Abstract
Ethics on animal use in science in Western society is based on utilitarianism, weighing the harms and benefits to the animals involved against those of the intended human beneficiaries. The 3Rs concept (Replacement, Reduction, Refinement) is both a robust framework for minimizing animal use and suffering (addressing the harms to animals) and a means of supporting high quality science and translation (addressing the benefits). The ambiguity of basic research performed early in the research continuum can sometimes make harm-benefit analysis more difficult since anticipated benefit is often an incremental contribution to a field of knowledge. On the other hand, benefit is much more evident in translational research aimed at developing treatments for direct application in humans or animals suffering from disease. Though benefit may be easier to define, it should certainly not be considered automatic. Issues related to model validity seriously compromise experiments and have been implicated as a major impediment in translation, especially in complex disease models where harms to animals can be intensified. Increased investment and activity in the 3Rs is delivering new research models, tools and approaches with reduced reliance on animal use, improved animal welfare, and improved scientific and predictive value.
Keywords: Animal welfare, Utilitarianism, Reduction, Replacement, Refinement, Drug development
1. Introduction
Scientific advances have made it possible to better diagnose and treat a number of diseases in both human and veterinary medicine bringing about substantial improvement in quality of life (QOL) for these patients. Because of the pivotal contribution of animal experiments to this work, animals are often used for biomedical research and development worldwide. Besides fundamental research on pathophysiological processes, the major part focuses on discovery and development of new medical entities such as drugs, biologicals, devices, and innovative medical procedures. The implicit agreement by the majority of society with the inherent value of animal use for scientific purposes is reflected in the laws permitting but protecting their use in almost every country. Underpinning this legislation is the consensus that animal experiments deserve major ethical consideration and such considerations should be in balance with the moral consideration of humans. This weighting of the costs (i.e., burden to the animal) and benefits for the welfare of animals against the costs and benefits for the welfare of humans (i.e., QOL) is described as a utilitarian viewpoint, and this viewpoint dominates in Western society. Such a viewpoint requires an understanding of the severity and magnitude of the disease state affecting humans, and also of the concept of animal welfare and its application to the species used in biomedical research. Consideration of the welfare of animals in biomedical research comprises the ethical responsibility of the scientific community to: (1) ensure the potential benefits arising from their use outweigh the burden placed on the animals while establishing a boundary of acceptable animal use; (2) ensure that any harm caused is as low as it can be and to strive to achieve the highest level of well-being where animal use is necessary. The concept of Replacement, Reduction and Refinement as guiding principles for humane in vivo research was first proposed in 1959 as a strategy to address this responsibility (Russell and Burch, 1959). Since that time, the ‘3Rs’ concept has become widely accepted as a robust ethical framework for reducing animal use and suffering, helping to address societal concerns about animal research. These guiding principles have prompted investigators to replace animal experiments with alternatives wherever possible, reduce the number of animals used per study to the minimum consistent with the scientific objective, and refine procedures or protocols to minimize any suffering that the animals may experience. Table 1 presents a more detailed definition of each ‘R’ in relation to various general examples. There is interplay between the 3Rs and conflicts can arise, such as when procedures that enable a reduction in animal numbers lead to greater harm for the fewer animals that are used (as might occur in a longitudinal study using imaging, for example). This conflict is usually resolved case-by-case by weighing the harms and benefits to the animals involved, or else by prioritizing the experience of the animals (i.e. refinement) over reduction.
Table 1.
Definitions and examples of replacement, reduction and refinement.
| ‘R’ | Definition | Examples |
|---|---|---|
| Replacement | Methods that avoid or replace the use of animals in areas where they would have otherwise been used. | Human volunteers, tissues and cells; mathematical and computer models; established animal cell lines, or cells and tissues taken from animals killed solely for this purpose (i.e. not having been subject to a regulated procedure); non-protected immature formsa of vertebrates; invertebrates, such as Drosophila and nematode worms. |
| In some cases, relative replacement (i.e. replacing the use of live ‘protected’ vertebrates with vertebrate cells or tissues, early life-stages or non-vertebrates) has been implemented as a first step to absolute replacement. | ||
| Reduction | Methods that minimize the number of animals used per experiment or test, either by enabling researchers to obtain comparable levels of information (of a given amount and precision) from fewer animals, or to obtain more information from the same number of animals (thereby avoiding further animal use). | Improved experimental design and statistical analysis; sharing of data and resources (e.g. animals and equipment) between research groups and organizations; use of technologies, such as imaging, that enable longitudinal studies in the same animals. |
| Refinement | Methods that minimize any pain, suffering, distress or lasting harm that may be experienced by the animals, and improve animal welfare. Refinement applies to all aspects of animal use, from the housing and husbandry used to the scientific procedures performed upon them. | Use of appropriate anesthetics and analgesics regimens; avoiding stress by training animals to cooperate with procedures such as blood sampling; providing animals with appropriate housing and environmental enrichment which allows the expression of species-specific behaviors. |
In the European Union, non-protected immature forms are embryonic and fetal mammals, birds and reptiles up to the last third of their gestation or incubation period, larval forms of amphibians and fish until they can feed independently, and cephalopods until the point at which they hatch.
Together the 3Rs provide a comprehensive means to reduce harm to animals, but also have steadily evolved into an especially meaningful tool in enhancing the overall scientific value for investigators. Owing to the growing concern over translation, there is increasing emphasis on animal model characterization to better understand the usefulness and limitations of animal studies, and strategies to improve agreement with the clinical situation to improve prediction – these efforts are aligned with the 3Rs, underscoring the importance of fully engaging with this ideology and methodology.
In this manuscript we present a brief overview of certain ethical principles underlying the use of animals in research and discuss the key multifactorial role of the 3Rs in shifting the ethical harm-benefit assessment. We give examples of how expertly designed 3Rs methods not only reduce harms to animals but can also expand our understanding of disease and strengthen scientific outcomes to accelerate translation to the clinic to benefit patients.
2. Animal welfare in biomedical research: ethical basis for the use of animals
There is general consensus with the view that animals do have “moral standing”, and the central question of animal ethics therefore concerns: “What is the basis of our duties towards animals? And what duties do we owe them?” (Sandøe et al., 1997). These perspectives in animal ethics drive the animal welfare science and public debate regarding the use of animals in biomedical research. Evidently, such discussion can be extended to the many other facets in which humans interact with animals, e.g., conservation in zoos, farming, hunting, companion use, entertainment use, and service function. The present overview is intended only for the context of sentient animals used in biomedical research. In accord with the moral status of animals, there are essentially two philosophical positions that dominate the debate over animal use, the utilitarianism view and the animal rights view.
2.1. Utilitarianism
Utilitarianism implies a weighing of harms and benefits for welfare of animals against harms and benefits for welfare of humans (Sandøe et al., 1997). Utilitarianism has been most forcefully defended by Singer (1989), who argued that what matters are the interests of those being affected by what we do, independent of whether it concerns the interests of humans or animals. According to the utilitarianism point of view, the optimal solution to an ethical dilemma is the solution in which the highest total welfare is gained by all sentient parties concerned. Fundamentally, utilitarianism is based on consequentialism, welfarism, and aggregationism (Hare, 2009). Principles of utilitarianism are often applied in medicine, ranging between the local level of institutional review boards in assessing the harm-benefit profile of an individual patient enrolling in a clinical trial, to a much higher level in government approving medical products or procedures based on harm-benefit profiles as applied to a population. As outlined by Hare (2009):
-
•
Consequentialism determines the moral quality of an action (that is, determines what is right or wrong) based on the consideration of its consequences,
-
•
Welfarism considers the consequences that are relevant to the morality of actions being the consequences that increase or diminish the welfare of all those affected,
-
•
Aggregationism refers to the distribution of welfare, i.e., a solution should be sought that maximizes total welfare (i.e., when one outcome produces more welfare that is unequally distributed, this outcome should be preferred above that with less welfare that is equally distributed).
