Abstract
Recent research has shown a widening gap in life expectancy at age 50 between the United States and Europe as well as large differences in the prevalence of diseases at older ages. Little is known about the processes determining international differences in the prevalence of chronic diseases. Higher prevalence of disease could result from either higher incidence or longer disease-specific survival. This article uses comparable longitudinal data from 2004 and 2006 for populations aged 50 to 79 from the United States and from a selected group of European countries to examine age-specific differences in prevalence and incidence of heart disease, stroke, lung disease, diabetes, hypertension, and cancer as well as mortality associated with each disease. Not surprisingly, we find that Americans have higher disease prevalence. For heart disease, diabetes, and cancer, incidence is lower in Europe when we control for sociodemographic and health behavior differences in risk, and these differences explain much of the prevalence gap at older ages. On the other hand, incidence is higher in Europe for lung disease and not different between Europe and the United States for hypertension and stroke. Our findings do not suggest a survival advantage conditional on disease in Europe compared with the United States. Therefore, the origin of the higher disease prevalence at older ages in the United States is to be found in higher prevalence earlier in the life course and, for some conditions, higher incidence between ages 50 and 79.
Keywords: Disease incidence, Disease-specific survival, Europe, United States
Introduction
Recent evidence indicates that the prevalence of chronic diseases in old age is higher in the United States than in Europe (Banks et al. 2006; Crimmins et al. 2010b; Thorpe et al. 2007). Americans have also been shown to have lower life expectancy at age 50 than those of most European countries, with the gap growing over time (Glei et al. 2010). The explanation for higher national levels of disease prevalence in the United States is not clear, however: higher prevalence could result from either higher disease incidence or longer survival with health conditions. The interpretation of the differences would be very different, depending on the underlying processes that prevail. The aim of this article is to decompose prevalence differences in chronic disease at older ages, using comparable longitudinal data from the United States and Europe.
There are indications that the sources of the gap in disease prevalence are diverse and that analysis of processes will provide insights. Cause-specific mortality rates show that differences in heart disease mortality account for about one-half of the difference between the United States and other low-mortality countries, and differences in mortality resulting from diabetes also account for some of the differences. Yet, the United States fares better in cancer mortality (Glei et al. 2010). However, the overall differences in mortality rates by cause do not provide information on how the relative likelihood of dying differs across countries among those with specific conditions because the rates are relative to the total population; thus, overall mortality differences by country do not give a complete picture of the dynamics involved.
Another important shortcoming of using mortality and prevalence data to understand health processes is the effect of heterogeneity or selection. For example, incidence rates could be invariant with age but differ across individuals. Omitting heterogeneity may bias inferences on the age pattern of incidence and mortality rates because of the potential dynamic selection of the fittest or less risk-prone groups over time (Manton et al. 1986). If the selection process differs across countries, incorrect inferences could be drawn from differences in average incidence and mortality rates.
Evidence on differences in incidence is scarce, in part because of the lack of comparable longitudinal data across countries. One exception is Banks et al. (2010), who compared the United States and England and found generally higher disease incidence rates after age 50 for Americans; they also found higher survival conditional on disease in the United States past age 65. However, it is unclear whether these patterns hold in other European countries where even larger prevalence differences with the United States are observed. Furthermore, the comparisons of incidence in Banks et al. (2010) focused on overall differences of diseases across age groups. In light of potential differences in conditional incidence and mortality rates by diseases, we investigate whether overall differences between Europeans and Americans are still observed when we control for sociodemographic and health behavior characteristics.
This article uses longitudinal data for populations aged 50 to 79 for the United States and five European countries to examine age-specific differences in disease incidence and disease-specific mortality from 2004 to 2006. We focus on the most common and frequent health conditions, which are major causes of mortality at older ages: heart disease, stroke, lung disease, diabetes, hypertension, and cancer. We investigate incidence of these conditions using survival models, which include indicators of sociodemographic factors and health behaviors to determine how these factors relate to disease onset and how they explain differences between Europe and the United States. We then investigate survival conditional on the presence of these conditions.
Data and Methods
This analysis uses two longitudinal data sets designed to be comparable: the Health and Retirement Study (HRS) and the Survey of Health, Ageing and Retirement in Europe (SHARE) collected in 2004 and 2006 for older individuals. HRS is an ongoing longitudinal survey of the U.S. population aged 50 and older, begun in 1992 and regularly refreshed at younger ages with new cohorts1. SHARE began collecting data in 2004 on the noninstitutionalized population aged 50 and older, with a target sample size of 2,000 respondents per country for 12 European countries. In our analysis, we use a subset of five European countries: Denmark, France, Italy, the Netherlands, and Spain. We choose these countries because of their relatively high response rate in the SHARE baseline interview and because of the close match between country-specific life table mortality from the Human Mortality Data Base (HMD n.d.) and mortality reported as occurring to survey respondents between Waves 1 and 2 of SHARE2. In our analysis, we will refer to these five countries collectively as “Europe.” We use data on those aged 50–79 because of a similar better match of mortality to the HMD.
For both the United States and Europe, we use 2004 as the base year and examine health transitions occurring in the interval between the initial interview and reinterview in 2006. Table 1 shows the national sample sizes for those aged 50–79 in 2004 and indicates the 2006 interview status of the 2004 respondents: interviewed, died, or lost to follow-up. Sample size in 2004 was about 16,000 individuals in the United States and more than 11,000 individuals in Europe. The proportion dying between the two waves is higher for HRS (2.9 %) than for SHARE (2.0 %). Survey response rates in Europe tend to be lower than in the United States: the initial response rate among the selected five SHARE countries at baseline was 62.7 %, and factoring the screening response rate yields overall response rates of 68.7 % for individuals in HRS (SHARE 2005). Analyses are weighted using sample weights provided in both surveys.
Table 1. Sample sizes (2004) and respondent status (2006) in five European countries (SHARE) and the United States (HRS): Individuals aged 50–79.
| Country | N (2004) | % Responded (2006) | % Died | % Lost |
|---|---|---|---|---|
| Denmark | 1,428 | 74.8 | 2.4 | 22.8 |
| France | 2,697 | 63.2 | 1.9 | 34.9 |
| Italy | 2,323 | 68.1 | 1.8 | 30.2 |
| Netherlands | 2,619 | 63.1 | 1.7 | 35.2 |
| Spain | 2,059 | 57.7 | 2.4 | 39.9 |
| Total European Countries | 11,126 | 64.0 | 2.0 | 34.0 |
| United States | 15,890 | 91.5 | 2.9 | 5.6 |
| Total | 27,016 | 79.6 | 2.5 | 17.9 |
Source: SHARE and HRS 2004, 2006.
