Abstract
Objective
Adrenocortical carcinoma (ACC) is a rare malignancy with a poor prognosis. Herein, we describe the clinical features and outcomes for a large series of ACC patients.
Design and Methods
Retrospective review of ACC patients seen at The University of Texas MD Anderson Cancer Center from 1998 through 2011.
Results
330 patients with median age at diagnosis of 48.5 years; 12 (3.6%) patients were under 18 years. Hormonally functioning tumors represented 41.8% (n=138) of all cases. Surgical resection for the primary tumor was done in 275 (83.3%) patients [45 at MD Anderson (16.4%)]. For those who had surgical resection, the median local-recurrence-free time was 1.04 years. Factors associated with local recurrence included positive surgical margins (P= 0.007) and advanced disease stage (P=0.026). Median overall survival time for all patients was 3.21 years. Median survival times were 24.1, 6.08, 3.47, and 0.89 years for stages I, II, III, and IV, respectively. In multivariable analysis, older age, functioning tumors, and higher disease stage remained significant prognostic factors associated with poor survival.
Conclusion
ACC prognosis remains poor with the use of currently available treatments. Older age, functioning tumors, and incomplete resections are clinical factors associated with poor survival. Surgical expertise is important to achieve complete resections and to improve outcome.
Keywords: Adrenocortical Carcinoma, Survival, Recurrence, secondary malignancies
INTRODUCTION
Adrenocortical carcinoma (ACC) is a rare and aggressive malignancy with an estimated annual incidence of about 2 cases per million people 1-3. Most of our knowledge about ACC is derived from case series that reflected national databases and tertiary referral centers experience 4-8. Since the first case series of malignant suprarenal tumors was described by Otto Ramsay in 18999, many milestones have been reached in the treatment and management of ACC, including the discovery of cortisone in the 1940s10 and the introduction of mitotane in the 1960s11. In the past few decades, better characterization of the molecular alterations that may occur in these tumors (TP53 mutation found in Li-Fraumeni syndrome12, the APC and CTNNB1 genes in familial adenomatous polyposis coli13, 14, and the CDKN1C15and IGF-216, 17genes associated with Beckwith-Wiedemann syndrome) have led to the proposal of therapeutic interventions and prognostic markers in ACC18-20. Since the publication of the last MDACC case series5, multiple important developments have occurred, including the introduction of two staging systems (by the International Union Against Cancer in 200421 and by the European Network for the Study of Adrenal Tumors [ENSAT] in 200922, 23 and the completion of the first phase III clinical trial in ACC24.
Despite these promising developments, the estimated 5-year overall survival rate for ACC patients remains poor at 15-44%7, 25-27. In this study, we summarize important clinical features of a large cohort of ACC patients to assess outcomes and treatment utilization in ACC over the past decade, including factors affecting prognosis. We also compared those findings with earlier reports from the same institution 4, 5, 25. Finally, we presented our current algorithms that we use to manage patients with ACC.
MATERIALS AND METHODS
Patient records and study design
With the approval of the institutional review board, we retrospectively reviewed the data for ACC patients from the Tumor Registry Database of the Department of Medical Informatics at The University of Texas MD Anderson Cancer Center from 1998 through 2011. We selected 1998 as our previous series was published in 2001 and included patients from 1980-19975. To ensure the accuracy of this retrospective analysis, data were extracted and entered into duplicate datasets by two independent groups of investigators (S.J, S.E, F.D, and M.A.H). Data fields for demographics, clinical outcomes, laboratory tests, imaging, pathologic diagnosis, and treatments were subsequently reviewed, verified, and reconciled into one database using Microsoft SQL Server version 2008 (Microsoft Corporation, Redmond, WA). It is our standard practice to confirm the diagnosis of ACC upon referral to MD Anderson for those patients who had outside surgery or biopsy prior to referral. The reporting of Weiss score and other markers of cell proliferation was not routinely performed and thus were not included in this report. Functional status of the tumor was determined through documentation in the medical records of cortisol, aldosterone, and/or androgen hypersecretion. Overall survival time was calculated from the date of tissue diagnosis to the date of death or to the last follow-up date. Time to local recurrence was calculated from the date of first surgical intervention. Patients who died without local recurrence were censored at the date of death. Patients were censored at the last follow-up if local recurrence or death had not occurred.
Resection margins were determined by reviewing pathology, operative reports, and perioperative records and defined as follows: R0, no evidence of tumor; R1, microscopically positive resection margins; R2, macroscopic residual disease; RX, status of resection margins is unknown. We used the ENSAT staging classification because of its better prognostic accuracy when compared to the International Union Against Cancer staging classification for adrenocortical carcinoma22, 23. The ENSAT staging system defines stage I as ACC measuring ≤5 cm in greatest dimension confined to the adrenal gland, stage II as tumor >5 cm without extra-adrenal invasion, stage III by the presence of positive lymph nodes, infiltration of surrounding tissue, or vascular tumor extension, while stage IV includes only patients with distant metastases22. This staging system has been in use in our institution since 1995 as proposed by Lee et al 28 and was done at the time of operation for the 275 patients who underwent surgery and at the time of diagnosis for the 55 patients who did not have resection of the primary tumor.
We also compared patients’ characteristics and outcomes from this cohort with those reported previously from the same institution. As we did not have the original datasets for patients reported before 1998, we defined disease burden as follows: local (stage I and stage II), regional (stage III), and distant (stage IV) to facilitate comparing the results of different series.
