Abstract
Infections caused by Staphylococcus aureus strains with Methicillin resistance are associated with increased mortality and morbidity, aggressive course, multiple drug resistance and hospital outbreaks. Several first and second line antibiotics are rapidly becoming ineffective for treatment due to emergence of resistance. Extracts of medicinal plants are rich source of unique phytochemicals. Plants used in traditional medicine have been reported to have significant anti-MRSA activity. The objective of this review is to provide a brief overview of antibiotics as well as anti-MRSA natural products and their future prospect.
Keywords: Methicillin resistant Staphylococcus aureus (MRSA), phytochemicals, plant extracts
INTRODUCTION
Staphylococcus aureus is one of the prominent medically important bacterial pathogen. Its potential to cause wide spectrum of pyogenic lesions involving several organs, hospital outbreaks and community acquired infections are well recognized. S. aureus infections are often fatal in nature and are associated resistance to several beta-lactam antibiotics used in hospitals.[1] These strains are known as MRSA (methicillin resistant S. aureus).[2] Historically, it had drawn special attention since 1970 due to its association with several nosocomial outbreaks and cross infections.[3] The epidemiology of this organism has changed over years. Life-threatening infections which were limited only in hospitals are now becoming widespread in community.[4] High usage of antibiotics in hospitals and selection pressure of these antibiotics has been implicated in development of multidrug resistance (MDR) in hospital acquired MRSA (HA-MRSA) strains. Likewise, increased use of antibiotics in animal feed has resulted in emergence of a new MRSA strain (livestock associated MRSA or LA-MRSA) with multiple non-beta lactam drug resistance.
Pertaining to the difference in virulence, pathogenicity, risk factors and drug resistance, MRSA strains are highly variable in different geographical areas. Even within a country, there may be local variation in MRSA strains.[5,6] MRSA prevalence varies from 20% to 54.8% in different parts of India,[7,8,9] while a recent study shows 29.1% prevalence in South India.[10] Antibiotic susceptibility pattern indirectly correlate with pathogenicity and therefore may be helpful in tracing the dissemination of the predominant MRSA strain or emergence of new MRSA strain. The transmission of Staphylococcal infection can be effectively reduced by stringent infection control measures and judicious use of antibiotics.[11] Conventional anti-MRSA antibiotics like Vancomycin, Teicoplanin, Linezolid and Daptomycin are currently in clinical use. However, development of resistance to many of these drugs has been identified worldwide. Vancomycin resistant and intermediate MRSA strains (VRSA and VISA) have been reported sporadically. Furthermore, it has also shown increase in the minimum inhibitory concentration (MIC) to Glycopeptides over years indicating reduced susceptibility. With the emergence of resistance to these drugs along with scarcity of newer anti-MRSA in the pipeline, the therapeutic options are likely to be further narrowed in future. Conversely, novel bioactive natural products have been identified to display anti-MRSA activity. Current research suggests these natural products have the prospect of being considered for treatment of MRSA infections. This review critically appraises conventional antibiotics and bioactive natural products with anti-MRSA activity.
Anti-MRSA antibiotics and their resistance mechanisms
Methicillin
Methicillin (originally called Celbenin) was the first beta lactamase resistant semisynthetic penicillin developed in 1960 to treat infections with penicillin resistant S. aureus. However, methicillin resistant strains of S. aureus emerged within one year of its clinical use.[12] The early reports of MRSA among European countries were from UK and Denmark.[13] MRSA has also been reported from India as early as 1964.[14] Methicillin exerts its antimicrobial activity by inhibiting transpeptidase mediated peptidoglycan cross-links by after binding with cell wall PBPs.
Methicillin resistance is mediated by an additional PBP (PBP2a) with low affinity for beta-lactam agents and it confers resistance to methicillin as well as other beta-lactam antibiotics. The mecA gene coding PBP2a along with two regulator genes (mecI and mecR1) is carried by SCCmec. Expression of mecA gene is usually inducible and regulated by mecI, mecR1 and additional genes like blaI, blaR1, femB, aux.[15] Apart from mecA mediated resistance, other resistance mechanisms which are not associated with treatment failure are also described.[16] Alteration of existing PBP by mutation in the beta lactam drug binding domain may give rise to resistance in S. aureus which are termed as MODSA (moderately oxacillin resistant S. aureus). Similarly few penicillinase hyper-producer S. aureus strains described as BORSA (borderline oxacillin resistant S. aureus) can slowly hydrolyse methicillin/oxacillin by type-A staphylococcal beta-lactamase resulting in borderline MIC and low level resistance.
