Abstract
Sugarcane (Saccharum officinarum Linn.) is an important perennial grass of Poaceae family, indigenous to tropical South Asia and Southeast Asia. It is cultivated worldwide due to the economical and medicinal value of its high yielding products. Sugarcane juice is well known as a raw material for the production of refined sugar and its wax is considered as a potential substitute for the expensive carnauba wax, which is of cosmetic and pharmaceutical interest. Refined sugar is the primary product of sugarcane juice, but during its processing, various other valuable products are also obtained in an unrefined form, such as, brown sugar, molasses, and jaggery. Sugarcane juice is widely used in India in the treatment of jaundice, hemorrhage, dysuria, anuria, and other urinary diseases. Herein, we have summarized the different phytoconstituents and health benefits of sugarcane and its valuable products. The phytochemistry of sugarcane wax (obtained from the leaves and stalks of sugarcane), leaves, juice, and its products has revealed the presence of various fatty acid, alcohol, phytosterols, higher terpenoids, flavonoids, -O- and -C-glycosides, and phenolic acids. The future prospective of some of the sugarcane products has been discussed, which needs a phytopharmacological study and has a great potential to be a valuable medicinal product.
Keywords: Fatty acid, phenolic acids, phytochemistry, Saccharum officinarum
INTRODUCTION
Sugarcane (Saccharum officinarum Linn.) is well-known crop of the family Poaceae. India is the second largest producer of sugarcane, after Brazil.[1] Saccharum is derived from the Greek word ‘Sakcharon,’ which means sugar especially sucrose. S. officinarum Linn, is a perennial grass, indigenous to tropical South Asia and Southeast Asia. It has a thick longitudinal stalk, which is generally three to five meters in height, approximately 5 cm in diameter, and is characterized by its sweet taste due to its high sucrose content. It is also known as chewing and noble cane. The sugarcane crop grows well in tropical and subtropical regions. It will require well-drained soil of pH 7.5 - 8.5 and high organic matter, along with a hot and humid environment.[2] Sugarcane has been used in various parts of world for curing various diseases. In the Ayurvedic system of medicine sugarcane is used either as a single drug or in combination with some other plant materials.[3,4] Some native and traditional healers of the world have recommended sugarcane juice for its diuretic property.[5,6] It is thought that regular use of sugarcane juice will keep the urinary flow clear and fast, which will further help the kidneys to perform their function properly. Sometimes it is used with lime juice and ginger juice for better results. It is also used as aphrodisiac, laxative, cooling, demulcent, antiseptic, and tonic.[7] According to the Unani system of medicine, sugarcane juice is considered good for patients with jaundice. It is considered beneficial for the liver and is recommended that jaundice patients take in a large amount of sugarcane juice for immediate relief. The assumptions of these traditional Indian medicinal systems have been supported by modern pharmacological studies, which have indicated that sugarcane has various bioactivities like anti-inflammatory, analgesic, antihyperglycemic, diuretic, and hepatoprotective effects. Although apigenin, tricin, and luteoline glycosides like orientin, vitexin, schaftoside, and swertisin were reported as the main constituents in sugarcane juice, various policosanols and steroids were also reported in different parts of S. officinarum. Owing to these bioactivities and chemical constituents, it has been noted that from the last few years great attention has been paid to the investigation of some lead moleculesthis cheapest crop for the various diseases. In the present study, we have tried to summarize the chemical profile and pharmacological aspects of sugarcane and its products. The aim and objective behind this review is to clear the picture of what scientists have done in this field and what the future focus of this field will be.
Sugarcane crop and its various products
Sugarcane crop is cultivated for the production of sugar, but the processing of sugarcane yields various valuable products such as bagasse,[8] Brown sugar, molasses, syrup, and jaggery, along with sugar (table sugar). The processing of sugarcane in a large scale industry for the production of sugar is shown in Figure 1. It is clearly indicated that sugar is obtained after refining of vacuum-concentrated cane juice. However, other sugarcane products such as jaggery, brown sugar, and molasses are obtained in an unrefined form.[9] On account of the unrefined form of these products, there must be a presence of some phenolic compounds, which enhance their nutritional and medicinal value.[10]
Figure 1.
