Abstract
Objective
Low-density lipoprotein cholesterol (LDL-C) is a risk factor for survival in middle-aged individuals, but conflicting evidence exists on the relationship between LDL-C and all-cause mortality among the elderly. The goal of this study was to assess the relationship between LDL-C and all-cause mortality among Chinese oldest old (aged 80 and older) in a prospective cohort study.
Methods
LDL-C concentration was measured at baseline and all-cause mortality was calculated over a 3-year period. Multiple statistical models were used to adjust for demographic and biological covariates.
Results
During three years of follow-up, 447 of 935 participants died, and the overall all-cause mortality was 49.8%. Each 1 mmol/L increase of LDL-C concentration corresponded to a 19% decrease in 3-year all-cause mortality (hazard ratio [HR] 0.81, 95% confidence interval [CI] 0.71–0.92). The crude HR for abnormally higher LDL-C concentration (≥3.37 mmol/L) was 0.65 (0.41–1.03); and the adjusted HR was statistically significant around 0.60 (0.37–0.95) when adjusted for different sets of confounding factors. Results of sensitivity analysis also showed a significant association between higher LDL-C and lower mortality risk.
Conclusions
Among the Chinese oldest old, higher LDL-C level was associated with lower risk of all-cause mortality. Our findings suggested the necessity of re-evaluating the optimal level of LDL-C among the oldest old.
Keywords: LDL-C, mortality, oldest old, epidemiology, China
Introduction
Low-density lipoprotein cholesterol (LDL-C) is a risk factor for cardiovascular and cerebrovascular diseases and is associated with increased mortality in middle-aged individuals [1]. For the elderly, however, there are three concerns that cast doubt on the applicability of general LDL-C recommendations. First, studies of older populations have led to conflicting conclusions on the relationship between LDL-C and all-cause mortality. Some studies showed that high concentrations of LDL-C were associated with higher risk of mortality and morbidity of cardiovascular and cerebrovascular diseases or all-cause mortality among the elderly [2, 3], while other studies found that low LDL-C concentrations were associated with increased mortality risk from non-cardiovascular disease [4], such as cancer [5], infection [6], liver diseases [7], and trauma [8] among elderly. Several studies also concluded that LDL-C was inversely associated with the risk of death in elderly people [9–14], which has attracted particular attention regarding the necessity for LDL-C lowering therapy in the aged population.
A second concern about the generalizability of lipid treatment recommendations is that most studies have been conducted in high-income countries. Understanding this relationship in low-income and heavily populated countries is particularly urgent as they confronting the challenge of a rapidly increasing aging population.
A third concern is that currently almost all the recommendations to lower the level of LDL-C were formulated for the general adult population [15–17], and there were very few studies that focused on the optimal LDL-C level of the oldest old. To investigate this question, we assessed the relationship between LDL-C and all-cause mortality in a longitudinal cohort of oldest old in China.
Methods
Study Design and Participants
We used data collected in the Chinese Longitudinal Healthy Longevity Survey (CLHLS). The study design of CLHLS has been described in detail elsewhere [18]. The baseline survey of this current study was conducted in 2009, in 7 longevity areas of China, and 935 aged 80 years or older participated in the baseline survey, including 319 octogenarians, 276 nonagenarians and 340 centenarians. The follow-up survey was conducted in 2012. The study was approved by the Ethics Committee of Peking University and the Ethics Committee of the National University of Singapore. Written consent was obtained from all participants.
Participants were followed-up for a length of 38 months from the baseline survey in June 2009 to August 2012. Participants’ survival status was ascertained during the follow-up survey. Dates of death were acquired and confirmed by participants’ family members or the village doctor. The information on cause-specific death was not collected in this study because (1) the seven survey fields are not covered by the death surveillance system in China, (2) reported conditions prior to death do not provide clear insight into the cause of death in the oldest old with a variety of chronic diseases, and (3) many of the oldest old die a natural death at home rather than in hospital where cause of death may be assessed. A ‘lost-to-follow-up’ status was assigned to those who could not be found and contacted.
Data Collection
Baseline assessments included a researcher-administered questionnaire, physical examination, and laboratory testing. All the questions were answered by the participants themselves or with their family members if the participants were unable to complete interviews. The following information was collected: socioeconomic and demographic characteristics, dietary behaviors and life style, diseases, cognitive function, and the performance of activities of daily living (ADL).