Statistical Analyses
Frequencies and percentages were reported for categorical variables. Fisher’s exact test or Chi-square test was used to evaluate association between two categorical variables. The Wilcoxon rank-sum test was used to evaluate the difference in the distribution of continuous variables between patient groups. The Kaplan-Meier method was used to analyze time-to-event endpoints, including overall survival and time to local recurrence. The log-rank test was used to evaluate differences in these endpoints between patient groups. Multivariable Cox proportional hazards models were fitted to include important demographic and clinical variables. All tests were two-sided. P-values less than 0.05 were considered statistically significant. Statistical software packages SAS 9.1.3 (SAS Institute, Cary, NC) and S-Plus 8.0 (TIBCO Software Inc., Palo Alto, CA) were used for all analyses.
RESULTS
Patients’ characteristics
330 patients with ACC were included in the current study. Most were Caucasian (n= 281, 85%) and female (n= 212, 64.2%). The median age was 48.5 years (range, 0-86 years); 12 patients (3.6%) were under the age of 18 years. ACC was associated with hormonal overproduction in 138 patients (41.8%) as follows: cortisol overproduction 76 patients (55.1%), aldosterone overproduction14 patients (10.1 %), androgens overproduction 21 patients (15.2 %), and overproduction of more than one hormone in 27 patients (19.6 %). Median tumor size was 11 cm (range, 1-27 cm) and median tumor weight was 308 grams (range, 4-3500 grams). There were no cases of bilateral ACC in our cohort. Table 1 summarizes the important clinical features of this cohort and Table 2 describes patients’ characteristics and outcomes in this study compared with those reported previously from the same institution.
Table 1.
Patients’ characteristics
| Characteristic | N=330 (%) |
|---|---|
| Race | |
| Caucasian | 281 (85.2) |
| Other | 49 (14.8) |
|
| |
| Gender | |
| Female | 212 (64.2) |
| Male | 118 (35.8) |
|
| |
| Hormonally functioning tumors | |
| Yes | 138 (41.8) |
| No | 192 (58.2) |
|
| |
| Anatomic site | |
| Left adrenal | 177 (53.8) |
| Right adrenal | 153 (46.2) |
|
| |
| ENSAT stage | |
| I | 11 (3.3) |
| II | 123 (37.3) |
| III | 111 (33.6) |
| IV | 85 (25.8) |
Table 2.
Comparison of patient characteristics and outcomes in this study with those reported previously from our institution
| Before 19805 | 1980-1997 5 |
1998-
2011 * |
|
|---|---|---|---|
| N=78(%) | N=139 (%) | N=330 | |
| Mean age (years) | 41.7 | 45.8 | 46.8 |
|
| |||
| Gender | |||
|
| |||
| Male | 34(44) | 55(39) | 118(36) |
|
| |||
| Female | 44(56) | 84(61) | 212(64) |
|
| |||
| Extent of disease | |||
|
| |||
| Local | 21(27) | 46(33) | 134(40) |
|
| |||
| Regional | 23(29) | 46(33) | 111(34) |
|
| |||
| Distant | 34(44) | 47(34) | 85(26) |
|
| |||
| Functioning tumor | 26(33) | 47(34) | 138(42) |
|
| |||
| Survival rate (%) | |||
| 2 years | NA | 61 | 64 |
| 5 years | NA | 47 | 38 |
This report
Associated malignancies and hereditary syndromes
Of the patients without hereditary cancer syndromes, 38 out of the 330 patients (11.5%), had other malignancies before or after their diagnosis with ACC. Breast and prostate cancers were the most common [7 patients with breast cancer (18.4%) and 7 patients with prostate cancer (18.4%)]. Other malignancies were skin cancer (4 patients, 10.5%); non-small cell lung cancer (4 patients, 10.5%), endometrial carcinoma (3 patients, 7.9%), papillary thyroid cancer (2 patients, 5.2%), renal cell carcinoma (2 patients, 5.2%), melanoma (2 patients, 5.2%), bladder cancer (1 patient, 2.6%), colorectal carcinoma (1 patient, 2.6%), cervical cancer (1 patient, 2.6%), ovarian carcinoma (1 patient, 2.6%), acute lymphoblastic leukemia (1 patient, 2.6%), and malignant tumors of undetermined etiology (2 patients, 5.2%).
Six patients had Li-Fraumeni syndrome (diagnosed clinically or through genetic testing), one had multiple endocrine neoplasia type 1, and another patient had a familial history of ACC. No patients had Beckwith-Wiedemann syndrome or familial adenomatous polyposis.
Treatment Utilization
Resection of the primary tumor was performed in 275 (83.3%) patients (n=45 [16.4%] at MD Anderson and n=230 [83.6%] outside MD Anderson). Open resection was performed in 244 (88.7%) patients and laparoscopic in 31 (11.2%) patients. Negative resection margins (R0) were achieved in 153 patients (55.6%). Positive margins (R1) were found in 47 patients (17.1%) and (R2) resection margins were found in 28 patients (10.2%). Margin status was unknown (RX) in 47 patients (17.1%).