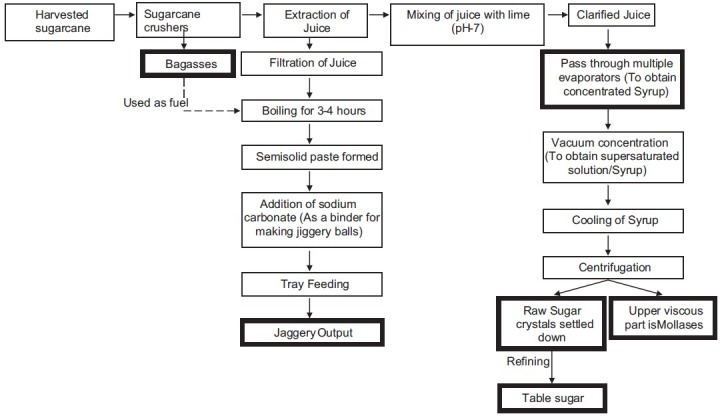
Processing of sugarcane products
Taxonomical classification
Kingdom: Plantae
Order: Poales
Family: Poaceae
Subfamily: Panicoideae
Tribe: Andropogoneae
Genus: Saccharum
Species: S. officinarum
Botanical description
Saccharum officinarum is a perennial plant that grows in clumps consisting of a number of strong unbranched stems. A network of rhizomes forms under the soil, which sends up secondary shoots near the parent plant. The stems vary in color being green, pinkish or purple and can reach 5 cm (16 ft) in height. They are jointed, nodes being present at the bases of the alternate leaves. The internodes contain a fibrous white pith, immersed in sugary sap. The elongated, linear, green leaves have thick midribs and saw-toothed edges that grow to a length of about 30 to 60 cm (12 to 24 ins) and width of 5 cm (2.0 ins). The terminal inflorescence is a panicle up to 60 cm (24 ins) long, a pinkish plume that is broadest at the base and tapering toward the top. The spikelets are borne on side branches and are about 3 mm (0.12 ins) long and are concealed in tufts of long, silky hair. The fruits are dry and each one contains a single seed.[11] Sugarcane harvesting typically occurs before the plant flowers, as the flowering process causes a reduction in sugar content.[12]
Phytochemical profiles of sugarcane and its various products
Chemistry of sugarcane wax
Sugarcane wax is a whitish to dark-yellowish powdery deposit on the surface of stalks and leaves of S. officinarum, which appears as a cuticle layer. It is necessary to consider sugarcane wax when reviewing the phytochemical profile of S. officinarum because of its widespread industrial application, and cosmetic and pharmaceutical interest.[13] It is a potential substitute for the expensive carnauba wax.[14] The amount of wax in sugarcane ranges between 0.1 and 0.3%, depending upon its variety.[15] Sugarcane wax is used as a commercial source of long chain fatty alcohols, acids, esters, aldehydes, and ketones. Policosanols and D-003 along with some steroids and terpenoids have also been identified and isolated from sugarcane wax. Policosanols are a mixture of long chain primary aliphatic alcohols (1 - 8) ranging from 2.5 - 80%. Octacosanol (1) constitutes 50 - 80% of the total policosanoles.[16] Other major pharmacologically active components of sugarcane wax are long chain aliphatic fatty acids (9 - 18) present at lower concentrations. The mixture of these acids is known as D-003 [Figure 2].[17] Although fatty acid and fatty alcohol are reported as major constituents[18,19,20,21,22] various phytosterols (19 - 22), steroids (23 - 28), and higher terpenoids (29 - 30) have also been reported in sugarcane wax[23,24] [Figure 3].
Figure 2.
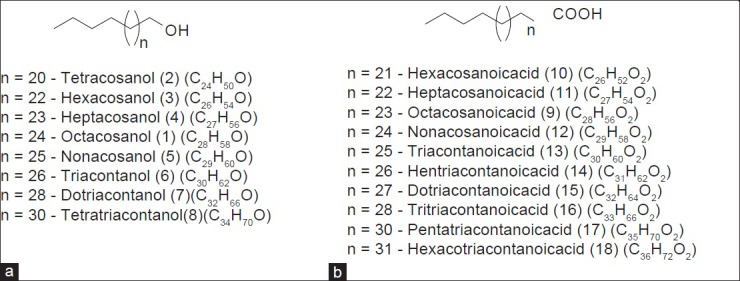
Chemistry of sugarcane wax (a) Long chain saturated fatty alcohols; (b) Long chain saturated fatty acids present in D-003
Figure 3.