Aggregationism often leads to objections from those that think equality of distribution alone matters, and cannot be sacrificed in the maximization of total welfare (Singer, 1989).
2.2. The animal rights view
The animal rights view is similar to utilitarianism in that it assumes humans and animals having comparable interests that should be respected in comparable ways. Unlike utilitarianism however, the animal rights view denies that we can justify beneficial results by using immoral means (Sandøe et al., 1997), which implies that the interests of one individual should never be sacrificed for the benefits of the other. For example, as defended by Regan, this ethical view would imply that one should never keep and/or use animals for biomedical research as it would violate the rights of the animal to be only used a ‘means to an end’ (i.e., for the sole purpose to achieve something else) (Regan, 1989). Both utilitarianism and animal rights views suggest that nothing other than the individual interests of humans and animals matter. A more moderate advocacy of the animal rights view was suggested by Sandøe et al. preserving a key notion “there are absolute, non-negotiable limits to what can be done to animals” (Olsson et al., 2010). They argue that one example of a non-negotiable limit would be barring procedures that would inflict suffering involving intense or prolonged pain or distress without relief and being outside the control of the animal.
2.3. Applying animal ethics in biomedical research – the hybrid view
The ethical debate has been dominated by disagreements between adherents to the utilitarian view (Singer) and adherents to the animal rights view (Regan). Despite these apparent disagreements, fundamental utilitarianism and animal rights views appear far more alike each other than different (DeGrazia, 1998), in agreement to provide the principle of equal consideration to both animals and humans. In either case, these ethical views when consequently enforced would result in radical changes in human-animal relationships that would go beyond what is generally considered “acceptable” in society. Thus, if the animal rights views were consequently enforced, it would result in total rejection of animal experiments. Utilitarianism is more nuanced: some adherents accept animal experiments in the case that alternatives are exhausted, and others like Singer suggest that an experiment be justified only under highly extraordinary circumstances for human health benefit.
Nowadays, utilitarianism is the dominating ethical approach in practicing animal ethics in Western society, but it is rarely applied in its “purist” form: thus, when ethical dilemma׳s in our moral duty towards animals occur, the interest of humans is invariably considered more important than that of animals (“speciesism”): there are different opinions on the perspective of how the relative importance of human interests relate to that of animals. Most people take a “hybrid view” (Sandøe et al., 1997), in which arguments from different ethical approaches are combined in a pluralist utilitarian approach, in which ethical arguments are weighed with respect to their relevance. In the example of biomedical research, one might argue that a hybrid view is one where elements from utilitarianism and animal rights are combined: an example is the perspective that animals might be used for disease research (utilitarianism), while at the same time a certain accepted level of welfare should be guaranteed to allow experimentation of animals irrespective of the benefit (animal rights view). From the hybrid perspective, variable weights are applied to different ethical arguments, and variable weights are applied to human as opposed to animal interests.
| What is the basis of our duties towards animals? |
|
3. Harm-benefit analysis
3.1. Assessment of animal welfare (the benefit factor)
The pluralist utilitarian approach is arguably easier to follow in translational research, where the main purpose is to develop treatments for direct application in humans or animals suffering from disease. In contrast, the practical benefits of using animals tend to be more difficult to predict in the case of fundamental or ‘basic science’ research, because applications of any results are further away from the research itself. Since the advancement of science and technology requires both varieties of research to be pursued, it is not necessarily fair to ask which type of research is likely to deliver more benefit in the case where there is obvious synergy. However, it is logical that different types of research are open for consideration in different ways. Almost always, translational research has the characteristics that the potential benefit to patients is well outlined, so that it should be more clear what burden is reasonable to be placed on the animal: in contrast, the ambiguity of basic research often makes judgment of harm-benefit more difficult. In the situation of basic research, the achievement of benefit is better limited to the likelihood of the research project meeting its specific aims in generating new scientific knowledge (Olsson et al., 2010). Because these experiments generally add incremental knowledge to the overall field it is important to relate the relevance of the research project to previous work, and any benefits generated, in connection with the original contribution or progress envisaged in the proposed work.
Even though the benefit anticipated from translational research is usually easier to define it should certainly not be considered automatic as issues related to model validity (e.g. improperly characterized models or those that fail to faithfully represent the clinical situation) can seriously compromise experiments (McGonigle and Ruggeri, 2014;Denayer et al., 2014; van der Worp et al., 2010). Evidently appropriate model selection, proper study design, an experienced research team, and transparent reporting of animal studies is necessary to realize the anticipated benefit. Recognizing this, the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines were developed in 2010 by the NC3Rs to improve the quality of reporting of in vivo research to maximize its value and minimize unnecessary animal use (Kilkenny et al., 2010).
3.2. Assessment of animal welfare (the harm factor)
Animal welfare is an inclusive concept since there is both the moral aspect of welfare reflecting the ethical use of animals and then the empirical aspect that directly concerns the well-being of the individual animal, as assessed by changes in physiology and behaviour. In animals several factors have been suggested to be included as relevant indications of well-being. Among the first scientific definitions proposed were those of the Brambell Committee known as the ‘five freedoms’ (Brambell, 1965):
-
•
freedom from thirst, hunger, and malnutrition
-
•
freedom from discomfort
-
•
freedom from pain, injury, and disease
-
•
freedom to express normal behavior
-
•
freedom from fear and distress.
The literal application of the five freedoms in biomedical research is complicated. For instance, there is a certain conflict with ‘freedom from disease’ when considering that, fundamentally, the model should closely resemble the disease it aims to study (or aspects of it). Also, in a well-fit model situation there is likely a conflict with ‘freedom from discomfort’, considering that animals are only to be used when the need is justifiable based on the suffering the disease inflicts on patients (e.g., it might be difficult to completely avoid suffering in a disease state worth modeling). The five freedoms are based on the suggestion that absence of harm determines the presence of welfare. The definition has since evolved to acknowledge the presence of positive and negative affective states in animals (Tannenbaum, 1991). Like in humans, not just the absence of suffering but also the presence of positive feelings is associated with well-being (Mench, 1998). Broom simplifies this view concisely as the animal׳s “state as regards its attempt to cope with its environment” (Broom, 1986) which is further extended by Ohl in the context of evolutionary adaption “Welfare as a biological function, embracing the continuum between positive and negative welfare, should take into account the dynamics of the individual׳s adaptive capacity” (Ohl and Van der Staay, 2012).
Also, continuing the discussion of ethics, several concepts have been developed that refer to the kind of moral concern for animals, going beyond the pure concept of animal welfare itself. For instance:
-
•
“intrinsic value” of animals refers to the idea that animals do not have more “instrumental value” than humans, but also a value in their own right (Brom, 1999).
-
•
“flourishing” or “self-realization” refers to an interpretation of animal welfare beyond that restricted to “absence of suffering”. Apart from the animal׳s interest “not to suffer”, it is advocated that animals should have the right to “flourish”, i.e., express all capacities that the animal was designed for in an evolutionary perspective (Brom, 1999).
With respect to the ethical concept of “intrinsic value”, the concept has fulfilled an important, persuasive function in the socio-political debate in the sense that introduction of the concept has facilitated the consensus that animals are not mere “instruments”, but have an importance and value of their own, irrespective of the value they have for humans and formed the basis for more complex definitions of welfare.
Unlike animals used in other interactions with humans, animals in biomedical research often are not in a normal state, but rather in a disease state relevant to the model or study interventions. Because the state of disease generally violates the ‘good health’ premise of welfare, it is relevant to examine welfare more broadly and to consider the animal׳s experiences. Spruijt et al. have described “Welfare is defined as the balance between positive (reward, satisfaction) and negative (stress) experiences or affective states. The state of this balance may range from positive (good welfare) to negative (poor welfare). These affective states are momentary or transient states which occur against the background of and are integrated with the state of this balancing system” (Spruijt et al., 2001). This definition has the important consequence that lowering the level of negative stimuli does not automatically bring the animal to a positive welfare state, but opens the possibility that lowering (but not eliminating) exposure to negative stimuli and increasing availability of positive stimuli will result in a net positive effect – proper application of refinement by investigators in animal models does exactly this (Buchanan-Smith et al., 2005). When refinement is approached in a multifactorial way that addresses the interests of the animal while also promoting the scientific objective it avoids that animals are simply used as a means to an end (i.e., the sole purpose of the action is to achieve something else); instead it inherently respects the intrinsic value of each animal as an individual with variable experiences.
