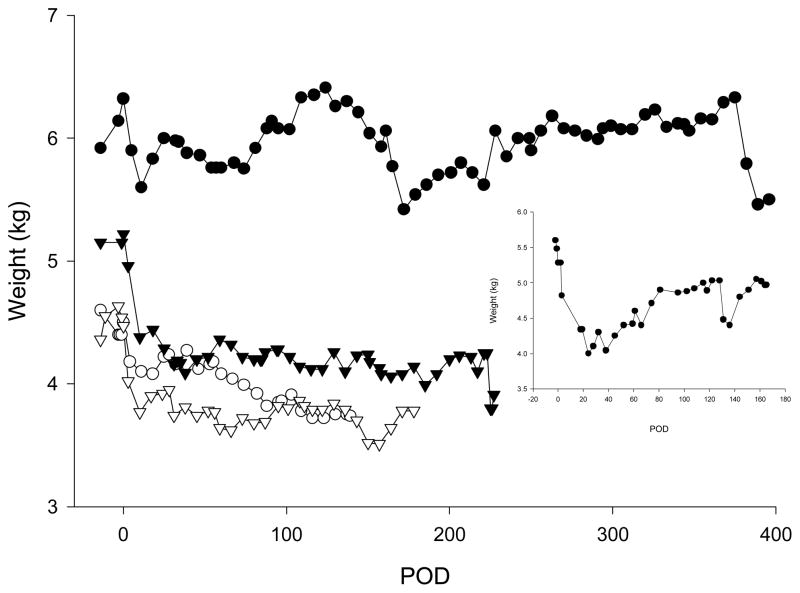
Post-transplant changes in body weight are shown in Fig. 3. Animal 1 (autologous islets) lost 19% body weight in the first month post-transplant and returned to 93–95% of the pre-transplant body weight by POD 81. In the first month post-transplant, body weight decreased by 5, 8, 20 and 16% for animals 2–4, respectively. Weight remained steady for animals 2, 4 and 5 but slowly continued to decrease for animal 3.
Figure 3. Body weight in an autologous (inset, animal 1) and in four allogeneic transplanted animals.
Closed circle: animal 2; open circle: animal 3; closed triangle: animal 4 and open triangle: animal 5.
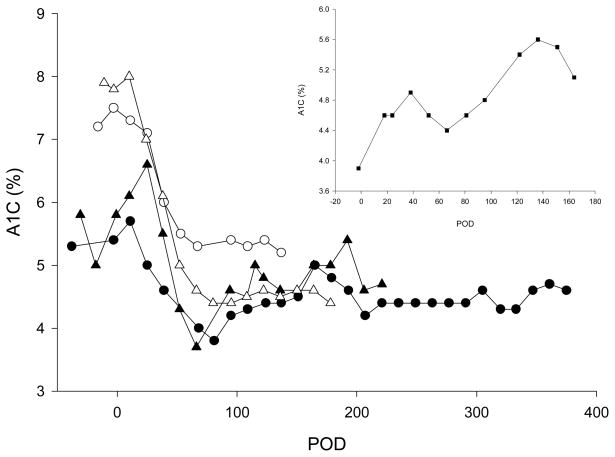
In healthy cynomolgus monkeys, the mean ± SD for A1C is 3.7 ± 0.2 % (range: 2.8 – 4.8%, n = 200), and in our STZ-induced diabetic monkeys, mean A1C is 5.8 ± 0.6 % (range = 4.4 – 7.6%, n = 62). All 4 allogeneic recipients shown in Fig. 4 experienced a gradual post-transplant drop in A1C, which was maximal between POD 66 and POD 81 and remained stable thereafter.
Figure 4. A1C levels in an autologous (inset, animal 1) and in four allogeneic recipients.
Closed circle: animal 2; open circle: animal 3; closed triangle: animal 4 and open triangle: animal 5.