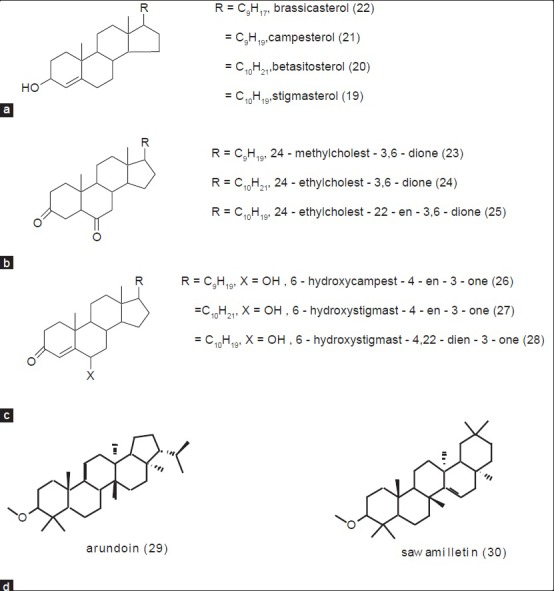
Chemistry of sugarcane wax (a) Simple phytosterols; (b) Ketosteroids; (c) Hydroxyketosteroids; (d) Higher terpenoids
Chemistry of sugarcane juice
Sugarcane juice is the first material used for the production of sugar and other various valuable products like raw sugar/brown sugar, jaggery, and molasses. Although these products are prepared from the same source, their method of processing is different, as shown in Figure 1. Furthermore, to understand the phytochemistry of jaggery (non-centrifugal sugar), brown sugar, and molasses, it is necessary to explain the phytochemical profile of sugarcane juice. Sugarcane juice is obtained by grinding the sugarcane culms. Basically it comprises of 70 - 75% water, 13 - 15% sucrose, and 10 - 15% fiber. Before 1971, it was assumed that the color of juice might be due to the presence of plant pigments. In 1971, several color components from sugarcane juice have been identified, with chlorogenic acid (31), cinnamic acid (32), and flavones being some of them.[25] Following that, all the colored components from sugarcane juice were classified into four major classes: Plant pigments, polyphenolic compounds, caramels, and degradation products of sugars condensed with amino derivatives. Sugarcane juice was then extensively studied for their flavonoid content. Thereafter, a large number of old and new flavonoids were isolated and identified.[26,27,28] High-Performance Liquid Chromatography with Diode-Array Detection (HPLC-DAD) analysis of phenolic compounds from sugarcane juice showed the presence of phenolic acids such as hydroxycinnamic acid (33), sinapic acid (34), and caffeic acid (35), along with flavones such as apigenin (36), luteolin (37), and tricin (38) [Figure 4]. Among the flavones, tricin derivatives accounted for the highest concentration.[29] Extensive chromatographic and spectroscopic studies indicated the presence of various -O- and -C- glycosides of the above-mentioned flavones, and 3947 were identified[30] [Figure 5]. Four new minor flavones swertisin (48), tricin-7-O-neohesperoside-4’-O-rhamnoside (49), tricin-7-O-methylglucuronate-4’-O-rhamnoside (50), and tricin-7-O-methylglucuronide (51) were isolated and identified from sugarcane juice.[31] In addition, some novel acylated flavone glycosides, such as, tricin-7-O-β-(6’-methoxycinnamic)-glucoside (52), luteolin-8-C-rhamnosyl glucoside (53), and tricin-4’-O-(erthroguaicylglyceryl)-ether (54) were isolated, along with orientin (47), from sugarcane juice[32] [Figure 6].
Figure 4.
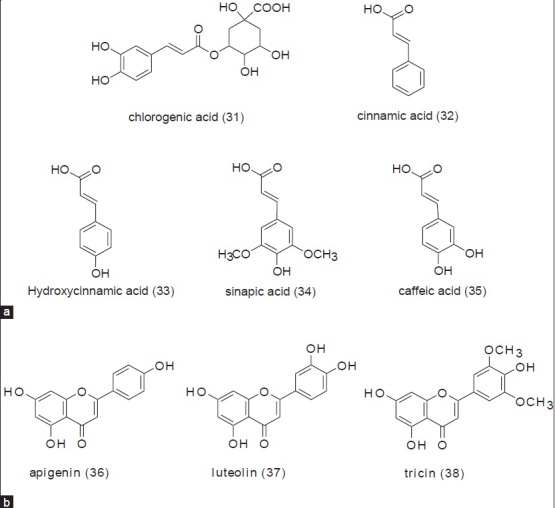
Phenolic compounds identified from sugarcane juice (a) Phenolic acids; (b) Flavones
Figure 5.
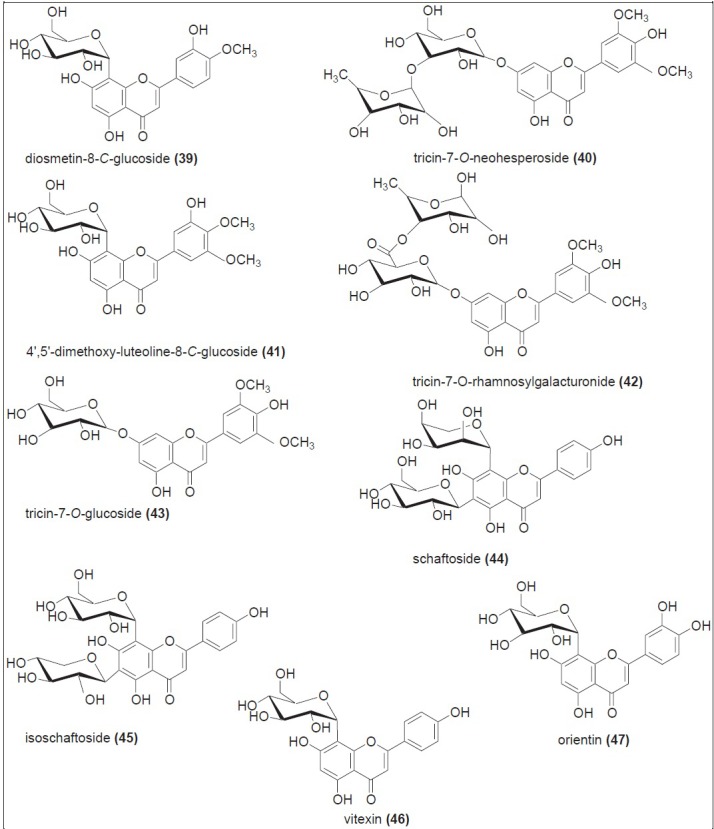
Flavone glycosides identified from sugarcane juice (39 – 47) and from sugarcane leaves (39, 40, 46, and 47)
Figure 6.