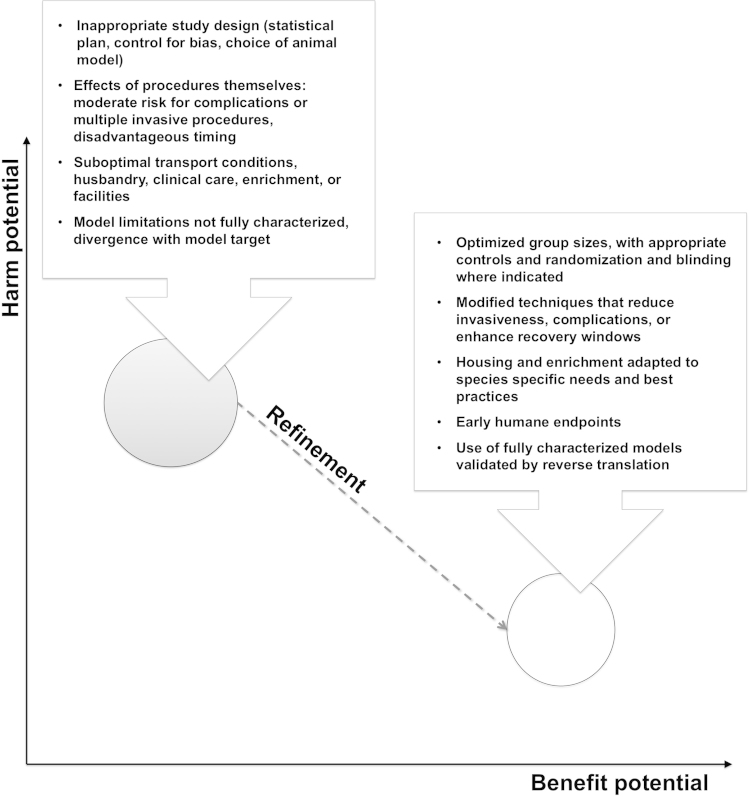
The practical assessment of harm should consider the severity and duration of all potential adverse effects, physical and psychological (Fig. 1). This can involve the animal species, experimental procedures, source of animals, transport, husbandry and care conditions, quality of the facilities involved, and expertise of the researchers (Smith et al., 2007). In animal models of disease, subjects may experience symptoms, complications, and repeat medical intervention similar to the clinical patient. In this situation the potential for harm may be great, and careful application of refined methods can decrease harms sufficiently in balance to justify proceeding (Fig. 1).
| Balancing harms against benefits |
|
Fig. 1.
Effect of refinement on the harm-to-benefit ratio.
4. Application of the 3Rs
4.1. Drivers for the 3Rs
Globally the practical protection of animals used in biomedical research has taken the consideration of welfare to a higher level than what is done in many other areas of human–animal interactions. The 3Rs principles (Table 1) are embedded in national and international legislation and compulsory guidelines regulating the use of animals for scientific purposes (e.g. European Parliament and the Council of the European Union, 2010; Administration of Quality Supervision, Inspection and Quarantine of the People׳s Republic of China and Standardization Administration of the People’s Republic of China, 2013; Ministry of Environment and Forestry India, 2013; United States Department of Agriculture, 2013) as well as local oversight (e.g., in the form of ethics committees), and also voluntary standards like in accreditation by Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) of an institution (AAALAC, 2014). International guidelines on the use of animals for regulatory purposes are also increasingly making recommendations and developing processes that contribute to the 3Rs (e.g. those from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use) (Ohno, 2002). Many industry companies now highlight the 3Rs as part of their corporate social responsibility (e.g. AstraZeneca, 2014; Unilever, 2014; Novartis, 2014; GlaxoSmithKline, 2014).
Regardless of positioning, either in industry or academic and knowledge institutions, it is the responsibility of the ethics committee to ensure the 3Rs are implemented locally. The overarching task of ethical review by ethics committees, for example the Institutional Animal Care and Use Committees (IACUC) in the US or Animal Welfare and Ethical Review Bodies (AWERB) in the UK, is to translate principles of animal welfare into the wellbeing of animals during experimental protocols as well as to act on behalf of the institution to critically review study protocols and ensure the research involving animals is justified by the benefits. The full charge of the IACUC is extensively detailed in the Guide for the Care and Use of Laboratory Animals (Janet et al., 2011). The “Guide” forms the basis also for institutions to pursue AAALAC accreditation, which is a voluntary process in which “research programs demonstrate that they meet the minimum standards required by law, and are also going the extra step to achieve excellence in animal care and use” (AAALAC, 2014).
Beyond the concern of the committees and government, opinion polls consistently show that important public support for in vivo research is conditional on the demonstration of the benefits of animal research in combination with humane experimental techniques and full application of the 3Rs (e.g. Ipsos MORI, 2014).
Historically the 3Rs were viewed primarily in the context of stewardship and animal welfare science. Nowadays the 3Rs principles are increasingly implemented within mainstream scientific practice as they are recognized as another tool that supports study design and interpretation (e.g. statistics). A key driver for this is the growing appreciation of the real opportunities provided by the 3Rs for supporting high quality science, improving business efficiency, and addressing some of the major challenges currently facing pharmaceutical and chemicals companies worldwide. The 3Rs can benefit not just animal welfare, but also human health, the environment and the economy.
The scientific imperative for developing new, robust approaches to research and development is very strong. Although the use of animals forms a major part of much biomedical research, success seen in animal studies has not always translated to the clinic. A high percentage of drug candidates are removed from development when tested in humans because of a lack of efficacy or safety that was not predicted in animal tests, with oncology, infectious disease and neuroscience indications having the highest attrition rates (Kola and Landis, 2004; Walker and Newell, 2008; Bailey et al., 2014). Reducing attrition, even by a small amount, can lead to huge financial savings and increased business growth. Hence initiatives are underway to develop new methods to screen failures out as early as possible and to select, with further research and development, those approaches most likely to succeed (e.g. Europe׳s Innovative Medicines Initiative and the FDA׳s Critical Path Initiative). Similarly, there are concerns about the utility of animal studies for testing environmental chemicals (Leist et al., 2008). For example, animals are invariably exposed to much higher doses than typical human exposures making interpretation difficult. Organizations such as the National Research Council and OECD (National Research Council, 2007; Leist et al., 2008; Organisation for Economic Co-operation and Development, 2013) have called for the development of mechanism-based assays that are more predictive of human biology, and increasingly attention has focused on in vitro and in silico approaches based on human material for solutions. There are also concerns about the reproducibility of academic science, which many pharmaceutical companies rely on for target identification and validation. For example, in 2012 scientists from Amgen and the University of Texas M.D. Anderson Cancer Center reported in Nature their attempts to confirm findings from 53 ‘landmark’ papers in the preclinical cancer field (Begley and Ellis, 2012). Eighty-nine percent of the studies described, the majority of which used animals, could not be reproduced with poor study design, investigator bias and incomplete reporting identified as major contributing factors. Similar findings have been reported for preclinical research in other disease areas (Table 2). The issues are not the same in every case but in general there is a need for greater methodological rigor (e.g. Randomization and blinding) to reduce bias and improve internal validity, more clinically relevant models, assays and outcome measures, and more comprehensive reporting within the literature. Funders such as the US National Institutes of Health and UK Medical Research Council, and journals such as the Nature and PLoS families, have committed to address the issues raised. As a first step, many have endorsed the ARRIVE guidelines (Kilkenny et al., 2010).
