SUMMARY
Objectives
Structural magnetic resonance imaging (MRI) studies have been inconsistent in demonstrating volumetric differences in subjects with bipolar disorder. Most studies have not found difference in total gray or white matter in bipolar patients compared with controls, but there have been several studies suggesting that regional abnormalities are present. These have predominately been located in the frontal and temporal lobes. Since age has been inversely correlated with total gray matter in patients, analyses of gray matter changes in older adults or in studies that have included older subjects have been difficult. This study assessed the presence of gray matter volume, and the potential for regional volumetric differences in older adults with bipolar disorder.
Methods
Fifty-six older adults with DSM-IV bipolar disorder (mean age 60.5) and 43 non-psychiatrically ill controls (mean age 58.1) had structured interviews and MRI scanning on a 1.5T GE Scanner. Image parcellation divided the cerebrum into 16 units. Volumetric differences were examined using the multivariate linear regression models with α = 0.05.
Results
Relative to controls, the older adults with bipolar disorder had significantly smaller gray matter volumes bilaterally in the inferior frontal areas. White matter volume was also reduced in these same areas but did not reach statistical significance when controlled for gender and age. No significant difference was noted in total gray or white matter volumes.
Conclusions
Older adults with bipolar disorder showed gray matter volumetric deficits in inferior frontal lobe regions which include structures identified as contributing to the anterior limbic network.
Keywords: bipolar, neuroimaging, gray matter, white matter, elderly, brain volume
INTRODUCTION
Bipolar disorder is a significant psychiatric illness that affects approximately 1–3% of the population (Kessler et al., 2005). The pathophysiology of this disease remains poorly understood, though a dysregulation in the anterior limbic network is thought to be involved. Neuroimaging studies have attempted to evaluate the network components by quantifying changes in both the gray and white matter of the brain. Functional neuroimaging has suggested differences in frontal and temporal cortex activity between bipolar subjects and controls; however, structural neuroimaging studies have not been consistent, and at times incongruent, in locating changes in these areas (Strakowski et al., 2000).
The majority of early structural MRI studies evaluating total cortical gray matter volume changes in adults did not find a difference relative to controls (Strakowski et al., 1993, 1999; Schlaepfer et al., 1994; Harvey et al., 1994; Dupont et al., 1995; Pearlson et al., 1997; Zipursky et al., 1997). This was recently substantiated by a meta-analysis of 98 structural imaging studies in bipolar disorder (Kempton et al., 2008). Therefore, the major focus of research has been in regional differences of specific cortical volumes. Early structural findings suggested that bipolar disorder may be associated with changes in the frontal and temporal cortex and subcortical structures (Strakowski et al., 2000), though findings are not consistent.
For example, several studies have examined prefrontal cortical volumes with mixed results. Coffman et al. (1990) found that there was a trend of smaller mean frontal areas in bipolar subjects. Sax et al. (1999) reported decreased prefrontal cortical volume that was associated with impaired performance on the Continuous Performance Test (CPT). In contrast, Strakowski et al. (1999) did not find any difference in frontal volumes. However, most of these studies used rather imprecise measurements of this brain region (Strakowski et al., 2000). In contrast, Drevets et al. (1997) and Hirayasu et al. (1999) evaluated a specific region in the frontal cortex, the subgenual prefrontal cortexes, and found it decreased in bipolar subjects.
Similarly, temporal lobe measurement results have also been mixed. In their preliminary results, Altshuler et al. (1991) noted decreased temporal volume in male bipolar patients that was associated with longer illness duration; however, this finding was not replicated in their larger sample (1998). Harvey et al. (1994) found increased left temporal volume in bipolar patients compared with healthy volunteers that appeared to be due to increases in gray matter. Pearlson et al. (1997) did not observe a difference in overall temporal lobe volume between bipolar subjects and healthy controls, but they did find an enlargement in the anterior, but not posterior, superior temporal gyrus (STG) in bipolar patients compared with schizophrenic and healthy comparison subjects. Jones et al. (2008) recently reported that subjects with bipolar disorder exhibited larger temporal lobe white matter bilaterally, and that antipsychotic use may be associated with increased white matter, but not gray matter, volume.