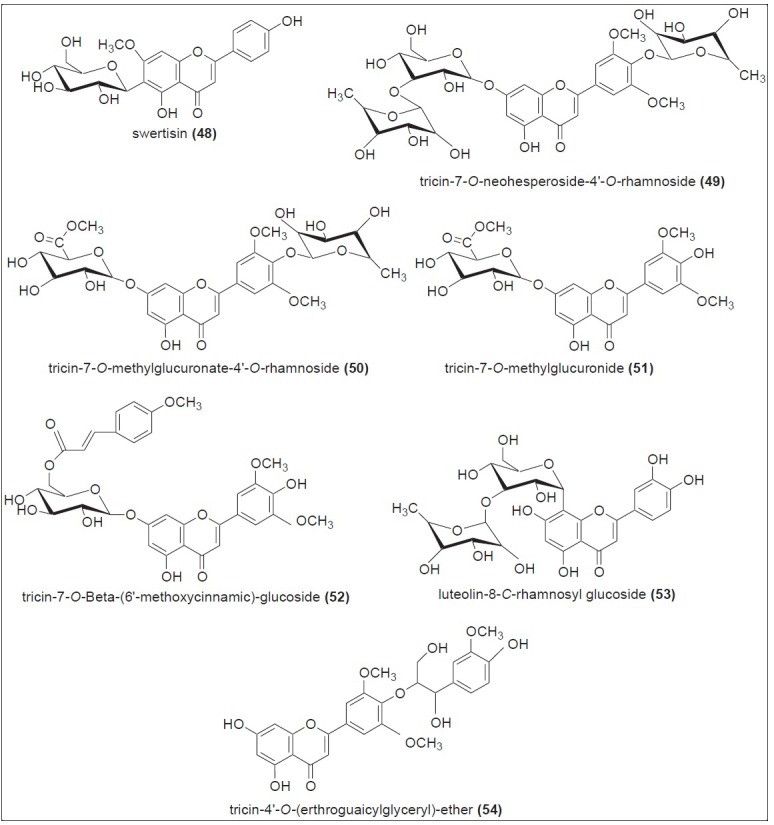
New Flavone glycosides identified from sugarcane juice (48 - 52) and from sugarcane leaves (53, 54)
Chemistry of sugarcane products
Chemistry of various sugarcane products like mill syrups, brown sugar, molasses, and non-centrifugal sugar were also extensively studied.[33] In addition to some known compounds of sugarcane juice (43 - 45 and 48), three new flavonoid glycosides, tricin7-(2’-rhamnosyl)-α-galacturonide (55), orientin-7, 3’-dimethyl ether (56), and iso-orientin-7,3’-O-dimethyl ether (57), were isolated and identified from mill syrups.[34] Mollases have also been studied for their polyphenolic content. One novel O-glycoside, dehydroconiferylalcohol-9’-O-β-D-glucopyranoside (58) along with the already reported isoorientin-7, 3’-O-dimethyl ether (57) were isolated as antibacterial compounds from sugarcane molasses[35] [Figure 7]. Brown sugars are also used commercially in Brazil for its nutraceutical value and other biological activities.[36,37] Liquid chromatography-mass spectrometry (LC-MS) analysis of aqueous and dichloromethane extracts of brown sugars confirmed the presence of various phenolic acids (59 - 63). In addition to phenolic acids, eight major volatile constituents (64 - 70) were also reported to be present in brown sugars [Figure 8].
Figure 7.
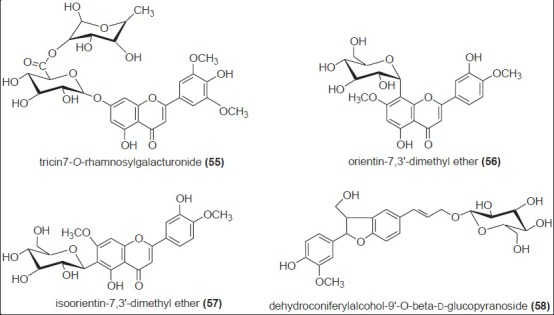
Phenolic glycosides of the sugarcane product
Figure 8.
