Abstract
Catharanthus roseus produces a wide range of terpenoid indole alkaloids (TIA). Many of them, such as vinblastine and vincristine, have significant bioactivity. They are valuable chemotherapy drugs used in combination with other drugs to treat lymphoma and leukemia. The TIA biosynthetic pathway has been investigated for many years, for scientific interest and for their potential in manufacturing applications, to fulfill the market demand. In this review, the progress and perspective of C. roseus TIA biosynthesis and its regulating enzymes are described. In addition, the culture condition, hormones, signaling molecules, precursor feeding on the accumulation of TIA, and gene expression are also evaluated and discussed.
Keywords: Biosynthesis pathway, enzymes, gene expression, regulation, terpenoid indole alkaloids, vinblastine
BIOSYNTHESIS OF BISINDOLE ALKALOIDS
The medicinal plant Catharanthus roseus (L.) G. Don is of enormous pharmaceutical interest because it contains about 130 terpenoid indole alkaloids (TIAs), some of which exhibit strong pharmacological activities.[1] Jamalicine is an antihypertensive alkaloid. Vinblastine and vincristine, which are bisindole alkaloids derived from coupling vindoline and catharanthine, were the first natural drugs used in cancer therapy and are still among the most valuable agents used in the treatment of cancer, to date.[2] All TIAs in C. roseus are derived from the central precursor strictosidine, which is a fusion product of the shikimate pathway–derived tryptamine moiety and the plastidic nonmevalonate pathway–derived secologanin moiety.[3] The anticancer agents vinblastine and vincristine are produced exclusively by C. roseus.[4] More importantly, the TIA biosynthetic pathway is under strict developmental and environmental control.[3]
The biosynthetic pathway of C. roseus TIA has been investigated for many years, but the whole process is not completely understood. Overall, three stages could be recognized in the biosynthesis of bisindole alkaloids:[3,5,6] (1) Formation of tryptamine and secologanin: Tryptamine is biosynthesized from the shikimate pathway and secologanin from the terpenoid pathway; (2) Formation of monomeric alkaloids: Tryptamine and secologanin are combined to form strictosidine, which is further converted to monomeric alkaloids like vindoline and catharanthine; (3) Formation of bisindole alkaloids: Vinblastine and vincristine synthesize from the coupling of catharanthine and vindoline [Figure 1].
Figure 1.
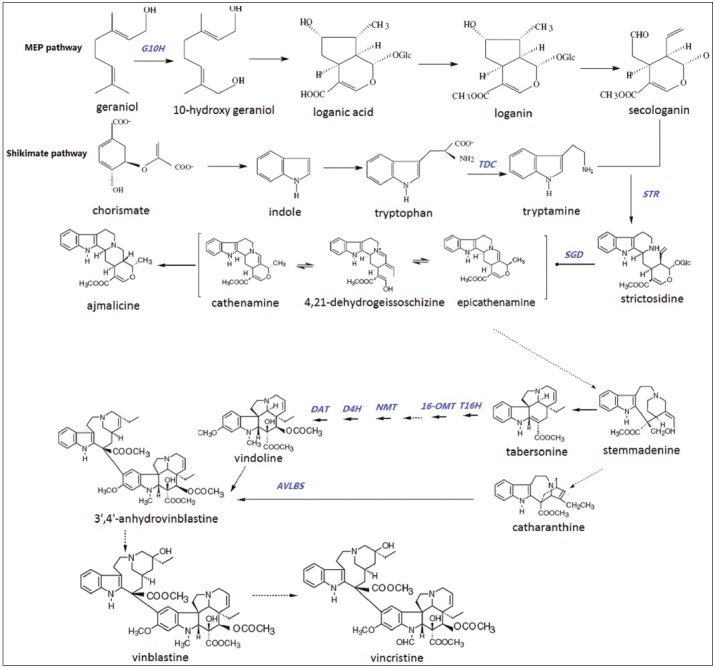
Biosynthesis of Catharanthus TIAs. Solid arrows indicate confirmed enzymatic conversions, whereas, the broken arrows indicate unknown enzymatic conversions. G10H: geraniol 10-hydroxylase; TDC: Tryptophan decarboxylase; STR: strictosidine synthase; SGD: Strictosidine β-D-glucosidase; T16H: 16-hydroxylase; OMT: O-methyltransferase; NMT: N-methyltransferase; D4H: Desacetoxyvindoline 4-hydroxylase; DAT: Deacetylvindoline-4-O-acetyltransferase; AVLBS: Anhydrovinblastine synthase
FORMATION OF STRICTOSIDINE
Strictosidine, the central intermediate in the TIA biosynthesis of C. roseus, is formed by the coupling of iridoid glycoside secologanin and tryptamine under the catalysis of strictosidine synthase (STR).[7,8]
FORMATON OF VINDOLINE