Physical examination was performed to measure systolic blood pressure (SBP), diastolic blood pressure (DBP), waist circumference (WC), and vision. Five milliliters of venous blood and 15 milliliters of urine were collected in the morning after an overnight fast for at least 12 hours. Serum creatinine was determined with the picric acid method and albuminuria was measured by dry chemistry reagent test strips (Siemens Diagnostics, NY, USA). Fasting plasma glucose, hemoglobin, LDL-C and high-density lipoprotein cholesterol (HDL-C) were measured by an Automatic Biochemistry Analyzer (Hitachi 7180, Japan) using commercially available diagnostic kits (Roche Diagnostic, Mannheim, Germany) at Capital Medical University in Beijing.
Key Variables and Definitions
The Mini-Mental Status Examination was used to define cognitive impairment (score: 0–17) [19]. Activity of daily living (ADL) was assessed based on self-reported performance of the following six self-care tasks: dressing, eating, toileting, bathing, indoor activities, and continence. ADL was defined as normal if an individual could deal with all six tasks independently; otherwise ADL was defined as restricted [20].
Hypertension was defined as systolic blood pressure≥140mmHg and/or diastolic blood pressure≥90mmHg, and/or being on antihypertensive therapy. Central obesity was defined as waist circumference ≥85cm in men or waist circumference ≥80cm in women participants. Type 2 diabetes mellitus was defined as fasting plasma glucose≥7.0 mmol/L or self-report of current diabetes related medication use. Anemia was defined as hemoglobin <130g/L in men or hemoglobin <120g/L in women. Abnormally high LDL-C level was defined as ≥3.37 mmol/L, and low HDL-C was defined as <1.04 mmol/L (15). Different LDL-C levels cut points were used to define higher LDL-C level, including LDL-C≥3.12 mmol/L and LDL-C≥2.85 mmol/L. Chronic kidney disease (CKD) was assessed from estimated glomerular filtration rate (eGFR) less than 60 ml/min per 1.73 m2 and/or albuminuria [21]; The eGFR was calculated with the modification of diet in renal disease (MDRD) equations for Chinese: eGFR=175 (serum creatinine)−1.234 ×age−0.179×0.79(if female) [22].
Statistical Analysis
The characteristics of participants were compared using t-test for continuous variables and Chi-square tests for categorical variables between death versus survival, and survival versus lost-to-follow-up, respectively. Mortality rate for 100-person-years was calculated by the life table method, which was partly adjusted for the influence of lost-to-follow-up.
The survival time was calculated from the date of the baseline survey to the date of death, and was defined as 38 months for those censored participants who survived or were lost-to-follow-up. Survival rate was estimated with the Kaplan-Meier product-limit method, and compared with the log-rank test. Schoenfold residual error method was used for the proportional hazards assumption test; the correlation coefficient was 0.04 and the p value was 0.35, which showed that it met the proportional hazards assumption. Crude and adjusted HRs for LDL-C concentrations were estimated using Cox proportional hazards models. In Model 1, HRs were adjusted for sex, age and marital status were adjusted. And current smoking, current alcohol drinking, and tea drinking were further included in Model 2. Central obesity, cognitive impairment, ADL restriction and blindness were further added in Model 3. Anemia, hypertension, type 2 diabetes mellitus, CKD and HDL-C were additionally added in the Model 4.
To address whether the associations between LDL-C and mortality were stable, models were performed using different LDL-C concentrations as cut points. To explore gender differences and the possibility that low LDL-C concentration may be a consequence of acute disease that causes increased mortality, these analyses were also stratified by gender and by excluding deaths in the first year of follow-up. To clarify the effect of lost-to-follow-up on the results, sensitivity analyses were conducted by censoring lost-to-follow-up at baseline (0 month) or follow-up assessment (38 months), or at the midpoint of follow-up (19 months).
Statistical analyses were performed with SAS, version 9.13 (SAS Institute Inc, Cary, NC, USA). 2-sided p value <0.05 was considered significant
Results
A total of 935 participants aged 80 years or older were enrolled in the baseline survey in 2009. The mean age of the participants was 94.2 years, and 69% of them were women. Approximately 8% (73/935) of participants were lost-to-follow-up. A total of 862 participants were successfully followed-up, and 447 died. Characteristics of those who survived, died or were lost-to-follow-up were compared (Table 1). The mean baseline age of those who died was significantly higher than those who survived. The mean baseline values of hemoglobin, systolic blood pressure, diastolic blood pressure and LDL-C were lower among those who died compared to those who survived. Participants who survived had a higher prevalence of central obesity, lower prevalence of cognitive impairment, ADL restrictions, blindness, and CKD than those who died. No significant difference was found for all covariates between those who survived and those who were lost-to-follow-up.