Measuring Incidence of Disease and Mortality
Disease presence in both SHARE and HRS is based on self-reports of major chronic conditions: heart disease, stroke, lung disease, diabetes, hypertension, and cancer. The surveys were specifically designed to harmonize their instruments so that most questions were worded identically. Information on the presence of six diseases is reported in response to the question, “Has a doctor ever told you that you had any of the following conditions?” Each condition is then asked about separately. Although diagnostic and reporting differences could affect national differences, Banks et al. (2006) showed that differences in self-reports of disease between England and the United States are not driven by differences in reporting. Moreover, they showed that the differences are generally confirmed by biomarkers when self-reports and relevant biomarkers can be compared. Thus, we will assume that reported differences in disease between Europe and the United States represent true differences.3
In SHARE Wave 1, the survey instrument assessed disease presence identically to HRS, but the questions and procedures differed somewhat in subsequent waves. In the 2006 HRS, responses provided at earlier waves about disease presence were preloaded. Those who did not have a condition in the previous wave were asked, “Has a doctor ever told you …” that you have a disease; we use a positive response to construct our measure of incidence. Those who previously said that they had a condition were reminded of this at the 2006 interview. Some respondents denied that they had reported the presence of disease. Respondents who denied having a condition in the past were then asked about their present state, and they were retroactively recoded as not previously having the disease at the earlier wave. The 2006 wave of SHARE did not allow respondents to dispute a condition reported in 2004. This difference in survey procedure could result in different U.S. incidence rates relative to European ones.
Further, respondents in SHARE who did not have a condition in 2004 were asked in 2006, “Has a doctor ever told you that you had/do you currently have any of these conditions? With this, we mean that a doctor has told you that you have this condition and that you are either currently being treated for or bothered by this condition.” Although we have no method of assessing the impact of the differences in questionnaires on incidence, we believe that overall, denials in HRS and the additional requirement of being bothered/treated in SHARE will have opposite effects on the gap between SHARE and HRS incidence rates.
Mortality is confirmed in HRS and SHARE through interviews that are conducted with family members or other close associates of deceased respondents, referred to as “exit interviews.” In addition, in HRS, deaths are identified in the National Death Index and Social Security records for those who allow record searches. We use a dichotomous variable indicating whether the respondent died between the 2004 and 2006 waves for both surveys (1 = deceased, 0 = alive).
Evaluation of Mortality Reports and Effect of Differential Response Rates
We compared probabilities of dying estimated from survey data with the corresponding values from the HMD life table for 2004. The probabilities of dying between age x and age x + 2 computed from the HMD life tables were compared with the corresponding values based on the surveys with a two-year average interval between interviews. Results indicate that survey estimates are relatively close to the life table estimates between ages 50 and 80 for the United States, the Netherlands, Spain, Italy, France, and Denmark. It is also true that the life expectancy for these five European countries represents fairly closely the entire set of SHARE countries in terms of life expectancy. Mortality above age 80 estimated from the survey is somewhat lower than that in the life tables, likely because of the exclusion of nursing home residents. For this reason, we limit our analysis to the age range 50–79.
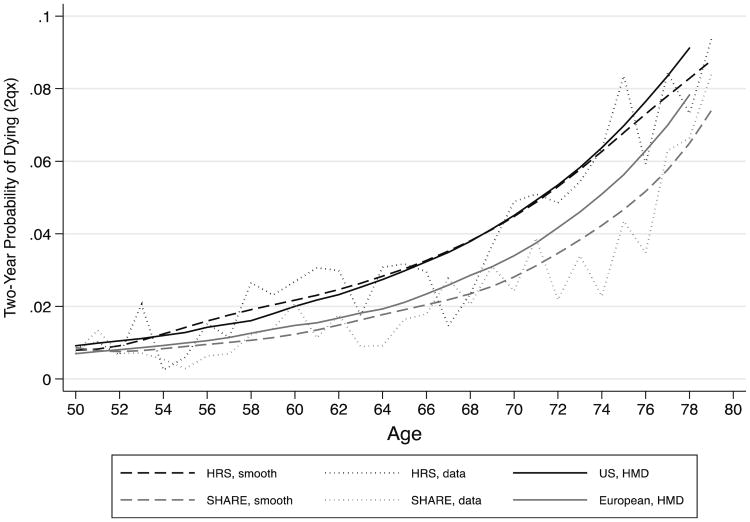
Figure 1 shows two-year probabilities of dying (2qx) in the United States and in the pooled five European countries (weighted by population size) from both the surveys and the HMD. The smoothed survey estimates for the United States are generally close to the life table values, although they are slightly higher between ages 55 and 65 and somewhat lower above age 75, whereas the European survey estimates are lower than the life table values, especially at the oldest ages. There are two reasons why the European rates based on the survey might differ more from rates based on vital statistics than the rates from the United States. First, SHARE is a new survey, but the HRS is a long-running longitudinal survey. Respondents who die do so an average of one year after the initial survey date, so those who are already very ill may not enter the initial wave of a survey; however, if the survey is long-running, they may be more likely to be included.4 In Online Resource 1, we show that differences in the mortality rates between the surveys and the HMD have only small effects on the expected number of years of life (partial life expectancies) between ages of 50 and 80.
Fig. 1.
Comparison of survey probabilities of dying (2qx) and HMD life table probabilities of dying (2qx) 2004 for U.S. and five European countries. Curve of survey probabilities of dying are smoothed using a lowess filter and are weighted using sampling weights. SHARE includes Denmark, France, Italy, the Netherlands, and Spain
Second, differential attrition could affect mortality and incidence rates. Nonresponse at follow-up is higher in SHARE than in HRS (about 5.6 % lost to follow-up in HRS vs. 34.0 % in the five SHARE countries; see Table 1). To determine appropriate adjustments for nonresponse, we analyzed the determinants of nonresponse in SHARE and developed inverse probability weights for nonresponse in Wave 2, which we compared with the weights provided with the survey data. We conclude that differential nonresponse is accounted for in the SHARE weights and is unlikely to bias the inferences we make on the two populations (for further details, see Online Resource 1). We use inverse probability weights when looking at transitions among survivors across waves. Hence, we use a “missing at random” assumption: that is, the non-follow-up is assumed random, conditional on a set of observables (Little and Rubin 1987).