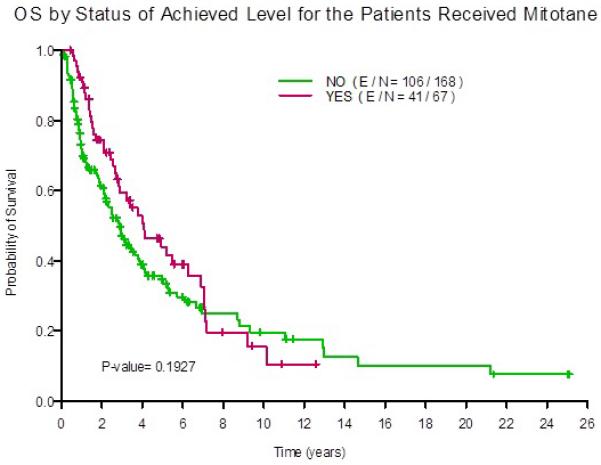
Mitotane was used in 235 (71.2%) of the 330 patients either as monotherapy or in combination with other systemic chemotherapy. Of these, 67 (28.5%) achieved serum mitotane level of 14 mg/l or higher. The median OS for those patients with levels of 14 mg/l and higher was 4.1 years (95% CI 2.8 , 7.0 years ) compared with 2.9 years (95% CI 2.2 , 3.8 years) for those who had lower mitotane levels. At 5-years after diagnosis, overall survival was 44% (95% CI 32%-60%)3 in patients with mitotane levels 14 mg/l or higher compared with 45% (95% CI 27%-44%) in those with lower mitotane levels. Figure 4 illustrates overall survival curves in both groups.
Figure 4. Overall survival (OS) in patients who achieve mitotane levels of 14 mg/l or higher.

(YES, red line) compared with the patients with lower levels (NO, green line).
Radiation therapy was used in 58 patients (18%) mostly as a palliative measure in metastatic disease while adjuvant radiation therapy after primary resection was used only in 16 patients (5%)29. More than 20 different traditional chemotherapy regimens were administered as first line systemic therapy and data were available for 163 patients (Table 3).
Table 3.
First-line chemotherapy regimens for patients with adrenocortical carcinoma
| Etoposide/cisplatin | Etoposide/doxorubicin/cispla tin |
Other regimens | No. of patients | |
|---|---|---|---|---|
| N=46 (28.2%) | N=58 (35.6%) | N=59 (36.1%) | N=163 | |
| Neoadjuvant | 6 | 10 | 8 | 24 |
|
| ||||
| For metastasis | 40 | 48 | 51 | 139 |
Recurrence and survival
In patients who had surgical resection (n=275), the median local-recurrence-free time was 1.0 year (95% confidence interval [CI]: 0.9–1.4). In univariate analysis, factors associated with local recurrence were surgical resection outside MD Anderson (P=0.035), positive surgical margins (P=0.007), and advanced disease stage (P=0.026) as shown in Table 4. On multivariate analysis, the Hazard Ratios for local recurrence (95% CI) including site of surgery (MDACC vs. outside MDACC), stage (1/2 vs. 3/4), and margin (negative vs. positive) were 0.603 (0.402-0.902), 0.749 (0.533-1.053), and 0.746 (0.541-1.030). Other factors were not associated with local recurrence and included gender, hormonal overproduction, and tumor location (right vs. left). Distant metastases were documented in 218 (66%) of the 330 patients during follow-up, with the lungs (66%), liver (57%), and bone (17%) being the most common sites.
Table 4.
Local-recurrence-free of patients with adrenocortical carcinoma
| Characteristic | Local-recurrence-free (95% CI) | P-value | ||
|---|---|---|---|---|
|
| ||||
| Median years | Rate at 3 years | Rate at 5 years | ||
| Surgery | ||||
| At MD Anderson | 1.56 (0.82–5.52) | 0.36 (0.24–0.55) | 0.32 (0.2–0.52) | 0.03 |
| Not at MD Anderson | 1.01 (0.83–1.37) | 0.26 (0.21–0.33) | 0.12 (0.08–0.18) | |
|
| ||||
| Margin Status | ||||
| R0 | 1.29 (1–1.79) | 0.29 (0.23–0.38) | 0.15 (0.1–0.23) | 0.007 |
| R1/R2 | 0.72 (0.42–1.03) | 0.18 (0.1–0.3) | 0.12 (0.06–0.24) | |
|
| ||||
| Disease Stage | ||||
| I/II | 1.37 (1.04–2.06) | 0.33 (0.25–0.42) | 0.18 (0.12–0.26) | 0.02 |
| III/IV | 0.84 (0.69–1.21) | 0.24 (0.17–0.33) | 0.13 (0.08–0.21) | |
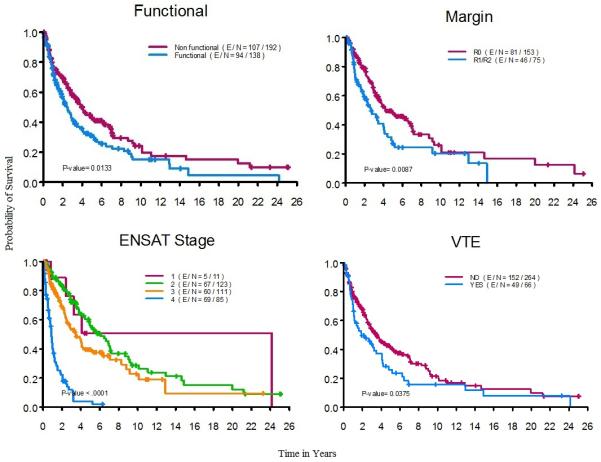
The median overall survival time for all patients (n=330) was 3.2 years (95% CI: 2.7–4.0 years). The median follow-up time for censored observations was 2.7 years (range: 0.1 – 25.1 years). The median overall survival times in relation to disease stage were as follows: Stage I (n=11, 3.3%), 24.1 years (95% CI: 3.2–Not reached); Stage II (n=123, 37.2%), 6.1 years (95% CI: 4.9–7.2); Stage III (n=111, 33.6%), 3.5 years (95% CI: 2.6–6.2); Stage IV (n=85, 25.7%), 0.9 years (95% CI: 0.7–1.1). In univariable analysis, factors associated with poor survival were functioning tumors, R1/R2 surgical resections, stage III or IV at diagnosis, and venous thromboembolism (Figure 1). Other factors such as gender and resection of 5 or more lymph nodes at time of initial surgery were not associated with overall survival. Older age, functioning tumors, and high disease stage at diagnosis remained significant prognostic factors associated with poor survival in a multivariable Cox proportional hazards model (Table 5).