Table 1.
Characteristics of 935 Chinese oldest old by the outcome over 3 years of follow-up
| Characteristic | Died, N=447 (%) | Survived, N=415 (%) | P-value* | Lost to follow-up, N=73 (%) | P-value† |
|---|---|---|---|---|---|
| Overall | 447(47.8) | 415(44.4) | 73(7.8) | ||
| Sex | |||||
| Male | 129(28.9) | 137(30.6) | 0.19 | 24(32.9) | 0.98 |
| Female | 318(71.1) | 278(79.4) | 49(67.1) | ||
| Age, year§ | 96.8±7.3 | 91.7±7.6 | 0.01 | 94.4±7.9 | 0.98 |
| Marital status | |||||
| In marriage | 49(11.0) | 99(23.9) | 0.01 | 16(21.9) | 0.72 |
| Not in marriage | 398(89.0) | 316(76.1) | 57(78.1) | ||
| Current smoking practice | 66(14.8) | 65(15.7) | 0.71 | 15(20.1) | 0.30 |
| Alcohol drinking habits | 71(15.9) | 69(16.0) | 0.77 | 7(9.6) | 0.13 |
| Tea drinking habits | 93(20.8) | 100(24.1) | 0.25 | 16(21.9) | 0.69 |
| Central obesity | 124(27.7) | 162(39.0) | 0.01 | 26(35.6) | 0.58 |
| Cognitive impairment | 274(61.3) | 147(35.4) | 0.01 | 31(18.5) | 0.52 |
| ADL restriction | 146(32.7) | 56(13.5) | 0.01 | 23(31.5) | 0.20 |
| Blindness | 72(16.1) | 40(9.6) | 0.01 | 5(6.9) | 0.45 |
| Hemoglobin (g/L) ╪ | 119.5±28.4 | 126.2±22.4 | 0.01 | 125.3±19.5 | 0.74 |
| Anemia | 251(56.2) | 178(42.9) | 0.01 | 37(50.7) | 0.22 |
| Systolic blood pressure (mmHg) ╪ | 140.3±26.1 | 143.3±25.7 | 0.09 | 139.6±26.1 | 0.27 |
| Diastolic blood pressure (mmHg) ╪ | 77.0±14.9 | 79.3±14.7 | 0.03 | 79.4±14.7 | 0.95 |
| Hypertension | 258(57.7) | 260(62.7) | 0.14 | 38(52.1) | 0.09 |
| Fasting plasma glucose (mmol/L) ╪ | 5.38±1.19 | 5.34±1.61 | 0.74 | 5.55±1.35 | 0.30 |
| Diabetes | 38(8.5) | 40(9.6) | 0.56 | 11(15.1) | 0.16 |
| CKD(chronic kidney disease) | 235(52.6) | 160(38.6) | 0.01 | 28(38.4) | 0.01 |
| HDL-C (mmol/L) ╪ | 1.23±0.32 | 1.20±0.32 | 0.19 | 1.22±0.29 | 0.61 |
| Abnormal HDL-C | 119(26.6) | 123(29.6) | 0.32 | 16(21.9) | 0.18 |
| LDL-C level§ | 2.00±0.74 | 2.10±0.79 | 0.04 | 2.15±0.88 | 0.67 |
| Abnormally higher LDL-C | 19(4.3) | 28(6.8) | 0.10 | 6(8.2) | 0.65 |
Abbreviations: ADL=activities of daily living; HDL-C=high-density lipoprotein cholesterol; LDL-C=low-density lipoprotein cholesterol; CKD= chronic kidney disease; Abnormal HDL-C level was defined as <1.04 mmol/L; Abnormally higher LDL-C level was defined as ≥3.37 mmol/L.
The dead compared with the “survived” group.
The lost-to-follow-up was compared with the “survived” group.
Data reported as means±SD for continuous variables.
The all-cause mortality rate in 3 years was 21.4 per 100 person-years for all subjects, 19.6 for males and 22.3 for females; and it was 12.1 per 100 person-years for octogenarians, 20.9 for nonagenarians and 33.1 for centenarians (Figure 1).
Figure 1.