Even focus on the smaller substructures of the temporal lobe has not shown consistencies in findings. Three MRI studies (Risch et al., 1992; Altshuler et al., 1998, 2000) evaluated the combined hippocampal–amygdalar complex volumes in bipolar subjects but did not find any substantive differences between patients with bipolar disorder, schizophrenia or controls. When the amygdala was evaluated separately, results again varied among studies. Pearlson et al. (1997) noted bipolar patients had smaller amygdala volumes than controls or patients with schizophrenia, while Altshuler et al. (1998, 2000) and Strakowski et al. (1999) found that the bipolar patients had increased amygdala volumes compared with patients with schizophrenia and controls.
Part of the difficulty in structural imaging research may be related to the effect of aging on the human brain. Postmortem findings have suggested that starting at the fourth decade, the brain begins to decrease in weight with a concomitant increase in the cerebrospinal fluid volume (Dekaban, 1978). Magnetic resonance imaging (MRI) studies confirmed these findings, showing that gray matter volume starts to decrease earlier in life (end of first decade) while white matter volume starts to decrease at the fourth decade (Pfefferbaum et al., 1994; Courchesne et al., 2000). However, there appears to be a large variability in aging effects among different areas of the brain. Several studies have noted preferential atrophy of the regions belonging to the prefrontal cortex (Coffey et al., 1992; Jernigan et al., 2001; Salat et al., 2001; Resnik et al., 2003) temporal lobe (especially the hippocampus) (Resnik et al., 2003; Tisserand et al., 2000; Tisserand et al., 2004; Raz et al., 2004), and posterior parietal cortex (Raz et al., 2004). Recent studies focusing on healthy subjects in the seventh and eighth decades of life have demonstrated similar patterns of regional gray matter volume loss, while white matter changes were noted to be uniformly wide spread (Resnik et al., 2003, Raz et al., 2004). Interestingly, the areas most sensitive to volumetric loss with aging are also structures associated with the anterior limbic network.
This study was undertaken to evaluate gray and white matter volumes in older subjects with bipolar disorder. We hypothesized that controlling for age and gender, there would be no differences in the total volume of either gray or white matter between older subjects with bipolar disorder and non-psychiatrically ill controls; however, we anticipated that there would be regional abnormalities in gray matter volume (decreased volume located in the temporal and frontal areas among bipolar subjects) but no regional abnormalities in white matter volume.
METHOD
Subjects
All subjects were participants in the NIMH-sponsored cross-sectional study for late life bipolar disorder. Participants were recruited from the psychiatric units at Duke University Medical Center (DUMC) and John Umstead Hospital (JUH), as well as community dwelling subjects with a history of bipolar disorder. All subjects completed a SCID interview and met diagnostic criteria for bipolar disorder as defined by the DSM-IV diagnostic criteria. Patients were excluded if they had evidence of dementia (scored 23 or lower on the Mini-Mental Status Examination [MMSE]), other primary psychiatric diagnosis, neurological or medical illnesses that contributed to the diagnosis, or a recent history of substance abuse. Control subjects were recruited by advertisement and had no active psychiatric illness. The study was approved by the Institutional Review Board of DUMC and the research committee of JUH.
Neuroimaging
Subjects were screened for implanted metal in their body or other contraindications to MRI. Axial images were acquired with a Signa 1.5 MR system (GE Healthcare, Milwaukee, WI) using the standard head (volumetric) radiofrequency coil. The scanner alignment light was used to adjust the head tilt and rotation so that the axial plane lights passed across the cantomeatal line and then sagittal lights were aligned with the center of the nose. A rapid sagittal localizer scan confirmed the alignment. The head position was adjusted and padded to minimized head rotation about the S–I axis. The subject’s rotation around the L–R axis was allowed to vary slightly according to the subject’s habitus in order to minimize discomfort and the resultant motion artifacts. After localization of slices to be imaged, the automated manufacturer algorithm adjusted the shim currents to obtain good magnetic field uniformity.
An axial dual-echo fast spin-echo acquisition was obtained. The pulse sequence parameters were repetition time = 4000 ms, echo time = 30 and 100 ms, 16 kHz full imaging bandwidth, echo train length = 16, a 256 × 256 matrix, 3 mm section thickness, 1 excitation and a 20 cm field of view. The images were acquired in two separate acquisitions with a 3-mm gap between sections. The second acquisition was offset by 3 mm from the first to create contiguous sections.