Figure 1. Factors associated with the overall survival of patients with adrenocortical carcinoma.
R0, no evidence of tumor; R1, microscopically positive resection margins; R2, macroscopic residual disease ENSAT, European Network for the Study of Adrenal Tumors; VTE, Venous Thromboembolism
Table 5.
Multivariable Cox proportional hazards model for overall survival of patients with adrenocortical carcinoma
| Prognostic factor | P-value | Hazard ratio (95% CI) |
|---|---|---|
| Age at diagnosis | 0.0089 | 1.013 (1.003–1.024) |
|
Functioning tumors vs.
non-functioning tumors |
0.0196 | 1.400 (1.055–1.857) |
| Stage I/II vs. III/IV | < 0.0001 | 0.438 (0.325–0.590) |
DISCUSSION
The current study investigates a large number of patients with ACC treated at a single institution. Compared with previous reports from our institution obtained during different time periods, patients in the current study tended to be diagnosed at an older age, have more localized tumors and more functioning tumors. Our cohort’s 5-year survival rate of 38% is similar to those reported by others6, 30-32 although we noticed a slightly lower 5-year survival rate than those reported from the same institution in the past. As we did not review the source documents from the older series, direct comparisons could not be done to assess if these differences are truly statistically significant. In addition, the variability in methodology may explain some of the differences in retrospectively collected data. The variation is likely small for certain outcomes (such as age, gender, date of diagnosis, death, and treatments received) but may be significant for other variables (such as performance status and certain operative complications)33-35. In this large cohort, patients were older than patients reported in previous reports from our institution and had higher percentage of localized disease and functioning tumors at the time of diagnosis. It remains unclear if these differences are due to increased detection of tumors at an earlier stage or a change in referral pattern. The presence of functioning tumors could lead to distinct clinical manifestations associated with excessive hormonal production and prompts further imaging studies. The theoretical benefit of earlier detection of functioning tumors is likely negated by the increased morbidity associated hormonally active tumors compared to non functioning tumors.
Surgery remains the treatment of choice for ACC 32, 36, 37, as it is the only therapeutic approach that can be curative for localized disease. In our study, complete resections of primary tumors were associated with both decreased disease recurrence and better overall survival36 , in agreement with findings reported in previous studies7, 26, 31. Median time to recurrence was about 1 year. Factors associated with local recurrence, other than incomplete resection, were resection performed outside of MD Anderson and advanced disease stage at diagnosis. In addition, we have recently described higher recurrence rates (especially peritoneal carcinomatosis) with laparoscopic resection when compared to open resection38. The improved survival in patients operated on at MD Anderson is in line of literature from Europe that suggested improved outcome in ACC patients who received their care in referral centers known for their expertise in ACC39. Adequate pre-operative imaging is crucial to plan initial surgical treatment as well as subsequent adjuvant therapy and should include imaging of the chest, abdomen, and pelvis. In the past few years, there has been an increasing use of markers of cell proliferation (such as Ki67%) as prognostic markers to help with treatment decisions especially after primary tumor resection40-42. In our study, almost 83% of the patients had their initial surgery outside our institution and this referral pattern resulted in lack of consistency in reporting proliferation markers (including Ki67%) and Weiss score. The absence of this information is a shortcoming of our study and similar large cohort studies published in the past decade 5,7, 8, 22. This limitation reinforces the need to have standardized pathological template that would contain key pathological features (e.g. Weiss score, Ki-67%, resection margins) to facilitate a uniform interpretation and generalization of pathological data in ACC.
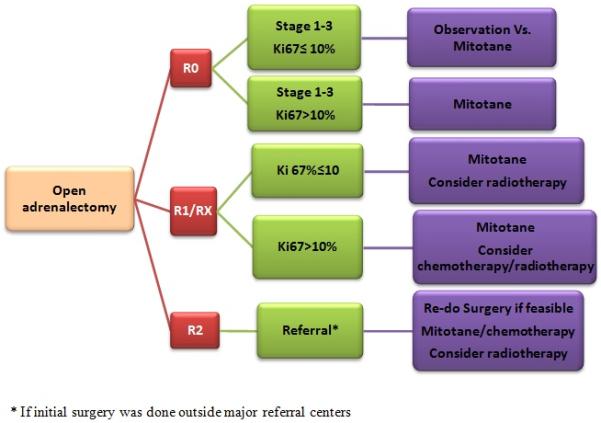
Figure 2 illustrates our current algorithm in approaching patients with localized ACC that combines the use of molecular markers (Ki67%) with other information such as resection margin and ENSAT stage for risk stratification and choosing adjuvant therapy.
Figure 2. A Suggested algorithm for the management of localized adrenocortical carcinoma.
R0, no evidence of tumor; R1, microscopic evidence of tumor; R2, macroscopic residual disease; RX, margins unknown. We recommend mitotane for 2-3 years.
The most common sites of distant metastasis in our study were the lungs and liver, followed by bone, in agreement with previous reports43-45. Although it would have been useful to include the extent of the disease in the survival analysis, the imaging studies utilized (CT, MRI, PET scan and bone scan) at the time of diagnosis varied between patients during the study period. The lack of uniformity in the imaging studies may lead to a difference in the number of metastases identified and therefore could estimate inaccurately the burden of disease at diagnosis.
