Abstract
Alpinia calcarata Roscoe (Family: Zingiberaceae), is a rhizomatous perennial herb, which is commonly used in the traditional medicinal systems in Sri Lanka. Alpinia calcarata is cultivated in tropical countries, including Sri Lanka, India, and Malaysia. Experimentally, rhizomes of Alpinia calcarata are shown to possess antibacterial, antifungal, anthelmintic, antinociceptive, anti-inflammatory, antioxidant, aphrodisiac, gastroprotective, and antidiabetic activities. Phytochemical screening revealed the presence of polyphenols, tannins, flavonoids, steroid glycosides and alkaloids in the extract and essential oil of this plant. Essential oil and extracts from this plant have been found to possess wide range of pharmacological and biological activities. This article provides a comprehensive review of its ethnomedical uses, chemical constituents and the pharmacological profile as a medicinal plant. Particular attention has been given to the pharmacological effects of the essential oil of Alpinia calcarata in this review so that the potential use of this plant either in pharmaceutics or as an agricultural resource can be evaluated.
Keywords: Alpinia calcarata, essential oil, hot alcoholic extract, pharmacological, phytochemical
INTRODUCTION
Alpinia calcarata Roscoe (Zingiberaceae) is a rhizomatous plant widely used as systemic medicinal sources in Sri Lanka.[1] The mature rhizomes are branched and dense with a light to dark brown color.[1,2] The leaf of the plant is simple, alternative, 25-32 cm long, 2.5-5 cm broad [Figure 1].[1,2] The flowers are irregular, bisexual and pendanculate. Terminal densed flowers are found in panicles 8.5cm long.[1,2] A. calcarata is cultivated in tropical countries including India, Sri Lanka and Malaysia.[2]
Figure 1.

Different parts of Alpinia calcarata. (a) Leafy plant (b) rhizome (c) flower of Alpinia calcarata. Photos are collected from cultivation area of Bangladesh Council of Scientific and Industrial Research (BCSIR), Chittagong, Bangladesh
The most important part of A. calcarata is its rhizome.[3,4] It is the major part of indigenous medicinal formulation for the treatment of indigestion, impurities of blood, throat inflammation, voice improvement and to marinate youthful vigor.[3,4] In Sri Lanka, It is also recommended as an aphrodisiac. The decoction of A. calcarata rhizome is widely used to treat cough, respiratory ailments, bronchitis, asthma, arthritis and diabetics.[2,5,6] Research has shown antibacterial, anthelmintic, antifungal, antinociceptive, antioxidant, gastroprotective, aphrodisiac and antidiabetic effects of both the ethanolic and aqueous extracts of A. calcarata rhizomes.[7,8,9,10,11,12,13,14,15,16] This herb is also used as traditional medicine for fever, stomachache and rheumatism.[16] Very recently the anti-inflammatory and antioxidative properties of A. calcarata hot ethanol extract and hot water extract have been reported by Arawwawala et al.,[17] Cytotoxic properties of A. calcarata rhizome alcoholic extract has been investigated against Ehrlich ascites carcinoma (EAC) tumor bearing Swiss Albino mice.[18] Pesticidal effect of A. calcarata essential oil has also been found against the American Cockroach, Periplanata Americana.[19] In a molecular study, genomic exploration of polyketide synthase (PKS) in A. calcarata identified the presence of an unusual intron at the region to form a second exon of typical PKSs.[20] It forms a gateway information of distribution of novel PKSs in Zingiberaceae.[20]
Roots, rhizomes and leaves of A. Calcarata have been investigated and revealed the presence of, protocatechinic acid, 1,8-cineole, quercetin, β-pinene, 4-O-methyl-syringic acid, methyl cinnamate, vanillic acid and a number of diterpenes.[21,22,23] Some diterpenes such as calcaratarins A-E, sesquiterpenes such as shyobunone and coumarins such as herniarin has been isolated by Kong et al. (2002) from the rhizomes of A. calcarata grown in China. The two bis-labdanic diterpenoids showed cytotoxic activity against human KB cells in vitro.[24] Apart from this, some benzenoids such as protocatechuic acid, alkaloids and different flavonoids were isolated from leaves of A. calcarata available in India.[25] A. calcarata, with such huge range of phytochemical diversity and pharmacological properties, has been ensured very safe and non-toxic in animal studies. This review presents very comprehensively the chemical constituents and pharmacological properties of A. calcarata so that the potential use of this plant either in pharmaceutics or as an agricultural resource can be readdressed.