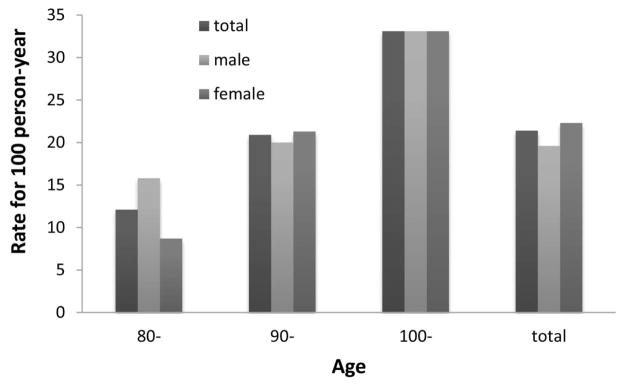
3-year all-cause mortality rate (person-year) among Chinese oldest old by gender
The average LDL-C level was 2.06 mmol/L for all participants, 1.87 and 2.14 mmol/L for men and women, respectively. The prevalence of abnormally high LDL-C (≥3.37 mmol/L) was 5.7% for all participants, and 3.4% for men and 6.6% for women. The average LDL-C level was lower among participants who died (2.00 mmol/L) than those who survived (2.10 mmol/L) (p=0.04).
When LDL-C was analyzed as a continuous variable, each 1 mmol/L increase in LDL-C corresponded to an 18% relative decrease in mortality risk for all participants with HR [95% CI] 0.81 [0.71–0.92] for all participants ( 0.71 [0.53–0.96] for men, and 0.80 [0.69–0.93] for women).
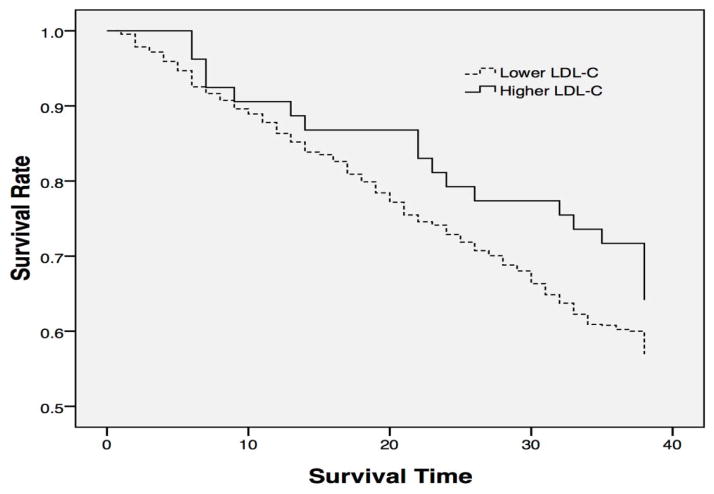
The crude mortality risk ratio for an abnormally high LDL-C concentration (≥3.37 mmol/L) was 0.65 (0.41–1.03). When demographic variables were adjusted (Model 1), those who had an abnormally higher LDL-C level had a 40% lower risk of mortality than those had a lower level of LDL-C (HR, 0.60; 95% CI, 0.38–0.96). The inverse association remained statistically significant after further adjusting for other variables (Table 2). Figure 2 shows the survival curves for the oldest old with lower and abnormally higher baseline LDL-C levels.
Table 2.
Cox’s proportional-hazards models for predicting 3-year all-cause mortality among 935 Chinese oldest old.
| Variables | Values | Crude | Model 1 OR(95%CI) |
Model 2 OR(95%CI) |
Model 3 OR(95%CI) |
Final model OR(95%CI) |
|---|---|---|---|---|---|---|
| Abnormally higher LDL-C | 0=low 1=abnormally higher | 0.65(0.41–1.03) | 0.60(0.38–0.96)* | 0.60(0.38–0.96)* | 0.64(0.40–1.00)* | 0.60(0.37–0.95)* |
| Sex | 0=female 1=male | - | 1.32(1.05–1.65)* | 1.32(1.03–1.67)* | 1.36(1.06–1.74)* | 1.40(1.09–1.79) â |
| Age | 0=80–89 1=90–99 2=100- | - | 1.69(1.49–1.91) â | 1.69(1.49–1.91) â | 1.42(1.24–1.63) â | 1.38(1.20–1.60) â |
| Marital status | 0=in marriage 1= not in marriage | - | 1.62(1.17–2.25) â | 1.62(1.17–2.25) â | 1.50(1.08–2.08)* | 1.49(1.07–2.08)* |
| Current smoking | 0=no 1=yes | - | - | 1.04(0.78–1.38) | 1.07(0.80–1.43) | 1.09(0.81–1.46) |
| Current alcohol drinking | 0=no 1=yes | - | - | 1.02(0.78–1.33) | 1.05(0.81–1.37) | 1.08(0.83–1.40) |
| Tea drinking | 0=no 1=yes | - | - | 0.91(0.72–1.15) | 0.93(0.73–1.17) | 0.85(0.67–1.09) |
| Central obesity | 0=no 1=yes | - | - | - | 0.65(0.53–0.80) â | 0.64(0.52–0.79) ** |
| Cognitive Impairment | 0=no 1=yes | - | - | - | 1.50(1.21–1.86) ** | 1.58(1.27–1.96) ** |
| ADL restriction | 0=no 1=yes | - | - | - | 1.56(1.25–1.95) ** | 1.57(1.26–1.97) ** |
| Blindness | 0=no 1=yes | - | - | - | 1.38(1.06–1.78)* | 1.42(1.09–1.83)** |
| Anemia | 0=no 1=yes | - | - | - | - | 1.13(0.93–1.34) |