Strictosidine-β-D-glucosidase (SGD) may be the enzyme playing an important role in steering the monoterpenoid indole alkaloid biosynthesis in a specific direction.[5] The removal of the glucose moiety of strictosidine by SGD leads to an unstable, highly reactive aglucon, which is thought to be converted to 4,21-dehydrogeissoschizine.[9] The latter is believed to be converted by cathenamine synthase to cathenamine.[5,10] Subsequently, the cathenamine is converted into tabersonine through several steps, which are not clearly understood. Finally, tabersonine is transformed into vindoline by a sequence of six steps.[4,5] The steps include: Aromatic hydroxylation, O-methylation, hydration of the 2,3-double bond, N(1)-methylation, hydroxylation at position 4, and 4-O-acetylation. The intermediates involved are 16-hydroxytabersonine, 16-methoxytabersonine, 16-methoxy-2,3-dihydro-3-hydroxy-tabersonine, desacetoxyvindoline, and deacetylvindoline. Most enzymes in the biosynthetic pathway from tabersonine to vindoline have been identified. They are tabersonine 16-hydroxylase (T16H), O-methyltransferase (OMT), N-methyltransferase (NMT), desacetoxyvindoline-4-hydroxylase(D4H), and deacetylvindoline-4-O-acetyltransferase (DAT). However, the enzyme that catalyzes the conversion of 16-methoxytabersonine to 16-methoxy-2,3-dihydro-3-hydroxy- tabersonine is still unknown.[4]
FORMATION OF CATHARANTHINE
The information on catharanthine biosynthesis is very limited. It may be derived from strictosidine through the intermediate of geissoschizine and stemmadenine. However, the enzymes involved are not isolated and the genes are not cloned.[5]
FORMATION OF BISINDOLE ALKALOIDS
The bisindole alkaloids vinblastine and vincristine are of great interest. They are synthesized from the coupling of the monomeric alkaloids catharanthine and vindoline. The product resulting from the coupling is α-3′,4′-anhydrovinblastine, which is converted into vinblastine and then further converted into vincristine. The coupling process is catalyzed by the enzyme anhydrovinblastine synthase (AVLBS).[11] However, the enzyme catalysis the formation of vinblastine from α-3′,4′-anhydrovinblastine is still unknown. Moreover, the enzyme that catalyzes the conversion of vinblastine to vincristine is also not isolated.
REGULATION FACTORS OF DIMERIC INDOLE ALKALOIDS DIAS AND THEIR PRECURSOR BIOSYNTHESIS
Light
Light is thought to have an effect on enzyme induction and activation. It has been shown that light significantly influences the biosynthesis of vindoline and other alkaloids, as well as acidic and basic peroxidase activities. Light promotes vindoline and serpentine biosynthesis, and stimulates plastid development and peroxidase activity.[12] After it is light-treated, the concentration of vindoline increases significantly in cultures of C. roseus, including in cultured cells, leaves, seedlings, and plants. The results of gene expression investigation have demonstrated that upregulation of tryptophan decarboxylase (TDC), D4H, and DAT has been observed in C. roseus cultures after light expression.[12,13,14,15,16,17] Researches have also shown that the content of catharanthine, vindoline, and vinblastine is markedly increased by ultraviolet (UV)-B radiation in C. roseus.[13,18]
Plant growth regulators
Plant growth regulators affect both culture growth and secondary metabolite production.
