Abstract
Background
Pyomyositis due to Escherichia coli (E. coli) is rarely reported in immunocompromised patients with hematological malignancy.
Case Report
We present a case report of a 34-year-old man who developed E. coli pyomyositis as a complication of acute myelogenous leukemia (AML). Magnetic resonance imaging (MRI) of the right hip suggested myofascial infection of the gluteal muscles, and a needle muscle aspiration grew E. coli phylogenetic group B2. The patient responded to intravenous piperacillin/tazobactam followed by prolonged oral levofloxacin.
Conclusion
Pyomyositis should be suspected in all immunocompromised patients complaining of muscle pain and may exhibit signs of localized muscle infection. Appropriate antibiotic therapy targeting fluoroquinolone-resistant E. coli should be considered for initial empiric therapy of pyomyositis in immunocompromised patients.
CASE REPORT
A 34-year-old man was transferred from a rural, community hospital following 4 days of severe right back pain radiating to his buttocks and right thigh. While his occupation required some light lifting, he did not recall any trauma. He denied rigors, feverishness, hemoptysis, dyspnea, polyuria, dysuria, or diarrhea. His muscle pain was severe, and he was unable to walk despite the use of ibuprofen. He denied any chronic medical problems or pertinent family history and did not use any illicit drugs. The patient was from Oxaca, Mexico, but had not traveled there within the last 9 months. He recently had a tooth extraction treated with amoxicillin and ibuprofen for a possible dental abscess.
Because of persistent muscular pain and the finding of pancytopenia, he was transferred to Mayo Clinic Health System. Subjective findings included severe right thigh pain worsened with minimal motion. Examination revealed extremely tender right paraspinous, gluteal, and thigh muscles without crepitus, fluctuance, or crackles on auscultation. The patient was unable to move his right leg due to severe pain, and passive motion localized the pain primarily to the right trochanteric area and anterior thigh. The skin above that area had small, superficial, broken bullae without skin inflammation due to his prior overzealous use of heating pads. There were no adenopathy or gross neurologic deficits.
Laboratory testing revealed hemoglobin of 8.6 gm/dl, platelet count of 178,000 cells/mm3, and a white blood cell count of 1800 cells/mm3, with 12% neutrophils, 2% bands, and 78% lymphocytes. Electrolytes were normal with creatinine of 0.9 mg/dl, aspartate transaminase of 27 units/L, alanine transaminase of 38 units/L, creatine phosphokinase of 75 units/L, and erythrocyte sedimentation rate >120 mm/hour. Urinalysis was negative for leucocyte esterase, nitrates, white cells, blood, and bacteria.
Additional laboratory tests included urine and blood cultures, quantiFERON-TB test for Mycobacterium tuberculosis, and antibody tests for HIV and Brucella, all of which were negative. Anemia evaluation revealed normal iron, iron binding capacity, vitamin B12, and folate levels, but an elevated ferritin of 1447 ng/ml. A bone marrow aspirate revealed >80% blasts compatible with acute myelogenous leukemia (AML), later found to be M1 type.
On the second day of hospitalization, his temperature rose to 39.3°C. After cultures of blood, urine, and sputum were obtained, the patient was started on cefepime; however, because of persistence of fever, severe pain, and immobility despite narcotic medication, he was switched to piperacillin/tazobactam. Magnetic resonance imaging (MRI) of the hip revealed increased T2 signal along the fascial planes in the vicinity of the right hip and within the anterior thigh muscles—the adductor group, gluteus minimus, and tensor fascia lata (Figure 1). There was no evidence of abscess or gas. He was switched to intravenous piperacillin/tazobactam. Aspiration of the adductor muscle in the right upper thigh grew E. coli that was susceptible to levofloxacin, cephalosporins, and piperacillin/tazobactam but resistant to amoxicillin. He was switched to levofloxacin and had gradual but progressive improvement in pain and mobility. He received a total of 6 weeks of oral levofloxacin and also was started on cytarabine and idarubicin induction chemotherapy.