Vancomycin
Vancomycin is currently the antibiotic of choice for treating MRSA infections. It is a branched glycosylated tricyclic peptide belonging to the glycopeptide antibiotic class. It binds to the growing ends of peptide chains and prevents their interaction with transpeptidase enzyme. Although reports of MRSA strains with diminished susceptibility to this antibiotic are not infrequent,[17] only few reports of vancomycin resistant S. aureus (VRSA) showing MIC ≥ 32 μg/ml have been documented. Four VRSA carrying vanA gene were reported from USA between 2002 and 2006.[18] Later Tiwari et al. reported vanA negative VRSA in 2006[19] and recently Saha et al. recovered one isolate of VRSA from Kolkata.[20] Vancomycin intermediate S. aureus (VISA) is characterized by MIC between 8-16 μg/ml. It was first reported in Japan in 1997.[21] Subsequently it was reported from United States and Europe. Intermediate sensitivity among MRSA has also been reported from South India.[22]
Linezolid
It is a new drug class, the Oxazolidinones. It binds to domain V of 23s RNA and prevents correct protein synthesis. Linezolid resistance occurs when at least 2 copies of 23s RNA genes are mutated specially with increased clinical use and the control measure is aggressive antibiotic stewardship (reducing its clinical use).[23] First case of linezolid resistance in MRSA was reported in 2001[24] and subsequently 8 cases in the US, 2 in Germany and 1 each in Brazil, Colombia and the UK. Spanish outbreak of linezolid resistant S. aureus had a different cause, the importation of the cfr gene carrying plasmid, which also mediates resistance to the older drugs clindamycin and chloramphenicol.[25]
Daptomycin
It is a calcium-dependent cyclic lipopeptide anti MRSA drug which act by depolarization of the bacterial cell membrane. However, due to its lipophilic nature it gets incorporated in alveolar surfactant and deposited in alveoli instead of bacterial cell membrane and results in eosinophilic pneumonia limiting its therapeutic use.[26] There is no defined resistance breakpoints for S. aureus, isolates are either categorized as susceptible or nonsusceptible.[27] Since vancomycin prolonged exposure is related to decreased daptomycin susceptibility, it should be ruled out by rechecking daptomycin MIC when a patient is unresponsive to this combination.[28]
Streptogramin antibiotics
These are derivative of Streptomyces pristinaespiralis. They are categorized into - group A (e.g. dalfopristin) and group B (e.g. quinupristin).[29] It is available for therapeutic use as combination of quinupristin and dalfopristin (30:70 ratio) which is more potent than single agent and may be active even when there is resistance to one component. Both quinupristin and dalfopristin bind to 50S ribosome at different sites to form a stable tertiary complex, and inhibit protein synthesis of bacteria. Genes coding Streptogramin inactivating enzymes (erm, msr, vat) can occur on transmissible elements. Drug elimination by efflux has also been described.[30] The first report of resistance to this antibiotic in MRSA was from France in 1975.[31] Diverse range of the resistance ranging from 0% to 31% has been detected in different studies globally.[32] Different groups in India have reported variable rate of resistance with the maximum of 64% as observed by Deep et al.[29,33]
Clindamycin and inducible clindamycin resistance
Clindamycin is a lincosamide antibiotic classically used for infections by aerobic Gram-positive cocci and anaerobes. Clindamycin resistance in S. aureus may be classified in one of the three phenotypes, designated as MLSBi, MLSBc and MS respectively.[34] Inducible resistance to Streptogramin B, macrolide and lincosamide in S. aureus is attributed to erm gene encoding an enzyme which methylate adenine residue of 23s rRNA.[34] This inducible clindamycin resistance has been found more frequently among MRSA strains and it often leads to treatment failure as it is not detected in routine antibiotic susceptibility tests.[35] It requires detection by a simple test, frequently described as D-test.