Auxin and cytokinin
Plant growth hormones inhibit the accumulation of alkaloid, while cytokinins act as accelerants. 2,4-D strongly inhibits alkaloid production, essentially during the growth phase.[19] More importantly, genes, such as, the 1-deoxy-D-xylulose-5-phosphate synthase (DXS) gene, 1-deoxy-D-xylulose-5-phosphate reductoisomerase (DXR) gene, and TDC gene are repressed in the suspension-cultured cells of C. roseus with 2,4-D.[20,21] Transferring these cell suspensions in a 2,4-D-free culture medium gradually increases the expression of these genes.[21,22] Moreover, 2,4-D depletion also enhances the 2C-methyl-D-erythritol 2,4-cyclodiphosphate synthase (CRMECS) mRNA steady-state level in C. roseus. This gene remains expressed at a low rate in the cell suspensions cultured with 2,4-D.[22] These investigations support the regulatory role of auxin on the methylerythritol phosphate (MEP) pathway, leading to the biosynthesis of the TIA terpene moiety, and auxin acts, at least in part, at the MEP pathway gene expression regulation level.[20]
Except for 2,4-D, other auxins such as, 1-Naphthaleneacetic acid (NAA) and indole-3-acetic acid (IAA), also downregulate the TDC gene in the biosynthetic pathway of alkaloids. Omission of NAA from the growth medium results in the accumulation of TDC.[21] Thus, auxin negatively regulates the expression of the genes associated with the terpenoid indole alkaloid biosynthesis in C. roseus
On the contrary, cytokinin remarkably enhances the accumulation of alkaloids in Catharanthus cultures. Genes experiments show that cytokinin greatly enhances the expression of the geraniol 10-hydroxylase (G10H) gene.[23]
Other plant growth regulators
Enhancement of catharanthine in the hairy roots and accumulation of vindoline in shoot cultures are observed after ethylene treatment.[15] In addition, ethylene applications promote the pathways toward ajmalicine, serpentine, and tabersonine.[24] Gibberellic acid (GA), similar to 2,4-D, has a strongly negative influence on the accumulation of vinblastine, vindoline, and catharanthine.[25] Our previous study has found that artemisinic acid, a cadinane-type of sesquiterpene, can stimulate the biosynthesis of catharanthine, vindoline, and vinblastine in C. roseus cultured cells. In addition, upregulation of G10H, SGD, TDC, T16H, and D4H was also observed.[26,27]
Signaling molecules
Jasmonates
Jasmonates are plant signaling molecules that play key roles in protection against certain pathogens and insects, by switching on the expression of gene-encoding defense proteins, including enzymes involved in the biosynthesis of toxic secondary metabolites.[28]
In the hairy roots, jasmonic acid (JA) is found to be a unique elicitor leading to an enhancement in the flux to several branches in the indole alkaloid pathway.[29] The accumulation of ajmalicine, serpentine, lochnericine, and hörhammericine are significantly increased after the addition of jasmonic acid.[29]
Methyl jasmonate (MJ) induces several alkaloids in C. roseus cultures. After treatment with MJ, ajmaline is increased in the cultured plant cells, while both ajmalicine and catharanthine are increased in the hairy roots. For shoot cultures, vindoline accumulation, induced by MJ, is observed, which is about 6.5-fold compared to the control.[15]
In C. roseus seedlings, the TIA genes exhibit a significant variation in the magnitude and timing of induction by MJ. ORCA3, a jasmonate-responsive APETALA2 (AP2)-domain transcription factor gene, exhibits the greatest increase in the transcript levels after MJ treatment.[30,31] MJ-induced increases in the transcript levels of the TIA genes occur in the following order: ORCA3, D4H, STR, TDC, G10H, and cytochrome P-450 reductase (CPR).[30]
Salicylic acid