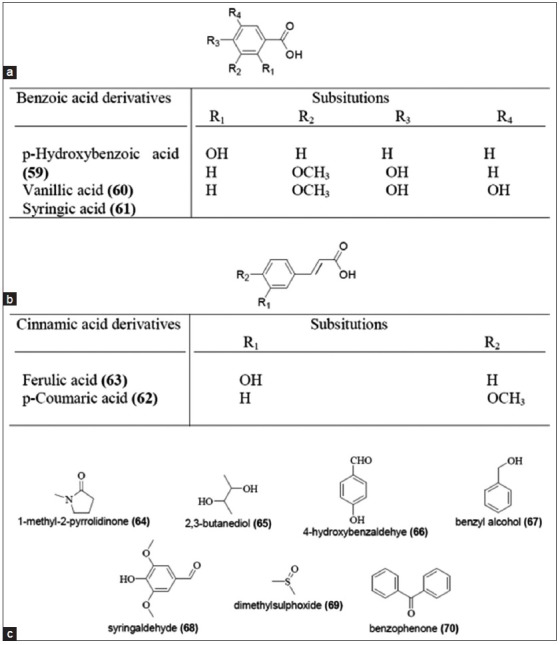
Chemical constituents of brown sugar (a) Benzoic acid derivatives; (b) Cinnamic acid derivatives; (c) Volatile constituents
A comparative study of polyphenolic compounds in various sugarcane products indicated that molasses were the richest source of phenolic acids as compared to clear juices and syrup.[38]
Chemistry of sugarcane leaves
Sugarcane leaves are also an important source of various policosanols and D-003 because of the presence of a thick coating of sugarcane wax. Along with the above-mentioned policosanols (1 - 8) and D-003 (9 - 18), sugarcane leaves are also reported to have some phenolic compounds like flavonoids. HPLC microfractionation of methanolic extract of sugarcane leaves was successfully done and various flavones -O- and -C- glycosides (39 - 40, 46 - 47, 53 - 54) were identified.
Pharmacological activity
Sugarcane contains various phytochemicals including phenolic compounds, plant sterols, and policosanols. Phenols help in the natural defense of plants against pests and diseases, while plant sterols and policosanols are the components of wax and plant oils. The phytochemicals have gained increased interest due to their antioxidant activity, cholesterol-lowering properties, and other potential health benefits. Several workers have reported the different biological activities of sugarcane in various in-vivo and in-vitro test models.
Analgesic activity
Ethanol extracts (95%) of both fresh leaves and shoots were administered intragastrically to mice at a dose of 1 g/kg. The leaf extracts were active against benzoyl peroxide-induced writhing and tail-flick response, but ethanol extract of shoots were active only against the tail-flick method.[39]
Antihepatotoxic activity
The aqueous extract of dried stems administered intraperitoneally to mice, at a dose of 25 mg/kg, was active against chloroform-induced hepatotoxicity.[40]
Antihyperglycemic activity
The ethanol extract of both dried leaves and stems was administered intragastrically to rabbits at a dose of 1 g/kg and 60 mg/animal, respectively. The ethanol extract of leaves produced weak activity against alloxan-induced hyperglycemia.[41] Furthermore, the juice of dried stems also exhibited hypoglycemic activity when administered intraperitoneally to mice at a dose of 200 mg/kg.[42]
Diuretic activity
The ethanol extract (50%) of fresh leaves administered intragastrically to rats at a dose of 40 ml/kg, was active, while its decoction did not exhibit any diuretic activity.[43,44]
Acetylcholine release
The effect of policosanols on the release of acetylcholine (ACh) at the neuromuscular junction in mice was examined. Results showed that policosanols enhanced either the spontaneous or the evoked ACh release to a small extent. Furthermore, it was also observed that the rate of conformational changes induced at the nicotinic receptor channel complex were also increased, which confirmed the release of ACh.[45]
Anti-inflammatory effect
Mixtures of fatty acids isolated from sugarcane wax were examined for their anti-inflammatory effect on both rats and mice. Oral administration of this mixture showed anti-inflammatory activity in the cotton pellet granuloma assay and in the carrageenan-induced pleurisy test, both in rats, as well as in the peritoneal capillary permeability test in mice.[46]
Antihypercholesterolemic effect
The antihypercholesterolemic effect of policosanols was examined on normocholesterolemic New Zealand rabbits. Policosanols were administered orally at a dose of 5 - 200 mg/kg for four weeks. Results showed that there was a significant decrease in the level of total cholesterol and low density lipoprotein cholesterol (LDL-C) in a dose-dependent manner. The serum triglyceride level was also reduced, but the reduction observed was not dose-dependent. The high-density lipoprotein level remained unchanged.[41] The policosanols were also examined for prevention of atherosclerosis in male New Zealand rabbits fed on a cholesterol-rich diet for 60 days at doses of 25 or 200 mg/kg. Policosanol-treated rabbits did not develop marked hypercholesterolemia and the intima thickness was also significantly less compared to the control animals.[47]
Antithrombotic activity