For patients with metastatic disease or unresectable tumors, the options are limited. Mitotane has been approved for ACC as adjuvant therapy and for treatment of advanced disease 46, 47. Moreover, the first randomized prospective study (ADIUVO trial, http://clinicaltrials.gov/ct2/show/study/NCT00777244) to assess efficacy of adjuvant mitotane in low-risk patients who underwent surgical resection, is open for patient recruitment. In our study, mitotane was used in 71% of the patients, were almost 29% achieved therapeutic levels. We observed that the survival curves of the patients who achieved mitotane level of 14 mg/l or higher, showed and early separation when compared to the patients with lower levels of mitotane. We believe this separation corresponds to the periods when most patients would have been receiving mitotane and suggests beneficial effect of reaching therapeutic level of 14-20 mg/l, as shown repeatedly in the literature. Unfortunately, the retrospective nature of this review limited our ability to capture the mitotane dose, side effect profile, or exact times of therapy.
Currently used systemic therapies often combine mitotane with systemic agents. In the only completed phase III trial of ACC, the combination of etoposide, doxorubicin, and cisplatin with mitotane was superior to the combination of streptozocin with mitotane in terms of progression-free survival (5 months versus 2.1 months); however, the two groups did not differ significantly in overall survival24. In our cohort, cisplatin/etoposide and etoposide/doxorubicin/cisplatin regimens were the most common. In a previous study from our group, neither regimen conferred a significant advantage 48. Therefore, there is an urgent need for more efficacious treatment for this lethal disease. In fact, we have recently published our experience of dual inhibition of the IGF1 receptor and mTOR pathway, were stable disease was achieved for more than 6 months in 42% of the patients 49. Figure 3 illustrates our current management plan for patients with advanced/metastatic ACC.
Figure 3.
An Algorithm for the management of locally advanced/metastatic adrenocortical carcinoma
The use of adjuvant radiotherapy in the context of ACC remains unclear. While some authors have proposed a decreased in local recurrences after adjuvant radiotherapy48, 50; in a recent study published by our group that included the 16 patients who received adjuvant radiotherapy, we were unable to demonstrate improved survival, recurrence rate or time to recurrence.29 Further prospective, multicenter study is needed to better determine the impact of radiotherapy on recurrence and survival.
ACC in children is extremely rare, with an estimated incidence of 19 new cases per year in the United States51. Carriers of TP53 mutations and some genetic syndromes are conditions that have been associated with ACC in children. In fact, it is estimated that about 50-80% of children with ACC carry a germline TP53 mutation52, 53, making Li-Fraumeni syndrome the most common inherited condition in young patients with ACC. In our cohort, 12 patients (3.6%) were younger than 18 years old at the age of diagnosis, and 50% of the pediatric patients (6 of 12) had Li-Fraumeni syndrome based upon clinical grounds or genetic testing.
Similar to older reports25, 54, approximately one out of ten patients with non-hereditary ACC patients had other malignancies, with breast and prostate cancer being the most frequent ones. Despite this apparent risk for other malignancies, we only recommend age- appropriate cancer screening that is adjusted per personal and family history in the few patients who have long-term survival.
This study was limited by the inherent shortcomings of retrospective reviews and potential referral bias. Referral bias is likely more pronounced in rare diseases that require special expertise compared to more common illnesses that require well set standards of care and commonly available treatments55. The main factors to cause this bias include the tendency to refer patients if they have unusual presentation, after failing prior treatments, and/or if they have advanced disease that requires special expertise. In ACC, referral bias is expected to result in worse outcome of the whole group if most included subjects were referred after failing prior treatments.
Also, it is unknown how many patients are treated outside major centers and never referred to be included in such study design. Some of these patients may have been cured and did not require further treatment or they may have accepted their diagnosis as being terminal and chose to stay in their local communities56. In our series, most of the patients had their initial surgical resection outside MDACC and then referred and it was often difficult to ascertain the cause of referral in all cases or the temporal relationship between recurrence and referral.
Nevertheless, the current study’s has multiple strengths including the summary of clinical experience with a large cohort of ACC patients treated at a single institution over the past decade and description our treatment approach in this rare disease.
The unchanged mortality observed over the decades in our cohort and that has been also described by a recent population study done in the Netherlands8 underscores the urgency to find better treatments for ACC.
Conclusions
Despite better understanding of molecular pathways involved in ACC and the availability of new classes of anti-cancer therapy, the prognosis of ACC remains poor. Older age at diagnosis, functioning tumors, and incomplete resections are clinical factors associated with worse survival. Surgical expertise is important to achieve complete resections and to improve outcome. There is an urgent need for more efficacious systemic treatments than what is currently used, as distant recurrence and ultimate death is very common despite the best efforts at locoregional control of disease.