Salicylic acid (SA) has been known to ameliorate the adverse effects of salinity by improving plant productivity through protecting the photosynthetic pigments and producing antioxidative compounds and enzymes.[32] It enhances vincristine and vinblastine alkaloid production in C. roseus by improving the antioxidant defense system.[32]
In seeding, salicylic acid treatment increased the production of tabersonine and a higher concentration of salicylic acid induced vindoline accumulation. Meanwhile, the activity of alkaline peroxidase increased 5-fold.[24]
Nitric oxide
Nitric oxide (NO) is known as a signaling molecule involved in the elicitor-induced defense responses of plants. Sodium nitroprusside (SNP), a donor of NO, stimulates catharanthine formation in C. roseus cells.[33] However, the yield of catharanthine decreases when treated with the JA inhibitor (such as ibuprofen). Therefore, NO stimulates the accumulation of catharanthine, which is JA-dependent.[33]
Precursor feeding
Feeding of precursors is one of the most effective strategies to increase the production of important secondary metabolites in cells and organ cultures.[34] The results of the addition of some precursors show positive results on the accumulation of alkaloids. For instance, the accumulation of ajmalicine and strictosidine is significantly enhanced when treated with tryptamine or loganic acid.[35]
CONCLUSION
The biosynthesis of the C. roseus terpenoid indole alkaloid has been studied for decades. Although there is tremendous progress, there are many steps that are still not profiled. To date, C. roseus is the only nature resource for antitumor agents, vinblastine and vincristine. However, their content in plants is very low. Furthermore, the chemical synthesis is far from being applicable for commercial-scale production. The plant cell culture of C. roseus is an alternative resource for these valuable medicinal compounds. However, before their commercial exploitation, their productivity has to be improved. Therefore, different approaches, such as optimization of culture conditions, feeding, and elicitation strategies have been tested, to enhance the production of these compounds. However, until now, the yields obtained from the cultures have been low. The unclear biosynthesis pathway of the TIAs is the main reason that blocks the progress. Therefore, elucidation of the TIA biosynthesis pathway must be emphasized on greatly and some new strategies, such as plant and microbial production, may also be employed for the manufacture of these valuable medicinal compounds.
ACKNOWLEDGMENTS
This research study was financially supported by the National Natural Science Foundation of China (No. 81073004, 81102771 and 81274045), the Natural Science Foundation of Guangdong Province (No. S2011040003028), Pearl River Scientific and Technological New Star Program of Guangzhou (No. 2014J2200004) and Science and Technology Specific Project of Guangzhou (No. 201300000138)
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.van Der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R. The Catharanthus alkaloids: Pharmacognosy and biotechnology. Curr Med Chem. 2004;11:607–28. doi: 10.2174/0929867043455846. [DOI] [PubMed] [Google Scholar]
- 2.Costa MM, Hilliou F, Duarte P, Pereira LG, Almeida I, Leech M, et al. Molecular cloning and characterization of a vacuolar class III peroxidase involved in the metabolism of anticancer alkaloids in Catharanthus roseus. Plant Physiol. 2008;146:403–17. doi: 10.1104/pp.107.107060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rischer H, Oresic M, Seppänen-Laakso T, Katajamaa M, Lammertyn F, Ardiles-Diaz W, et al. Gene-to-metabolite networks for terpenoid indole alkaloid biosynthesis in Catharanthus roseus cells. Proc Natl Acad Sci U S A. 2006;103:5614–19. doi: 10.1073/pnas.0601027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liscombe DK, OConnor SE. A virus-induced gene silencing approach to understanding alkaloid metabolism in Catharanthus roseu s. Phytochemistry. 2011;72:1969–77. doi: 10.1016/j.phytochem.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Sayed M, Verpoorte R. Catharanthus terpenoid indole alkaloids: Biosynthesis and regulation. Phytochem Rev. 2007;6:277–305. [Google Scholar]