Ethnopharmacology
Alpinia calcarata Roscoe (Family: Zingiberaceae) is widely distributed in the tropical countries such as Sri Lanka, Malaysia and India. Rhizomes of this herb are frequently used as traditional remedies for cough, bronchitis, respiratory ailments, asthma, arthritis and diabetics in Sri Lanka.[2,5] It is a very common medicinal herb available in Sri Lanka. It is usually cultivated in Sri Lankan village gardens for medicinal purposes.[1] A. calcarata is named as Kulanjan in Hindi, Heen-araththa in Sinhala, Amkolinji in Tamil and Kattuchena in Malyalam In India.[26] It is widely used for treating colds, reducing swellings, relieving stomachache and invigorating the circulatory systems in Indian region.[26] It is also grown in different parts of Bangladesh Figure 1 and also used in inflammatory diseases, cough and respiratory problems.[27] The beneficial effects of this plant are widely varied with the region of cultivation. Arambewela and co-workers (2005a) reported that the major constituents in the essential oils of root, rhizome and leaves of A. calcarata grown in Sri Lanka are different from that of Indian cultivars.[11]
Phytochemistry
Numbers of benzenoids such as vanillic acid, protocatechuic acid, syringic acid, alkaloids and flavonoids were isolated from Indian A. calcarata leaves.[25] Kong and co-researchers have isolated some diterpenes such as calcaratarins A-E, sesquiterpenes such as shyobunone and coumarins such as herniarin from the rhizomes of A. calcarata grown in China.[22,24] According to Arambewela and coworkers, at least eighteen volatile compounds were identified from the essential oil of rhizomes, roots and leaves of A. calcarata.[11] Among the isolated compounds, 1, 8 - cineol was found to be the major constituent in rhizomes and leaves oil while fenchyl acetate was in the roots. Novel bis-labdanic diterpenoids such as calcaratarin D and calcaratarin E were isolated and identified from the rhizomes of A. calcarata. Spectral evidence showed that structurally they were a pair of stereoisomers.[24] Qualitative phytochemical analysis, of the hot ethanol extract and hot water extract, carried out by Farnsworth method revealed the presence of tannins, polyphenols, steroid glycosides, flavonoids and alkaloids in A. calcarata rhizomes.[11] The relative percentages of total ash, moisture, water soluble ash, acid insoluble ash, water extractable matter and ethanol extractable matter were of 8.3-8.8, 5.5-6.8, 7.2-7.8, 0.036-0.040, 18.6-20.5 and 22.6-24.8, respectively.[28]
Essential oil composition
Pungent odoriferous oils were known to be present in A. calcarata and the major components of the rhizome volatile oil are 1,8-cineol (42%), camphene (7.6%), α and β-pinene (11.30%), α-fenchyl acetate (14.7%), camphor (5%), borneol (2.5%).[10,21,29] Volatile oils isolated from different parts of A. calcarata Rosc., growing in South India, Hyderabad, were analyzed by GC/MS and capillary GC. The yields of oil were: Flower 0.06%, leaf sheath 0.03%, stems 0.05% and root 0.18%. Sixty-two compounds accounting for 92.3-98.3% of the oils were identified. The oil of flower contained 1,8-cineole (12.8%), β-pinene (12.5%) and (E)-methyl cinnamate (12.3%) as the major constituents. The major components of the leaf sheath oil were 1,8-cineole (23.2%) and humulene epoxide I (10.6%). The stem oil contained 1,8-cineole (33.2%) and β-pinene (11.2%) as the prominent compounds. On the contrary, the root oil contained α-fenchyl acetate (37.6%), 1,8-cineole (15.6%) and camphene (13.6%) as the main constituents [Table 1].[11] According to Rout et al. (2005), the leaf oil contained 1,8-cineole (21.9-24.7%), β-pinene (16.8-29.1%), camphor (4.9-8.0%), while the root oil had α-fenchyl acetate (39.1-45.2%), 1,8-cineole (15.1-15.5%) and camphene (9.0-12.3%) as the main constituents.[29] Other analysis of A. calcarata has displayed the presence of 4-O-methyl-syringic acid, methyl cinnamate, protocatechinic acid, quercetin, vanillic acid and several diterpenes and terpenes as the main constituents.[25] Most of the researchers reported the 1,8-Cineole as the major chemical component in the essential oil of A. calcarata.[21,23,30,31] However, rhizome oil constituents of A. calcarata from Bangladesh was found to contain α-fenchyl acetate (51.4%) and 1,8-cineole (15.1%) as main constituents [Table 2].[27,32]
Table 1.
Constituents of Alpinia calcarata rhizome essential oil (Arambewela et al. 2005)
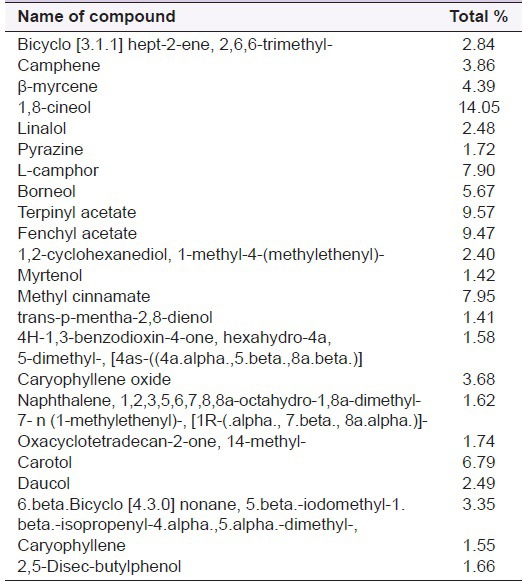
Table 2.