Images were processed in DUMC’s Neuropsychiatric Imaging Research Laboratory (NIRL) on SUN workstations. Volume measurements were performed with a NIRL-modified version of MrX software, which was created by GE Corporate Research and Development (Schenectady, NY) and originally modified by Brigham and Women’s Hospital for image segmentation (Boston, MA). The segmentation protocol and process for converting image intensity to segmented tissue types has been previously described (Payne et al., 2002). Briefly, it is a semi-automated method that uses the multiple MR contrasts available to identify different tissue classifications through a ‘seeding’ process, in which a trained analyst manually selects pixels in each tissue type to be identified (gray matter, white matter, cerebrospinal fluid, lesions or background). The seeding protocol identifies the range of signal intensities that characterize each tissue type. Once the brain is segmented into tissue types and the non-brain tissue stripped away through a masking procedure, the cerebrum is separated out (from cerebellum and brainstem) using tracing and connectivity functions.
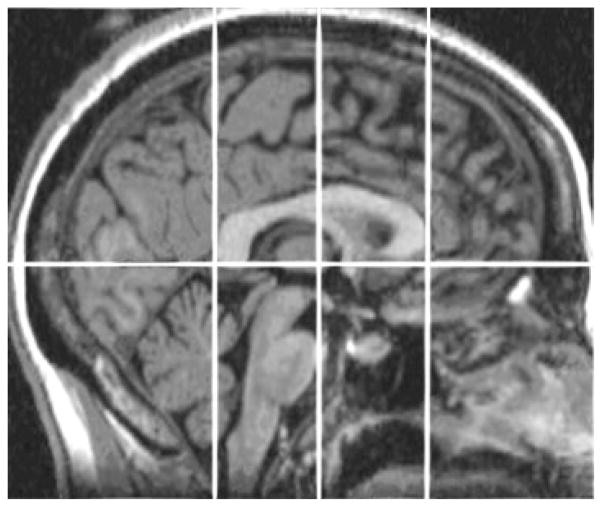
Additional procedures were required to obtain regional measures of gray and white matter volume in the cerebrum. This subdivision was performed using the GRID program which was developed by NIRL. Each scan was re-aligned to a standard orientation, including making the anterior commissure–posterior commissure (AC–PC) plane horizontal. Following this, the planes were defined. First, a mid-sagittal plane was used to divide the left and right cerebral hemispheres. Then, an axial plane was created along the AC–PC plane, dividing superior regions from inferior. Next, coronal planes were created perpendicular to the axial plane at the anterior and posterior extent of the corpus callosum. Finally, a third coronal plane was created at the midpoint between the first two coronal planes, which divided the brain into anterior and posterior halves in each hemisphere. See Figures 1 and 2 for illustration of these divisions and numbering of regions.
Figure 1.
Placement of dividing planes. In this mid-sagittal view, AC–PC and corpus callosum (anterior, posterior, and middle) planes are visible.
Figure 2.
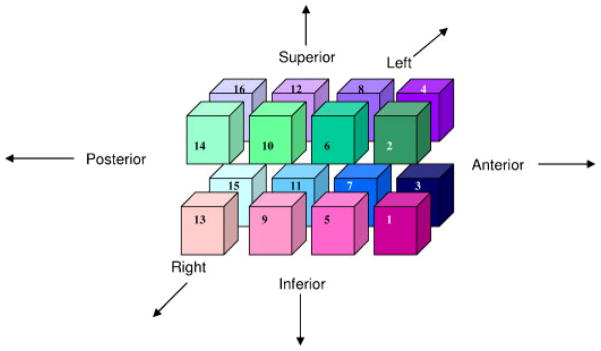
Numbered diagram of the 16 parcellation regions. Regions 1–4 are anterior to corpus callosum. Regions 13–16 are posterior to corpus callosum. Odd-numbered regions are inferior to AC–PC plane, while even-numbered are above. Plane separating regions 5–8 from 9–12 is midway between anterior and posterior of corpus callosum.