Policosanols and D-003 were examined for their platelet aggregation and antithrombotic activity in rats. Oral administration of D-003 at a single dose of 200 mg/kg and policosanols at a concentration of 25 mg/kg in rats, significantly increased the plasma level of 6 keto-PGF1-α (a stable metabolite of prostacyclin PGI (2) as compared to the control group. Furthermore, D-003 also significantly reduced the thromboxane, TxB (2), plasma levels and weight of venous thrombus in collagen-stimulated whole blood of rats[48] The pharmacokinetic study showed that the effect of D-003 was observed after 0.5 hours of dosing and the maximal effect exhibited after one to two hours of treatment.[49]
Toxicity profile of sugarcane juice
There is some presence of polycyclic aromatic hydrocarbons (PAHs) in sugarcane juice. PAHs are formed during incomplete combustion of the organic matter and their presence originates mainly from processing and cooking of food. At harvesting season most of the sugarcane plantation is burnt and this burning is an important source of PAHs. HPLC analysis of sugarcane juice collected at different periods was done, which confirmed the presence of four PAHs: Benz (a) anthracene, benzo (b) fluoranthene, benzo (k) fluoranthene, and benzo (a) pyrene in the juices collected in the harvested period.[50]
CONCLUSION
The modern phytochemical and pharmacological reports on the sugarcane crop and its different products, used as food in India, were reviewed. Sugarcane juice is commonly known as a nutritional drink in India and is considered a unique source of variable contents of different hydrophilic components, with significant biological activities. Sugarcane juice and its unrefined products such as brown sugar, molasses, and jaggery are the richest source of phenolic compounds, such as, phenolic acids, flavonoids, and different glycosides. These components justified their presence in the juice by showing significant pharmacological results. As sugarcane juice contains 70 - 75% of water, the probability of having a lipophilic compound is very less. Although the chemistry of sugarcane juice is mostly associated with sugarcane leaves and shoots in the presence of phenolic compounds, they differ from sugarcane juice in having various policosanols, D-003, and phytosterols. These lipophilic compounds are the important components of sugarcane wax and are reported to have various pharmacological effects like sympathomimetic, antihypercholesterolemic, and antithrombotic activities.
As future investigations continue, S. officinarum and its products may prove to be a rich source of new compounds and there is a wide scope for investigating more activities from the compounds isolated from this cheapest source. Although few reports have shown the presence of carcinogenic compounds such as polycyclic aromatic hydrocarbons in S. officinarum, there is still a need for more advanced studies to confirm the presence of these hydrocarbons.
Discussing about the future, three prospective goals seem to be largely open for future exploitation. Once the accurate and precise chemical composition of the compounds in S. officinarum is understood, it will lead to further studies, to understand the metabolic pathways of these useful compounds. Second, understanding the phytochemistry of certain unrefined products of sugarcane such as jaggery will further verify the thermostable chemical components of sugarcane juice, which remain in it. The third goal will be to understand the phytopharmacological studies. In addition, despite the large number of identified compounds in S. officinarum, there is a scarcity of detailed examinations of their pharmacological activities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.James G. 2nd ed. London: Blackwell Publishing Ltd; 2004. Sugarcane; pp. 152–7. [Google Scholar]
- 2.Koh HL, Chua TK, Tan CH. Singapore: World Scientific Publishing; 2009. A Guide to Medicinal Plants: An Illustrated Scientific and Medical Approach; p. 13. [Google Scholar]
- 3.Anis M, Iqbal M. Antipyretic utility of some Indian plants in traditional medicine. Filoterpia. 1986;57:52–5. [Google Scholar]
- 4.Vedavthy S, Rao KN, Rajiah M, Nagarajun N. Folklore information from Rysalasenna region, Andhra Pradesh for family planning and birth control. Int J Pharmacognosy. 1991;29:113–6. [Google Scholar]
- 5.Karthikeyan J, Simipillai SS. Sugarcane in therapeutics. J Herb Med Toxicol. 2010;4:9–14. [Google Scholar]