Constituents of Alpinia calcarata rhizome essential oil (Bshuiyan et al. 2011)
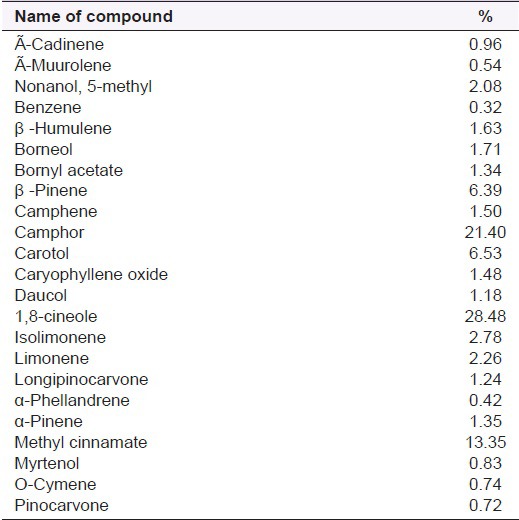
Pharmacological properties
Antioxidative effects
The antioxidative properties of cold ethanolic extract (CEE), hot water extract (HWE) and hot ethanolic extract (HEE) A. calcarata (Zingiberaceae) rhizomes were investigated by TBARS (thiobarbituric acid reactive substances) assay and DPPH (2,2-diphenyl-l-picrylhydrazyl) free radical scavenging assay. Among the tested extracts, highest antioxidant potential was evident in CEE. In TBARS assay antioxidant potential of CEE was comparable to that of positive control BHT (butylated hydroxy toluene). On the other hand in DPPH assay the free radical scavenging ability of CEE was lower than BHT.[28] However, the researchers concluded the moderate antioxidant activity of A. calcarata rhizome at least in the above-mentioned in vitro studies.
Hypoglycemic and anti-hyperglycemic effects
Alpinia calcarata has been evidenced with potential antidiabetic effects. Two different groups of researchers have examined the antidiabetic effects of A. calcarata. Arambewela et al., investigated the effects of hot ethanolic extract and hot water extract in normal glycemic and streptozotocin (STZ)-induced diabetic rats with an oral administration of the extract.[15] In normoglycemic rats, administration (each dose in a volume of 1 mL DW) of a daily oral dose of 250, 500, 750, 1000 and 1500 mg/kg hot HEE and HWE significantly (P < 0.05) decreased the blood glucose levels in a dose dependent manner. Oral glucose tolerance in rats was also improved very markedly with both the hot ethanol extract and hot water extract. The hypoglycemic effect of hot ethanol extract was usually higher than that of the hot water extract. However, in STZ-induced diabetic rats, the hot ethanol extract or the hot water extract failed to reduce blood glucose levels. Apart from this, glucose absorption from the small intestine was significantly decreased and the glycogen accumulation in both skeletal muscle and liver was increased by the hot ethanol extract. The study concluded the strong antihyperglycemic effects of A. calcarata rhizomes. Raj et al. (2011) studied the ameliorative property of ethanol extract A. calcarata rhizomes in alloxan-induced diabetic rodent model.[16] The rhizome extract of A. calcarata significantly (P < 0.05) reduced the blood glucose level, body weight gain, plasma triglyceride and total cholesterol levels when with an oral dose of 100, 200 and 300 mg/kg/day for 21 days. From the results of the above-mentioned studies, it is clear that A. calcarata has better effect on improving glucose tolerance rather than reducing blood glucose when the effects of ethanolic extract might be better than the aqueous extract in this regard. Further study can only clarify this confusion.
Effect of A. calcarata in gastric ulcers and gastroprotection
Arambewela and co-workers evaluated the gastroprotective effect of hot water extract of A. calcarata Roscoe rhizomes.[13] They carried out the study in ethanol induced gastric ulcerative albino rats using three oral doses (500, 750, 1000 mg/kg) of hot water extract. Ethanol induced gastric damage was significantly (P < 0.05) and dose dependently protected by the oral administration of hot water extract.[13] The gastroprotective effect of hot water extract was better than that of the reference H2-receptor antagonist, cimetidine. The hot water extract significantly inhibited gastric volume, acidity (total and free) and significantly (P < 0.05) increased the gastric pH when the gastric mucosal secretion was remained unaltered. Furthermore, the significant (P < 0.05) antihistaminic activity of the hot water extract has also reported in the same study. The hot water extract was also well tolerated: It did not show any overt sign of toxicity, hepatotoxicity (in terms of liver function marker such as aspartate transaminase, alanine transaminase) or renal toxicity (in terms serum urea and creatinine). The research concluded that hot water extract of A. calcarata rhizome has potent and safer gastroprotective effect.[13]
Antibacterial and antifungal effect
Methanol and hexane extract of A. calcarata shared significant activity against E. coli, Staphyloccus aureus, Streptococcus pneumoniae, Pseudomonas aeroginosa, Enterobactraerogenes, Salmonella paratyphi, Vibrio cholerae and Bacillus subtilis.[33] A. Calcarata rhizome essential oil was tested against ten pathogenic bacteria and seven fungi. In terms of antibacterial activity, essential oil showed remarkable activity with zone of inhibition of 15 mm each against E. coli and Bacillus subtilis, followed by Klebsiella pneumoniae (12 mm), Acetobacter pasteurianus (10 mm) and Agrobacterium rhizogenes (11 mm). Three bacteria Lactobacillus lactis, Lactobacillus acidophilus and Staphylococcus aureus displayed the inhibition zone of 8 mm each and Bradyrhizobium and Flavobacterium species showed each 7 mm of inhibitory activity against essential oil.