Statistical analysis
Descriptive statistics, means, standard deviations, and percentages were used for continuous variables and categorical variables, respectively. Individual parcellation regions were standardized using the values of the total brain volume. Non-parametric, Wilcoxon tests were used to determine bivariate differences between the subject and control groups on values of volumetric variables of the image parcellation. Association between cases and categorical variables (age and gender) was examined using Chi-squared statistic. Logistic regression analysis was used was used to examine the case vs control status on the image parcellation data dichotomized at their median. These models were controlled for age and gender.
RESULTS
Demographic data
Fifty-six older adults with DSM-IV bipolar disorder (mean age 60.5 ± 9.1; range 51–89) and 49 non-psychiatrically ill controls (mean age 58.1 ± 6.3; range 51–77) were recruited for this study. No significant differences were noted in age (p = 0.4038), gender (bipolar70%female;controls59%female;p = 0.2665), or race (bipolar 79% white; controls 89% white; p = 0.1600).
Gray matter volume
There were no differences in total gray matter volume between bipolar subjects and controls (see Table 1). Chi-square test found a decrease in gray matter in bipolar subjects in the anterior half of the brain (inclusive of parcellation areas 1–8) (p = 0.0375). The primary areas in which regional deficits between the two groups were noted in the anterior–inferior brain regions (parcellation areas 1 (p = 0.0150) and 3 (p = 0.0057)). This deficit in gray matter volume in bipolar subjects compared to non-psychiatrically ill controls remained significant when controlled for age and gender (parcellation areas 1 (p = 0.0089), 3 (p = 0.0004)).
Table 1.
Regional gray matter findings
| Parcellation area | Older bipolar cases | Older controls | Wilcoxon test | Odds ratios (95% CI) |
|---|---|---|---|---|
| 1 | 7.92 ± 3.0 | 9.30 ± 2.9 | 0.0150 | 0.297 (0.120–0.738) |
| 2 | 25.20 ± 4.0 | 26.94 ± 5.3 | 0.2495 | — |
| 3 | 7.09 ± 2.6 | 8.31 ± 2.4 | 0.0057 | 0.165 (0.060–0.451) |
| 4 | 21.34 ± 3.4 | 22.49 ± 4.2 | 0.3399 | — |
| 5 | 29.99 ± 5.0 | 32.21 ± 5.2 | 0.0796 | — |
| 6 | 52.12 ± 6.8 | 55.49 ± 7.2 | 0.0571 | — |
| 7 | 28.02 ± 4.4 | 29.80 ± 3.8 | 0.0534 | — |
| 8 | 44.91 ± 5.9 | 47.08 ± 5.3 | 0.1568 | — |
| 9 | 25.26 ± 4.0 | 26.03 ± 4.4 | 0.4725 | — |
| 10 | 53.77 ± 8.4 | 57.71 ± 8.7 | 0.0562 | — |
| 11 | 25.17 ± 3.7 | 26.25 ± 3.8 | 0.3361 | — |
| 12 | 51.60 ± 7.7 | 54.75 ± 8.6 | 0.1264 | — |
| 13 | 15.14 ± 4.6 | 15.40 ± 4.6 | 0.5109 | — |
| 14 | 45.57 ± 9.6 | 47.37 ± 8.7 | 0.3101 | — |
| 15 | 19.77 ± 5.6 | 20.28 ± 5.4 | 0.4094 | — |
| 16 | 50.80 ± 8.8 | 52.72 ± 8.7 | 0.3881 | — |
White matter volume
There were no differences noted in total white matter volume between older bipolar subjects and non-psychiatrically ill controls (see Table 2). Chi-square tests did find a decrease in white matter volume in anterior brain regions (parcellation areas 1( p = 0.0318), 3 (p = 0.0456), and 8 (p = 0.0473)) of bipolar subjects compared with non-psychiatrically ill controls. However, these differences did not remain significant when controlled for age and gender.
Table 2.