Other Variables
Because selection may affect the age patterns of survival and incidence we observe, we control for sociodemographic characteristics and health behaviors in most of the analysis. Sociodemographic controls for age, sex, and years of education are included. We also include measures of overweight and smoking in our regression analyses. Smoking is self-reported as former smoker, current smoker, or never smoked. Body mass index (BMI) is categorized using self-reports of height and weight provided in both surveys and classified into two groups: class II obesity and higher (BMI ≥ 35), and those with lower BMI. The average number of years of education is higher for Americans than Europeans (12.9 years vs. 8.1 years). However, Europeans experience better health behaviors: 26.0 % of the Europeans but 58.3 % of Americans are past smokers; and 19.0 % of Europeans versus 25.4 % of Americans are current smokers. The class II obesity level for Americans is double that of Europeans (11.9 % vs. 5.2 %).
Methods
A clear relationship exists between prevalence of disease and the incidence and duration of diseases. Prevalence is the proportion of individuals who have a health condition at a specified point in time (point prevalence). Incidence is the rate of occurrence of new disease within a specified period. Prevalence depends on not only prior incidence but also on survival or the duration of disease.5 If the incidence of a disease is low but the duration of disease—that is, until death—is long, the prevalence will be high relative to the incidence. Conversely, if the incidence of a disease is high and the duration of the disease is short, the prevalence will be low relative to the incidence. A change in the duration of a disease resulting from the development of a new treatment, which prevents death but does not cure, will lead to an increase in prevalence.
We first examine descriptive data on prevalence and incidence of diseases and mortality by age groups in Europe and the United States. Then we examine two-year disease incidence rates among those who did not have each condition at the initial interview. We estimate incidence from hazards models with age as the underlying time metric for each of the six diseases, using a set of pooled samples for the United States and Europe for those without specified diseases at the initial interview. Model 1 includes an indication of being in the European sample and controls for sex; health behaviors and years of education are then added to the model (Model 2). We use the Weibull distribution for the age-based hazards models in these analyses to address the nonmonotonic pattern of onset with age observed in the descriptive data.6 This approach allows us to determine whether any differences between European and U.S. incidence rates differ and whether these rates are eliminated or reduced when health behaviors and education are controlled.
To study differences in mortality rates by disease across countries, we estimate survival models with age as the time metric, pooling U.S. and European data for those with specified diseases at the initial interview. We present three models: Model 1 includes an indicator of being in the European sample and controls for sex, health conditions, and interactions between the European indicator and health conditions. The interaction terms capture the difference between Europe and the United States in mortality rates conditional on having a disease, and the European sample dummy variable captures differences in baseline mortality; Model 2 adds controls for years of education; and Model 3 adds controls for health behaviors (former/current smoker and BMI ≥ 35). In estimating the survival models, we take into account right-censoring as well as differential exposure time between interviews. Although average time between interviews does not vary much between the United States and Europe, considerable heterogeneity exists among participants. We use weights in estimating the models. Online Resource 1 gives additional details on the estimation of the models.
Results
Prevalence and Incidence
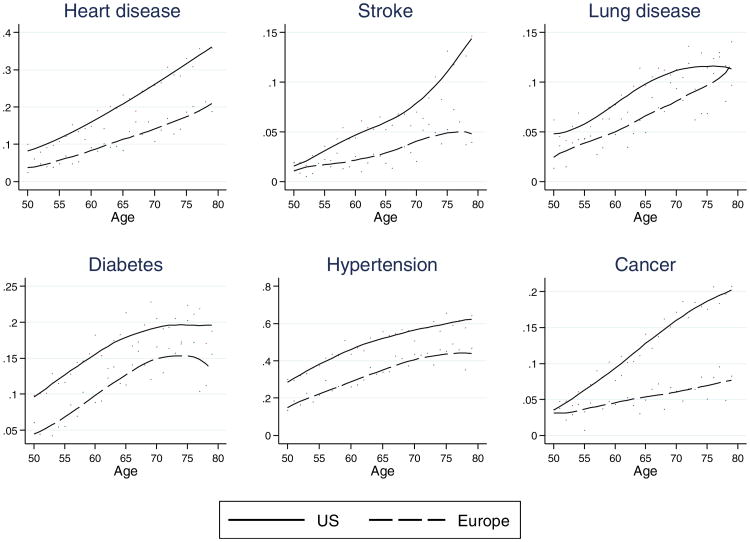
In Fig. 2, we document differences in the prevalence of diseases by age using the cross-sectional data from Wave 1. As expected, the prevalence of each disease is significantly higher in the United States than in Europe. Close to one-fifth (18.3 %) of Americans in this age range have heart disease, compared with one-tenth (10.4 %) of the Europeans. Prevalence of most conditions is 40 % to 100 % higher in the United States than in Europe. For some conditions (such as heart disease, stroke and cancer), the gap widens with age. For other conditions (lung disease, diabetes, and hypertension), the gap remains relatively constant or declines. However, this cross-sectional view of the data does not allow us to understand the processes that drive these differences or how they evolve with age.
Fig. 2.
Prevalence of disease in Europe and the United States by age in 2004. Prevalence by age and region at Wave 1 are computed using sample weights. A lowess filter is used to smooth the prevalence rates obtained
In Table 2, we show the prevalence (or percentage) of disease at the initial interview in the age range 50–54 as well as the incidence or the percentage who acquire the health condition by 2006 among those who did not have the condition in 2004 in five age groups from 50 to 79 for individual diseases in the United States and Europe. Focusing first on the prevalence at the initial age group in our sample, we observe that levels are consistently higher for the United States and that the differences are large for a number of conditions at age 50. The differences are smaller for stroke, lung disease, and cancer. This clarifies that part of the difference in disease prevalence at older ages begins well before age 50.