Table 2.
Examples of papers citing the limitations of animal studies.
| Disease/research area | Reference |
|---|---|
| Asthma | Holmes et al. (2011), Abbott-Banner et al. (2013), Mullane and Williams (2014), Mercer et al. (2015) |
| Cancer | De Bono and Ashworth (2010), Begley and Ellis, (2012), Moreno and Pearson (2013), Ruggeri et al. (2014) |
| CNS disorders | McGonigle (2014) |
| Emetic liability | du Sert et al. (2012) |
| Epilepsy | Löscher (2011) |
| Multiple sclerosis | Friese et al. (2006), Mix et al. (2010), Pachner (2011), Baker et al. (2011) |
| Pain | Percie du Sert and Rice (2014) |
| Sepsis | Webb (2014) |
| Stroke | Crossley et al. (2008), Mergenthaler and Meisel (2012), Sena et al. (2010), van der Worp et al. (2010), Howells et al. (2014) |
| Transplantation | Graham and Schuurman (2013), Wijkstrom et al. (2013) |
4.2. An emphasis on 3Rs science
There are a number of organizations globally that focus on the 3Rs as an aspect of laboratory animal care (e.g. American Association for Laboratory Animal Science, Institute for Laboratory Animal Research, International Council for Laboratory Animal Science, AAALAC, and Federation of European Laboratory Animal Science Associations) (Griffin et al., 2014). In the UK, the pioneering National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) has been central to shifting mindsets within the scientific community and accelerating the development and application of all 3Rs. It directly appeals to scientists by funding research and early career development, supporting open innovation and the commercialization of 3Rs technologies, and providing opportunities to partner to address specific challenges faced by scientists in specific fields (e.g. use of chronic implants in neuroscience studies with non-human primates).
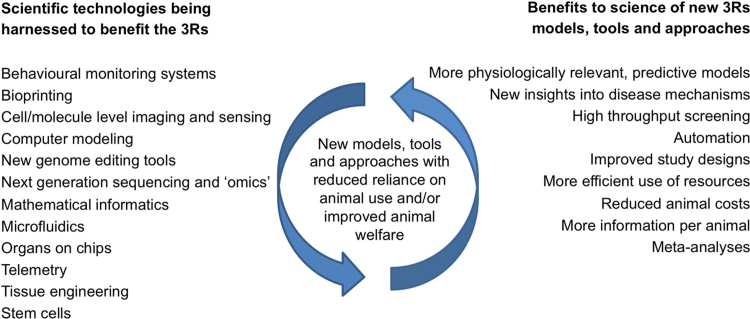
There is now much more focus with academia and industry on developing alternative approaches that avoid the use of animals and provide better tools for modeling human biology and disease. An exciting pipeline of technologies with 3Rs potential is emerging from the academic science base, including stem cell technologies, 3D tissue constructs and bioprinting, organ-on-chips and microfluidics, advanced in vitro and in vivo imaging, and mathematical and in silico modeling. These are benefiting animals but also the scientific community, facilitating scientific progress in a virtuous circle (Fig. 2). The need to improve the design, conduct and analysis of in vivo research is also gathering momentum, with greater emphasis on minimizing animal use and improving animal welfare. Knowledge about animals׳ physical and behavioral requirements, and the welfare impact of scientific procedures, is expanding rapidly and being translated into practical information to minimize pain and distress and improve the robustness and reproducibility of animal experiments. For example, novel handling methods for mice which avoid the high anxiety and variation associated with traditional methods (Hurst and West, 2010), use of ‘grimace scales’ to assess post-surgical pain in animals, so that it can be alleviated and its potentially confounding effects removed (Keating et al., 2012; Leach et al., 2012), and provision of environmental enrichment to satisfy species-typical needs, reduce abnormal behaviour, and improve environmental construct validity (Martin et al., 2010; Burrows et al., 2011; Bayne and Wurbel, 2014).
| Importance of the 3Rs |
|
Fig. 2.
Virtuous circle of the 3Rs and scientific progress.
5. The essential role of the investigator
Tannebaum suggests that reluctance by investigators to engage in ethical assessment of animal research follows from thinking that their scientific background does not qualify them, but reminds us “the pain and distress minimization principle cannot be applied correctly to an animal research project without knowledge and expertise possessed uniquely by scientists who are familiar with the kinds of questions asked by the project, the applicability to these questions of various kinds of experiments or research techniques, the nature and effects of possible ways of using the animals on what they experience, and techniques for preventing or minimizing their pain or distress. These are all matters of science and not ethical theory (Tannenbaum, 2013)”. The 3Rs principles cannot be applied correctly to an animal experiment without the knowledge base possessed uniquely by the scientists familiar with the research question, underscoring their critical role to realize the full potential of the 3Rs. All scientists should appreciate the relevance of the 3Rs to their work, especially from the perspective of validity, and to be aware of the latest developments in the 3Rs that relate to their research field and how they can contribute to these.
As individuals responsible for the design and conduct of research, investigators have a crucial role to play in implementation of the 3Rs. Most regulatory systems for the protection of animals in science place the onus on the investigator to apply the 3Rs when selecting models and approaches to be used for basic and applied research, regulatory testing, and education and training, with assistance and oversight provided by the institutional ethics committee (IACUC or AWERB). In a well-run animal facility, investigators, their scientific peers (not necessarily within the same discipline), the attending veterinarian and animal care staff will adopt a team approach, working together on the identification, application and review of 3Rs methods. Ideally this should begin with an evaluation of the availability of approaches to avoid or limit animal use. As new knowledge, technologies and approaches emerge there should be timely assessment and evolution of scientific and husbandry practices, research strategies and study designs to meet best practice. Sources of contemporary information and advice include the NC3Rs website (www.nc3rs.org.uk), Altweb site from John Hopkins Center for Alternatives to Animal Testing (http://altweb.jhsph.edu/) and the European Commission׳s database on alternative methods, DB-ALM (http://ecvam-dbalm.jrc.ec.europa.eu/beta/) is one.
In addition to the implementation of existing 3Rs methods, there are exciting opportunities open to investigators to contribute to the ambitious challenge of developing novel research models and tools aimed at reducing animal use and improving animal welfare. Such research is now a legitimate scientific goal in its own right, and provides new opportunities for funding, technological innovation, multidisciplinary collaboration and publishing. Investigators in the biosciences should consider applying to the competitive funding schemes available within their region (e.g. UK NC3Rs, www.nc3rs.org.uk, EU Horizon 2020, http://ec.europa.eu/programmes/horizon2020/). Nor is progress in the 3Rs limited to technological development and hypothesis driven research. There are opportunities to join in with pre-competitive data sharing to identify optimized study designs and protocols and to generate an evidence base to stimulate changes in policy, regulations and practice (Chapman et al., 2013; Robinson et al., 2008; Prescott et al., 2010).
Once developed these new research models and tools need to be published, disseminated and widely adopted in order to achieve major reductions in animal use and improvements in animal welfare. This requires investigators reviewing manuscripts and grant applications, and those conducting in vivo research, to have an open mind and be receptive towards novel approaches. For academic scientists in particular, who have built their careers on specific animal models, changing to a new, gold standard model (animal or non-animal) can be daunting, even where there is evidence to suggest the alternative approach is superior. However, the incentives include more informative and/or clinically relevant models (Tymvios et al., 2008; Moore and Emerson, 2012; Lidster et al., 2013), more rapid screening tools with improved sensitivity and/or specificity (Persaud et al., 2010; Redhead et al., 2012; Vinci et al., 2012; Walmsley and Tate, 2012) and the possibility of discoveries that would not otherwise be made using traditional models. For example, Williams and colleagues have used the social amoeba, Dictyostelium discoideum, to elucidate the mechanism of action of sodium valproate, the most widely prescribed drug for epilepsy treatment, and to identify new fatty acids and fatty acid derivatives with more potent anti-epileptic activity (Terbach et al., 2011; Chang et al., 2012).