Bioactive natural products anti-MRSA activity
Indian medicinal plants
Indian continent is blessed with 120 families and 130000 species of plants. Many of these are known to have medicinal properties. From historical time, various parts of these plants have been used in treatment of communicable as well as non-communicable diseases. However, the bioactive phytoconstituents contributing to antimicrobial properties are yet to be discovered.
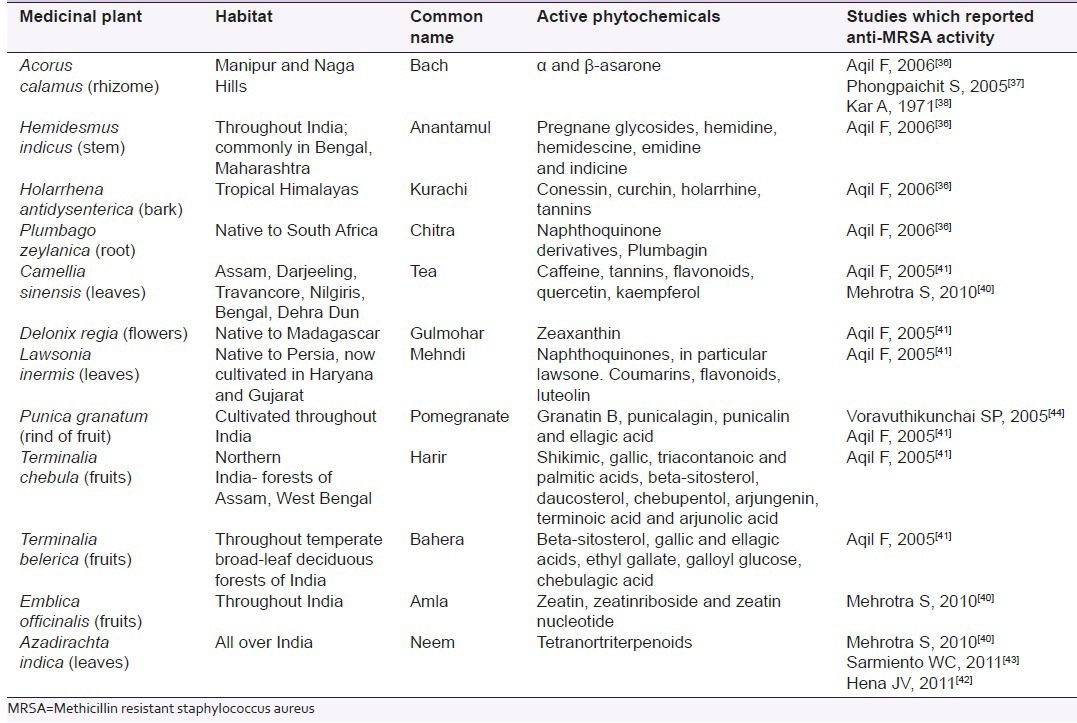
Recent research has identified Acorus calamus, Lawsonia inermis, Hemidesmus indicus, Holarrhena antidysenterica, Punica granatum, Plumbago zeylanica, Camellia sinensis, Delonix regia, Terminalia chebula, Emblica officinalis and Terminalia belerica have in vitro antimicrobial action against MRSA. A. calamus also known as sweet flag, calamus and bach, grows throughout India, especially in hills of Manipur and Nagaland. Its rhizome has effect on nervous system and has been used as antihypertensive, sedative, antianxiety, antispasmodic, anticonvulsant as well as for bronchial infection, chronic diarrhoea and dysentery. Several studies reported bactericidal activity of its rhizome and leaf extracts.[36,37,38]
Emblica officinalis, Terminalia chebula and Terminalia bellirica fruits are constituents of a polyherbal Ayurvedic medicinal formulation known as Triphala. Its antioxidant, antimicrobial, anticancer, anti-allergic, cardiotonic, hypocholesterolemic and hepatoprotective properties are well recognized and it has been used in treatment of malabsorption, constipation, dyspepsia and hyperglycemia for its multiple health benefits.[39] Although human clinical trials have not been conducted to support its use in infections, evidence from in vitro studies and animal models suggests each component of Triphala have anti-MRSA property.[39,40,41]
Likewise, leaves of Camellia sinensis (tea),[40,41] Lawsonia inermis (mehndi)[41] and Azadirachta indica (neem),[40,42,43] Holarrhena antidysenterica (Kurachi) bark,[36] Delonix regia (Gulmohar) flowers,[41] Punica granatum (Pomegranate) rind,[41,44] Hemidesmus indicus (Anantamul) stem[36] and Plumbago zeylanica (Chitra) root[36] has shown in vitro activity against MRSA [Table 1].