Acknowledgments
Funding Sources: This paper is supported in part by the National Institutes of Health through The University of Texas MD Anderson Cancer Center Support Grant, CA016672, and The Beverlin Fund for Adrenal Cancer Research
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures
Financial disclosures: None
References
- 1.Gerhardt PR, Handy VH, Ferber B. Trends in cancer incidence, mortality, and probability in the state of New York. N Y State J Med. 1957;57:1387–1390. [PubMed] [Google Scholar]
- 2.Griswold MH, Cutler SJ. The Connecticut cancer register. Seventeen years of experience. 1956. Conn Med. 2006;70:323–328. [PubMed] [Google Scholar]
- 3.Soreide JA, Brabrand K, Thoresen SO. Adrenal cortical carcinoma in Norway, 1970-1984. World J Surg. 1992;16:663–667. doi: 10.1007/BF02067349. discussion 668. [DOI] [PubMed] [Google Scholar]
- 4.Nader S, Hickey RC, Sellin RV, Samaan NA. Adrenal cortical carcinoma. A study of 77 cases. Cancer. 1983;52:707–711. doi: 10.1002/1097-0142(19830815)52:4<707::aid-cncr2820520424>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Vassilopoulou-Sellin R, Schultz PN. Adrenocortical carcinoma. Clinical outcome at the end of the 20th century. Cancer. 2001;92:1113–1121. doi: 10.1002/1097-0142(20010901)92:5<1113::aid-cncr1428>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Abiven G, Coste J, Groussin L, Anract P, Tissier F, Legmann P, Dousset B, Bertagna X, Bertherat J. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91:2650–2655. doi: 10.1210/jc.2005-2730. [DOI] [PubMed] [Google Scholar]
- 7.Icard P, Goudet P, Charpenay C, Andreassian B, Carnaille B, Chapuis Y, Cougard P, Henry JF, Proye C. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001;25:891–897. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 8.Kerkhofs TM, Verhoeven RH, Van der Zwan JM, Dieleman J, Kerstens MN, Links TP, Van de Poll-Franse LV, Haak HR. Adrenocortical carcinoma: A population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer. 2013 doi: 10.1016/j.ejca.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Ramsay O. Malignant tumors of the suprarrenal glands. Johns Hopkins Hosp Bull. 1899;94-96:20–29. [Google Scholar]
- 10.Hench PS, Slocumb CH, et al. The effects of the adrenal cortical hormone 17-hydroxy-11-dehydrocorticosterone (Compound E) on the acute phase of rheumatic fever; preliminary report. Proc Staff Meet Mayo Clin. 1949;24:277–297. [PubMed] [Google Scholar]
- 11.Hutter AM, Jr., Kayhoe DE. Adrenal cortical carcinoma. Results of treatment with o,p’DDD in 138 patients. Am J Med. 1966;41:581–592. doi: 10.1016/0002-9343(66)90220-8. [DOI] [PubMed] [Google Scholar]
- 12.Varley JM, McGown G, Thorncroft M, Cochrane S, Morrison P, Woll P, Kelsey AM, Mitchell EL, Boyle J, Birch JM, Evans DG. A previously undescribed mutation within the tetramerisation domain of TP53 in a family with Li-Fraumeni syndrome. Oncogene. 1996;12:2437–2442. [PubMed] [Google Scholar]
- 13.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 14.Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagnere AM, Rene-Corail F, Jullian E, Gicquel C, Bertagna X, Vacher-Lavenu MC, Perret C, Bertherat J. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65:7622–7627. doi: 10.1158/0008-5472.CAN-05-0593. [DOI] [PubMed] [Google Scholar]
- 15.Hatada I, Ohashi H, Fukushima Y, Kaneko Y, Inoue M, Komoto Y, Okada A, Ohishi S, Nabetani A, Morisaki H, Nakayama M, Niikawa N, Mukai T. An imprinted gene p57KIP2 is mutated in Beckwith-Wiedemann syndrome. Nat Genet. 1996;14:171–173. doi: 10.1038/ng1096-171. [DOI] [PubMed] [Google Scholar]
- 16.Gicquel C, Raffin-Sanson ML, Gaston V, Bertagna X, Plouin PF, Schlumberger M, Louvel A, Luton JP, Le Bouc Y. Structural and functional abnormalities at 11p15 are associated with the malignant phenotype in sporadic adrenocortical tumors: study on a series of 82 tumors. J Clin Endocrinol Metab. 1997;82:2559–2565. doi: 10.1210/jcem.82.8.4170. [DOI] [PubMed] [Google Scholar]
- 17.Weksberg R, Shen DR, Fei YL, Song QL, Squire J. Disruption of insulin-like growth factor 2 imprinting in Beckwith-Wiedemann syndrome. Nat Genet. 1993;5:143–150. doi: 10.1038/ng1093-143. [DOI] [PubMed] [Google Scholar]
- 18.Heaton JH, Wood MA, Kim AC, Lima LO, Barlaskar FM, Almeida MQ, Fragoso MC, Kuick R, Lerario AM, Simon DP, Soares IC, Starnes E, Thomas DG, Latronico AC, Giordano TJ, Hammer GD. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and beta-catenin. Am J Pathol. 2012;181:1017–1033. doi: 10.1016/j.ajpath.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soon PS, Gill AJ, Benn DE, Clarkson A, Robinson BG, McDonald KL, Sidhu SB. Microarray gene expression and immunohistochemistry analyses of adrenocortical tumors identify IGF2 and Ki-67 as useful in differentiating carcinomas from adenomas. Endocr Relat Cancer. 2009;16:573–583. doi: 10.1677/ERC-08-0237. [DOI] [PubMed] [Google Scholar]
- 20.Ozata DM, Caramuta S, Velazquez-Fernandez D, Akcakaya P, Xie H, Hoog A, Zedenius J, Backdahl M, Larsson C, Lui WO. The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocr Relat Cancer. 2011;18:643–655. doi: 10.1530/ERC-11-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLellis RA, LR, Heitz PU, Eng C. Pathology and genetics of tumours of endocrine organs. World Health Organization Classification of Tumours. IARC Press; 2004. [Google Scholar]