- 6.Liu D, Jin H, Chen Y, Cui L, Ren W, Gong Y, et al. Terpenoid indole alkaloids biosynthesis and metabolic engineering in Catharanthus roseus. J Integr Plant Biol. 2007;49:961–74. [Google Scholar]
- 7.Treimer JF, Zenk MH. Purification and properties of strictosidine synthase, the key enzyme in indole alkaloid formation. Eur J Biochem. 1979;101:225–33. doi: 10.1111/j.1432-1033.1979.tb04235.x. [DOI] [PubMed] [Google Scholar]
- 8.Mizukami H, Nordlöv H, Lee SL, Scott AI. Purification and properties of strictosidine synthetase (an enzyme condensing tryptamine and secologanin) from Catharanthus roseus cultured cells. Biochemistry. 1979;18:3760–3. doi: 10.1021/bi00584a018. [DOI] [PubMed] [Google Scholar]
- 9.Geerlings A, Ibañez MM, Memelink J, van Der Heijden R, Verpoorte R. Molecular cloning and analysis of strictosidine beta-D-glucosidase, an enzyme in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. J Biol Chem. 2000;275:3051–6. doi: 10.1074/jbc.275.5.3051. [DOI] [PubMed] [Google Scholar]
- 10.Rüffer M, Kan-Fan C, Husson H, Stöckigt J, Zenk MH. 4,21-Dehydrogeissoschizine, an intermediate in heteroyohimbine alkaloid biosynthesis. J Chem Soc Chem Commun. 1979;22:1016–8. [Google Scholar]
- 11.Sottomayor M, Lopez-Serrano M, DiCosmo F, Ros Barceló A. Purification and characterization of alpha-3′,4′-anhydrovinblastine synthase (peroxidase-like) from Catharanthus roseus (L.) G. Don. FEBS Lett. 1998;428:299–303. doi: 10.1016/s0014-5793(98)00551-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Zhu WH, Hu Q. Effects of light and plant growth regulators on the biosynthesis of vindoline and other indole alkaloids in Catharanthus roseus callus cultures. Plant Growth Regul. 2001;33:43–9. [Google Scholar]
- 13.Liu Y, Zhao D, Zu Y, Tang Z, Zhang Z, Jiang Y, et al. Effects of low light on terpenoid indole alkaloid accumulation and related biosynthetic pathway gene expression in leaves of Catharanthus roseus seedlings. Bot Stud. 2011;52:191–6. [Google Scholar]
- 14.He L, Yang L, Tan R, Zhao S, Hu Z. Enhancement of vindoline production in suspension culture of the Catharanthus roseus cell line C20hi by light and methyl jasmonate elicitation. Anal Sci. 2011;27:1243–8. doi: 10.2116/analsci.27.1243. [DOI] [PubMed] [Google Scholar]
- 15.Vázquez-Flota F, Hernández-Dominguez E, de Lourdes Miranda-Ham M, Monforte-González M. A differential response to chemical elicitors in Catharanthus roseus in vitro cultures. Biotechnol Lett. 2009;31:591–5. doi: 10.1007/s10529-008-9881-4. [DOI] [PubMed] [Google Scholar]
- 16.Schröder G, Unterbusch E, Kaltenbach M, Schmidt J, Strack D, De Luca V, et al. Light-induced cytochrome P450-dependent enzyme in indole alkaloid biosynthesis: Tabersonine 16-hydroxylase. FEBS Lett. 1999;458:97–102. doi: 10.1016/s0014-5793(99)01138-2. [DOI] [PubMed] [Google Scholar]
- 17.Ouwerkerk PB, Hallard D, Verpoorte R, Memelink J. Identification of UV-B light-responsive regions in the promoter of the tryptophan decarboxylase gene from Catharanthus roseus. Plant Mol Biol. 1999;41:491–503. doi: 10.1023/a:1006321100550. [DOI] [PubMed] [Google Scholar]
- 18.Ramani S, Jayabaskaran C. Enhanced catharanthine and vindoline production in suspension cultures of Catharanthus roseus by ultraviolet-B light. J Mol Signal. 2008;3:9. doi: 10.1186/1750-2187-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arvy MP, Imbault N, Naudascher F, Thiersault M, Doireau P. 2,4-D and alkaloid accumulation in periwinkle cell suspensions. Biochimie. 1994;76:410–6. doi: 10.1016/0300-9084(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 20.Hedhili S, Courdavault V, Giglioli-Guivarc’h N, Gantet P. Regulation of the terpene moiety biosynthesis of Catharanthus roseus terpene indole alkaloids. Phytochem Rev. 2007;6:341–51. [Google Scholar]