Table 1.
Medicinal plants in Indian traditional medicine with anti-MRSA properties
Miscellaneous medicinal plants
With the increase in awareness of medicinal value of plants and herbal component, several studies have investigated the antibacterial properties of herbal plants against MDR pathogens in current years. This trend is seen worldwide and developed countries are no exception. Significant activity against MRSA have been documented in studies which used extract of plants mentioned in traditional medicine of Thailand (Garcinia mangostana, Quercus infectoria),[44] Nigeria (Terminalia avicennioides, Phyllantus discoideus, Ocimum gratissimum, Acalypha wilkesiana)[45] and Australia (Eremophila alternifolia, Amyema quandong, Eremophila duttonii, Lepidosper maviscidum).[46] Voravuthikunchai et al. found that Quercus infectoria, Garcinia mangostana and Punica granatum had highest antibacterial activity and MICs for MRSA were 0.2-0.4 mg/mL, 0.05-0.4 mg/mL and 0.2-0.4 mg/mL respectively.[44] In another study, the MICs of ethanol extracts of four Nigerian plants i.e. A. wilkesiana, P. discoideus, T. avicennioides and O. gratissimum ranged from 18.2 to 24.0 mcg/ml.[45] While the leaf extract from Eremophila duttonii was most bactericidal among five Australian medicinal plants in reducing the number of viable cells of MRSA,[46] ten Italian plants exhibited MRSA biofilm inhibition with minuscule bacteriostatic activity.[47]
Active phytoconstituents with anti MRSA activity
Despite ample research evidence of anti-MRSA activity of various plant products, there is lack of adequate information about precise phytoconstituents possessing anti MRSA activity. Most studies have investigated anti-MRSA activity of different plant parts (leaf, bark, flower, rind, fruit etc.) extracted in various solvents (aqueous, methanol, ethanol, ethyl acetate etc.) and expressed the result of phytochemical analysis of these extracts in terms of presence of alkaloids, terpenoids, flavonoids, phenols, steroids and glycosides. Since in most instances isolation of each phytochemical in pure form and re-testing their anti-MRSA activity not attempted and these phytochemicals are often unnamed or named generically (as alkaloids, terpenoids, flavonoids, etc.), it is difficult to assign the anti-MRSA activity to a particular component.
Among the compounds with reported anti-MRSA activity, β-asarone from Acorus calamus rhizome, mansonone F from Ulmus davidiana var. japonica, galloylated flavonol rhamnosides from Calliandra tergemina leaves, Prenylated flavonoids from Desmodium caudatum root, eupomatenoid-5 from Piper regnellii leaves are important.
Beta-asarone is cis-isomer of 2, 4, 5-trimethoxy-l-propenylbenzene, and the active constituent of A. calamus. Sujina et al. found that beta-asarone was the major constituent (92.4%) in A. calamus essential oil and showed 12 mm zone of inhibition and 2.5 mg/ml MIC value against MRSA.[48] In contrast, Devi et al. reported minimal antibacterial activity of its extracts and β-asarone.[49]
Mansonone F is an anti-MRSA sesquiterpenoid quinone compound of U. davidiana var. japonica present in the fourth fraction of root extract of U. davidiana obtained by silica gel column chromatography.[50] It has been found to have potent antimicrobial activity against gram positive bacteria including MRSA. However, its activity against gram negative bacteria is insignificant.
Kaempferol-3-O-(2″,3″,4″-tri-O-galloyl)-α-L-rhamnopyranoside, quercetin-3-O-(3″,4″-di-O-galloyl)-α-L-rhamnopyranoside, and quercetin-3-O-(2″,3″,4″-tri-O-galloyl)-α-L-rhamnopyranoside are three novel galloylated flavonol rhamnosidesides from C. tergemina leaves. Chan et al. found these phytocompounds exert lytic effect on MRSA.[51] Moreover, acylation of these rhamnosidesides is critical for their anti-MRSA activity. Likewise, seven prenylated flavonoids and one prenylated chromanochroman isolated from Desmodium caudatum also showed in vitro anti-MRSA activity.[52] Prenyl or a 2,2-dimethylpyran group in these compounds are essential for their antimicrobial action.