| Hypertension | 0=no 1=yes | - | - | - | - | 0.94(0.78–1.14) |
| Diabetes | 0=no 1=yes | - | - | - | - | 0.87(0.62–1.22) |
| CKD | 0=no 1=yes | - | - | - | - | 1.55(1.28–1.89)** |
| Abnormal HDL-C | 0=normal 1=abnormal | - | - | - | - | 1.09(0.88–1.36) |
Abbreviations: ADL=activities of daily living; CKD=chronic kidney disease; HDL-C=high-density lipoprotein cholesterol; LDL-C=low-density lipoprotein cholesterol; Abnormal HDL-C level was defined as <1.04 mmol/L; Abnormally high LDL-C level was defined as ≥3.37 mmol/L.
P<0.05
P<0.01.
Figure 2.
Survival curves for Chinese oldest old stratified by baseline LDL-C level
We further examined the mortality risk for different cutoff values of LDL-C concentrations (Table 3). At all cutoff values, participants with higher LDL-C levels had a significantly lower risk of mortality compared to those with lower LDL-C levels. After excluding those who died during the first year of follow-up, censoring at 19 months, or deleting all lost-to-follow-up, the significant associations were marginally significant but the significant association of higher concentrations of LDL-C with mortality risk remained. The results for each 1 mmol/L increase of LDL-C concentration with mortality risk also remained significant or marginally significant (Table 3 and 4).
Table 3.
Sensitivity analysis of the association between LDL-C level and mortality by censoring time for loss-to-follow-up.
| Cut-off | Censoring time | HR(95%CI) (adjusted with final model) †
|
||
|---|---|---|---|---|
| Men | Women | Total | ||
| LDL-C≥3.37mmol/L (130mg/dL) | Censoring at 38 months | 0.41(0.12–1.34) | 0.59(0.35–0.98)* | 0.60(0.37–0.958)* |
| Censoring at 19 months | 0.40(0.12–1.31) | 0.63(0.38–1.0) | 0.62(0.39–0.99)* | |
| Deleting all lost-to-follow-up | 0.40(0.12–1.32) | 0.66(0.40–1.11) | 0.64(0.40–1.03) | |
| LDL-C≥3.12mmol/L (120mg/dL ) | Censoring at 38 months | 0.43(0.15–1.21) | 0.57(0.38–0.85) ** | 0.59(0.41–0.85) ** |
| Censoring at 19 months | 0.41(0.15–1.17) | 0.57(0.38–0.85) ** | 0.59(0.41–0.85) ** | |
| Deleting all lost-to-follow-up | 0.41(0.15–1.16) | 0.58(0.39–0.87) ** | 0.59(0.41–0.86) ** | |
| LDL-C≥2.85mmol/L (110mg/dL) | Censoring at 38 months | 0.67(0.35–1.29) | 0.69(0.51–0.94)* | 0.72(0.55–0.95)* |
| Censoring at 19 months | 0.69(0.36–1.34) | 0.67(0.50–0.92)* | 0.71(0.54–0.94)* | |
| Deleting all lost-to-follow-up | 0.71(0.37–1.38) | 0.68(0.50–0.92)* | 0.72(0.54–0.94)* | |
| Each 1 mmol/L increase in LDL-C | Censoring at 38 months | 0.71(0.53–0.96)* | 0.80(0.69–0.93) ** | 0.81(0.71–0.92) ** |
| Censoring at 19 months | 0.73(0.54–0.98)* | 0.80(0.69–0.93) ** | 0.82(0.71–0.93) ** | |
| Deleting all lost-to-follow-up | 0.74(0.56–0.99)* | 0.81(0.69–0.94) ** | 0.82(0.72–0.94)* | |
Abbreviations: LDL-C=low-density lipoprotein cholesterol;
P<0.05,
P<0.01.