In order to evaluate the antifungal effect of the chemicals present in the essential oil of A. calcarata, seven fungi were tested. Of these, Aspergillus aculeatus, and Aspergillus awomori exposed the maximum inhibitory zones of 10 mm and 7 mm respectively. Aspergillus niger, Candida albican, Fusarium oxysporum, Rhodotorula species and Trichoderma virideae showed the inhibitory zone of 12mm each. The overall inhibitory effect of A. calcarata extract was better activity against the pathogenic bacteria than fungus.[34] Antifungal activities against crop pathogens Curvularia spp. and Colletorichum spp. using the agar plate method were also investigated. The degree of inhibition of the growth of crop pathogens Curvularia spp. and Colletorichum spp. by essential oil was varied with time period and its effects were better than the positive control drug, daconil. A promising antifungal effect of A. calcarata essential oil is suggested in this study.[28] From the results of the above-mentioned studies, it is evident that A. calcarata has a strong anti-bacterial and anti-fungal effects even sometimes better than the reference drug.
Antinociceaptive effect
Hot water extract and hot ethanol extract of A. calcarata rhizome were carried out for antinociceptive activity in three models of nociception (tail flick, hot plate and formalin tests) in albino rats.[10,29] hot water extract at the doses of 100, 250, 500, 750, 1000 mg/kg) and hot ethanol extract at the doses of 100, 250, 500, 750, 1000 mg/kg were orally administrated to the experimental rats and the reaction time was counted. A strong and dose dependent antinociceptive activity was observed in the hot plate and the formalin tests. But no significant antinociceptive effect was recorded in the tail flick test. The antinociceptive effect in hot ethanol extract was slightly higher than that in hot water extract. Finally, the result remarked an opioid mediated antinociceptive effect of A. calcarata.
Anti-inflammatory effect
Alpinia calcarata Roscoe rhizomes are often used in Sri Lankan traditional medicines as a remedy for bronchitis, cough, respiratory ailments, diabetics, asthma and arthritis. Generally the anti-arthritic drugs have anti-inflammatory and antinociceptive properties. Arawwawala et al., carried out the anti-inflammatory activity of hot water extract and hot ethanol extract of A. calcarata rhizomes in carrageenan-induced inflammatory model.[17] The administered doses of hot water extract and hot ethanol extract (250, 500, 750, and 1000 mg/kg) significantly (P < 0.05) inhibited the carrageenan-induced inflammation. The most potent and pronounced effect was noticed 4 h after carrageenan injection. The anti-inflammatory effect of hot ethanol extract (obtained by 500 mg/kg) was superior compared to the reference drug, indomethacin at 4 h time period. The findings concluded that the inhibitions of histamine and prostaglandin synthesis are the probable mechanisms by which A. calcarata mediates its anti-inflammatory action. The study has ascertained the previous report of Sharma and Singh on the traditional uses of A. calcarata as an anti-inflammatory medicinal plant.[4] Rahman et al., has also researched and concluded the effective anti-inflammatory nature of A. calcarata essential oil.[29]
Insects and pests repellant properties
Plant extracts with known effects on insects could be useful as complementary or alternative treatments to the classical chemical originated insecticides.[35] These sorts of plants usually contain allelochemical compounds such as essential oil. Commonly, insects inhale, absorbe or ingest essential oil via skin. Thus, these oils may show repellant as well as toxic effects on insects.[36] Paranagama and Ekanayake carried out a study to identify the bioactive components of rhizome of A. calcarata as a repellant for the common household pests, Periplanata americana in Sri Lanka.[35]
Six bioactive compounds α-pinene, β-pinene, camphene, 1,8-cineole, camphor and fenchyl acetate containing crude essential oil showed repellant properties when compared with the known attractants such as camphor, pheromone etc., Responses of the essential oil were compared with the known attractants, the highest gas chromatography-electroantennographic detection (GC-EAD) amplitudes were shown by the essential oil under the laboratory conditions used. The electrophysical responses of the A. calcarata essential oil also showed the highest activity compared to known attractants and repellants. In the behavioral bioassay, the essential oil elicited significantly higher repellant properties in P. americana than in the control.[36]
Reproductive competence
Aphrodisiac action of A. calcarata rhizome was investigated in a sexual competence study.[14] The study examined the effects of A. calcarata rhizomes on male rats for fertility and sexual competence with a hot water extract. Three doses of hot water extract (150, 250 and 500 mg/kg bw) were orally administrated to male rats and 3 h later using receptive females their sexual behavior was monitored for 15 min. Fertility was determined in a separate group (with the highest dose) using a noncompetitive copulation test. Sexual behavior study noted that the hot water extract of A. calcarata improved the ejaculating and latency for ejaculation was prolonged markedly. Moreover, the counts for rats mounting and intromitting, and the latencies for mounting and intromission were inhibited. These observations collectively noticed a strong aphrodisiac action of A. calcarata.[14] The other indexes remained unchanged indicating sexual arousability, non-impairment in libido, sexual performance, sexual vigor or penile erection. However, a high dose of hot water extract slightly impaired the sexual motivation in a partner preference test. In fertility test, profound oligozoospermia was induced although fertility was uninhibited. With the administration of the highest dose of hot water extract, the serum testosterone level was elevated. The number of spontaneous penile erections was also elevated rapidly and markedly. The study concluded that rhizomes of A. calcarata possess a strong and safe oral aphrodisiac effects and it has been considered as a non-toxic.[14]
Anticancer effect
Perveen et al., carried out the cytotoxic assay of ethanol extract of A. calcarata rhizome against Ehrlich ascites carcinoma (EAC) tumor bearing Swiss Albino mice.[18] It was found that intraperitoneal dosing of 8 mg/kg/day ethanol extract significantly (P < 0.01) reduced tumor cell growth rate (85.70%), increased life span (70.25%) and decreased tumor weight (62.00%) in comparison to those of EAC bearing mice. The rhizome extract also normalized the depleted hematological parameters such as RBC, WBC, Hb%, differential blood cell counts (e.g. monocytes, lymphocytes, neutrophils etc.) of EAC bearing mice. The toxic effects of host were not very serious to be recovered gradually towards normal within a few days after treatment. This research concluded the potent cytotoxic activity of rhizome ethanol extract of A. calcarata against EAC tumor bearing Swiss Albino mice. The data suggested that the plant could be recommended as a newer and potential source of antitumor agents.