Marcal et al. spectroscopically identified eupomatenoid-6, eupomatenoid-5, eupomatenoid-3 and conocarpan as the major constituents of P. regnellii leaf extract.[53] Among these components, eupomatenoid-5 only had antibacterial properties and was responsible for anti-MRSA activity of P. regnellii.
Future prospects
The lack of newer antibiotics under development, emergence of drug resistance among several pathogenic bacterial species and their world wide spread along with limited therapeutic options with antibiotics attributed to their higher toxicity and comorbidities in patients have heralded the futility of antibiotics in near future. Although natural herbal products are in use for centuries for treatment of infective ailments, not much is known about their active principles, scientific basis of use, pharmacological and safety profiles. A vast area of traditional medicine has remained undiscovered. Hence, phytochemistry became more relevant in treatment of MDR pathogens like MRSA. However, some aspect needs to be addressed. Difference in extraction method, instruments and raw materials (plants grown on different regions) may lead to wide variation in results and needs standardization.[43] Most studies utilize diffusion methods for determining antibacterial activity and interpret the results in terms of size of zone of inhibition (ZOI). Unlike antibiotics phytochemicals do not have universally accepted guidelines on ZOI cut offs for diffusion method and also ZOI cannot reflect the concentration of phytochemicals in infected tissues required to inhibit bacterial growth. Hence, it may be more appropriate to determine the MIC and MBC. Since plant extract may contain numerous substances and one or more components may contribute to antibacterial activity, the identification of the active phytoconstituent from a plethora of alkaloids or glycosides of the extract often become challenging. Finally, the in vitro antibacterial action needs further support by animal studies and human clinical trials to determine the safety profile, therapeutic window and optimum dosage schedule in addition to its therapeutic efficacy before considering for routine use.
CONCLUSION
The threat of MRSA in developing as well as in developed countries is on the rise. Plants used in traditional medicine the rich source of bioactive phytoconstituents. Evidence suggests that several medicinal plants have demonstrable anti-MRSA activity and have a prospect of being considered as a potential treatment option for MRSA infections.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Pavillard R, Harvey K, Douglas D, Hewstone A, Andrew J, Collopy B, et al. Epidemic of hospital-acquired infection due to methicillin-resistant Staphylococcus aureus in major Victorian hospitals. Med J Aust. 1982;1:451–4. [PubMed] [Google Scholar]
- 2.Lucet JC, Paoletti X, Demontpion C, Degrave M, Vanjak D, Vincent C, et al. Staphylococcus aureusResistant a la Meticilline en Hospitalisation A Domicile (SARM HAD) Study Group. Carriage of methicillin-resistant Staphylococcus aureus in home care settings: Prevalence, duration, and transmission to household members. Arch Intern Med. 2009;169:1372–8. doi: 10.1001/archinternmed.2009.217. [DOI] [PubMed] [Google Scholar]
- 3.Shanson DC, Kensit JC, Duke R. Outbreak of hospital infection with a strain of Staphylococcus aureus resistant to gentamicin and methicillin. Lancet. 1976;2:1347–8. doi: 10.1016/s0140-6736(76)91986-3. [DOI] [PubMed] [Google Scholar]
- 4.Pantosti A, Venditti M. What is MRSA? Eur Respir J. 2009;34:1190–6. doi: 10.1183/09031936.00007709. [DOI] [PubMed] [Google Scholar]