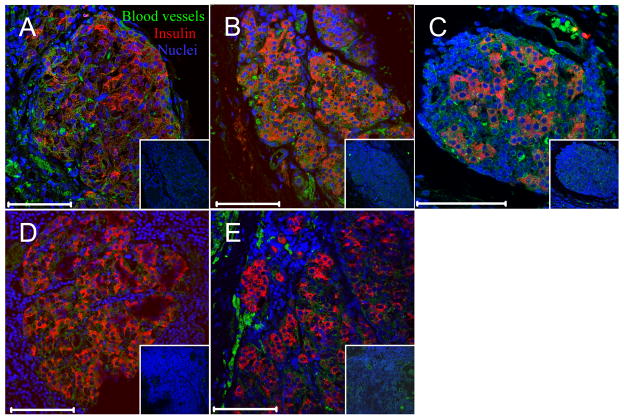
Figures 5A and B show histopathological assessment of omental pouch grafts explanted from an autologous (animal 1) and an allogeneic recipient (animal 5), respectively. Histological sections (H&E) revealed mixed cellular aggregates within adipose tissue. These aggregates contained small lymphoid cells arranged in nodules, without germinal center formation, but with occasional histiocytes. Focal areas showed multinucleated giant cells and refractile material compatible with suture, representative of a foreign body reaction. In addition, there were eosinophilic uniform cells that were present in varying size aggregates that resembled pancreatic islets of Langerhans. There was a consistent increased vascularity within grafted tissue and around islets (Fig. 6), and much less vascularization in surrounding adipose tissue. Rare inflammatory cells were present within the islet, but there was no evidence of isletitis. Immunohistochemical analysis showed that insulin was uniformly expressed within the islets in a pattern reminiscent of insulin in normal pancreatic islets. There was focal loss of intensity in some areas. Multicolor immunofluorescence staining and analysis by confocal microscopy was performed to examine the presence of several hematopoietic cell populations along with insulin. There were populations of CD4+, CD8+ and CD 68+ (macrophages) cells surrounding the islets. However, there was no significant infiltration of the islets by either of these T cells or macrophages, confirming the impression seen in the H&E sections. Occasional areas showed cell populations that were devoid of hematopoietic cell markers (not shown) and which likely represented fibrogranulomatous tissue. Figure 6 shows immunofluorescence staining representative of 3 tissue sections from each animal for the presence of blood vessels within islets from the autologous implant and the 4 allogeneic recipients that were followed long-term. Excellent vascularization was evident for all but animal 4 (Fig. 6D), which had the poorest post-transplant metabolic control, despite evidence of good c-peptide levels.
Figure 5. Histopathological assessment of omental pouch explants from an autologous (A, animal 1, explanted on POD 124) and from an allogeneic islet recipient (B, animal 5, explanted on POD 145).
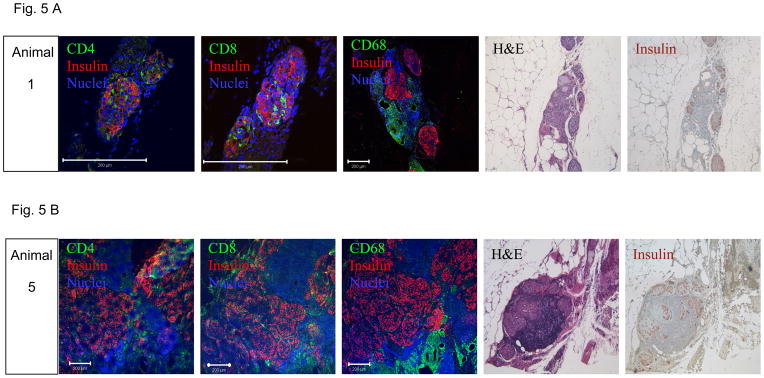
Confocal immunofluorescence showing expression of insulin (red) and CD4+, CD8+ or CD68+ T cells (green) at a magnification of 40X (bar indicates 200 μm), H&E staining at 50X and immunohistochemistry staining for insulin (brown, 50X).
Figure 6. Vascularization of transplanted islets.
Confocal fluorescent images of transplanted islets. Insulin (red), blood vessels (combination of anti-CD31 and anti-CD34, green), nuclei (blue). White bar indicates 100 μm, insets are control staining with primary antibodies omitted. A: animal 1; B: animal 2; C: animal 3; D: animal 4; and E: animal 5.