Enzymatic effect of A. calcarata
Fungal strain of A. calcarata has been identified for amylase production. Among thirty isolates of endophytic fungi of A. calcarata, isolate number seven identified as Cylindrocephalum sp. (Ac-7) showed highest amylolytic activity and was taken for further study. Influence of various physical and chemical factors such as pH, temperature, carbon and nitrogen sources on amylase production in liquid media were studied. The maximal amylase production was found to be at 30°C and at pH 7.0 of the growth medium. Among the various carbon and nitrogen sources tested, maltose at 1.5% and Sodium nitrate at 0.3% respectively gave optimum amylase production.[37]
Toxic effect and safety profile
Several complementary and alternative uses of A. calcarata have been investigated. An effective but unsafe drug, nonetheless, becomes abandoned. Due to many important and effective uses, an evaluation has been made whether A. calcarata rhizomes possess any toxic effect in albino rats.[35] In the toxicity study, 1500 mg/kg/day of hot ethanol extract and hot water extract of A. calcarata rhizome were orally administrated to Wistar Albino rats for 42 consecutive days. Experimental rats did not show any acute or chronic toxic effects as evident from their effects on hepatic function, nephritic function, RBC count, WBC count and hemoglobin concentration. Even no toxicity was noticed on external morphology and wet weights of selected organs. Apart from these, no evidence was appeared to mediate any unacceptable effects on water and food intake, body weight gain, consistency of feces and color of urine by both the extracts. The researchers concluded that hot ethanol extract and hot water extract of A. calcarata produced no serious toxic or side effect at the dose used in this study for animal model.
Genetic diversity of A. Calcarata
Plant polyphenols contribute as valuable secondary metabolites containing a wide spectrum of biological activities. Most of the genes coding for the biosynthetic machineries of plant secondary metabolism are encoded by small families of genes originated via the duplication over evolutionary time.[35] Type III polyketide synthase (PKS) or chalcone synthase is a multigene family available in most of the plants.[38] A highly conserved stretches of amino acid residues of Polyketide synthases form catalytic pockets. The amino acid residues forming these pockets are located mostly within the second exon.[39] As the substitution of key amino acid residues can have tremendous impact on the PKS reaction mechanism, the genomic information derived from such studies can give molecular insights into functional novelties. However, the genomic distribution of the PKS superfamily in medicinal plants is still little investigated; Radhakrishnan et al., carried out a PCR based investigation of A. calcarata PKS gene to unveil its genomic features.[40]
The genomic status of PKS in A. calcarata carried out in this study explored the presence of an unusual intron at the region forming the second exon of typical PKSs. This forms gateway information of distribution of novel PKSs in Zingiberaceae. The sequence analysis of the second exon of A. calcarata PKS identified in this study displayed the unusual occurrence of an intron that can be considered as a novelty. The sequence identified in this study opens a perspective for further molecular investigation, their metabolite richness, the distribution of structurally diverse phenolic compounds throughout the A. calcarata species. The study concluded the higher probability that PKSs in Zingiberaceae might have been subject to remarkable genomic changes.
Standardization of A. calcarata
Some pharmacognostic parameters are useful to be determined quantitatively for setting standards for crude drugs. An investigation was carried out to standardize the rhizomes of A. calcarata by (a) phytochemicals screening (b) physicochemical parameters determination and (c) Densitogram development. Phytochemical screening revealed the presence of polyphenols, tannins, flavonoids, steroid glycosides and alkaloids in A. calcarata rhizomes. The percentages of moisture, total ash, acid insoluble ash, water soluble ash, ethanol extractable matter and water extractable matter were of 5.5-6.8, 8.3-8.8, 0.036-0.040, 7.2-7.8, 22.6-24.8 and 18.6-20.5 respectively. To detect the improper handling and adulteration of drugs their physicochemical analysis is very important. The total ash is especially important for the evaluation of quality and purity of drugs. Usually the total ash includes both the physiologic and non-physiologic ash such as carbonates, phosphates, silicates, and silica. High ash value indicates an adulteration, contamination, substitution or carelessness in preparing the crude drug for marketing.[41] Acid-insoluble ash is an indicative of contamination with silica, for example, earth and sand. Comparison of the values for total ash and acid-soluble ash of the same sample will differentiate between contaminants and variations of the natural ash of the drug. Water soluble ash indicates the water soluble salts in the drug.[41] Furthermore, the degree of adulteration in powdered raw materials of A. calcarata and its comparative powder microscopy has also been revealed to identify two similar medicinal plant materials microscopically.[42]
Extractive values also represent the presence of polar and/or nonpolar compounds in a plant material. The water soluble extractive value can help the best to identify the quality, adulteration, or incorrect processing of the crude drug during the process of storage and drying.[41] Moisture is an inevitable component of crude drugs, which must be eliminated as far as practicable. Insufficient drying can lead to spoilage of the plant materials by molds and bacteria. It also makes possible the enzymatic destruction of active principles.[41] The results of phytochemical screening studies and physico-chemical parameters can be used as important tool for standardizing the A. calcarata rhizomes.