Adjusted for sex, age, marital status, current smoking, current alcohol drinking, tea drinking, central obesity, cognitive impairment, ADL restriction, blindness, chronic kidney disease, anemia, hypertension, type 2 diabetes mellitus and abnormal HDL-C level.
Table 4.
The mortality risk for higher LDL-C after the exclusion of mortality in the first year.
| Cut-off | HR(95%CI) (adjusted with final model) †
|
||
|---|---|---|---|
| Men | Women | Total | |
| LDL-C≥3.37mmol/L(130mg/dL) | 0.41(0.10–1.77) | 0.59(0.32–1.07) | 0.59(0.34–1.02) |
| LDL-C≥3.12mmol/L (120mg/dL ) | 0.48(0.15–1.58) | 0.61(0.39–0.95)* | 0.64(0.43–0.97)* |
| LDL-C≥2.85mmol/L (110mg/dL) | 0.71(0.33–1.51) | 0.63(0.44–0.91)* | 0.69(0.50–0.95)* |
| Each 1mmol/L increase in LDL-C | 0.73(0.51–1.03)) | 0.82(0.69–0.98)* | 0.83(0.71–0.96)* |
Abbreviations: LDL-C=low-density lipoprotein cholesterol.
P<0.05.
Adjusted for sex, age, marital status, current smoking, current alcohol drinking, tea drinking, central obesity, cognitive impairment, ADL restriction, blindness, chronic kidney disease, anemia, hypertension, type 2 diabetes mellitus and abnormal HDL-C level
Discussion
Our results indicated that a higher level of LDL-C was inversely associated with 3-year all-cause mortality among the Chinese oldest old. Compared with participants who had a lower LDL-C, those with high concentrations had a 40% lower mortality risk, which was consistent with several other studies [9–14, 21]. A follow-up study in France reported that lower level of LDL-C was associated with increased mortality risk for hospitalized elderly patients [11]. Studies further demonstrated that higher LDL-C was associated with reduced risk of mortality for both Japanese very elderly [13] and oldest old [23]. This phenomenon was also found in an elderly Brazilian cohort [9], elderly Italian women [14], non-demented elderly [11] and elderly patients with heart failure [12] in the United States.
There are several possible explanations for the inverse association between LDL-C level and mortality risk, First, evidence from animal experiments and epidemiological studies suggested that higher LDL-C affects the immune system, which may improve survival through enhanced defense against bacteria and viruses, or by raising delivery of lipids to cells which may promote the immune response as well as tissue repair [24]. Furthermore, a cohort study demonstrated that high LDL-C may improve the odds of survival in the context of fever and sepsis [6].
Second, epidemiological studies suggest that low LDL-C in the oldest old may exert an indirect effect to increase non-cardiovascular mortality [4]. It was not possible to establish a direct link of low LDL-C to an adverse effect on vascular mortality or non-vascular mortality because of the unavailable cause-specific mortality in our study. Some studies found low LDL-C was an independent predictor of depression [25], cancer [5], injury, homicides and suicide [26]. In other studies, LDL-C was reported to have an inverse association with death from liver diseases [7], intra parenchymal haemorrhage [27], and advanced chronic kidney disease [28].
Third, LDL-C was highly correlated to total cholesterol and among the oldest old, low total cholesterol was associated with increased mortality in the oldest old [29]. This phenomenon was also found by other studies of elderly [30] and middle aged [31] people.
Fourth, perhaps individuals susceptible to the vascular effects of high LDL-C had already died before aged 80 years, and thus were not included in the sample. The individuals who remained would be a select group with lower cholesterol whose genetic makeup or other factors protect them from the effects of high LDL-C level and thereby enhance survival. To a certain extent our data supported this hypothesis, the concentrations of LDL-C in the oldest old was very low in our study.
Several studies show that higher LDL-C increased all-cause mortality and lower LDL-C is beneficial in the elderly [3, 4]. A possible explanation for the different results in the present study is that, unlike the studies noted, we focus on the oldest old and have sufficient numbers aged 80 years and older to make meaningful conclusions. It is likely that successful aging of the oldest old may depend on a complex restructuring of physiological mechanism and adaptation of metabolism, it is likely that their metabolism is able to adapt to the challenges of aging, detrimental factors during early and middle ages may become protective in later life.