- 22.Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, Terzolo M, Mueller HH, Hahner S, Allolio B, German Adrenocortical Carcinoma Registry G & European Network for the Study of Adrenal T Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer. 2009;115:243–250. doi: 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
- 23.Lughezzani G, Sun M, Perrotte P, Jeldres C, Alasker A, Isbarn H, Budaus L, Shariat SF, Guazzoni G, Montorsi F, Karakiewicz PI. The European Network for the Study of Adrenal Tumors staging system is prognostically superior to the international union against cancer-staging system: a North American validation. Eur J Cancer. 2010;46:713–719. doi: 10.1016/j.ejca.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, Sorbye H, Torpy DJ, Stepan V, Schteingart DE, Arlt W, Kroiss M, Leboulleux S, Sperone P, Sundin A, Hermsen I, Hahner S, Willenberg HS, Tabarin A, Quinkler M, de la Fouchardiere C, Schlumberger M, Mantero F, Weismann D, Beuschlein F, Gelderblom H, Wilmink H, Sender M, Edgerly M, Kenn W, Fojo T, Muller HH, Skogseid B, Group F-AS Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366:2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 25.Venkatesh S, Hickey RC, Sellin RV, Fernandez JF, Samaan NA. Adrenal cortical carcinoma. Cancer. 1989;64:765–769. doi: 10.1002/1097-0142(19890801)64:3<765::aid-cncr2820640333>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Pommier RF, Brennan MF. An eleven-year experience with adrenocortical carcinoma. Surgery. 1992;112:963–970. discussion 970-961. [PubMed] [Google Scholar]
- 27.Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol. 1999;6:719–726. doi: 10.1007/s10434-999-0719-7. [DOI] [PubMed] [Google Scholar]
- 28.Lee JE, Berger DH, el-Naggar AK, Hickey RC, Vassilopoulou-Sellin R, Gagel RF, Burgess MA, Evans DB. Surgical management, DNA content, and patient survival in adrenal cortical carcinoma. Surgery. 1995;118:1090–1098. doi: 10.1016/s0039-6060(05)80119-9. [DOI] [PubMed] [Google Scholar]
- 29.Habra MA, Ejaz S, Feng L, Das P, Deniz F, Grubbs EG, Phan A, Waguespack SG, Ayala-Ramirez M, Jimenez C, Perrier ND, Lee JE, Vassilopoulou-Sellin R. A retrospective cohort analysis of the efficacy of adjuvant radiotherapy after primary surgical resection in patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:192–197. doi: 10.1210/jc.2012-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, Kebebew E, Sturgeon C. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130–3136. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 31.Paton BL, Novitsky YW, Zerey M, Harrell AG, Norton HJ, Asbun H, Kercher KW, Heniford BT. Outcomes of adrenal cortical carcinoma in the United States. Surgery. 2006;140:914–920. doi: 10.1016/j.surg.2006.07.035. discussion 919-920. [DOI] [PubMed] [Google Scholar]
- 32.Kendrick ML, Lloyd R, Erickson L, Farley DR, Grant CS, Thompson GB, Rowland C, Young WF, Jr., van Heerden JA. Adrenocortical carcinoma: surgical progress or status quo? Arch Surg. 2001;136:543–549. doi: 10.1001/archsurg.136.5.543. [DOI] [PubMed] [Google Scholar]
- 33.Shiloach M, Frencher SK, Jr., Steeger JE, Rowell KS, Bartzokis K, Tomeh MG, Richards KE, Ko CY, Hall BL. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210:6–16. doi: 10.1016/j.jamcollsurg.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 34.Hutter MM, Rowell KS, Devaney LA, Sokal SM, Warshaw AL, Abbott WM, Hodin RA. Identification of surgical complications and deaths: an assessment of the traditional surgical morbidity and mortality conference compared with the American College of Surgeons-National Surgical Quality Improvement Program. J Am Coll Surg. 2006;203:618–624. doi: 10.1016/j.jamcollsurg.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Beard CM, Yunginger JW, Reed CE, O’Connell EJ, Silverstein MD. Interobserver variability in medical record review: an epidemiological study of asthma. J Clin Epidemiol. 1992;45:1013–1020. doi: 10.1016/0895-4356(92)90117-6. [DOI] [PubMed] [Google Scholar]
- 36.Grubbs EG, Callender GG, Xing Y, Perrier ND, Evans DB, Phan AT, Lee JE. Recurrence of adrenal cortical carcinoma following resection: surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol. 2010;17:263–270. doi: 10.1245/s10434-009-0716-x. [DOI] [PubMed] [Google Scholar]
- 37.Schteingart DE, Doherty GM, Gauger PG, Giordano TJ, Hammer GD, Korobkin M, Worden FP. Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr Relat Cancer. 2005;12:667–680. doi: 10.1677/erc.1.01029. [DOI] [PubMed] [Google Scholar]
- 38.Cooper AB, Habra MA, Grubbs EG, Bednarski BK, Ying AK, Perrier ND, Lee JE, Aloia TA. Does laparoscopic adrenalectomy jeopardize oncologic outcomes for patients with adrenocortical carcinoma? Surg Endosc. 2013 doi: 10.1007/s00464-013-3034-0. [DOI] [PubMed] [Google Scholar]