Figure 1.
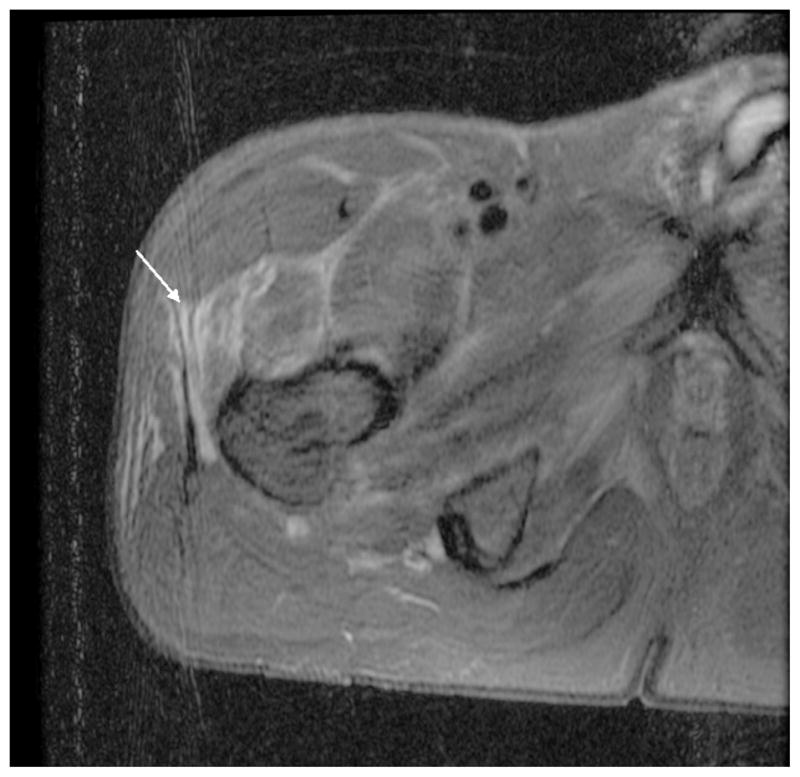
MRI of hip revealing increased T2 signal intensity along the fascial planes in the vicinity of right hip, involving anterior upper thigh, the adductor group, gluteus minimus, and the tensor fascia lata (white arrow) suggesting myositis with no evidence of abscess, soft tissue gas, or septic hip or osteomyelitis.
Characterization of the Myositis E. Coli Isolate
The E. coli isolate was positive for β-lactamase using an acidometric screening and was nonhemolytic when grown on a sheep blood agar plate. It was negative for 0157:H7 by serologic testing. Phylogenetic grouping of the isolate was done according to Clermont et al.1 The strain was shown to be part of the B2 phylogenetic group. A virulence factor gene analysis then was performed targeting several virulence factor genes common among extraintestinal E. coli strains.2 The myositis E. coli isolate was positive for the aer, fimH, fyuA, and usp genes, but was negative for the cnfI, papGI, papGII, papGIII, hlyC, and hra genes.
DISCUSSION
Escherichia coli is the most common cause of urinary tract infections and gram-negative bacteremia in the United States.3–5 E. coli sepsis causes approximately 40,000 deaths per year in the United States and substantial morbidity and health care costs.5 Extraintestinal infection with community-acquired E. coli is associated typically with multiple virulence factors.6 Pyomyositis is a rare extraintestinal manifestation of deep tissue E. coli infection.
While infectious pyomyositis can be caused by a variety of pathogens, including viral and parasitic, it usually is caused by gram-positive bacteria, especially Staphylococcus aureus and, less frequently, Streptococcus pyogenes.7 In tropical countries, pyomyositis may occur due to synergistic co-infections with tissue parasites.2 In these regions, bacterial pyomyositis occurs most frequently in the upper back paraspinous muscles or the anterior thighs. In contrast, in non-tropical countries, the thigh and trunk muscles, as found in our case, are most commonly involved.