Moreover, it is also notable that a relatively lower LDL-C in the oldest old is extensively advertised in some communities even though this age-group is not well represented in epidemiological or clinical trials. But according to our study, and those of others, keeping higher LDL-C rather than lower concentration is beneficial to their survival for those aged 80 years or older. More epidemiological and clinical data are needed for primary prevention of mortality in the oldest old.
Strengths and Limitations
This is the first study using a relatively large sample of oldest old to investigate the associations of LDL-C and all-cause mortality. It is a longitudinal prospective study, which has a greater power to assess the epidemiological association between LDL-C and mortality.
This study has several limitations. We did not investigate treatment with lipid-lowering drugs, but it likely had little effect on our results because only a few participants reported being diagnosed by a doctor as having dyslipidemia. Exploration of potential intermediate variables, such as the diseases causing death, was not performed because of the unavailable cause-specific mortality data. In addition, our study was focused on Chinese oldest old, so our results may not be applicable to other ethnic or age groups.
Conclusions
In conclusion, our cohort study provides epidemiological evidence that higher levels of LDL-C were associated with better survival among the oldest old. We suggest that more interventional studies are needed to elucidate the clinical effects of higher LDL-C level in the oldest old.
Acknowledgments
The authors thank the staff from provincial and county CDCs in the seven longevity areas in this study for their contributions in data collection.
Funding Sources
This work was supported by the National Natural Science Foundation of China (81273160 to X.M.S), the National Institute of Health/National Institute of Aging, (RO1AG023627 to Z.Y. and the Claude D. Pepper Older Americans Independence Centers grant 5P30 AG028716 from NIA to VBK), and the Singapore Ministry of Health’s National Medical Research Council under its STaR Award Grant as part of the project “Establishing a Practical and Theoretical Foundation for Comprehensive and Integrated Community, Policy and Academic Efforts to Improve Dementia Care in Singapore” (NMRC/STaR/0005/2009 to DB Matchar), NSFC grants (71110107025 and 81273160).
Footnotes
Disclosure statement
The authors have nothing to disclose.
References
- 1.Pedersen TR, Kjekshus J, Berg K, et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 2.Aronow WS, Ahn C. Correlation of serum lipids with the presence or absence of coronary artery disease in 1,793 men and women aged ≥62 years. Am J Cardiol. 1994;73:702–703. doi: 10.1016/0002-9149(94)90938-5. [DOI] [PubMed] [Google Scholar]
- 3.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 4.Razzolini R, Tarantini G, Ossena G, et al. Non-cardiovascular mortality, low-density lipoprotein cholesterol and statins: a meta-regression analysis. Cardiology. 2007;109:110–116. doi: 10.1159/000105551. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 6.Shor R, Wainstein J, Oz D, et al. Low serum LDL cholesterol levels and the risk of fever, sepsis, and malignancy. Ann Clin Lab Sci. 2007;37:343–348. [PubMed] [Google Scholar]
- 7.Sritara P, Patoomanunt P, Woodward M, et al. Associations between serum lipids and causes of mortality in a cohort of 3,499 urban Thais: The Electricity Generating Authority of Thailand (EGAT) study. Angiology. 2007;58:757–63. doi: 10.1177/0003319707304042. [DOI] [PubMed] [Google Scholar]
- 8.Delgado-Rodríguez M, Medina-Cuadros M, Gómez-Ortega A, et al. Cholesterol and serum albumin levels as predictors of cross infection death, and length of hospital stay. Arch Surg. 2002;137:805–812. doi: 10.1001/archsurg.137.7.805. [DOI] [PubMed] [Google Scholar]
- 9.Cabrera MA, de Andrade SM, Dip RM. Lipids and all-cause mortality among older adults: a 12-year follow-up study. The Scientific World J. 2012;2012:930139. doi: 10.1100/2012/930139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Protogerou AD, Iaria P, et al. Prognosis in the hospitalized very elderly: The PROTEGER study. Int J Cardiol. 2013;168:2714–2719. doi: 10.1016/j.ijcard.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Schupf N, Costa R, Luchsinger J, et al. Relationship between plasma lipids and all-cause mortality in nondemented elderly. J Am Geriatr Soc. 2005;53:219–226. doi: 10.1111/j.1532-5415.2005.53106.x. [DOI] [PubMed] [Google Scholar]