Clonal propagation of A. calcarata
It has been estimated that approximately 1.70 tons of A. calcarata dried rhizome were required for the northern districts of Kerala state in India.[42] The plant is propagated conventionally by rhizome cuttings which are insufficient for a commercial scale production to meet the current demand. Moreover, it is impractical and uneconomic to utilize the rhizomes for cultivation purposes as it constitute the raw material for the drug preparation. Furthermore, improvement through conventional breeding is difficult due to its rare flowering and lack of seed set. Prolonged maturity period is yet another barrier for the continuous availability of propagules for cultivation. In vitro propagation techniques through resident meristems favor the production of true plants.
It is well accepted and proven technology to generate genuine raw materials of medicinal plants. Qualitative and quantitative analysis of secondary metabolites are advantageous for the micropropagation of medicinal plants and it has been reported for many medicinal plant species.[43,44] Production of plants in vitro from small to commercial scale has forced many researchers to think about important issues such as cost efficiency, higher productivity, automation and optimization of minor environment.[45] Although there is a previous report on preliminary information on micropropagation of A. calcarata there has been no study for the establishment of a cost effective rapid production of uniform plants.[46] By considering the importance of large scale cultivation of A. calcarata, Sudha et al., established an efficient and cost effective rapid clonal propagation on Murashige and Skoog medium and assessed the chemical fidelity of the in vitro raised plants.[47]
The rooted plants were hardened in mist chamber showed 95% survival and well established in the field. The acclimatized plants showed rhizome formation after 4-6 weeks of growth under shade house. Volatile chemicals profile of the leaves, rhizome and root of the in vitro and conventionally propagated plants analyzed by gas chromatography-mass spectrometry were qualitatively and quantitatively similar. The analysis of growth characteristics of 36 months old in vitro and conventionally propagated plants showed a 50% increment of rhizome fresh biomass with prolific root and leaf growth in the former than the latter one.
MATERIALS AND METHODS
Relevant literatures were collected by searching the major scientific databases including Pubmed, Science direct, Medline and Google scholar for phytochemical and phytopharmacological as well as medicinal importance of A. calcarata. Some articles were found through tracking citations from other publications or by directly accessing the journals’ web-site. They were considered on the basis of the geographical region of their origin. The literature considered are those available covering the period, 2000 to 2013. The keywords combinations for the search were: A. calcarata; pharmacological, phytochemical; essential oil; hot alcoholic extract. Supplementary information was obtained by using some other keywords combination such as plant extract, medicinal uses and phytomedicinal. A total of 48 research articles that reports on the in vivo and not in vitro activity were recovered and presented in this review.
CONCLUSIVE REMARKS
The genus Alpinia is a rapidly spreading plant and is presently considered as an alternative source of medication in many common and rare health problems. A number of studies have been carried out on the uses and applications of Alpinia. Of all the species, A. calcarata has been widely studied. It is believed that detailed information as presented in this review on its phytochemistry and various pharmacological properties of the different extracts, essential oil and the constituents might provide incentive for proper evaluation of the use of the plant in medicine. Some research organizations in India are cultivating this plant for commercial supply as raw material for phytochemicals. Test conducted in animal models for anti-inflammatory, analgesic, antinociceptive, reproductive capability, anticancer, gastroprotective and other biological activities have shown promising results without any adverse effect. The major constituents of the essential oil have been shown to produce precocious effects in insect control as well as in bacterial and fungal infection. Further works, however, still need to be carried out on the dose response relationship and mechanisms of actions of all the effect observed so far. This is particularly significant and calls for a detailed study bearing in mind that the plant is very prevalent in the tropics. Additional studies in this area could serve as a means of using the plant in multi-syndromes in human diseases.