Table 2. Prevalence and incidence (percentages) by age in the United States (HRS) and five European countries (SHARE): Individuals aged 50–79.
| Heart Disease | Stroke | Lung Disease | Diabetes | Hypertension | Cancer | |
|---|---|---|---|---|---|---|
| A. United States: Prevalence at Ages 50–54 and Incidence Rates by Age | ||||||
| Prevalence 50–54 | 9.02 | 2.21 | 4.98 | 11.04 | 32.71 | 4.85 |
| N, 50–54 | 2,473 | 2,473 | 2,473 | 2,472 | 2,469 | 2,472 |
| Incidence | ||||||
| Ages 50–54 | 2.18 | 0.65 | 1.04 | 3.07 | 8.11 | 1.24 |
| Ages 55–59 | 3.30 | 0.93 | 1.59 | 3.99 | 9.65 | 1.99 |
| Ages 60–64 | 3.89 | 0.80 | 1.73 | 4.27 | 11.71 | 2.63 |
| Ages 65–69 | 5.03 | 1.22 | 1.57 | 3.60 | 11.49 | 3.20 |
| Ages 70–74 | 5.33 | 2.56 | 2.06 | 3.19 | 12.28 | 3.37 |
| Ages 75–79 | 7.24 | 3.16 | 2.71 | 3.08 | 11.44 | 4.20 |
| Total Incidence (Ages 50–79) HRS | 3.91 | 1.28 | 1.65 | 3.59 | 10.18 | 2.45 |
| N, Ages 50–79 | 11,559 | 13,604 | 13,263 | 11,939 | 7,053 | 12,801 |
| B. Europe: Prevalence at Ages 50–54 and Incidence by Age | ||||||
| Prevalence, Ages 50–54 | 4.31 | 1.19 | 3.31 | 5.11 | 18.00 | 3.34 |
| N, Ages 50–54 | 2,187 | 2,187 | 2,187 | 2,187 | 2,187 | 2,187 |
| Incidence | ||||||
| Ages 50–54 | 1.81 | 0.61 | 1.55 | 3.46 | 9.61 | 0.64 |
| Ages 55–59 | 2.09 | 0.81 | 2.51 | 3.33 | 13.32 | 1.54 |
| Ages 60–64 | 5.06 | 0.93 | 3.90 | 4.16 | 17.00 | 1.48 |
| Ages 65–69 | 5.80 | 1.66 | 3.46 | 4.94 | 17.67 | 1.84 |
| Ages 70–74 | 9.00 | 3.35 | 6.40 | 4.83 | 24.12 | 1.85 |
| Ages 75–79 | 6.43 | 4.36 | 5.81 | 5.48 | 19.42 | 2.64 |
| Total Incidence (Ages 50–79) SHARE | 4.47 | 1.65 | 3.57 | 4.16 | 15.51 | 1.55 |
| N, Ages 50–79 | 6,417 | 6,929 | 6,702 | 6,456 | 4,911 | 6,783 |
Notes: Europe includes Denmark, France, Italy, the Netherlands, and Spain. The significance of differences is shown in Fig. 3.
Source: HRS 2004–2006. HRS weights are used in this table. SHARE, 2004–2006. SHARE weights are used in this table.
Surprisingly, aggregate incidence levels for most diseases are higher in Europe. For example, average incidence of lung disease in Europe after age 50 is 3.57 %, compared with 1.65 % in the United States. Stratifying by age shows that incidence rates are significantly higher in Europe after age 60 for many conditions. For three conditions—lung disease, diabetes, and hypertension (and to some extent stroke) —the differences increase with age, suggesting an initial delay in the age at onset of these conditions in Europe but then acceleration in onset at later ages.
However, part of the explanation for these observed differences in age patterns of disease may be underlying differences in the characteristics of the population at risk. As diagnosis of cases increases, the undiagnosed population is composed of more people with less inherent risk for the condition because those most susceptible have had earlier incidence. Because the U.S. prevalence is higher at the earlier ages, the undiagnosed population in the United States at older ages may be less at risk than the undiagnosed population in Europe, perhaps leading to a negative relationship between prevalence and incidence.
Because aggregate age patterns can be misleading owing to selection, we analyze incidence models, which control for sociodemographic and health behavior differences (Table 3). When we adjust for demographic status, the onset of lung disease and hypertension is higher in Europe than in the United States; the onset of diabetes and cancer is lower in Europe than in the United States; and no significant differences are seen for the onset of heart disease and stroke between Europe and the United States (Model 1). For cancer, we have reason to believe that high cancer incidence in the United States is related to more robust cancer screening in the United States, particularly at older ages (Crimmins et al. 2010a). Age and the onset of all conditions are positively related.7 Males are also more likely to experience the onset of most conditions.
Table 3. Disease incidence model estimates: Individuals aged 50–79.
| Heart Disease | Stroke | Lung Disease | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Sociodemographic | ||||||
| Europe | −0.08 | −0.24* | −0.03 | −0.17 | 0.54** | 0.22* |
| Age | 1.58** | 1.65** | 1.74** | 1.68** | 1.38** | 1.49** |
| Male | 0.23** | 0.18* | 0.36** | 0.27* | 0.04 | −0.06 |
| Years of education | −0.03* | −0.03† | −0.06** | |||
| Health Behaviors | ||||||
| Former smoker | −0.04 | −0.08 | 0.43** | |||
| Current smoker | 0.21† | 0.46* | 1.19** | |||
| BMI ≥ 35 | 0.47** | 0.29 | 0.79** | |||
| Constant | −5.91** | −5.77** | −7.57** | −7.03** | −6.05** | −5.99** |
| Diabetes | Hypertension | Cancer | ||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Sociodemographic | ||||||
| Europe | −0.15† | −0.27* | 0.14** | −0.02 | −0.50** | −0.36** |
| Age | 1.16** | 1.17** | 1.30** | 1.26** | 1.52** | 1.60** |
| Male | 0.13† | 0.18* | −0.02 | 0.10† | 0.37** | 0.19† |
| Years of education | −0.06** | −0.05** | 0.02 | |||
| Health Behaviors | ||||||
| Former smoker | −0.03 | −0.11 | 0.23 | |||
| Current smoker | −0.04 | −0.12 | 0.43* | |||
| BMI ≥ 35 | 1.08** | 0.57** | 0.30 | |||
| Constant | −4.56** | −4.07** | −3.82** | −3.12** | −6.28** | −6.89** |
Notes: Weibull model estimates obtained by maximum likelihood. Age is the shape parameter (α, see Online Resource 1). Model 1 includes controls for age and sex. Model 2 adds controls for years of education and health behaviors. Europe includes the Netherlands, Italy, France, Denmark, and Spain. Spain is the reference country in Europe. Sample weights are used.
Source: SHARE and HRS 2004, 2006.
p<.10;
p < .05;
p < .01
When years of education and health behaviors are added to the model (Model 2), Europeans have significantly higher onset of lung disease only, and have lower onset of heart disease, diabetes, and cancer; no significant differences exist between Europe and the United States for the onset of stroke and hypertension. This may indicate that the higher incidence of hypertension among Europeans without these controls is partly linked to the educational and health-behavioral compositions of the populations. Significantly higher disease onset for males persists for heart disease, stroke, diabetes, and cancer, and appears for hypertension. Having more years of education is negatively related to the onset of all health conditions except cancer.