Review of current literature reveals our case is very unusual. Pyomyositis due to enteric, gram-negative rods is quite rare, and even fewer reports of its occurrence in hematologic malignancies are noted in the literature (Table 1).8–14
Table 1.
A Literature Review of Cases of E. Coli Pyomyositis in Immunocompromised Hosts
| Reported by | Number of Cases | Age (years) | Sex | Site | Underlying Condition | E. Coli Susceptibility | Treatment |
|---|---|---|---|---|---|---|---|
| Hall7 (1990) | 1 | 62 | Female | Gluteus maximus | Trauma/unknown | Unknown | Unknown |
| Lortholary9 (1994) | 1 | 42 | Male | Psoas | HIV-AIDS | B-lactamase + | Ceftazidime, amikacin, fosfomycin × 16 days |
| Vilades10 (1994) | 1 | 28 | Male | Gluteus | HIV-AIDS | Sensitive | Ciprofloxacin × 6 weeks |
| Cone11 (1997) | 1 | 68 | Male | Anterior tibial compartment | Metastatic prostate cancer | Fluoroquinolone -resistant | Ampicillin and gentamicin (unknown duration) |
| Jou12 (1998) | 3 | Unknown | Unknown | Intra/extra pelvic | Unknown | Unknown | Unknown |
| Johnson13 (2003) | 1 | 56 | Male | Psoas, erector spinae | Diabetes mellitus | Sensitive | Piperacillin/tazobactam × 6 weeks |
| Chiu14 (2008) | 1 | 48 | Female | Calf | Neutropenia due to chemotherapy for acute myelogenous leukemia | B-lactamase + | Meropenem × 3 weeks |
| Vigil8 (2010) | 6 | 38–67 years | Male (4), Female (2) | Calf (5) Thigh (2) |
Leukemia (5), Lymphoma (1) | Fluoroquinolone- resistant | Carbapenum and amikacin |
This is the first case of E. coli pyomyositis at our 345-bed secondary referral center, and, in a period of 30 years, only 1 other case of bacterial pyomyositis, also a gram-negative rod (Salmonella enterica), was diagnosed. Interestingly, in the latter case, which occurred in a gastrocnemius muscle, the patient also was suffering from AML (observation by third author). Since this was a case report, IRB approval was not obtained.
In the present report, several host factors allowed the initial infection to ensue. First, the patient had recently received amoxicillin to which the isolate was resistant, predisposing him to selective colonization; second, he was immunocompromised (neutropenic) due to AML.
The E. coli isolate collected from the patient phylogenetically typed as a B2 group that is common among extraintestinal E. coli isolates, but displayed few virulence factor genes, which is unusual for these E. coli isolates.6 When compared to uropathogenic E. coli isolates, the myositis isolate mapped closely with 1 broad group of uropathogenic E. coli identified through optical mapping.15 What is interesting about this broad group of extraintestinal E. coli strains is that they are missing many key virulence factors normally associated with extraintestinal E. coli. The loss of several virulence factors may have allowed this isolate to initiate and sustain myositis because of the absence of toxins that would trigger an immune response and the lack of adherence structures that could have targeted the E. coli cells for elimination by his diminished number of white blood cells. We believe the source of infection could be transmigration of E. coli from the gut, and earlier exposure to amoxicillin may have contributed to its resistance to amoxicillin.
Pyomyositis has been classified into 3 stages: Stage I, initial muscle inflammation that is not associated with abscess; Stage II, associated with early abscess, usually occurring approximately 2 to 3 weeks into illness; and Stage III, with signs of toxicity and systemic infection.8 We believe that our patient, presenting 2 to 4 days after onset of symptoms, did not mount a full inflammatory response due to severe neutropenia. Hence, abscess formation, as often noted in tissue infections of patients with severe neutropenia, did not occur.
Based on a report from MD Anderson Cancer Center at the University of Texas, the clinical course of E. coli myositis can be severe. Fifty percent of its patients required Intensive Care Unit management due to sepsis, and 33% died.8 All of these isolates were fluoroquinolone resistant. Fortunately, our patient’s strain was fluoroquinolone-susceptible, and, with prompt diagnosis and therapy of 6 total weeks, the infection responded despite his underlying acute leukemia.