ACKNOWLEDGEMENT
Authors wish to thank Dr. Shaikh Bokhear Uddin, Associate Professor, Department of Botany, University of Chittagong, for his kind cooperation in supplying the necessary information on A. calcarata.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Dassanayake MD, Fosberg FR. New Delhi: Amreind; 1981. A revised hand book to the flora of Ceylon; pp. 517–8. [Google Scholar]
- 2.Jayaweera DM. Colombo: National Science Council of Sri Lanka; 1982. Medicinal Plants Used in Ceylon; p. 213. [Google Scholar]
- 3.Kaul PN, Rao BR, Singh K, Bhattacharya AK, Ramesh GR. Volatile constituents of essential oils isolated from different parts of A. calcarata Rosc. J Essent Oil Res. 2005;17:7–9. [Google Scholar]
- 4.Sharma AK, Singh RH. Screening of anti-inflammatory activity of certain indigenous drugs on carrageenin induced bind paw oedema in rats. Bull Med Res. 1980;11:262–71. [Google Scholar]
- 5.Ramanayake L. Publication of Department of Ayurveda. Colombo, Sri Lanka: 1994. Osu Visithuru; pp. 68–71. [Google Scholar]
- 6.Arambewela LS, Basnayake CS, Serasinghe P, Tissera MS, Dias S, Weerasekara DR. NARESA Printing Unit. Colombo, Sri Lanka: 1995. 1995. Traditional treatment in Sri Lanka for chronic Arthritis; pp. 645–51. [Google Scholar]
- 7.George M, Pandalai KM. Investigations on plant antibiotics. Ind J Med Res. 1949;37:169–81. [Google Scholar]
- 8.Kaleysa RR. Screening of indigenous plants for anthelmintic action against human Ascaris lumbricoides. Indian J Physiol Pharmacol. 1975;19:47–9. [PubMed] [Google Scholar]
- 9.Pushpangadan P, Atal CK. Ethno-medico-botanical investigations in Kerala I. Some primitive tribals of Western Ghats and their herbal medicine. J Ethnopharmacol. 1984;11:59–77. doi: 10.1016/0378-8741(84)90096-5. [DOI] [PubMed] [Google Scholar]
- 10.Arambewela LS, Arawwawala LD, Ratnasooriya WD. Antinociceptive activities of aqueous and etahnolic extracts of Alpinia calcarata rhizomes in rats. J Ethnopharmacol. 2004;95:311–6. doi: 10.1016/j.jep.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Arambewela LS, Arawwawala LD. Antioxidant activities of ethanolic and hot aqueous extracts of Alpinia calcarata rhizomes. Aust J Med Herbalism. 2005;17:91–4. [Google Scholar]
- 12.Arambewela LS, Arawwawala LD, Ratnasooriya WD. Gastroprotective activity of hot ethanolic extract of Alpinia calcarata rhizomes in rats. Ceylon J Med Sci. 2005;48:1–11. [Google Scholar]
- 13.Arambewela LS, Arawwawala LD, Ratnasooriya WD. Effect of Alpinia calcarata rhizomes on ethanol-induced gastric ulcers in rats. Phcog Mag. 2009;4:226–31. [Google Scholar]
- 14.Ratnasooriya WD, Jayakody JR. Effects of aqueous extract of Alpinia calcarata rhizomes on reproductive competence of male rats. Acta Biol Hung. 2006;57:23–35. doi: 10.1556/ABiol.57.2006.1.3. [DOI] [PubMed] [Google Scholar]
- 15.Arambewela LS, Arawwawala LD, Ratnasooriya WD. Hypoglycemic and antihyperglycemic activities of the aqueous and the ethanolic extracts of Alpinia calcarata rhizomes in rats. Phcog Mag. 2009;5:412–8. [Google Scholar]
- 16.Raj N, Nadeem S, Jain S, Raj C, Nandi CK. Ameliorative effects of Alpinia calcarata in alloxan-induced diabetic rats. Digest J Nanomat Biost. 2011;6:991–7. [Google Scholar]
- 17.Arawwawala DA, Arambewela SR, Ratnasooriya DW. Alpinia calcarata Roscoe: A Rich Source of Phytopharmaceuticals in Sri Lanka. Nat Prod J. 2012;2:263–9. [Google Scholar]
- 18.Perveen R, Islam F, Khanum J, Yeasmin T. Preventive effect of ethanol extract of Alpinia calcarata Rosc on Ehrlich's ascitic carcinoma cell induced malignant ascites in mice. Asian Pac J Trop Med. 2012;5:121–5. doi: 10.1016/S1995-7645(12)60009-1. [DOI] [PubMed] [Google Scholar]
- 19.Paranagama PA, Ekanayake S. Repellant properties from essential oil of Alpinia calcarata Rosc. Against the American Cockroach Periplanata Americana. J Natn Sci Foundat Sri Lanka. 2004;32:1–12. [Google Scholar]
- 20.Radhakrishnan EK, Varghese RT, Vasudevan SE. Unusual intron in the second exon of a type III polyketide synthase gene of Alpinia alcarata Rosc. Genet Mol Biol. 2010;33:141–5. doi: 10.1590/S1415-47572010000100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tewari A, Pant AK, Mathela CS, Mengi N, Kohl E, Bestmann HJ. Volatile constituents of A. calcarata. J Essen Oil Res. 1999;11:739–41. [Google Scholar]
- 22.Kong LY, Qin MJ, Niwa M. Diterpenoids from the rhizomes of Alpinia calcarata. J Nat Prod. 2000;63:939–42. doi: 10.1021/np9904962. [DOI] [PubMed] [Google Scholar]
- 23.Arambewela LS, Kumaratunga A, Arawwawala M, Owen NL, Du L. Volatile oils of Alpinia calcarata grown in Sri Lanka. J Essent Oil Res. 2005;17:124–5. [Google Scholar]