- 5.Simor AE, Louie L, Watt C, Gravel D, Mulvey MR, Campbell J, et al. Canadian Nosocomial Infection Surveillance Program. Antimicrobial susceptibilities of health care-associated and community-associated strains of methicillin-resistant Staphylococcus aureus from hospitalized patients in Canada 1995 to 2008. Antimicrob Agents Chemother. 2010;54:2265–8. doi: 10.1128/AAC.01717-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivasan S, Sheela D, Shashikala Mathew R, Bazroy J, Kanungo R. Risk factors and associated problems in the management of infections with methicillin resistant Staphylococcus aureus. Indian J Med Microbiol. 2006;24:182–5. [PubMed] [Google Scholar]
- 7.Daskalaki M, Otero JR, Chaves F. Molecular characterization of resistance to mupirocin in methicillin-resistant Staphylococcus aureus isolates in a tertiary hospital in Spain. J Antimicrob Chemother. 2009;63:826–8. doi: 10.1093/jac/dkp025. [DOI] [PubMed] [Google Scholar]
- 8.Mathanraj S, Sujatha S, Sivasangeetha K, Parija SC. Screening for methicillin-resistant Staphylococcus aureus carriers among patients and health care workers of a tertiary care hospital in south India. Indian J Med Microbiol. 2009;27:62–4. [PubMed] [Google Scholar]
- 9.Anupurba S, Sen MR, Nath G, Sharma BM, Gulati AK, Mohapatra TM. Prevalence of methicillin resistant Staphylococcus aureus in a tertiary referral hospital in eastern Uttar Pradesh. Indian J Med Microbiol. 2003;21:49–51. [PubMed] [Google Scholar]
- 10.Pai V, Rao VI, Rao SP. Prevalence and antimicrobial susceptibility pattern of methicillin-resistant Staphylococcus aureus (MRSA) isolates at a tertiary care hospital in Mangalore, South India. J Lab Physicians. 2010;2:82–4. doi: 10.4103/0974-2727.72155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Struelens MJ. Guidelines and indicators for methicillin-resistant Staphylococcus aureus control in hospitals: Toward international agreement? Curr Opin Infect Dis. 2009;22:337–8. doi: 10.1097/QCO.0b013e32832dbae9. [DOI] [PubMed] [Google Scholar]
- 12.Jevons MP. “Celbenin ”-resistant Staphylococci. BMJ. 1961;1:124–5. [Google Scholar]
- 13.Tattevin P, Diep BA, Jula M, Perdreau-Remington F. Methicillin-resistant Staphylococcus aureus USA300 clone in long-term care facility. Emerg Infect Dis. 2009;15:953–5. doi: 10.3201/eid1506.080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pal SC, Ray BG. Methicillin-Resistant Staphylococci. J Indian Med Assoc. 1964;42:512–7. [PubMed] [Google Scholar]
- 15.Chambers HF. Methicillin resistance in staphylococci: Molecular and biochemical basis and clinical implications. Clin Microbiol Rev. 1997;10:781–91. doi: 10.1128/cmr.10.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown DF, Edwards DI, Hawkey PM, Morrison D, Ridgway GL, Towner KJ, et al. Joint Working Party of the British Society for Antimicrobial Chemotherapy;Hospital Infection Society;Infection Control Nurses Association. Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphylococcus aureus (MRSA) J Antimicrob Chemother. 2005;56:1000–18. doi: 10.1093/jac/dki372. [DOI] [PubMed] [Google Scholar]
- 17.Hanaki H, Hiramatsu K. Evaluation of reduced vancomycin susceptibility of MRSA strain Mu50 with various conditions of antibiotic susceptibility tests. Jpn J Antibiot. 1997;50:794–8. [PubMed] [Google Scholar]
- 18.Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States 2002-2006. Clin Infect Dis. 2008;46:668–74. doi: 10.1086/527392. [DOI] [PubMed] [Google Scholar]
- 19.Tiwari HK, Sen MR. Emergence of vancomycin resistant Staphylococcus aureus (VRSA) from a tertiary care hospital from northern part of India. BMC Infect Dis. 2006;6:156. doi: 10.1186/1471-2334-6-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha B, Singh AK, Ghosh A, Bal M. Identification and characterization of a vancomycin-resistant Staphylococcus aureus isolated from Kolkata (South Asia) J Med Microbiol. 2008;57:72–9. doi: 10.1099/jmm.0.47144-0. [DOI] [PubMed] [Google Scholar]