Intrahepatic allogenic islet transplantation
Results of intrahepatic transplantation of different numbers of allogeneic islets into 20 diabetic cynomolgus monkeys treated with steroid free immune suppression are shown in Table 2. Transient periods of insulin independence were observed in 2/2 animals receiving a full islet mass (≥ 10,000 IEQ/kg), and also in 1/13 animals receiving between 5,000 and 10,000 IEQ/kg, with no insulin independence in animals receiving < 5,000 IEQ/kg. Measurement of c-peptide values on POD 3 – 7 in 19 animals showed that 17/19 animals had early post-transplant c-peptide values ≥ 1.0 ng/ml; for the other two animals, one (E) never achieved c-peptide levels > 0.9 ng/ml post-transplant, and the other (R) achieved c-peptide levels > 1.0 ng/ml 18 days post-transplant. The maximum c-peptide achieved was 4.8 ng/ml, with a range of 1.2 – 4.8 ng/ml. The majority of animals attained adequate levels of rapamycin in the first 2 weeks post-transplant, with the exception of one monkey (animal D), which had negligible rapamycin levels and rejected the graft by POD 25. All animals experienced progressive decreases in c-peptide and increases in EIR over time; 11/20 monkeys had partial function at the end of the experiment (17/20 underwent elective necropsy, 2/20 were found expired in their cages - with evidence of CMV infection - and 1 died subsequent to multiple episodes of hypoglycemia that were resistant to treatment).
Table 2.
Intrahepatic Islet Allotransplant Data
| ID | Days post-STZ | IEQ/kg | EIR pre-tx | % Drop EIR on POD 14 | POD 50% Drop in EIR | Max % Drop EIR | POD c-peptide >0.2/value | POD c-peptide >1.0/value | Max c-peptide (ng/ml) | Days off insulin | Days c-peptide positive |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 87 | 13,550 | 3.6 | 91 | 14 | 100 | 7/2.2* | 7/2.2* | 2.8 | 11 | 243** |
| B | 67 | 10,909 | 3.4 | 87 | 14 | 100 | 27/2.9* | 27/2.9* | 2.0 | 46 | 92** |
| C | 304 | 7,961 | 3.4 | 53 | 14 | 91 | 3/1.7 | 3/1.7 | 4.0 | 0 | 73 |
| D | 150 | 7,042 | 2.0 | 56 | 14 | 88 | 4/1.4 | 4/1.4 | 1.4 | 0 | 11 |
| E∫ | 111 | 5,000 | 2.8 | 17 | N/A | 20 | 5/0.7 | N/A | 0.9 | 0 | 104 |
| F∫ | 113 | 5,000 | 5.2 | 27 | N/A | 27 | 4/1.9 | 4/1.9 | 4.4 | 0 | 101 |
| G | 172 | 5,000 | 3.5 | 58 | 14 | 67 | 3/2.4 | 3/2.4 | 3.6 | 0 | 164 |
| H∫ | 98 | 5,000 | 4.1 | 70 | 14 | 88 | 4/1.9 | 4/1.9 | 3.9 | 0 | 347 |
| I | 227 | 5,000 | 3.1 | 74 | 14 | 74 | 4/2.3 | 4/2.3 | 3.4 | 0 | 46** |
| J | 145 | 5,406 | 2.9 | 44 | 15 | 58 | 4/2.1 | 4/2.1 | 2.1 | 0 | 46** |
| K∫ | 115 | 5,000 | 3.8 | 87 | 14 | 100 | 3/1.9 | 3/1.9 | 2.9 | 20 | 515** |
| L∫ | 136 | 5,000 | 3.0 | 26 | N/A | 40 | 5/3.9 | 5/3.9 | 3.9 | 0 | 410** |
| M | 172 | 5,000 | 2.9 | 77 | 14 | 77 | 4/4.5 | 4/4.5 | 4.8 | 0 | 67 |
| N∫ | 51 | 5,000 | 2.9 | 72 | 14 | 94 | 4/2.3 | 4/2.3 | 3.4 | 0 | 291** |
| O | 105 | 5,000 | 2.3 | 94 | 14 | 94 | 4/3.5 | 4/3.5 | 4.6 | 0 | 88** |
| P∫ | 56 | 4,864 | 3.2 | 7 | N/A | 49 | 4/1.2 | 4/1.2 | 1.2 | 0 | 47 |
| Q∫ | 374 | 4,700 | 7.2 | 68 | 14 | 73 | 4/2.0 | 4/2.0 | 3.2 | 0 | 102** |
| R | 51 | 4,100 | 3.7 | 11 | N/A | 19 | 3/0.6 | 18/1.5 | 1.7 | 0 | 52 |
| S | 46 | 3,329 | 2.7 | 62 | 14 | 76 | 3/1.4 | 3/1.4 | 2.6 | 0 | 213 |
| T | 220 | 3,000 | 2.8 | 27 | N/A | 45 | 3/1.6 | 3/1.6 | 2.8 | 0 | 136** |
Indicates animals for which data has been previously published (13);
First c-peptide sample measured;