Former and current smokers have a significantly higher likelihood of lung disease onset. Being a current smoker is related to a higher likelihood of developing heart disease, stroke, and cancer. The effect of obesity on the risk of onset for heart disease, lung disease, diabetes, and hypertension is strong. Overall, models show that observed sociodemographic differences explain some of the differences in incidence and that the remaining unexplained differences tend to favor Europe relative to the United States.
Mortality
We now focus on mortality among those with diseases. Indeed, if individuals with a condition survive longer in one country compared with another, prevalence may be higher without higher incidence of disease. We report the proportion dying by age among those with each of the six diseases at the initial interview in Table 4: heart disease, stroke, lung disease, diabetes, hypertension, and cancer. We examine the significance of the difference in mortality by age between Europe and the United States. We then compute the U.S./European mortality ratio for those with a condition. For all diseases, we find higher mortality risks in the United States than Europe. For stroke, lung disease, diabetes, and hypertension, the ratio (U.S./Europe) is highest at age 50–59, indicating that the relative mortality differences are greater at younger old ages; however, the relative difference for heart disease is greatest at oldest ages.
Table 4. Probability of dying (qx) in two years among those with a disease.
| United States | Europe | Ratio (United States/Europe) | |
|---|---|---|---|
| Heart Disease | |||
| 50–59 | 0.036 | 0.025 | 1.44 |
| 60–69 | 0.054* | 0.037 | 1.46 |
| 70–79 | 0.102** | 0.054 | 1.89 |
| Total | 0.067** | 0.041 | 1.63 |
| N | 3,273 | 1,128 | |
| Stroke | |||
| 50–59 | 0.047 | 0.012 | 3.92 |
| 60–69 | 0.087* | 0.053 | 1.64 |
| 70–79 | 0.122 | 0.121 | 1.01 |
| Total | 0.092* | 0.073 | 1.26 |
| N | 1,026 | 353 | |
| Lung Disease | |||
| 50–59 | 0.046 | 0.018 | 2.56 |
| 60–69 | 0.079* | 0.048 | 1.65 |
| 70–79 | 0.146† | 0.079 | 1.85 |
| Total | 0.090** | 0.052 | 1.73 |
| N | 1,405 | 668 | |
| Diabetes | |||
| 50–59 | 0.038† | 0.008 | 4.75 |
| 60–69 | 0.061* | 0.042 | 1.45 |
| 70–79 | 0.100 | 0.079 | 1.27 |
| Total | 0.064** | 0.047 | 1.36 |
| N | 2,852 | 1,124 | |
| Hypertension | |||
| 50–59 | 0.020 | 0.005 | 4.00 |
| 60–69 | 0.033** | 0.027 | 1.22 |
| 70–79 | 0.073** | 0.045 | 1.62 |
| Total | 0.039** | 0.027 | 1.44 |
| N | 8,158 | 3,334 | |
| Cancer | |||
| 50–59 | 0.031 | 0.047 | 0.66 |
| 60–69 | 0.079† | 0.033 | 2.39 |
| 70–79 | 0.110 | 0.104 | 1.06 |
| Total | 0.078 | 0.018 | 4.33 |
| N | 1,905 | 588 |
Notes: The first two columns for both regions report the two-year probability of dying for those diagnosed with a condition. The ratio of the two (United States/Europe) gives the relative mortality risk of those with a condition.
Source: HRS and SHARE, age 50–79, sample weights used.
Test of significance with condition (United States/Europe):
p < .10;
p < .05;
p < .01
The mortality differences may be at least partly explained by the fact that compared with Europeans, Americans are more likely to have multiple chronic diseases (Crimmins et al. 2010b), are more likely to be obese, and have a different smoking history. To account for these differences, we estimate survival models with a Gompertz specification, using a pooled sample of Americans and Europeans. We allow the effect of each condition on mortality to differ between the United States and Europe by adding an interaction between prevalence of a disease at baseline and the dummy variable for being in the European sample. We also include a dummy variable for being in the European sample, which captures baseline differences in mortality among those without a particular disease. After the initial model, which includes age and sex, sequential models control for years of education, current and past smoking experience, and obesity (Table 5).
Table 5. Adjusted coefficients from the hazard model, by disease.
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| United States | Difference in the Effect Between Europe and the United States | United States | Difference in the Effect Between Europe and the United States | United States | Difference in the Effect Between Europe and the United States | |
| Baseline | — | 0.13 | — | 0.02 | — | −0.17 |
| Heart Disease | 0.52** | −0.20 | 0.53** | −0.24 | 0.45** | −0.14 |
| Stroke | 0.63** | 0.32 | 0.61** | 0.31 | 0.50** | −0.38 |
| Lung Disease | 0.91** | −0.15 | 0.89** | −0.17 | 0.55** | 0.15 |
| Diabetes | 0.57** | −0.02 | 0.54** | −0.02 | 0.54** | 0.00 |
| Hypertension | 0.20† | −0.17 | 0.20** | −0.18 | 0.14 | −0.10 |
| Cancer | 0.91** | 0.29 | 0.93** | 0.32 | 0.95** | 0.27 |
Notes: Gompertz models fitted by maximum likelihood. Model 1 includes controls for age, sex, health conditions, and interactions between health conditions and the European versus American indicator. Model 2 adds controls for years of education. Model 3 adds controls for health behaviors. For each model, the first column reports the hazard coefficient for the United States, and the second column reports the coefficient on the interaction between the European indicator and each disease.
Source: HRS and SHARE, ages 50–79, sample weights used.
p<.10;
p<.01
The hazard of mortality is associated with all conditions when we control for age and sex (Table 5, Model 1). For four diseases—heart disease, lung disease, diabetes, and hypertension—mortality is lower in Europe than in the United States with controls for age and sex, but differences are not statistically different. For stroke and cancer, the hazard of mortality is higher in Europe than in the United States, but the difference is not statistically significant. The size of the mortality difference does not change much with controls for education (Table 5, Model 2). When health behaviors are introduced in Model 3, the size of the mortality coefficients of our health conditions remains the same except for stroke, for which Europeans now have lower but not statistically significantly lower mortality; lung disease, for which Europeans now have a higher hazard ratio of mortality; and diabetes, for which there is now no effect of place of residence. Overall, we do not find evidence for survival differences between the United States and Europe conditional on disease prevalence and on sociodemographic differences that would help explain the pattern of prevalence observed in the cross-section. Interestingly, baseline differences in the hazard of mortality between the United States and Europe are positive in Model 1 and become negative in Model 3, but these associations are not statistically significant. Hence, we find no residual differences in mortality between the United States and Europe after we account for diseases and sociodemographic differences.