- 21.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–6. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 22.Menezes GA, Harish BN, Sujatha S, Vinothini K, Parija SC. Emergence of vancomycin-intermediate Staphylococcus species in Southern India. J Med Microbiol. 2006;57:911–2. doi: 10.1099/jmm.0.47829-0. [DOI] [PubMed] [Google Scholar]
- 23.Rehm SJ. Two new treatment options for infections due to drug-resistant gram-positive cocci. (405-13).Cleve Clin J Med. 2002;69:397–401. doi: 10.3949/ccjm.69.5.397. [DOI] [PubMed] [Google Scholar]
- 24.Wong H, Louie L, Watt C, Sy E, Lo RY, Mulvey MR, et al. Characterization of ermA in macrolide-susceptible strains of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:3602–3. doi: 10.1128/AAC.00313-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morales G, Picazo JJ, Baos E, Candel FJ, Arribi A, Peláez B, et al. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus. Clin Infect Dis. 2010;50:821–5. doi: 10.1086/650574. [DOI] [PubMed] [Google Scholar]
- 26.Miller BA, Gray A, Leblanc TW, Sexton DJ, Martin AR, Slama TG. Acute eosinophilic pneumonia secondary to daptomycin: A report of three cases. Clin Infect Dis. 2010;50:e63–8. doi: 10.1086/652656. [DOI] [PubMed] [Google Scholar]
- 27.CLSI. CLSI approved standard M100-S23. Wayne, PA: CLSI; 2013. Performance standards for antimicrobial susceptibility testing; p. 204. [Google Scholar]
- 28.Ahmad NM, Rojtman AD. Successful treatment of daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus bacteremia with the addition of rifampin to daptomycin. Ann Pharmacother. 2010;44:918–21. doi: 10.1345/aph.1M665. [DOI] [PubMed] [Google Scholar]
- 29.Deep A, Goel N, Sikka R, Chaudhary U, Yadav S, Gupta A. Quinpristin dalfopristin resistance in gram Positive bacteria: Experience from a tertiary care referral center in North India. J Infect Dis Antimicrob Agents. 2008;25:117–21. [Google Scholar]
- 30.Keshari SS, Kapoor AK, Kastury N, Singh DK, Bhargava A. Emergence of pristinamycin resistance in India. Indian J Pharmacol. 2009;41:47–8. doi: 10.4103/0253-7613.48884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goffic FL, Capmau ML, Bonnet D, Cerceau C, Soussy C, Dublanchet A, et al. Plasmid-mediated pristinamycin resistance. PACIIA: A new enzyme which modifies pristinamycin IIA. J Antibiot (Tokyo) 1977;30:665–9. doi: 10.7164/antibiotics.30.665. [DOI] [PubMed] [Google Scholar]
- 32.Mendes RE, Sader HS, Deshpande LM, Diep BA, Chambers HF, Jones RN. Characterization of baseline methicillin-resistant Staphylococcus aureus isolates recovered from phase IV clinical trial for linezolid. J Clin Microbiol. 2010;48:568–74. doi: 10.1128/JCM.01384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma DN, Dwivedi JN. Pulmonary schistosomiasis in sheep and goats due to Schistosoma indicum in India. J Comp Pathol. 1976;86:449–54. doi: 10.1016/0021-9975(76)90013-x. [DOI] [PubMed] [Google Scholar]
- 34.Fiebelkorn KR, Crawford SA, McElmeel ML, Jorgensen JH. Practical disk diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulasenegative staphylococci. J Clin Microbiol. 2003;41:4740–4. doi: 10.1128/JCM.41.10.4740-4744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prabhu K, Rao S, Rao V. Inducible clindamycin resistance in Staphylococcus aureus isolated from clinical samples. J Lab Physicians. 2011;3:25–7. doi: 10.4103/0974-2727.78558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aqil F, Ahmad I, Owais M. Evaluation of anti-methicillin-resistant Staphylococcus aureus (MRSA) activity and synergy of some bioactive plant extracts. Biotechnol J. 2006;1:1093–102. doi: 10.1002/biot.200600130. [DOI] [PubMed] [Google Scholar]
- 37.Phongpaichit S, Pujenjob N, Rukachaisirikul V, Ongsakul M. Antimicrobial activities of the crude methanol extract of Acorus calamus Linn. Songklanakarin J Sci Technol. 2005;27(Suppl 2):517–23. [Google Scholar]