- 12.Charach G, Rabinovich A, Ori A, et al. Low levels of low-density lipoprotein cholesterol: a negative predictor of survival in elderly patients with advanced heart failure. Cardiology. 2013;127:45–50. doi: 10.1159/000355164. [DOI] [PubMed] [Google Scholar]
- 13.Tikhonoff V, Casiglia E, Mazza A, et al. Low-density lipoprotein cholesterol and mortality in older people. J Am Geriatr Soc. 2005;53:2159–2164. doi: 10.1111/j.1532-5415.2005.00492.x. [DOI] [PubMed] [Google Scholar]
- 14.Brescianini S, Maggi S, Farchi G, et al. Low total cholesterol and increased risk of dying: are low levels clinical warning signs in the elderly? Results from the Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2003;51:991–996. doi: 10.1046/j.1365-2389.2003.51313.x. [DOI] [PubMed] [Google Scholar]
- 15.Joint committee for developing Chinese guidelines on prevention and treatment of dyslipidemia in adults. Chinese guidelines on prevention and treatment of dyslipidemia in adults. Chin J Cardiol. 2007;35:390–419. [PubMed] [Google Scholar]
- 16.De Backer G, Ambrosioni E, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: Third Joint Task Force of European and other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of eight societies and by invited experts) Eur J Cardiovasc Prev Rehabil. 2003;10 (Suppl):1–10. doi: 10.1097/01.hjr.0000087913.96265.e2. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:27–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 18.Gu D. Healthy longevity in China. Springer; Netherlands: 2008. General data quality assessment of the CLHLS; pp. 39–60. [Google Scholar]
- 19.Li LW, Zhang J, Liang J. Health among the oldest-old in China: Which living arrangements make a difference? Soc Sci Med. 2009;68:220–227. doi: 10.1016/j.socscimed.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng Y, Shen K. Main dimensions of health status among the Chinese elderly. Chin J Prev Med. 2010;44:108–114. [PubMed] [Google Scholar]
- 21.Eknoyan G, Levin NW. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 22.Chinese eGFR Investigation Collaboration. Modification and evaluation of MDRD estimating equation for Chinese patients with chronic kidney disease. Chinese Journal of Nephrology. 2006;22:589–595. [Google Scholar]
- 23.Takata Y, Ansai T, Soh I, et al. Serum total cholesterol concentration and 10-year mortality in an 85-year-old population. Clin Interv Aging. 2014;9:293–300. doi: 10.2147/CIA.S53754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Netea MG, Demacker PN, Kullberg BJ, et al. Low density lipoprotein receptor-deficient mice are protected against lethal endotoxemia and severe Gram-negative infections. J Clin Invest. 1996;97:1366–1372. doi: 10.1172/JCI118556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aijänseppä S, Kivinen P, Helkala EL, et al. Serum cholesterol and depressive symptoms in elderly Finnish men. Int J Geriatr Psychiatry. 2002;17:629–634. doi: 10.1002/gps.666. [DOI] [PubMed] [Google Scholar]
- 26.Cummings P, Psaty BM. The association between cholesterol and death from injury. Ann Intern Med. 1994;120:848–855. doi: 10.7326/0003-4819-120-10-199405150-00006. [DOI] [PubMed] [Google Scholar]
- 27.Wassertheil-Smoller S, Applegate WB, Berge K, et al. Low-density lipoprotein cholesterol concentrations and death due to intra parenchymal hemorrhage: the Ibaraki Prefectural Health Study. Circulation. 2009;119:2136–2145. doi: 10.1161/CIRCULATIONAHA.108.795666. [DOI] [PubMed] [Google Scholar]
- 28.Fu S, Yi S, Zhu B, et al. Prevalence, clinical predictors, and prognostic impact of chronic renal insufficiency in very old Chinese patients with coronary artery disease. Aging Clin Exp Res. 2013;25:385–391. doi: 10.1007/s40520-013-0059-0. [DOI] [PubMed] [Google Scholar]
- 29.Weverling-Rijnsburger AW, Blauw GJ, Lagaay AM, et al. Total cholesterol and risk of mortality in the oldest old. Lancet. 1997;350:1119–1123. doi: 10.1016/s0140-6736(97)04430-9. [DOI] [PubMed] [Google Scholar]
- 30.Schatz IJ, Masaki K, Yano K, et al. Cholesterol and all-cause mortality in elderly people from the Honolulu Heart Program: a cohort study. Lancet. 2001;358:351–355. doi: 10.1016/S0140-6736(01)05553-2. [DOI] [PubMed] [Google Scholar]
- 31.Smith George Davey, Shipley Martin J, Marmot Michael G, Rose Geoffrey. Plasma cholesterol concentration and mortality. JAMA. 1992;267:70–76. [PubMed] [Google Scholar]