- 24.Kong LY, Qin MJ, Niwa M. New cytotoxic bis-labdanic diterpenoids from Alpinia calcarata. Planta Med. 2002;68:813–7. doi: 10.1055/s-2002-34404. [DOI] [PubMed] [Google Scholar]
- 25.Merh PS, Daniel M, Sabnis SD. Chemistry and taxonomy of some members of the Zingiberaceae. Curr Sci. 1986;55:835–9. [Google Scholar]
- 26.Ahemed AD, Medine G, Meryem S, Hatice O, Fikerettin S, Isa K. Antimicrobial effects of Ocimum basciliam (Labiatea) extract. Turkey J Biol. 2005;29:155–60. [Google Scholar]
- 27.Chowdhury JU, Yusuf M, Husain MM. Composition of the rhizome oil of Alpinia calcarata. Ind Perfumer. 2003;47:355–77. [Google Scholar]
- 28.Arambewela LS, Arawwawala LD, Athauda N. Antioxidant and antifungal activities of essential oil of Alpinia calcarata Roscoe rhizomes. J Ayurveda Integr Med. 2010;1:199–202. doi: 10.4103/0975-9476.72621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman MA, Rahman MM, Ahmed NU. Essential oil of Alpinia alcarata Rosc. rhizome: Heals inflammation and nociception in animal models. J Biologically Active Products from Nature. 2013:365–76. [Google Scholar]
- 30.Rout PK, Sahoo S, Rath SP, Rao YR. Analysis of the leaf, rhizome and root oils of two accessions of Alpinia calcarata rosc. cultivated at Bhubaneswar. J Essen Oil Res. 2005;17:398–400. [Google Scholar]
- 31.Rath SP, Sahoo SB, Sviniva SC. Analysis of cultivated Alpinia calcarata. Indian J Natural Prod. 1994;10:12–3. [Google Scholar]
- 32.Bhuiyan MN, Begum J, Nandi NC. Volatile constituents of essential oils isolated from different parts of Alpinia calcarata Rosc. Afr J Plant Sci. 2011;5:349–52. [Google Scholar]
- 33.Robinson JP, Balakrishnan V, Raj S, Britto SJ. Antimicrobial activity of Alpinia calcarata and characterization of new ?, b unsaturated carbonyl compound. Adv Biol Res. 2009;3:185–7. [Google Scholar]
- 34.Joseph B, George G, Helen M. Analysis of phytochemical constituents and antimicrobial activities of Alpinia calcarata against clinical pathogens. Asian Pac J Trop Biomed. 2012;5:1–4. [Google Scholar]
- 35.Arambewela M, Ratnasooriya WD, Arawwawala L. Safety profile of Alpinia calcarata Roscoe, used in traditional medicine in Sri Lanka. Boletin Latinoamericano y del Caribe de Plantas Medicinales y Aromaticas. 2011;10:435–42. [Google Scholar]
- 36.Don-Pedro KN. Fumigant toxicity is the major route of insecticidal activity of citrus peel essential oils. Pesticide Sci. 1996;46:71–8. [Google Scholar]
- 37.Durbin ML, McCaig B, Clegg MT. Molecular evolution of the chalcone synthase multigene family in the morning glory genome. Plant Mol Biol. 2000;42:79–92. [PubMed] [Google Scholar]
- 38.Radhakrishnan EK, Soniya EV. Molecular analysis of type III polyketide synthase (PKS) gene family from Zingiber officinale Rosc. Afr J Plant Sci. 2009;3:44–8. [Google Scholar]
- 39.Brand S, Hölscher D, Schierhorn A, Svatos A, Schröder J, Schneider B. A type III polyketide synthase from Wachendorfia thyrsiflora and its role in diarylheptanoid and phenylphenalenone biosynthesis. Planta. 2006;224:413–28. doi: 10.1007/s00425-006-0228-x. [DOI] [PubMed] [Google Scholar]
- 40.Radhakrishnan EK, Sivakumar KC, Soniya EV. Molecular characterization of novel form of Type III polyketide synthase from Zingiber Officinale Rosc. and its analysis using bioinformatics method. J Proteom Bioinform. 2009;2:310–5. [Google Scholar]
- 41.Mukherjee PK. Quality Control of Herbal Drugs. New Delhi, India: 2002. Business horizons; pp. 1–6. [Google Scholar]
- 42.Sasidharan N, Muraleedhara PK. Research Report No. 193, ISBN 0970-8103. Thrissur, Kerala: Kerala Forest Research Institute; 2000. Survey on the Commercial Exploitation and Consumption of Medicinal Plants by the Drug Industry in Northern Kerala; pp. 1–26. [Google Scholar]
- 43.Mohanty S, Parida R, Singh S, Joshi RK, Subudhi E, Nayak S. Biochemical and Molecular Profiling of Micropropagated and Conventionally Grown Kaempferia galanga. Plant Cell Tissue Org Cult. 2010;106:39–46. [Google Scholar]
- 44.Singh M, Chaturvedi R. Improved Clonal Propagation of Splilanthes acmella Murr. for Production of Scopoletin. Plant Cell Tissue Org Cult. 2010;103:243–53. [Google Scholar]
- 45.Pati PK, Kaur J, Singh P. A Liquid culture sys-tem for shoot proliferation and analysis of pharmaceuti-cally active constituents of Catharanthus roseus (L.). G. Don. Plant Cell Tissue Org Cult. 2011;105:299–307. [Google Scholar]
- 46.Agretious TK, Martin KP, Hariharan M. In Vitro Clonal Multiplication of Alpinia calcarata Rosc. Phytomorphol. 1996;46:133–8. [Google Scholar]
- 47.Sudha CG, George M, Rameshkumar KB, Nair GM. Improved Clonal Propagation of Alpinia calcarata Rosc., a commercially Important medicinal plant and evaluation of chemical fidelity through comparison of volatile compounds. Am J Plant Sci. 2012;3:930–40. [Google Scholar]