Simulated Prevalence
Prevalence, survival, and incidence are tightly linked. Changes in prevalence are attributable to survival among those who had the condition and incidence among those who survived and did not have the condition at baseline. We want to use our estimated models of incidence and survival to perform counterfactual simulations to find the relative contributions of survival and incidence to prevalence, so we first demonstrate that our models perform well in predicting actual prevalence. Beginning with age-specific prevalence rates in the follow-up sample in 2004, we use predicted incidence and survival for each condition based on the models in Tables 3 and 5 to estimate 2006 prevalence for the follow-up sample. We find that actual 2006 prevalence of health conditions and predicted 2006 prevalence line up well. For further details, see Online Resource 1.
Counterfactual Simulations
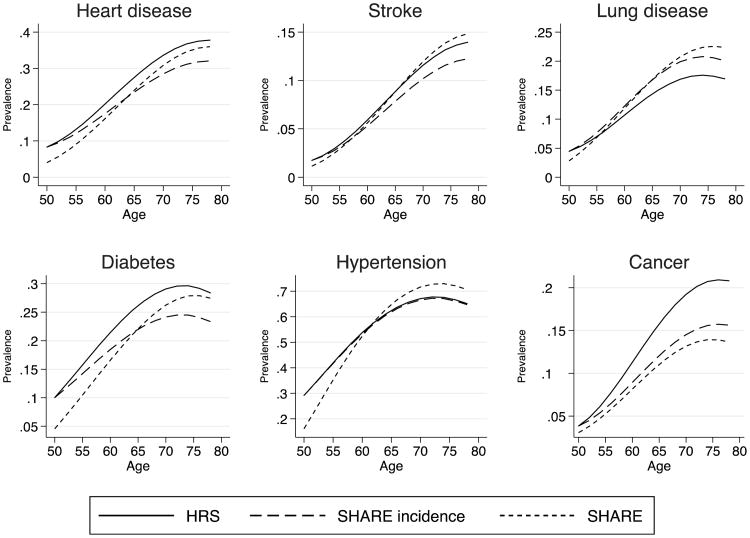
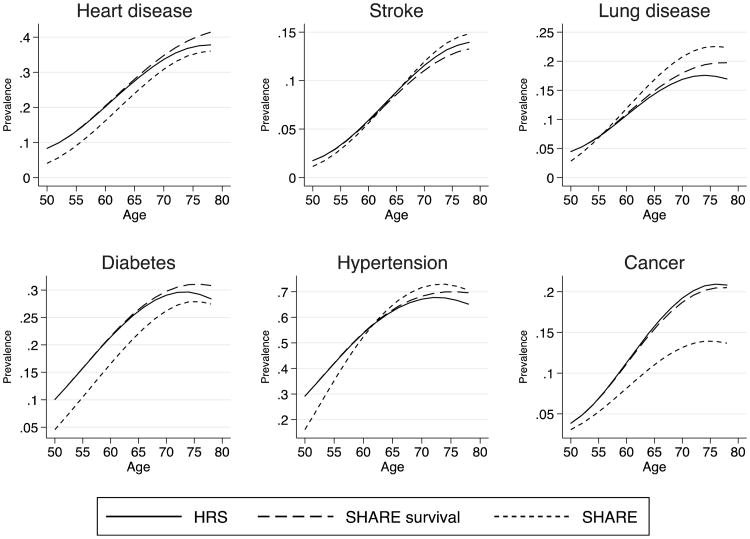
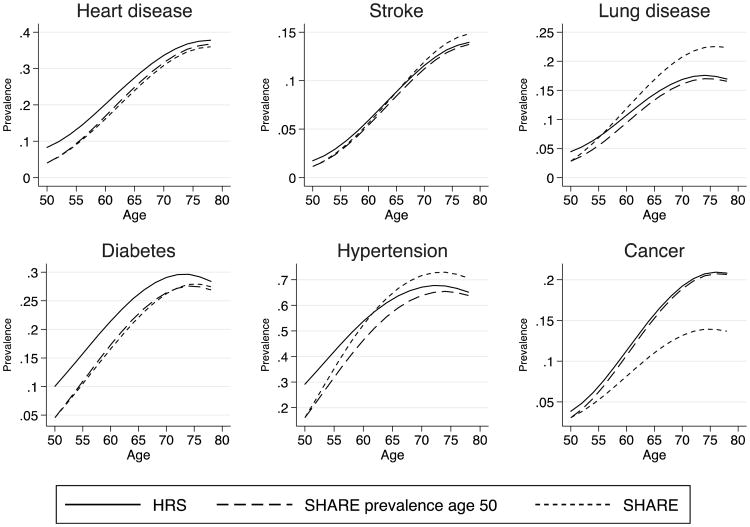
To better understand whether differences in incidence and survival can explain differences in prevalence by age observed between Europe and the United States, we constructed counterfactual scenarios. Our baseline simulation begins with American prevalence at age 50, and then simulates disease prevalence to age 80 based on American age-specific incidence and mortality models (Tables 3 and 5). For the counterfactual simulations, we sequentially replace American levels of incidence, survival, and prevalence with European levels. Figure 3 is based on European incidence rates (and American mortality and initial prevalence), Fig. 4 is based on European mortality (and American incidence and initial prevalence), and Fig. 5 is based on European prevalence at age 50 (and American incidence and mortality). Because of some differences in mortality rates between survey and HMD life tables, we scale up mortality rates from HRS and SHARE to match the HMD life tables for each set of countries, and we use inverse probability weights to correct for non-follow-up.
Fig. 3.
Counterfactual simulation of prevalence by age estimated by varying incidence rates. We project prevalence by age starting with initial prevalence from each country and then apply incidence and survival rates keeping characteristics constant. The HRS scenario uses initial prevalence, incidence, and survival rates from the HRS, with average American characteristics. The SHARE-incidence scenario uses U.S. initial prevalence and survival rates but applies SHARE incidence rates evaluated at average characteristics from the United States. Finally, the SHARE scenario is analogous to the HRS scenario for Europeans. Incidence rates and survival rates are weighted. Aggregate baseline survival rates are also weighted to match period life tables
Fig. 4.