5.1. Experimental design and reporting standards
Numerous surveys have documented serious omissions in the reporting of animal-based studies (e.g. Kilkenny et al., 2009). To make their work more transparent and reproducible, investigators should report in accordance with the ARRIVE guidelines (Kilkenny et al., 2010), as is recommended/required by over 430 scientific journals and the major UK bioscience funding bodies. The guidelines and supporting resources, such as a checklist and presentation with speaker notes, are available on the NC3Rs website (www.nc3rs.org.uk/arrive-guidelines). More comprehensive reporting should have the added benefit of making systematic reviews and meta-analyses of in vivo research more feasible (Hooijmans et al., 2011; Leenaars et al., 2011). This can lead to 3Rs impacts, such as supporting a reduction in animal numbers, determining whether high severity tests, multiple tests or higher species are necessary, and avoiding the use of uninformative models or those that do not translate. The NC3Rs is facilitating the wider use of systematic reviews and meta-analyses by researchers for 3Rs purposes by supporting the CAMARADES consortium (www.dcn.ed.ac.uk/camarades/default.htm).
Scientists, especially those trained extensively in in vitro techniques, may not have access to training for or expertise in experimental design or statistical analysis of animal experiments and as a result may use too many or too few animals, both of which are unethical, or analyze and interpret data incorrectly. Often group sizes are based on what has previously been used or what has been reported in the literature without a rigorous evaluation, and there is a lack of awareness of strategies to avoid bias. To help address these issues and support investigators that lack institutional access to professional statistical support, the NC3Rs is developing the Experimental Design Assistant, an online, knowledge-based system available to all scientists and amenable to a wide range of research areas (www.nc3rs.org.uk/experimental-design-assistant-eda). The NIH is supporting training courses targeted at graduate scientists, postdoctoral fellows and beginning investigators (http://grants.nih.gov/grants/guide/rfa-files/RFA-GM-15-006.html).
| Investigators and the 3Rs |
|
6. 3Rs use to reduce harm to animals and increase translational value
There are many examples of application of the 3Rs that demonstrate improved animal well-being and scientific benefit do not conflict with each other, but can act in synergy to improve the translational value of the model. This is highly relevant in convincing the scientific community that model design, support, and validation is worthy of at least as much attention as the scientific question they study.
6.1. 3D tumor spheroids for target validation and drug evaluation
Substantial advances in three-dimensional (3D) culture systems have improved agreement with the tumor microenvironment in vivo to replace use of mice in early screening of anticancer agents. Eccles and colleagues have developed a toolkit of 3D tumor spheroid models to support high throughput preclinical studies (Vinci et al., 2012). Prior to their development of the 3D culture toolkit many animals were used without legitimate in vitro validation since 2D tumor cell cultures are not sufficiently predictive of in vivo response. The toolkit provides more predictive in vitro functional assays of cell growth, motility, tissue invasion and angiogenesis that have improved early drug evaluation and replaced a significant proportion of animals used alongside 2D.
6.2. Moving away from thromboembolic mortality as a model of pulmonary embolism
A striking example of reduction of animals has been achieved in the mouse model of pulmonary embolism by Emerson and colleagues. Platelet-dependent thrombosis is a major factor in heart attack and stroke and studied extensively in mouse models capable of modeling physical factors like blood flow, shear stress, and vascular endothelial cell mediators (Tymvios et al., 2009). Conventional modeling relies on injection of thrombogenic substances in conscious animals that often results in paralysis and death. In contrast, Emerson׳s refined model is performed under general anesthesia using radiolabeled platelets and imaging to measure platelet function in real time during non-fatal thromboembolism (Tymvios et al., 2009). Not only were they successful in strengthening the model by broadening the spectrum beyond a single extreme (i.e. fatal pulmonary embolism) and measuring a specific biological response rather than events with non-specific causes, but also they were able to reduce the number of mice per experiment by around 90% (Emerson, 2010).
6.3. Neuroprotection in a novel mouse model of multiple sclerosis
Disease models typically place a considerable burden on animals from the perspective of symptoms resulting from the disease, but also in disease monitoring and application of experimental therapies. Extensive characterization of animal models (Table 2) is a key driver for refinement. Baker and colleagues recently developed a highly innovative refined mouse model of multiple sclerosis (MS) that avoids substantial suffering of animals (e.g. progressive ascending paralysis) associated with conventional autoimmune encephalomyelitis models (Lidster et al., 2013). Likewise they identified limitations in the conventional model, in that it primarily represented central nervous system inflammation but not other immune-independent mechanisms of neurodegeneration (Baker et al., 2011). Their approach induces optic neuritis (ON), the presenting feature in the majority of MS patients, to model axonal loss and neurodegeneration characteristic in MS. This has special scientific relevance, as disease progression can be monitored serially using non-invasive clinically relevant techniques, key in evaluating neuroprotective strategies. From the perspective of animal wellbeing, instead of paralysis the resulting disability from disease is visual sensory loss that is much better tolerated in rodents already evolved for nocturnal behaviors (Lidster et al., 2013).
6.4. Holistic refinement in nonhuman primate diabetes models
Diabetic animal models are another example of disease models where the burden to animals is substantial since animals require intensive clinical monitoring and medical care. These experiments should be run under conditions of optimal refinement. In induced models the method for disease induction must reliably result in disease while minimizing risk to the animal (Graham et al., 2011a, 2011b). Refinements techniques should also be used improve disease management, introduce features into the model to make it more ‘clinical trial-like’, or to avoid model-induced confounding, e.g. preventing nephro- and hepato- toxicity in streptozotocin-induced animals (Graham et al., 2012, 2010). The primary outcome measure in diabetes studies is often a stress sensitive metabolic parameter (e.g. blood glucose) so refined animal handling techniques are imperative (Lapin et al., 2013; Shirasaki et al., 2013;Gartner et al., 1980). Nonhuman primates are extensively used in to study β-cell replacement strategies (e.g. islet cell transplantation), for reasons described in depth elsewhere in this issue, and can be trained to cooperate with and facilitate their medical care while remaining in the familiar homecage using counter conditioning and positive reinforcement techniques (Graham et al., 2010). The scientific community is relatively clear on the aspect of refinement that attempts to lower the negative experiences of the animal but less so in the more progressive interpretation that seeks to also increase positive experiences for the animal to flourish. Training complex behaviors to NHPs is an important opportunity for the animals to engage in challenge, apply cognitive skills to decide actions, be active participants in their environments and reduce stress. Noteworthy is the fact that most ‘stress hormones’ have immunosuppressive activity and certainly considered a confound in studies aimed at evaluating immune response to transplanted tissue (Graham and Schuurman, 2013). Glucocorticosteroids might be among the best examples and are well known for their direct and chronic effect on thymus histology. Interestingly the presence of acute or chronic involution in thymic histology was significantly reduced in diabetic and immunosuppressed NHPs trained for cooperation. The use of refinement techniques in this model was successful both in significantly reducing model-induced adverse events affecting animal wellbeing and also in eliminating certain confounding variables that interfere with proper safety and efficacy evaluation of cell therapy products and immunosuppressive regimens (Graham and Schuurman, 2013).
| Application of the 3Rs |
| Practical application of the 3Rs can accelerate and improve translation. The model design, application, and validation is worthy of at least as much attention as the scientific question under study. |
7. Conclusion and perspectives
While focused primarily on the ethical imperative to minimize harm to animals in science, in developing the 3Rs, Russell and Burch also maintained that scientific excellence and the humane use of laboratory animals are inextricably linked. Although there is general agreement that improving the welfare of the animal enhances the quality of research, the 3Rs should be viewed even one level higher; this level includes the possibility that proper application of the 3Rs not only improves animal welfare but also enhances the ‘model agreement’ or translational value of the research. This combination approach towards the 3Rs seems essential to engage scientists in a more meaningful way with the 3Rs in practice. It is reasonable to expect that any animal model will have some degree of limitation, but proper experimental design and characterization plus detailed understanding of limitations allows for development of replacement alternatives or refinement of in vivo models towards a closer agreement with the human situation. Taking this step further can improve the predictive value of models, such that the translational power is increased and, in the case of animal based models, the contribution of the animal is maximized.