animal electively euthanatized with positive c-peptide. Days post-STZ: duration of diabetes pre-transplant; EIR: exogenous insulin requirements; POD c-peptide > 0.2 ng/ml/value: POD when the c-peptide values detected were >0.2 ng/ml/corresponding c-peptide value; POD c-peptide > 1.0 ng/ml/value: POD when the c-peptide values detected were >1.0 ng/ml/corresponding c-peptide value.
Discussion
The greater omentum has several of the characteristics sought in an extrahepatic site for islet transplantation, and our results illustrate the feasibility and efficacy of one autologous and five allogeneic islet transplants in an omental pouch in diabetic nonhuman primates. Independent of IEQ/kg, animals transplanted with allogeneic islets in an omental pouch experienced significant improvement in metabolic control post-transplant, as evidenced by a decrease in the EIR of 66–92%. For recipients of intrahepatic allogeneic islets, 6/20 animals never achieved a 50% reduction in EIR, although these were all recipients of a marginal islet mass. Only one of the omental pouch monkeys received less than 5,000 IEQ/kg. In contrast to NHP recipients of intrahepatic islets, 90% of which achieved CP levels of >1.0 ng/ml in the first week post-transplant, 100% of these omental pouch recipients experienced a delay in function, with 5/5 animals that were followed long-term requiring ≥ 24 days to achieve CP levels > 1.0 ng/ml. After this period, c-peptide levels at least doubled and remained positive for the duration of the experiment (from 124 to 376 days), with improved A1C levels. Elective explant of the omental pouch was followed by negative c-peptide values in 4/4 animals, demonstrating that the transplanted islets were the source of c-peptide and responsible for the reduction in EIR and A1C.
The omentum has been shown to promote neovascularization in ischemic tissues due to its angiogenic properties (35–37). Histologically (H&E and immunofluorescence), the grafts appeared to be well vascularized at the time of explant; however, we did not undertake a time-course study of vascularization. It is possible that the observed delay in graft function could be due to delayed vascularization and exposure of omental pouch islets to hypoxia in the early posttransplant period.
Ferguson and Scothorne (38) suggested the omentum may have an element of privilege as a transplant site, after showing that a small number (12–15) of allogeneic free-floating islets implanted in the greater omentum of non-immunosupressed guinea pigs were able to survive for long periods of time, with no rejected grafts observed. In our experiments using immunosuppressed monkeys, histopathological examination of omental pouches removed after prolonged periods of time (from a minimum of 124 days to a maximum of 376 days) showed well granulated islets surrounded by varying amounts of lymphocytes, with minimal signs of lymphocytic infiltration. The animal with the most prominent lymphocytic infiltrate (Table 1, animal 4, Fig. 6D) experienced sub-immunosuppressive levels of rapamycin from POD 18–38, which may have allowed for increased migration of leukocytes and potential depression of function due to increased inflammatory cytokines locally. There was an equivalent distribution of T cell subsets and macrophages. The pattern resembles a chronic inflammatory response; however, we explanted prior to increasing dysfunction in order to verify return to diabetes.