Counterfactual simulation of prevalence by age estimated by varying survival rates. We project prevalence by age starting with initial prevalence from each country and then apply incidence and survival rates, keeping characteristics constant. The HRS scenario uses initial prevalence, incidence, and survival rates from the HRS, with average American characteristics. The SHARE-survival scenario uses U.S. initial prevalence and incidence rates but applies SHARE survival rates evaluated at average characteristics from the United States. Finally, the SHARE scenario is analogous to the HRS scenario for Europeans. Incidence rates and survival rates are weighted. Aggregate survival rates are also weighted to match period life tables
Fig. 5.
Counterfactual simulation of prevalence by age estimated by varying initial prevalence rates. We project prevalence by age starting with initial prevalence from each country and then apply incidence and survival rates keeping characteristics constant. The HRS scenario uses initial prevalence, incidence, and survival rates from the HRS, with average American characteristics. The SHARE-prevalence scenario uses SHARE initial prevalence but applies HRS incidence and survival rates evaluated at average characteristics from the United States. Finally, the SHARE scenario is analogous to the HRS scenario for Europeans. Incidence rates and survival rates are weighted. Aggregate baseline survival rates are also weighted to match period life tables
Figure 3 projects prevalence by age for Americans using initial prevalence and survival curves from the United States (average observable characteristics) but applying counterfactual European incidence rates evaluated at U.S. observed characteristics (“SHARE incidence”). For comparison, we also plot American and European prevalence by age predicted using American and European characteristics, respectively. It is important to note that both U.S. and European predicted prevalence profiles are now different from those in Fig. 2. The key difference is that we now use prevalence at age 50 and incidence at each successive age to reconstruct the age path of prevalence rather than use the cross-sectional profile of prevalence. The resulting profile suggests a different picture from that observed with the cross-section. First, the gap in prevalence with age is not as wide, suggesting that the underlying process is nonstationary (perhaps because of cohort effects). Second, the results suggest that U.S. prevalence of heart disease and stroke are reduced for all ages when European incidence rates are applied, resulting in lower prevalence of heart disease (at older ages) and stroke in the counterfactual U.S. estimate than in Europe. Smaller differences in prevalence between the United States and Europe are seen for cancer and lung diseases. For all conditions except cancer, Europe is actually predicted to catch up with the United States as the population ages—an effect that is most dramatic for hypertension and lung disease. Therefore, the gap that we need to explain is vastly different when we adopt a longitudinal view as opposed to a cross-sectional view.
We perform a similar exercise for survival. Figure 4 shows prevalence by age for Europeans and for Americans, as well as results for a third scenario using initial American prevalence and incidence rates but applying European survival rates evaluated with U.S. characteristics. Our results indicate that the use of European survival with the U.S. characteristics reduces the prevalence of stroke at older ages; increases the prevalence of heart disease, lung disease, and diabetes at older ages; and reveals no differences in the prevalence of cancer. These simulations confirm that survival does not play a significant role in explaining difference in prevalence at older ages between Europe and the United States.
Finally, it is interesting to ask what would happen to U.S. prevalence at older ages if Americans started the process with the European prevalence at age 50. In Fig. 5, we report results from this scenario. For heart disease, starting Americans with European prevalence and then having them experience American incidence and survival would almost entirely explain the gap in predicted prevalence at ages above 50. A similar story can be told for diabetes. For stroke, lung disease, and cancer, initial prevalence does not play a large role in affecting differences. For hypertension, the initial prevalence explains differences at younger ages.
Discussion
Our results reaffirm that disease prevalence is significantly higher in the United States than in Europe and that these differences begin well before age 50. However, longitudinal analysis of the process affecting this prevalence adds to our understanding of how they arise. Selection plays a role. Indeed, unconditional incidence rates for the diseases are higher in Europe; however, most of these differences disappear and are reversed after we use models that adjust for differences in sociodemographic characteristics and health behaviors. One motivation for using comparable longitudinal data in the United States and Europe was to examine the contributions to greater prevalence of disease at older ages in the United States: initial conditions (prevalence at age 50), incidence between age 50 and older ages, and survival conditional on having the disease. Our results suggest that a mix of differences in initial prevalence and lower incidence in Europe at older ages may explain that gap. However, we do not find evidence suggesting that there is a survival advantage in the United States for those with a condition, which would increase prevalence relative to Europe. Results for heart disease illustrate our findings well. At ages 50–54, heart disease prevalence is 9 % in the United States and 4 % in Europe—a large gap in initial conditions. Unconditionally, incidence in the United States is lower in the age range 50–79, which should reduce the gap. Again in the aggregate, mortality conditional on having heart disease is higher in the United States, which should also reduce the gap. Yet, prevalence at ages 50-79 in the United States remains about twice as high as prevalence in Europe.
To reconcile these patterns, we first have to compare incidence and survival rates for a population that has the same characteristics. Our models suggest that after we account for sociodemographic differences and differences in health behaviors, incidence is generally lower in Europe relative to the United States. We do not find differences in survival after we account for differences in characteristics. We then show that reconstructing prevalence at older ages in the United States using European initial prevalence at age 50, and subsequent incidence explains the gap in prevalence we observe. The patterns for diabetes and heart disease are similar. Some differences emerge for other conditions. In particular, the widening gap for cancer can be almost entirely explained by differences in incidence after age 50. For lung disease and hypertension, differences in incidence are actually unfavorable to Europeans and thus cannot explain the gap that we observe in the data. For both conditions, differences are mostly explained by differences in initial conditions.
Some limitations in the present analysis could affect our findings. SHARE underestimates population mortality, whereas HRS mortality is very close to life table mortality. If survey mortality rates were higher and closer to the HMD life table mortality rates for the European population, survival rates conditional on having a disease would be higher and closer to those in the United States. Additionally, because SHARE respondents who were neither bothered by nor treated for a condition may have failed to report it, incidence in SHARE might have been even higher than we observed.
Because we do not consider the nursing home population, our results do not reflect the entire population. The nursing home population in both the United States and in Europe would contain persons with relatively high risk of mortality and morbidity. Nursing home residence is generally relatively low in these countries at ages below 80. In the HRS, the nursing home population is 0.8 % of the sample at ages 50–80. Estimates among those in a similar age range in Europe indicate relatively low nursing home usage for two of the five countries we considered: the Netherlands (2 % of those aged 65–80) and Denmark (1 % of persons aged 60–79). Usage appears somewhat higher for France (6 % for those aged 65–80). In the entire population aged 65 and older, nursing home usage is low in Italy (2 %) and higher in Spain (5.8 %) (Huber et al. 2009). We believe that because the percentages in nursing homes are relatively similar in Europe and the United States, differential use of nursing homes is unlikely to influence the differences that we observe. The combination of effects from these data issues might result in a slight increase of the prevalence rates for the European population; however, it is unlikely that this increase would be sufficient to change the direction of our findings. In addition, we use data for a relatively short time interval from both surveys. Using data for more waves over a longer time interval would add stability to some estimates and would verify the direction of our estimates.