Better and more consistent application of the 3Rs is considered a major opportunity for “scientific, economic, and humanitarian” cross-benefit (Zurlo et al., 1996). Already in the mid-1990s participants in the Sheringham workshop made several very good recommendations towards this goal, especially highlighting the need to harmonize the incorporation of the 3Rs into various legal frameworks across nations, provide 3Rs-specific training, and also the need for international discussion and agreement on practical implementation of harm-benefit analysis (Zurlo et al., 1996). Considering the urgent need to accelerate translation, application of the 3Rs should be given a very high priority by scientists and regulators.
Authorship contributions
Design and outline: Melanie Graham, Mark Prescott
Writing: Melanie Graham, Mark Prescott
Draft review and finalization: Melanie Graham, Mark Prescott
Conflict of interest
The authors declare that there is no conflict of interest.
References
- AAALAC, 2014. The AAALAC International Accreditation Program. 〈http://www.aaalac.org/accreditation/〉.
- Abbott-Banner K.H., Holmes A., Adcock I., Rao N.L., Barrett E., Knowles R. Models of respiratory disease symposium. J. Inflamm. 2013;10:I1. doi: 10.1186/1476-9255-10-S1-I1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Administration of Quality Supervision, Inspection and Quarantine of the People׳s Republic of China and Standardization Administration of the People׳s Republic of China, 2013. National Standard of the People׳s Republic of China, Laboratory Animal Facilities – General Requirements for Quality and Competence, Draft Standard for Approval 12th April, 2013.
- AstraZeneca, 2014. Our 3Rs Commitment. 〈http://www.astrazeneca.com/Responsibility/Research-ethics/Animal-research/Our-3Rs-commitment〉.
- Bailey J., Thew M., Balls M. An analysis of the use of animal models in predicting human toxicology and drug safety. Altern. Lab. Anim. 2014;42:181–199. doi: 10.1177/026119291404200306. [DOI] [PubMed] [Google Scholar]
- Baker D., Gerritsen W., Rundle J., Amor S. Critical appraisal of animal models of multiple sclerosis. Mult. Scler. 2011;17:647–657. doi: 10.1177/1352458511398885. [DOI] [PubMed] [Google Scholar]
- Bayne K., Wurbel H. The impact of environmental enrichment on the outcome variability and scientific validity of laboratory animal studies. Rev. Sci. Tech. 2014;33:273–280. doi: 10.20506/rst.33.1.2282. [DOI] [PubMed] [Google Scholar]
- Begley C.G., Ellis L.M. Drug development: raise standards for preclinical cancer research. Nature. 2012;483:531–533. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- Brambell, F.W.R., 1965. Report of the Technical Committee to Enquire into the Welfare of Animals Kept Under Intensive Livestock Husbandry Systems: Presented to Parliament by the Secretary of State for Scotland and the Minister of Agriculture, Fisheries and Food by Command of Her Majesty December, 1965. HM Stationery Office.
- Brom, F., 1999. The use of ׳intrinsic value of animals׳ in the Netherlands. in: Dol, M., Fentener van Vlissingen, M., Kasanmoentalib, S. (Eds.). Van Gorcum, Assen, the Netherlands, pp. 15–28.
- Broom D.M. Indicators of poor welfare. Br. Vet. J. 1986;142:524–526. doi: 10.1016/0007-1935(86)90109-0. [DOI] [PubMed] [Google Scholar]
- Buchanan-Smith, H.M., Rennie, A., Vitale, A., Pollo, S., Prescott, M.J., Morton, D.B., 2005. Harmonising the Definition of Refinement.
- Burrows E.L., McOmish C.E., Hannan A.J. Gene-environment interactions and construct validity in preclinical models of psychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:1376–1382. doi: 10.1016/j.pnpbp.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Chang P., Orabi B., Deranieh R.M., Dham M., Hoeller O., Shimshoni J.A., Yagen B., Bialer M., Greenberg M.L., Walker M.C., Williams R.S. The antiepileptic drug valproic acid and other medium-chain fatty acids acutely reduce phosphoinositide levels independently of inositol in Dictyostelium. Dis. Model. Mech. 2012;5:115–124. doi: 10.1242/dmm.008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K.L., Holzgrefe H., Black L.E., Brown M., Chellman G., Copeman C., Couch J., Creton S., Gehen S., Hoberman A. Pharmaceutical toxicology: designing studies to reduce animal use, while maximizing human translation. Regul. Toxicol. Pharmacol. 2013;66:88–103. doi: 10.1016/j.yrtph.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Crossley N.A., Sena E., Goehler J., Horn J., van der Worp B., Bath P.M., Macleod M., Dirnagl U. Empirical evidence of bias in the design of experimental stroke studies: a metaepidemiologic approach. Stroke. 2008;39:929–934. doi: 10.1161/STROKEAHA.107.498725. [DOI] [PubMed] [Google Scholar]
- De Bono J., Ashworth A. Translating cancer research into targeted therapeutics. Nature. 2010;467:543–549. doi: 10.1038/nature09339. [DOI] [PubMed] [Google Scholar]
- DeGrazia D. Animal ethics around the turn of the twenty-first century. J. Agric. Environ. Ethics. 1998;11:111–129. doi: 10.1023/a:1009504617295. [DOI] [PubMed] [Google Scholar]
- Denayer T., Stöhr T., Van Roy M. Animal models in translational medicine: validation and prediction. New Horiz. Transl. Med. 2014;2:5–11. [Google Scholar]
- du Sert N.P., Holmes A.M., Wallis R., Andrews P.L. Predicting the emetic liability of novel chemical entities: a comparative study. Br. J. Pharmacol. 2012;165:1848–1867. doi: 10.1111/j.1476-5381.2011.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson M. Refinement, reduction and replacement approaches to in vivo cardiovascular research. Br. J. Pharmacol. 2010;161:749–754. doi: 10.1111/j.1476-5381.2010.00959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Parliament and the Council of the European Union, 2010. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals used for Scientific Purposes OJ L276/33. Off. J. Eur. Union 276.
- Friese M.A., Montalban X., Willcox N., Bell J.I., Martin R., Fugger L. The value of animal models for drug development in multiple sclerosis. Brain. 2006;129:1940–1952. doi: 10.1093/brain/awl083. doi: awl083 [pii] [DOI] [PubMed] [Google Scholar]
- Gartner K., Buttner D., Dohler K., Friedel R., Lindena J., Trautschold I. Stress response of rats to handling and experimental procedures. Lab. Anim. 1980;14:267–274. doi: 10.1258/002367780780937454. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline, 2014. The 3Rs in Medicine Research. 〈http://www.gsk.com/en-gb/research/our-use-of-animals/the-3rs-in-medicine-research/〉.