Acknowledgments
We wish to thank the clinical microbiology laboratory for identifying the bacterial isolate. The phylogenetic grouping and virulence factor gene detection was supported by NIH grant AI065432-01A2 to W. R. S.
Funding/Support: None declared.
Footnotes
Financial Disclosures: None declared.
References
- 1.Clermont O, Bonacorsi A, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000;66:4555–4558. doi: 10.1128/aem.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swartz MN. Myositis. In: Mandell GL, Douglas RG, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Disease. 5. Philadelphia, PA: Churchill Livingstone; 2000. [Google Scholar]
- 3.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1A):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 4.Laupland KB, Gregson DB, Church DL, Ross T, Pitout JD. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin Microbiol Infect. 2008;4:1041–1047. doi: 10.1111/j.1469-0691.2008.02089.x. [DOI] [PubMed] [Google Scholar]
- 5.Russo TA, Johnson JR. Medical and economic consequences of infections due to extraintestinal pathogenic Escherichia coli (ExPEC) Microb Infect. 2003;5(5):449–456. doi: 10.1016/s1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 6.Cooke NM, Smith SG, Kelleher M, Rogers TR. Major differences exist in frequencies of virulence factors and multidrug resistance between community and nosocomial Escherichia coli bloodstream isolates. J Clin Microbiol. 2010;48:1099–1104. doi: 10.1128/JCM.02017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall RL, Callahan JJ, Moloney E, et al. Pyomyositis in a temperate climate: presentation, diagnosis, and treatment. J Bone Joint Surg Am. 1990;72:124–1244. [PubMed] [Google Scholar]
- 8.Vigil KJ, Johnson JR, Johnston BD, et al. Escherichia coli pyomyositis: an emerging infectious disease among patients with hematologic malignancies. Clin Infect Dis. 2010;50:374–380. doi: 10.1086/649866. [DOI] [PubMed] [Google Scholar]
- 9.Lortholary O, Jehl F, Petitjean O, et al. Polymicrobial pyomyositis and bacteremia in a patient with AIDS. Clin Infect Dis. 1994;19(3):552–553. doi: 10.1093/clinids/19.3.552. [DOI] [PubMed] [Google Scholar]
- 10.Vilades C, Garcia-Queralt R, Rivas I, et al. Pyomyositis due to Escherichia coli in a patient infected by HIV. Br J Rheumatol. 1994;33(4):404–405. doi: 10.1093/rheumatology/33.4.404. [DOI] [PubMed] [Google Scholar]
- 11.Cone LA, Lamb RB, Graff-Radford A, et al. Pyomyositis of the anterior tibial compartment. Clin Infect Dis. 1997;25(1):146–148. doi: 10.1086/514497. [DOI] [PubMed] [Google Scholar]
- 12.Jou IM, Chiu NT, Yang CY, et al. Pyomyositis with special reference to the comparison between extra- and intrapelvic muscle abscess. Southeast Asian J Trop Med Public Health. 1998;12:1002–1005. [PubMed] [Google Scholar]
- 13.Johnson JR, Gajewski A, Lesse AJ, et al. Extraintestinal pathogenic Escherichia coli as a cause of invasive nonurinary infections. J Clin Microbiol. 2003;41(2):5798–5802. doi: 10.1128/JCM.41.12.5798-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu SK, Chang FY. Pyomyositis caused by extended-spectrum beta-lactamase-producing Escherichia coli in a patient with acute myeloid leukemia. Int J Infect Dis. 2008;13(3):e85–e87. doi: 10.1016/j.ijid.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 15.Schwan WR, Briska A, Stahl B, et al. Use of optical mapping to sort uropathogenic Escherichia coli strains into distinct subgroups. Microbiology. 2010;56:2124–2135. doi: 10.1099/mic.0.033977-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