Although we recognize these limitations, the analysis presented here is an important first step in understanding the complex health and survival differences between Europe and the United States. Overall, the origin of the higher prevalence of health conditions in the United States results from a higher incidence of diseases and prevalence earlier in the life course. Higher American prevalence does not appear to result from higher survival with disease.
Supplementary Material
Acknowledgments
The authors acknowledge funding from NIA R01 AG040176-02, funding provided from the Spanish Ministry of Science and Innovation (ECO2010-21787-C03-01), and the Beatriu de Pinós Grant 2010–2012. An earlier version of this article was presented at the annual meeting of the Population Association of America, held in San Francisco, May 3–5, 2012. The SHARE data collection has been primarily funded by the European Commission through the fifth framework program (project QLK6-CT-2001-00360 in the thematic program Quality of Life). Further support by the European Commission through the sixth framework program (projects SHARE-I3, RII-CT-2006-062193, as an Integrated Infrastructure Initiative, COMPARE, CIT5-CT-2005-028857, as a project in Priority 7, Citizens and Governance in a Knowledge Based Society, and SHARE-LIFE (CIT4-CT-2006-028812)), and through the seventh framework program (SHARE-PREP (No 211909) and SHARE-LEAP (No 227822)) is gratefully acknowledged. Substantial co-funding for add-ons, such as the intensive training program for SHARE interviewers, came from the U.S. National Institute on Aging (U01 AG09740-13S2, P01 AG005842, P01 AG08291, P30 AG12815, R21 AG025169, Y1-AG-4553-01, IAG BSR06-11, and OGHA 04-064). Support for the HRS data collection was primarily provided by the National Institute on Aging (U01 AG009740).
Footnotes
The original sampling frame for each entering HRS cohort is the noninstitutionalized population; however, respondents who moved to nursing homes are retained in the sample (HRS 2011). We eliminated nursing home residents to keep the sample comparable to the SHARE sample; because almost none of the respondents in SHARE live in nursing homes, we eliminated the small numbers who do.
We further explain the selection of these European countries in Online Resource 1.
A possible exception is cancer, for which more aggressive diagnostic procedures in the United States might explain some of the higher prevalence observed there (Crimmins et al. 2010b).
Adams et al. (2003) showed that the match to life table mortality for older HRS respondents (age 70+ in 1993) improves in later waves after the first follow-up.
Another mechanism is recovery, which affects prevalence of some health conditions. However, we examine lifetime prevalence and thus rule out recovery.
See the methodology section Estimation of Incidence and Mortality Models in Online Resource 1.
An age parameter greater than 2 indicates a rising age profile; a parameter between 1 and 2 indicates a positive relationship with age; and a parameter less than 1 indicates a negative relationship with age.
Electronic supplementary material The online version of this article (doi:10.1007/s13524-015-0372-7) contains supplementary material, which is available to authorized users.
Contributor Information
Aïda Solé-Auró, Email: aida.sole@upf.edu, Pompeu Fabra University, c/Ramon Trias Fargas 25-27, 08005 Barcelona, Spain.
Pierre-Carl Michaud, Département des sciences économiques, Université du Québec à Montréal and RAND, Case postale 8888, Succ. Centre-Ville, Montréal, Québec, Canada H3C 3P8.
Michael Hurd, RAND and NBER, 1776 Main Street, Santa Monica, CA 90407, USA.
Eileen Crimmins, Davis School of Gerontology, University of Southern California, 3715 McClintock Ave, Room 218, Los Angeles, CA 90089-0191, USA.
References
- Adams P, Hurd MD, McFadden D, Merrill A, Ribeiro T. Healthy, wealthy, and wise? Tests for direct causal paths between health and socioeconomic status. Journal of Econometrics. 2003;112:3–56. [Google Scholar]
- Banks J, Marmot M, Oldfield Z, Smith JP. Disease and disadvantage in the United States and in England. Journal of the American Medical Association. 2006;295:2037–2045. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- Banks J, Muriel A, Smith JP. Disease prevalence, disease incidence, and mortality in the United States and in England. Demography. 2010;47:211–231. doi: 10.1353/dem.2010.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Garcia K, Kim JK. Are international differences in health similar to international differences in life-expectancy? In: Crimmins EM, Preston SH, Cohen B, editors. International differences in mortality at older ages: Dimensions and sources. Washington, DC: National Academies Press; 2010a. pp. 68–102. [PubMed] [Google Scholar]
- Crimmins EM, Preston SH, Cohen B. International differences in mortality at older ages: Dimensions and sources. Washington, DC: National Academies Press; 2010b. [PubMed] [Google Scholar]
- Glei DA, Meslé F, Vallin J. Diverging trends in life expectancy at age 50: A look at causes of death. In: Crimmins EM, Preston SH, Cohen B, editors. International differences in mortality at older ages: Dimensions and sources. Washington, DC: National Academies Press; 2010. pp. 17–67. [PubMed] [Google Scholar]
- Health and Retirement Study. Sample sizes and response rates. 2011 Retrieved from http://hrsonline.isr.umich.edu/sitedocs/sampleresponse.pdf.
- Huber M, Rodrigues R, Hoffmann F, Gasior K, Marin B. Vienna, Austria: European Centre for Social Welfare Policy and Research; 2009. Facts and figures on long-term care in Europe and North America. Retrieved from http://www.euro.centre.org/data/1258467686_61318.pdf. [Google Scholar]
- Human Mortality Database. (n.d.). University of California, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany). Retrieved from www.mortality.org or www.humanmortality.de.
- Little RJ, Rubin DB. Statistical analysis with missing data. New York, NY: Wiley; 1987. [Google Scholar]
- Manton KG, Stallard E, Vaupel JW. Alternative models for the heterogeneity of mortality risks among the aged. Journal of the American Statistical Association. 1986;81:635–644. doi: 10.1080/01621459.1986.10478316. [DOI] [PubMed] [Google Scholar]
- SHARE. 2005 Retrieved from http://www.share-project.org/uploads/tx_sharepublications/SHARE_BOOK_METHODOLOGY_Wave1.pdf.
- Thorpe KE, Howard DH, Galactionova K. Differences in disease prevalence as a source of the U.S.-European health care spending gap. Health Affairs. 2007;26(6):w678–w686. doi: 10.1377/lthaff.26.6.w678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.