- 6.Cáceres A, Girón LM, Alvarado SR, Torres MF. Screening of antimicrobial activity of plants popularly used in Guatemala for the treatment of dermatomucosal diseases. J Ethnopharmacol. 1987;20:223–37. doi: 10.1016/0378-8741(87)90050-x. [DOI] [PubMed] [Google Scholar]
- 7.Khare CP. New York: Springer Science; 2007. Indian Medicinal Plants: An Illustrated Dictionary. [Google Scholar]
- 8.Xu F, Sun RC, Sun JX, Liu CF, He BH, Fan JS. Determination of cell wall ferulic and p-coumaric acids in sugarcane bagasse. Analytica Chimica Acta. 2005;552:207–17. [Google Scholar]
- 9.Harish Nayaka MA, Sathisha UV, Manohar MP, Chandrashekar KB, Dharmesh MS. Cytoprotective and antioxidant activity studies of jaggery sugar. Food Chem. 2009;115:113–8. [Google Scholar]
- 10.Chen ZY, Jiao R, Ma KY. Cholesterol-lowering nutraceuticals and functional foods. J Agric Food Chem. 2008;56:8761–73. doi: 10.1021/jf801566r. [DOI] [PubMed] [Google Scholar]
- 11.Kew: Royal Botanic Gardens; 2012. Saccharum officinarum; pp. 9–21. [Google Scholar]
- 12.Australian Government, Department of Health and Ageing, Office of the Gene Technology Regulator; 2004. The Biology and Ecology of Sugarcane (Saccharum spp. hybrids) in Australia; p. 10. [Google Scholar]
- 13.Hoepfner SW, Botha FC. Purification and characterisation of fructokinase from the culm of sugarcane. Plant Sci. 2004;167:645–54. [Google Scholar]
- 14.Taylor AK. From raw sugar to raw materials. Chem Innov. 2000;30:45–8. [Google Scholar]
- 15.Laguna Granja A, Hernandez JM, Quintana DC, Valmana LA, Ferreiro RM, Mesa MG. Mixture of higher primary aliphatic alcohols, its obtention from sugar cane wax and its pharmaceutical uses. US Patent No. 585. 1999;6316 [Google Scholar]
- 16.Awika JM, Rooney LW. Sorghum phytochemicals and their potential impact on human health. Phytochemistry. 2004;65:1199–221. doi: 10.1016/j.phytochem.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Mas R. D-003: A new substance with promising lipid modifying and pleiotropic effects for atherosclerosis management. Drugs Future. 2004;29:773–86. [Google Scholar]
- 18.Guita BK, Gupta GK, Atal CK. Sugarcane press mud: A potential source of phytosterols, fatty alcohols, fatty acid and hard wax. Res Ind. 1985;30:95–101. [Google Scholar]
- 19.Lamberton JA, Redcliffe AH. The chemistry of sugar-cane wax. I. Nature of sugar-cane wax. Aust J Chem. 1960;13:261–8. [Google Scholar]
- 20.Warth AH. New York: Reinhold Publishing Corporation; 1956. The Chemistry and Technology of Waxes; p. 92. [Google Scholar]
- 21.Deshmane SS, Dev S. Higher isoprenoids-II: Triterpenoids and steroids of Saccharum officinarum Linn. Tetrahedron. 1971;27:1109–18. [Google Scholar]
- 22.Goswami PC, Singh HD, Bareuh JN. Isolation of phytosterols from sugarcane press mud and microbial conversion of the phytosterols to 17- ketosteroids. Curr Sci. 1984;53:917–9. [Google Scholar]
- 23.Georges P, Sylvestre M, Ruegger H, Bourgeois P. Ketosteroids and hydroxyketosteroids, minor metabolites of sugarcane wax. Steroids. 2006;71:647–52. doi: 10.1016/j.steroids.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Bryce TA, Martin-Smith M, Osske G, Schreiber K, Subramanian G. Sterols and triterpenoids-XI. Isolation of arundoin and sawammilletin from Cuban sugar cane wax. Tetrahedron. 1967;23:1283–96. [Google Scholar]
- 25.Farber L, Carpenter FG, McDonald EJ. Separation of colorants from cane sugar. Int Sugar J. 1971;69:323–8. [Google Scholar]
- 26.Smith P, Paton NH. Sugarcane flavonoids. Sugar Technol Rev. 1985;12:117–42. [Google Scholar]
- 27.McGhie TK. Analysis of sugar cane flavonoids by capillary zone electrophoresis. J Chromatogr. 1993;634:107–12. [Google Scholar]
- 28.de Armas R, Martinez M, Vincente C, Legaz ME. Free and conjugated polyamines and phenols in raw and alkaline-clarified sugarcane juice. J Agric Food Chem. 1999;47:3086–92. doi: 10.1021/jf980715+. [DOI] [PubMed] [Google Scholar]
- 29.Maurício Duarte-Almeida J, Novoa AV, Linares AF, Lajolo FM, Inés Genovese M. Antioxidant activity of phenolic compounds from sugar cane (Saccharum officinarum L.) juice. Plant Foods Hum Nutr. 2006;61:187–92. doi: 10.1007/s11130-006-0032-6. [DOI] [PubMed] [Google Scholar]
- 30.Vila FC, Colombo R, de Lira TO, Yariwake JH. HPLC microfractionation of flavones and antioxidant (radical scavenging) activity of Saccharum officinarum L. J Braz Chem Soc. 2008;19:903–8. [Google Scholar]
- 31.Colombo R, Yariwake JH, Queroz EF, Ndjoko K, Hostettmann K. On-line identification of minor flavones from sugarcane juice by LC/UV/MS and post-column derivatization. J Braz Chem Soc. 2009;20:1574–9. [Google Scholar]