- 39.Hermsen IG, Kerkhofs TM, den Butter G, Kievit J, van Eijck CH, Nieveen van Dijkum EJ, Haak HR, Dutch Adrenal N. Surgery in adrenocortical carcinoma: Importance of national cooperation and centralized surgery. Surgery. 2012;152:50–56. doi: 10.1016/j.surg.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Terzolo M, Boccuzzi A, Bovio S, Cappia S, De Giuli P, Ali A, Paccotti P, Porpiglia F, Fontana D, Angeli A. Immunohistochemical assessment of Ki-67 in the differential diagnosis of adrenocortical tumors. Urology. 2001;57:176–182. doi: 10.1016/s0090-4295(00)00852-9. [DOI] [PubMed] [Google Scholar]
- 41.Stojadinovic A, Ghossein RA, Hoos A, Nissan A, Marshall D, Dudas M, Cordon-Cardo C, Jaques DP, Brennan MF. Adrenocortical carcinoma: clinical, morphologic, and molecular characterization. J Clin Oncol. 2002;20:941–950. doi: 10.1200/JCO.2002.20.4.941. [DOI] [PubMed] [Google Scholar]
- 42.Morimoto R, Satoh F, Murakami O, Suzuki T, Abe T, Tanemoto M, Abe M, Uruno A, Ishidoya S, Arai Y, Takahashi K, Sasano H, Ito S. Immunohistochemistry of a proliferation marker Ki67/MIB1 in adrenocortical carcinomas: Ki67/MIB1 labeling index is a predictor for recurrence of adrenocortical carcinomas. Endocr J. 2008;55:49–55. doi: 10.1507/endocrj.k07-079. [DOI] [PubMed] [Google Scholar]
- 43.Datrice NM, Langan RC, Ripley RT, Kemp CD, Steinberg SM, Wood BJ, Libutti SK, Fojo T, Schrump DS, Avital I. Operative management for recurrent and metastatic adrenocortical carcinoma. J Surg Oncol. 2012;105:709–713. doi: 10.1002/jso.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erdogan I, Deutschbein T, Jurowich C, Kroiss M, Ronchi C, Quinkler M, Waldmann J, Willenberg HS, Beuschlein F, Fottner C, Klose S, Heidemeier A, Brix D, Fenske W, Hahner S, Reibetanz J, Allolio B, Fassnacht M, on behalf of the German Adrenocortical Carcinoma Study G The Role of Surgery in the Management of Recurrent Adrenocortical Carcinoma. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-2559. [DOI] [PubMed] [Google Scholar]
- 45.Bellantone R, Ferrante A, Boscherini M, Lombardi CP, Crucitti P, Crucitti F, Favia G, Borrelli D, Boffi L, Capussotti L, Carbone G, Casaccia M, Cavallaro A, Del Gaudio A, Dettori G, Di Giovanni V, Mazziotti A, Marrano D, Masenti E, Miccoli P, Mosca F, Mussa A, Petronio R, Piat G, Marazano L, et al. Role of reoperation in recurrence of adrenal cortical carcinoma: results from 188 cases collected in the Italian National Registry for Adrenal Cortical Carcinoma. Surgery. 1997;122:1212–1218. doi: 10.1016/s0039-6060(97)90229-4. [DOI] [PubMed] [Google Scholar]
- 46.Haak HR, Hermans J, van de Velde CJ, Lentjes EG, Goslings BM, Fleuren GJ, Krans HM. Optimal treatment of adrenocortical carcinoma with mitotane: results in a consecutive series of 96 patients. Br J Cancer. 1994;69:947–951. doi: 10.1038/bjc.1994.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, Reimondo G, Bollito E, Papotti M, Saeger W, Hahner S, Koschker AC, Arvat E, Ambrosi B, Loli P, Lombardi G, Mannelli M, Bruzzi P, Mantero F, Allolio B, Dogliotti L, Berruti A. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356:2372–2380. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 48.Fareau GG, Lopez A, Stava C, Vassilopoulou-Sellin R. Systemic chemotherapy for adrenocortical carcinoma: comparative responses to conventional first-line therapies. Anticancer Drugs. 2008;19:637–644. doi: 10.1097/CAD.0b013e328300542a. [DOI] [PubMed] [Google Scholar]
- 49.Naing A, Lorusso P, Fu S, Hong D, Chen HX, Doyle LA, Phan AT, Habra MA, Kurzrock R. Insulin growth factor receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with metastatic adrenocortical carcinoma. Br J Cancer. 2013 doi: 10.1038/bjc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polat B, Fassnacht M, Pfreundner L, Guckenberger M, Bratengeier K, Johanssen S, Kenn W, Hahner S, Allolio B, Flentje M. Radiotherapy in adrenocortical carcinoma. Cancer. 2009;115:2816–2823. doi: 10.1002/cncr.24331. [DOI] [PubMed] [Google Scholar]
- 51.Lack EE, Mulvihill JJ, Travis WD, Kozakewich HP, Adrenal cortical neoplasms in the pediatric and adolescent age group Clinicopathologic study of 30 cases with emphasis on epidemiological and prognostic factors. Pathol Annu. 1992;27(Pt 1):1–53. [PubMed] [Google Scholar]
- 52.Varley JM, McGown G, Thorncroft M, James LA, Margison GP, Forster G, Evans DG, Harris M, Kelsey AM, Birch JM. Are there low-penetrance TP53 Alleles? evidence from childhood adrenocortical tumors. Am J Hum Genet. 1999;65:995–1006. doi: 10.1086/302575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat. 2003;21:313–320. doi: 10.1002/humu.10185. [DOI] [PubMed] [Google Scholar]
- 54.Didolkar MS, Bescher RA, Elias EG, Moore RH. Natural history of adrenal cortical carcinoma: a clinicopathologic study of 42 patients. Cancer. 1981;47:2153–2161. doi: 10.1002/1097-0142(19810501)47:9<2153::aid-cncr2820470908>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 55.Layde PM, Broste SK, Desbiens N, Follen M, Lynn J, Reding D, Vidaillet H. Generalizability of clinical studies conducted at tertiary care medical centers: a population-based analysis. J Clin Epidemiol. 1996;49:835–841. doi: 10.1016/0895-4356(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 56.Sutherland LR, Verhoef MJ. Patients who seek a second opinion: are they different from the typical referral? J Clin Gastroenterol. 1989;11:308–313. doi: 10.1097/00004836-198906000-00013. [DOI] [PubMed] [Google Scholar]