Since we did not transplant free-floating islets in the omental pouch, we cannot determine whether seeding islets on a scaffold is or is not essential for graft function and survival in an omental pouch in nonhuman primates. Previous experiments in rodents compared the effectiveness of transplanting islets extrahepatically in diabetic mice using synthetic polymer scaffolds or free-floating islets (39, 40) to restore normoglycemia. Both studies (39, 40) showed that transplanting islets on a synthetic scaffold improved islet function and survival, perhaps by providing an appropriate extracellular matrix. However, using a larger animal model, Ao et al. (21) reported a 71% success restoring euglycemia when transplanting free-floating islets in an omental pouch in diabetic dogs.
In summary, in our clinically relevant large animal model, auto and allogeneic islets seeded on a synthetic biodegradable scaffold and transplanted in an omental pouch engrafted and survived for long periods of time, with sustained improvement in metabolic control. Graft explants were accompanied by increased EIR, similar to those before islet transplant, with negative CP values. Histopathological analysis of the explants showed well granulated and well vascularized islets with insulin positive cells surrounded by varying degrees of T cell subsets and macrophages. Common to all transplanted animals was a delay in graft function, possibly due to delays in revascularization. Although the numbers are small, 4/4 recipients of allogeneic islets in an omental pouch maintained graft function through POD 139, but 7/9 recipients of intrahepatic islets experienced complete loss of function by POD 104. Our results suggest that a greater number of IEQ/kg may be required to achieve insulin independence in the omental pouch as compared to the liver; this is difficult to confirm as the number of full mass transplants was limited for both models. The results for tolerability of the steroid free immunosuppressive regimen were similar for both the omental pouch and the liver in terms of weight loss and incidence of infection. Duration of graft function for both sites is similar to what is observed clinically (4). Many factors contribute to graft outcome, including levels of immunosuppression, the varying effects of the drugs on individual recipients, islet quality and number engrafted and whether or not the recipient experiences infection. With this pilot study, we have demonstrated that the omental pouch allows for auto- and allogeneic islet engraftment and survival. Further studies will be necessary to define and understand the parameters that might contribute to attainment of insulin independence and long-term survival in the omental pouch, including choice of scaffold material, numbers of islets and methods to enhance revascularization. Based on the similarity between the omentum of humans and nonhuman primates (24), our results suggest that this extrahepatic transplant site has potential for clinical islet cell transplantation.
Acknowledgments
This work was funded in part by the Diabetes Research Institute Foundation, NIH U19AI43900, JDRFI 2004-808 and LifeScan, Inc. This study was presented in part at the 2007 American Transplant Congress, San Francisco, CA and at the International Pancreas and Islet Transplant Association meeting, 2007, Minneapolis, MN. Codman, a Johnson & Johnson company, distributes the biodegradable scaffold described in this manuscript and J.J.O. is an employee of LifeScan, Inc, also a Johnson & Johnson company.
The authors wish to thank Waldo Diaz and James Geary for their excellent care of the monkeys utilized in the transplant studies; Alex Rabassa for his excellent assistance with islet isolation and animal care and Kevin Johnson for his excellent assistance with sample processing.