- 21.Pasquali G, Goddijn OJ, de Waal A, Verpoorte R, Schilperoort RA, Hoge JH, et al. Coordinated regulation of two indole alkaloid biosynthetic genes from Catharanthus roseus by auxin and elicitors. Plant Mol Biol. 1992;18:1121–31. doi: 10.1007/BF00047715. [DOI] [PubMed] [Google Scholar]
- 22.Veau B, Courtois M, Oudin A, Chénieux JC, Rideau M, Clastre M. Cloning and expression of cDNAs encoding two enzymes of the MEP pathway in Catharanthus roseus. Biochim Biophys Acta. 2000;1517:159–63. doi: 10.1016/s0167-4781(00)00240-2. [DOI] [PubMed] [Google Scholar]
- 23.Papon N, Bremer J, Vansiri A, Andreu F, Rideau M, Crèche J. Cytokinin and ethylene control indole alkaloid production at the level of the MEP/terpenoid pathway in Catharanthus roseus suspension cells. Planta Med. 2005;71:572–4. doi: 10.1055/s-2005-864163. [DOI] [PubMed] [Google Scholar]
- 24.El-Sayed M, Verpoorte R. Growth, metabolic profiling and enzymes activities of Catharanthus roseus seedlings treated with plant growth regulators. Plant Growth Regul. 2004;44:53–8. [Google Scholar]
- 25.Pan Q, Chen Y, Wang Q, Yuan F, Xing S, Tian Y, et al. Effect of plant growth regulators on the biosynthesis of vinblastine, vindoline and catharanthine in Catharanthus roseus. Plant Growth Regul. 2010;60:133–41. [Google Scholar]
- 26.Wen W, Zhu J, Liu J, Yu RM. Impact of artemisinic acid on the growth and catharanthine production in Catharanthus roseus cultured cells. J Med Plants Res. 2012;6:2019–28. [Google Scholar]
- 27.Liu J, Zhu J, Tang L, Wen W, Lv S, Yu R. Enhancement of vindoline and vinblastine production in suspension-cultured cells of Catharanthus roseus by artemisinic acid elicitation. World J Microbiol Biotechnol. 2014;30:175–80. doi: 10.1007/s11274-013-1432-z. [DOI] [PubMed] [Google Scholar]
- 28.Montiel G, Zarei A, Körbes AP, Memelink J. The Jasmonate-responsive element from the ORCA3 promoter from Catharanthus roseus is active in Arabidopsis and is controlled by the transcription factor AtMYC2. Plant Cell Physiol. 2011;52:578–87. doi: 10.1093/pcp/pcr016. [DOI] [PubMed] [Google Scholar]
- 29.Rijhwani SK, Shanks JV. Effect of elicitor dosage and exposure time on biosynthesis of indole alkaloids by Catharanthus roseus hairy root cultures. Biotechnol Prog. 1998;14:442–9. doi: 10.1021/bp980029v. [DOI] [PubMed] [Google Scholar]
- 30.Wei S. Methyl jasmonic acid induced expression pattern of terpenoid indole alkaloid pathway genes in Catharanthus roseus seedlings. Plant Growth Regul. 2010;61:243–51. [Google Scholar]
- 31.Goklany S, Rizvi NF, Loring RH, Cram EJ, Lee-Parsons CW. Jasmonate-dependent alkaloid biosynthesis in Catharanthus roseus hairy root cultures is correlated with the relative expression of Orca and Zct transcription factors. Biotechnol Prog. 2013;29:1367–76. doi: 10.1002/btpr.1801. [DOI] [PubMed] [Google Scholar]
- 32.Idrees M, Naeem M, Aftab T, Khan MM, Moinuddin Salicylic acid mitigates salinity stress by improving antioxidant defence system and enhances vincristine and vinblastine alkaloids production in periwinkle [Catharanthus roseus (L.) G. Don] Acta Physiol Plant. 2011;33:987–99. [Google Scholar]
- 33.Li M, Peebles CA, Shanks JV, San KY. Effect of sodium nitroprusside on growth and terpenoid indole alkaloid production in Catharanthus roseus hairy root cultures. Biotechnol Prog. 2011;27:625–30. doi: 10.1002/btpr.605. [DOI] [PubMed] [Google Scholar]
- 34.Baldi A, Dixit VK. Yield enhancement strategies for artemisinin production by suspension cultures of Artemisia annua. Bioresource Technol. 2008;99:4609–14. doi: 10.1016/j.biortech.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 35.Zeng Y, Yan F, Tang L, Chen F. Increased crocin production and induction frequency of stigma-like structure from floral organs of Crocus sativus by precursor feeding. Plant Cell Tiss Organ. 2003;72:185–91. [Google Scholar]