- Graham M.L., Mutch L.A., Kittredge J.A., Rieke E.F., Robinson N.A., Zolondek E.K., Faig A.W., DuFour T.A., Munson J.W., Schuurman H.J. Management of adverse side-effects after chemotherapy in macaques as exemplified by streptozotocin: case studies and recommendations. Lab. Anim. 2012;46:178–192. doi: 10.1258/la.2012.011077. [DOI] [PubMed] [Google Scholar]
- Graham M.L., Janecek J.L., Kittredge J.A., Hering B.J., Schuurman H.J. The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp. Med. 2011;61:356. [PMC free article] [PubMed] [Google Scholar]
- Graham M.L., Mutch L.A., Rieke E.F., Kittredge J.A., Faig A.W., DuFour T.A., Munson J.W., Zolondek E.K., Hering B.J., Schuurman H.J. Refining the high-dose streptozotocin-induced diabetic non-human primate model: an evaluation of risk factors and outcomes. Exp. Biol. Med. 2011;236:1218–1230. doi: 10.1258/ebm.2011.011064. [DOI] [PubMed] [Google Scholar]
- Graham M.L., Rieke E.F., Mutch L.A., Zolondek E.K., Faig A.W., DuFour T.A., Munson J.W., Kittredge J.A., Schuurman H.J. Successful implementation of cooperative handling eliminates the need for restraint in a complex non-human primate disease model. J. Med. Primatol. 2010;60:479–485. doi: 10.1111/j.1600-0684.2011.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M.L., Schuurman H. The usefulness and limitations of the diabetic macaque model in evaluating long-term porcine islet xenograft survival. Xenotransplantation. 2013;20:5–17. doi: 10.1111/xen.12012. [DOI] [PubMed] [Google Scholar]
- Graham M.L. Refinement of vascular access port placement in nonhuman primates: complication rates and outcomes. Comp. Med. 2010;60:479. [PMC free article] [PubMed] [Google Scholar]
- Griffin G., Clark J.M., Zurlo J., Ritskes-Hoitinga M. Scientific uses of animals: harm-benefit analysis and complementary approaches to implementing the three Rs. Rev. Sci. Tech. 2014;33:265–272. doi: 10.20506/rst.33.1.2283. [DOI] [PubMed] [Google Scholar]
- Hare R. In: A Utilitarian Approach. 2 ed. Kuhse H., Singer P., editors. Blackwell Publishing; Malden, MA, USA: 2009. pp. 85–90. [Google Scholar]
- Holmes A.M., Solari R., Holgate S.T. Animal models of asthma: value, limitations and opportunities for alternative approaches. Drug Discov. Today. 2011;16:659–670. doi: 10.1016/j.drudis.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Hooijmans C.R., de Vries R., Leenaars M., Curfs J., Ritskes‐Hoitinga M. Improving planning, design, reporting and scientific quality of animal experiments by using the Gold Standard Publication Checklist, in addition to the ARRIVE guidelines. Br. J. Pharmacol. 2011;162:1259–1260. doi: 10.1111/j.1476-5381.2010.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells D.W., Sena E.S., Macleod M.R. Bringing rigour to translational medicine. Nat. Rev. Neurol. 2014;10:37–43. doi: 10.1038/nrneurol.2013.232. [DOI] [PubMed] [Google Scholar]
- Hurst J.L., West R.S. Taming anxiety in laboratory mice. Nat. Methods. 2010;7:825–826. doi: 10.1038/nmeth.1500. [DOI] [PubMed] [Google Scholar]
- Ipsos MORI, 2014. Attitudes to Animals in Research: A Report by Ipsos MORI for the Department for Business, Innovation & Skills.
- Janet, C., Barbee, R., Bielitzki, J., Clayton, L., Donovan, J., Hendriksen, C., Kohn, D., Lipman, N., Locke, P., Melcher, J., 2011. Guide for the Care and Use of Laboratory Animals.
- Keating S.C., Thomas A.A., Flecknell P.A., Leach M.C. Evaluation of EMLA cream for preventing pain during tattooing of rabbits: changes in physiological, behavioural and facial expression responses. PloS One. 2012;7:e44437. doi: 10.1371/journal.pone.0044437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C., Parsons N., Kadyszewski E., Festing M.F., Cuthill I.C., Fry D., Hutton J., Altman D.G. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PloS One. 2009;4:e7824. doi: 10.1371/journal.pone.0007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola I., Landis J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004;3:711–716. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- Lapin B., Gvozdik T., Klots I. Blood glucose levels in Rhesus Monkeys (Macaca mulatta) and Cynomolgus Macaques (Macaca fascicularis) under moderate stress and after recovery. Bull. Exp. Biol. Med. 2013;154:497–500. doi: 10.1007/s10517-013-1986-7. [DOI] [PubMed] [Google Scholar]
- Leach M.C., Klaus K., Miller A.L., Di Perrotolo M.S., Sotocinal S.G., Flecknell P.A. The assessment of post-vasectomy pain in mice using behaviour and the Mouse Grimace Scale. PloS One. 2012;7:e35656. doi: 10.1371/journal.pone.0035656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenaars, M., Ritske-Hoitinga, M., Griffin, G., Ormandy, E., 2011. Background to the Montréal Declaration on the Synthesis of Evidence to Advance the 3Rs Principles in Science, as Adopted by the 8th World Congress on Alternatives and Animal Use in the Life Sciences, Montréal, Canada, on August 25, 2011. and Animal Use in the Life Sciences, Montreal, 35.
- Leist M., Hartung T., Nicotera P. The dawning of a new age of toxicology. ALTEX. 2008;25:103–114. [PubMed] [Google Scholar]
- Lidster K., Jackson S.J., Ahmed Z., Munro P., Coffey P., Giovannoni G., Baker M.D., Baker D. Neuroprotection in a novel mouse model of multiple sclerosis. PloS One. 2013;8:e79188. doi: 10.1371/journal.pone.0079188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20:359–368. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Martin B., Ji S., Maudsley S., Mattson M.P. “Control” laboratory rodents are metabolically morbid: why it matters. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonigle P. Animal models of CNS disorders. Biochem. Pharmacol. 2014;87:140–149. doi: 10.1016/j.bcp.2013.06.016. [DOI] [PubMed] [Google Scholar]
- McGonigle P., Ruggeri B. Animal models of human disease: challenges in enabling translation. Biochem. Pharmacol. 2014;87:162–171. doi: 10.1016/j.bcp.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Mench J.A. Thirty years after Brambell: whither animal welfare science? J. Appl. Anim. Welf. Sci. 1998;1:91–102. doi: 10.1207/s15327604jaws0102_1. [DOI] [PubMed] [Google Scholar]
- Mercer P.F., Abbott-Banner K., Adcock I.M., Knowles R.G. Translational models of lung disease. Clin. Sci. 2015;128:235–256. doi: 10.1042/CS20140373. [DOI] [PubMed] [Google Scholar]
- Mergenthaler P., Meisel A. Do stroke models model stroke? Dis. Model. Mech. 2012;5:718–725. doi: 10.1242/dmm.010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Environment and Forestry India, 2013. Standard Operation Procedure (SOP) for Institutional Animal Ethics Committee (IAEC), Guidelines on the Regulation of Scientific Experiments on Animals, Guidelines for Laboratory Animal Facility and Breeding of and Experiments on Animals (Control and Supervision) Rules, Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA).
- Mix E., Meyer-Rienecker H., Hartung H., Zettl U.K. Animal models of multiple sclerosis—potentials and limitations. Prog. Neurobiol. 2010;92:386–404. doi: 10.1016/j.pneurobio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, C., Emerson, M., 2012. Assessment of Platelet Aggregation Responses In Vivo in the Mouse. In: Anonymous. Springer, pp. 21–28. [DOI] [PubMed]
- Moreno L., Pearson A.D. How can attrition rates be reduced in cancer drug discovery? Expert Opin. Drug Discov. 2013;8:363–368. doi: 10.1517/17460441.2013.768984. [DOI] [PubMed] [Google Scholar]
- Mullane K., Williams M. Animal models of asthma: reprise or reboot? Biochem. Pharmacol. 2014;2014;8787:131–139. 131–139. doi: 10.1016/j.bcp.2013.06.026. [DOI] [PubMed] [Google Scholar]
- National Research Council, 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy. Committee on Toxicity and Assessment of Environmental Agents.
- Novartis, 2014. Animal Welfare at Novartis. 〈http://www.novartis.com/innovation/responsibly-tackling-the-challenging-issues/animal-research/animal-welfare/the-3rs.shtml〉.