Abbreviations
- AUC
area under the curve
- CP
fasting c-peptide
- EIR
exogenous insulin requirement
- FBG
fasting blood glucose
- IEQ
islet equivalents
- IVGTT
intravenous glucose tolerance test
- NHP
nonhuman primate
- NPO
nothing per oral
- POD
post operative day
- SFIS
steroid- free immune suppression
- SI
stimulation index
- STZ
streptozotocin
References
- 1.Hering BJ. Achieving and maintaining insulin independence in human islet transplant recipients. Transplantation. 2005;79(10):1296–7. doi: 10.1097/01.tp.0000157321.55375.86. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto S, Okitsu T, Iwanaga Y, Noguchi H, Nagata H, Yonekawa Y, et al. Successful islet transplantation from nonheartbeating donor pancreata using modified Ricordi islet isolation method. Transplantation. 2006;82(4):460–5. doi: 10.1097/01.tp.0000231710.37981.64. [DOI] [PubMed] [Google Scholar]
- 3.Toso C, Baertschiger R, Morel P, Bosco D, Armanet M, Wojtusciszyn A, et al. Sequential kidney/islet transplantation: efficacy and safety assessment of a steroid-free immunosuppression protocol. Am J Transplant. 2006;6(5 Pt 1):1049–58. doi: 10.1111/j.1600-6143.2006.01303.x. [DOI] [PubMed] [Google Scholar]
- 4.Froud T, Ricordi C, Baidal DA, Hafiz MM, Ponte G, Cure P, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant. 2005;5(8):2037–46. doi: 10.1111/j.1600-6143.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 5.Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50(4):710–9. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro AM, Ricordi C, Hering B. Edmonton’s islet success has indeed been replicated elsewhere. Lancet. 2003;362(9391):1242. doi: 10.1016/S0140-6736(03)14526-6. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–30. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 9.Benhamou PY, Oberholzer J, Toso C, Kessler L, Penfornis A, Bayle F, et al. Human islet transplantation network for the treatment of Type I diabetes: first data from the Swiss-French GRAGIL consortium (1999–2000). Groupe de Recherche Rhin Rhjne Alpes Geneve pour la transplantation d’Ilots de Langerhans. Diabetologia. 2001;44(7):859–64. doi: 10.1007/s001250100571. [DOI] [PubMed] [Google Scholar]
- 10.Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48(10):1907–14. doi: 10.2337/diabetes.48.10.1907. [DOI] [PubMed] [Google Scholar]
- 11.Johansson H, Lukinius A, Moberg L, Lundgren T, Berne C, Foss A, et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54(6):1755–62. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 12.Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Kallen R, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360(9350):2039–45. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 13.Berman DM, Cabrera O, Kenyon NM, Miller J, Tam SH, Khandekar VS, et al. Interference with tissue factor prolongs intrahepatic islet allograft survival in a nonhuman primate marginal mass model. Transplantation. 2007;84(3):308–15. doi: 10.1097/01.tp.0000275401.80187.1e. [DOI] [PubMed] [Google Scholar]
- 14.Bottino R, Fernandez LA, Ricordi C, Lehmann R, Tsan MF, Oliver R, et al. Transplantation of allogeneic islets of Langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation. Diabetes. 1998;47(3):316–23. doi: 10.2337/diabetes.47.3.316. [DOI] [PubMed] [Google Scholar]
- 15.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77(5):587–97. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 16.Ryan EA, Lakey JR, Paty BW, Imes S, Korbutt GS, Kneteman NM, et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51(7):2148–57. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 17.Desai NM, Goss JA, Deng S, Wolf BA, Markmann E, Palanjian M, et al. Elevated portal vein drug levels of sirolimus and tacrolimus in islet transplant recipients: local immunosuppression or islet toxicity? Transplantation. 2003;76(11):1623–5. doi: 10.1097/01.TP.0000081043.23751.81. [DOI] [PubMed] [Google Scholar]
- 18.Markmann JF, Rosen M, Siegelman ES, Soulen MC, Deng S, Barker CF, et al. Magnetic resonance-defined periportal steatosis following intraportal islet transplantation: a functional footprint of islet graft survival? Diabetes. 2003;52(7):1591–4. doi: 10.2337/diabetes.52.7.1591. [DOI] [PubMed] [Google Scholar]
- 19.Liebermann-Meffert D, White H, Vaubel E. The greater omentum: anatomy, physiology, pathology, surgery, with an historical survey. Berlin: Springer-verlag; 1983. [Google Scholar]