- 38.Kar A, Jain S. Antibacterial evaluation of some indigenous medicinal volatile oils. Qualitas Plantarum et Materiae Vegetabiles. 1971;20:231–7. [Google Scholar]
- 39.Kirubanandan S, Swethkamal K, Renganathan S. Activities of triphala towards promoting collagen synthesis at wound site and inhibiting methicillin-resistant Staphylococcus aureus and its enzymes. Int J Pharm Pharm Sci. 2013;5:54–62. [Google Scholar]
- 40.Mehrotra S, Srivastava AK, Nandi SP. Comparative antimicrobial activities of Neem, Amla, Aloe, Assam Tea and Clove extracts against Vibrio cholerae, Staphylococcus aureus and Pseudomonas aeruginosa. J Med Plants Res. 2010;4:2473–8. [Google Scholar]
- 41.Aqil F, Khan MS, Owais M, Ahmad I. Effect of certain bioactive plant extracts on clinical isolates of beta-lactamase producing methicillin resistant Staphylococcus aureus. J Basic Microbiol. 2005;45:106–14. doi: 10.1002/jobm.200410355. [DOI] [PubMed] [Google Scholar]
- 42.Hena JV, Sudha S. Studies on community acquired MRSA (Methicillin resistant Staphylococcus aurees) and its treatment regimen. Indian J Applied and Pure Bio. 2011;26:63–6. [Google Scholar]
- 43.Sarmiento WC, Maramba CC, Gonzales ML. An in-vitro study on the antibacterial effect of neem (Azadirachta indica) leaf extract on methicillin-sensitive and Methicillin-resistant Staphylococcus aureus. PIDSP J. 2011;12:40–5. [Google Scholar]
- 44.Voravuthikunchai SP, Kitpipit L. Activity of medicinal plant extracts against hospital isolates of methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect. 2005;11:510–2. doi: 10.1111/j.1469-0691.2005.01104.x. [DOI] [PubMed] [Google Scholar]
- 45.Akinyemi KO, Oladapo O, Okwara CE, Ibe CC, Fasure KA. Screening of crude extracts of six medicinal plants used in South- West Nigerian unorthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. BMC Complement Altern Med. 2005;5:6. doi: 10.1186/1472-6882-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palombo EA, Semple SJ. Antibacterial activity of Australian plant extracts against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) J Basic Microbiol. 2002;42:444–8. doi: 10.1002/1521-4028(200212)42:6<444::AID-JOBM444>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 47.Quave CL, Plano LR, Pantuso T, Bennett BC. Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus. J Ethnopharmacol. 2008;118:418–28. doi: 10.1016/j.jep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sujina I, Prabhu V, Hemlal H, Ravi S. Essential oil composition, Isolation of β-asarone and its antibacterial and MRSA activity from the rhizome of Acorus calamus. J Pharm Res. 2012;5:3437–40. [Google Scholar]
- 49.Devi S, Ganjewala D. Antimicrobial activity of Acorus calamus (L.). rhizome and leaf extract. Acta Biol Szeged. 2009;53:45–9. [Google Scholar]
- 50.Shin DY, Kim HS, Min KH, Hyun SS, Kim SA, Huh H, et al. Isolation of a potent anti-MRSA sesquiterpenoid quinone from Ulmus davidiana var. japonica. Chem Pharm Bull (Tokyo) 2000;48:1805–6. doi: 10.1248/cpb.48.1805. [DOI] [PubMed] [Google Scholar]
- 51.Chan EW, Gray AI, Igoli JO, Lee SM, Goh JK. Galloylated flavonol rhamnosides from the leaves of Calliandra tergemina with antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA) Phytochemistry. 2014;107:148–54. doi: 10.1016/j.phytochem.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 52.Sasaki H, Kashiwada Y, Shibata H, Takaishi Y. Prenylated flavonoids from Desmodium caudatum and evaluation of their anti-MRSA activity. Phytochemistry. 2012;82:136–42. doi: 10.1016/j.phytochem.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Marçal FJ, Cortez DA, Ueda-Nakamura T, Nakamura CV, Dias Filho BP. Activity of the extracts and neolignans from Piper regnellii against methicillin-resistant Staphylococcus aureus (MRSA) Molecules. 2010;15:2060–9. doi: 10.3390/molecules15042060. [DOI] [PMC free article] [PubMed] [Google Scholar]