- Ohl F., Van der Staay F. Animal welfare: at the interface between science and society. Vet. J. 2012;192:13–19. doi: 10.1016/j.tvjl.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Ohno Y. ICH guidelines—implementation of the 3Rs (refinement, reduction, and replacement): incorporating best scientific practices into the regulatory process. ILAR J. 2002;43:S95–S98. doi: 10.1093/ilar.43.suppl_1.s95. [DOI] [PubMed] [Google Scholar]
- Olsson J., Robinson P., Sandøe P. In: Ethics of animal research. 3 ed. Hau J., Schapiro S., editors. CRC Press, Taylor & Francis Group; Boca Raton, FL, USA: 2010. pp. 21–38. [Google Scholar]
- Organisation for Economic Co-operation and Development, 2013. Guidance Document on Developing and Assessing Adverse Outcome Pathways: Series on Testing and Assessment, No. 184.
- Pachner A.R. Experimental models of multiple sclerosis. Curr. Opin. Neurol. 2011;24:291–299. doi: 10.1097/WCO.0b013e328346c226. [DOI] [PubMed] [Google Scholar]
- Percie du Sert N., Rice A. Improving the translation of analgesic drugs to the clinic: animal models of neuropathic pain. Br. J. Pharmacol. 2014;171:2951–2963. doi: 10.1111/bph.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud, S.J., Arden, C., Bergsten, P., Bone, A.J., Brown, J., Dunmore, S., Harrison, M., Hauge-Evans, A.C., Kelly, C., King, A., 2010. Pseudoislets as Primary Islet Replacements for Research: Report on a Symposium at King׳s College London, London UK. Islets 2, 236–239. [DOI] [PubMed]
- Prescott M.J., Brown V.J., Flecknell P.A., Gaffan D., Garrod K., Lemon R.N., Parker A.J., Ryder K., Schultz W., Scott L. Refinement of the use of food and fluid control as motivational tools for macaques used in behavioural neuroscience research: report of a Working Group of the NC3Rs. J. Neurosci. Methods. 2010;193:167–188. doi: 10.1016/j.jneumeth.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Redhead K., Wood K., Jackson K. Testing of veterinary clostridial vaccines: from mouse to microtitre plate. Dev. Biol. 2012;134:45–50. [PubMed] [Google Scholar]
- Regan T. In: The case for animal rights. 2 ed. Regan T., Singer P., editors. Prentice Hall; Englewood Cliffs, NJ, USA: 1989. pp. 105–114. [Google Scholar]
- Robinson S., Delongeas J., Donald E., Dreher D., Festag M., Kervyn S., Lampo A., Nahas K., Nogues V., Ockert D. A European pharmaceutical company initiative challenging the regulatory requirement for acute toxicity studies in pharmaceutical drug development. Regul. Toxicol. Pharmacol. 2008;50:345–352. doi: 10.1016/j.yrtph.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Ruggeri B.A., Camp F., Miknyoczki S. Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem. Pharmacol. 2014;87:150–161. doi: 10.1016/j.bcp.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Russell W., Burch R. Universities Federation for Animal Welfare; Potters Bar, England: 1959. The Principles of Humane Experimental Technique. [Google Scholar]
- Sandøe P., Crisp R., Holtug N. In: Ethics. Appleby M., Hughes B., editors. CAB International; Wallingford, Oxon, UK: 1997. pp. 3–18. [Google Scholar]
- Sena E.S., Van Der Worp H Bart, Bath P.M., Howells D.W., Macleod M.R. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8:e1000344. doi: 10.1371/journal.pbio.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki Y., Yoshioka N., Kanazawa K., Maekawa T., Horikawa T., Hayashi T. Effect of physical restraint on glucose tolerance in cynomolgus monkeys. J. Med. Primatol. 2013;42:165–168. doi: 10.1111/jmp.12039. [DOI] [PubMed] [Google Scholar]
- Singer P. In: All animals are equal. 2 ed. Regan T., Singer P., editors. Prentice Hall; Englewood Cliffs, NJ, USA: 1989. pp. 73–86. [Google Scholar]
- Smith J.A., van den Broek F.A., Martorell J.C., Hackbarth H., Ruksenas O., Zeller W., FELASA Working Group on Ethical Evaluation of Animal Experiments Principles and practice in ethical review of animal experiments across Europe: summary of the report of a FELASA working group on ethical evaluation of animal experiments. Lab. Anim. 2007;41:143–160. doi: 10.1258/002367707780378212. [DOI] [PubMed] [Google Scholar]
- Spruijt B.M., van den Bos R., Pijlman F.T.A. A concept of welfare based on reward evaluating mechanisms in the brain: anticipatory behaviour as an indicator for the state of reward systems. Appl. Anim. Behav. Sci. 2001;72:145–171. doi: 10.1016/s0168-1591(00)00204-5. [DOI] [PubMed] [Google Scholar]
- Tannenbaum J. Ethics and animal welfare: the inextricable connection. J. Am. Vet. Med. Assoc. 1991;198:1360–1376. [PubMed] [Google Scholar]
- Tannenbaum J. In: Chapter 1 – Ethics in Biomedical Animal Research: The Key Role of the Investigator. Conn P.M., editor. Academic Press; Boston: 2013. pp. 3–36. [Google Scholar]
- Terbach N., Shah R., Kelemen R., Klein P.S., Gordienko D., Brown N.A., Wilkinson C.J., Williams R.S. Identifying an uptake mechanism for the antiepileptic and bipolar disorder treatment valproic acid using the simple biomedical model Dictyostelium. J. Cell Sci. 2011;124:2267–2276. doi: 10.1242/jcs.084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymvios C., Moore C., Jones S., Solomon A., Sanz-Rosa D., Emerson M. Platelet aggregation responses are critically regulated in vivo by endogenous nitric oxide but not by endothelial nitric oxide synthase. Br. J. Pharmacol. 2009;158:1735–1742. doi: 10.1111/j.1476-5381.2009.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymvios C., Jones S., Moore C., Pitchford S.C., Page C.P., Emerson M. Real-time measurement of non-lethal platelet thromboembolic responses in the anaesthetized mouse. Thromb. Haemost. 2008;99:435–440. doi: 10.1160/TH07-07-0479. [DOI] [PubMed] [Google Scholar]
- Unilever, 2014. Developing Alternative Approaches to Animal Testing. 〈http://www.unilever.com/sustainable-living-2014/our-approach-to-sustainability/responding-to-stakeholder-concerns/developing-alternative-approaches-to-animal-testing/〉.
- United States Department of Agriculture, 2013. Animal Welfare Act and Animal Welfare Regulations, November 2013.
- Bart van der Worp H., Howells D.W., Sena E.S., Porritt M.J., Rewell S., O׳Collins V., Macleod M.R. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinci M., Gowan S., Boxall F., Patterson L., Zimmermann M., Court W., Lomas C., Mendiola M., Hardisson D., Eccles S.A. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012;10 doi: 10.1186/1741-7007-10-29. 29-7007-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker I., Newell H. Do molecularly targeted agents in oncology have reduced attrition rates? Nat. Rev. Drug Discov. 2008;8:15–16. doi: 10.1038/nrd2758. [DOI] [PubMed] [Google Scholar]
- Walmsley, R.M., Tate, M., 2012. The GADD45a-GFP GreenScreen HC assay. In: Anonymous. Springer. pp. 231–250. [DOI] [PubMed]
- Webb D.R. Animal models of human disease: inflammation. Biochem. Pharmacol. 2014;87:121–130. doi: 10.1016/j.bcp.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Wijkstrom M., Bottino R., Cooper D.K. Limitations of the pig to non human primate islet transplantation model. Xenotransplantation. 2013;20:2–4. doi: 10.1111/xen.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurlo J., Rudacille D., Goldberg A.M. The three Rs: the way forward. Environ. Heal. Perspect. 1996;104:878–880. doi: 10.1289/ehp.96104878. [DOI] [PMC free article] [PubMed] [Google Scholar]