- 20.al-Abdullah IH, Anil Kumar MS, Kelly-Sullivan D, Abouna GM. Site for unpurified islet transplantation is an important parameter for determination of the outcome of graft survival and function. Cell Transplant. 1995;4(3):297–305. doi: 10.1177/096368979500400308. [DOI] [PubMed] [Google Scholar]
- 21.Ao Z, Matayoshi K, Lakey JR, Rajotte RV, Warnock GL. Survival and function of purified islets in the omental pouch site of outbred dogs. Transplantation. 1993;56(3):524–9. doi: 10.1097/00007890-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Gustavson SM, Rajotte RV, Hunkeler D, Lakey JR, Edgerton DS, Neal DW, et al. Islet auto-transplantation into an omental or splenic site results in a normal beta cell but abnormal alpha cell response to mild non-insulin-induced hypoglycemia. Am J Transplant. 2005;5(10):2368–77. doi: 10.1111/j.1600-6143.2005.01041.x. [DOI] [PubMed] [Google Scholar]
- 23.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103(7):2334–9. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaffanjon PC, Kenyon NM, Ricordi C, Kenyon NS. Omental anatomy of non-human primates. Surg Radiol Anat. 2005;27(4):287–91. doi: 10.1007/s00276-005-0329-4. [DOI] [PubMed] [Google Scholar]
- 25.Nehlsen-Cannarella SL, Bohn ML. A direct approach to determine the ABH phenotype of baboons. Immunol Invest. 1987;16(1):57–62. doi: 10.3109/08820138709055712. [DOI] [PubMed] [Google Scholar]
- 26.Wijkstrom M, Kenyon NS, Kirchhof N, Kenyon NM, Mullon C, Lake P, et al. Islet allograft survival in nonhuman primates immunosuppressed with basiliximab, RAD, and FTY720. Transplantation. 2004;77(6):827–35. doi: 10.1097/01.tp.0000116390.76425.20. [DOI] [PubMed] [Google Scholar]
- 27.Kenyon NS, Fernandez LA, Lehmann R, Masetti M, Ranuncoli A, Chatzipetrou M, et al. Long-term survival and function of intrahepatic islet allografts in baboons treated with humanized anti-CD154. Diabetes. 1999;48(7):1473–81. doi: 10.2337/diabetes.48.7.1473. [DOI] [PubMed] [Google Scholar]
- 28.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. Bmj. 1990;300(6719):230–5. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenyon NS, Chatzipetrou M, Masetti M, Ranuncoli A, Oliveira M, Wagner JL, et al. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc Natl Acad Sci U S A. 1999;96(14):8132–7. doi: 10.1073/pnas.96.14.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–20. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 31.Ricordi C, Gray DW, Hering BJ, Kaufman DB, Warnock GL, Kneteman NM, et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27(3):185–95. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- 32.Latif ZA, Noel J, Alejandro R. A simple method of staining fresh and cultured islets. Transplantation. 1988;45(4):827–30. [PubMed] [Google Scholar]
- 33.Johnson JE. Methods for studying cell death and viability in primary neuronal cultures. Methods Cell Biol. 1995;46:243–76. doi: 10.1016/s0091-679x(08)61932-9. [DOI] [PubMed] [Google Scholar]
- 34.Idahl LA. A micro perifusion device for pancreatic islets allowing concomitant recordings of intermediate metabolites and insulin release. Anal Biochem. 1972;50(2):386–98. doi: 10.1016/0003-2697(72)90047-4. [DOI] [PubMed] [Google Scholar]
- 35.Goldsmith HS, Griffith AL, Kupferman A, Catsimpoolas N. Lipid angiogenic factor from omentum. Jama. 1984;252(15):2034–6. [PubMed] [Google Scholar]
- 36.Hockel M, Schlenger K, Doctrow S, Kissel T, Vaupel P. Therapeutic angiogenesis. Arch Surg. 1993;128(4):423–9. doi: 10.1001/archsurg.1993.01420160061009. [DOI] [PubMed] [Google Scholar]
- 37.Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res. 1997;67(2):147–54. doi: 10.1006/jsre.1996.4983. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson J, Scothorne RJ. Further studies on the transplantation of isolated pancreatic islets. J Anat. 1977;124(Pt 1):9–20. [PMC free article] [PubMed] [Google Scholar]
- 39.Blomeier H, Zhang X, Rives C, Brissova M, Hughes E, Baker M, et al. Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation. 2006;82(4):452–9. doi: 10.1097/01.tp.0000231708.19937.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dufour JM, Rajotte RV, Zimmerman M, Rezania A, Kin T, Dixon DE, et al. Development of an ectopic site for islet transplantation, using biodegradable scaffolds. Tissue Eng. 2005;11(9–10):1323–31. doi: 10.1089/ten.2005.11.1323. [DOI] [PubMed] [Google Scholar]