- 32.Duarte-Almeida JM, Negri G, Salatino A, de Carvalho JE, Lajolo FM. Antiproliferative and antioxidant activities of a tricin acylated glycoside from sugarcane (Saccharum officinarum) juice. Phytochemistry. 2007;68:1165–71. doi: 10.1016/j.phytochem.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. [Google Scholar]
- 34.Mabry TM, Liu YL, Pearce J, Dellamonica G, Chopin J, Markham KR, et al. New flavonoids from sugarcane (Saccharum) J Nat Prod. 1984;47:127–30. [Google Scholar]
- 35.Takara K, Ushijima K, Wada K, Iwasaki H, Yamashita M. Phenolic compounds from sugarcane molasses possessing antibacterial activity against carcinogenic bacteria. J Oleo Sci. 2007;56:611–4. doi: 10.5650/jos.56.611. [DOI] [PubMed] [Google Scholar]
- 36.Payet B, Shum Cheong Sing A, Smadja J. Assessment of antioxidant activity of cane brown sugars by ABTS and DPPH radical scavenging assays: Determination of their polyphenolic and volatile constituents. J Agri Food Chem. 2005;53:10074–9. doi: 10.1021/jf0517703. [DOI] [PubMed] [Google Scholar]
- 37.Godshell MA, Roberts EJ. New Orleans: SPRI; 1982. Phenolics in sugar products: Their role in flavour and color production. Proceedings of the 1982 Sugar Processing Research Conference; pp. 47–72. [Google Scholar]
- 38.Payet B, Shum Cheong Sing A, Smadja J. Comparison of the concentrations of phenolic constituents in cane sugar manufacturing products with their antioxidant activities. J Agric Food Chem. 2006;54:7270–6. doi: 10.1021/jf060808o. [DOI] [PubMed] [Google Scholar]
- 39.Costa M, Di Stasi LC, Kirizawa M, Mendaçolli SL, Gomes C, Trolin G. Screening in mice of some medicinal plants used for analgesic purposes in the state of São Paulo. J Ethnopharmacol. 1989;27:25–33. doi: 10.1016/0378-8741(89)90074-3. [DOI] [PubMed] [Google Scholar]
- 40.Jin YF, Liang HZ, Cao CY, Wang ZW, Shu RS, Li XY. Immunological activity of bagasse polysaccharides (author's transl) Zhongguo Yao Li Xue Bao. 1981;2:269–75. [PubMed] [Google Scholar]
- 41.Arruzazabala ML, Carbajal D, Mas R, Molina V, Valdes S, Laguna A. Cholesterol-lowering effects of policosanol in rabbits. Biol Res. 1994;27:205–8. [PubMed] [Google Scholar]
- 42.Takahashi M, Konno C, Hikino H. Isolation and hypoglycemic activity of saccharin A, B, C, D, E and F glycans of Saccharum officinarum stalks. Planta Med. 1985;3:258–60. [Google Scholar]
- 43.Ribeiro Rde A, Fiuza de Melo MM, De Barros F, Gomes C, Trolin G. Acute antihypertensive effect in conscious rats produced by some medicinal plants used in the state of São Paulo. J Ethnopharmacol. 1986;15:261–9. doi: 10.1016/0378-8741(86)90164-9. [DOI] [PubMed] [Google Scholar]
- 44.Cáceres A, Girón LM, Alvarado SR, Torres MF. Screening of antimicrobial activity of plants popularly used in the Guatemala for the treatment of dermatomucosal diseases. J Ethnopharmacol. 1987;20:223–37. doi: 10.1016/0378-8741(87)90050-x. [DOI] [PubMed] [Google Scholar]
- 45.Re L, Barocci S, Capitaini C, Vivani C, Ricci M, Rinaldi L, et al. Effects of some natural extracts on the acetylcholine release at the mouse neuromuscular junction. Pharmacol Res. 1999;39:239–45. doi: 10.1006/phrs.1998.0433. [DOI] [PubMed] [Google Scholar]
- 46.Ledón N, Casacó A, Rodríguez V, Cruz J, González R, Tolón Z, et al. Anti-inflammatory and analgesic effects of a mixture of fatty acids isolated and purified from sugarcane wax oil. Planta Med. 2003;69:367–9. doi: 10.1055/s-2003-38880. [DOI] [PubMed] [Google Scholar]
- 47.Arruzazabala ML, Noa M, Menéndez R, Más R, Carbajal D, Valdés S, et al. Protective effect of policosanol on atherosclerotic lesions in rabbits with exogenous hypercholesterolemia. Braz J Med Biol Res. 2000;33:835–40. doi: 10.1590/s0100-879x2000000700015. [DOI] [PubMed] [Google Scholar]
- 48.Molina V, Arruzazabala ML, Carbajal D, Más R. D-003, a potential antithrombotic compound isolated from sugar cane wax with effects on arachidonic acid metabolites. Prostaglandins Leuko Essent Fatty Acids. 2002;67:19–24. doi: 10.1054/plef.2002.0376. [DOI] [PubMed] [Google Scholar]
- 49.Molina V, Arruzazabala ML, Carbajal D, Más R, Valdés S. Antiplatelet and antithrombotic effect of D-003. Pharmacol Res. 2000;42:137–43. doi: 10.1006/phrs.2000.0664. [DOI] [PubMed] [Google Scholar]
- 50.Silvia AV, Natali GS, Milton BN. Polycyclic aromatic hydrocarbons in sugarcane juice. Food Chem. 2009;116:391–4. [Google Scholar]