Regional white matter findings
| Parcellation area | Older bipolar cases | Older controls | Wilcoxon test | Odds ratios (95% CI) |
|---|---|---|---|---|
| 1 | 3.03 ± 1.9 | 3.61 ± 1.6 | 0.0318 | 0.481 (0.203–1.137) |
| 2 | 11.28 ± 3.2 | 12.45 ± 4.0 | 0.2059 | — |
| 3 | 3.49 ± 2.1 | 4.07 ± 1.7 | 0.0456 | 0.511 (0.218–1.198) |
| 4 | 13.31 ± 3.3 | 14.48 ± 3.9 | 0.1568 | — |
| 5 | 7.56 ± 2.6 | 7.99 ± 2.5 | 0.4269 | — |
| 6 | 35.49 ± 7.7 | 37.97 ± 8.9 | 0.1546 | — |
| 7 | 9.24 ± 3.0 | 9.91 ± 2.7 | 0.5689 | — |
| 8 | 39.71 ± 7.5 | 42.84 ± 8.3 | 0.0473 | 0.425 (0.172–1.049) |
| 9 | 14.82 ± 3.7 | 14.39 ± 3.7 | 0.4820 | — |
| 10 | 55.90 ± 11.0 | 58.51 ± 10.9 | 0.1927 | — |
| 11 | 16.24 ± 3.94 | 16.39 ± 3.7 | 0.7422 | — |
| 12 | 55.05 ± 10.8 | 58.89 ± 12.2 | 0.0534 | — |
| 13 | 4.91 ± 3.0 | 16.95 ± 7.0 | 0.6518 | — |
| 14 | 46.95 ± 11.23 | 46.55 ± 9.3 | 0.8363 | — |
| 15 | 17.32 ± 7.5 | 16.05 ± 6.5 | 0.8844 | — |
| 16 | 41.28 ± 11.1 | 41.55 ± 10.0 | 0.7654 | — |
DISCUSSION
We did not find any significant differences in total gray or white matter brain volumes between older bipolar subjects and controls. However, using a parcellation technique to examine volumetric regions of gray and white matter in the brain, we did observe a bilateral decrease in cortical gray matter volume in the anterior, inferior brain regions of older bipolar subjects compared with control subjects. White matter volume in these areas also appeared to be decreased, though this did not remain statistically significant when age and gender were controlled in the analyses. No statistical differences were noted in the temporal or parietal areas for either gray or white matter.
While our techniques differ from other structural neuroimaging studies, we note our findings are consistent with the hypothesized mood regulating network as well as other reports of structural abnormalities in bipolar disorder. Few studies have found decreases in total gray or white matter in the brains of subjects with bipolar disorder; however, when considered regionally, specific differences have been noted in the prefrontal cortex, subcortical, and medial temporal structures (Strakowski et al., 2000; Strakowski et al., 2005; Nugent et al., 2006). Thus, the major focus of structural neuroimaging has been on defining regional changes either through “region of interest”, “voxel-based mapping”, or density studies.
The prefrontal cortex is comprised of several discrete brain regions that are difficult to delineate by currently available resolution techniques. Five studies have evaluated the relatively large prefrontal brain regions in bipolar subjects with mixed findings. Three studies (Coffman et al., 1990; Sax et al., 1999; Haznedar et al., 2005) noted a decrease in prefrontal volumes in bipolar subjects, while four studies (Strakowski et al., 1993; Schlaepfer et al., 1994; López-Larson et al., 2002; McDonald et al., 2005) did not identify any changes. These discrepancies may be related to differences in MRI acquisition or segmentation protocols, subregion delineation, or patient populations.
Interestingly, even studies that did not identify volumetric changes in the larger area may still have noted changes in specific subregions. For example, López-Larson et al. (2002) did not find any significant differences in prefrontal volumes overall in bipolar subjects, but they did find smaller gray matter volume in the left superior and middle and right prefrontal subregions. Other researchers have identified specific prefrontal subregions, such as the subgenual prefrontal cortex (Drevets et al., 1997; Hirayasu et al., 1999) and the left anterior cingulate (Sassi et al., 2004), with decreased gray matter volume in bipolar subjects. The areas in our comparable parcellation region include the middle frontal gyrus, inferior frontal gyrus, orbitofrontal gyrus, gyrus rectus and the anterior cingulate. Thus, our findings are consistent with the hypothesis that changes in the structures that comprise the proposed anterior limbic network (consisting of prefrontal–striatal–thalamic structures that are believed to modulate socioemotional behaviors) may be associated with bipolar disorder (Strakowski et al., 2005). Our findings are also consistent with a larger literature in structural neuroimaging in late life depression that has found significant prefrontal cortex abnormalities (with significant decreased volumes in the anterior cingulated, gyrus rectus, and orbitofrontal areas) (see Ballmaier et al., 2004).
On a further note, López-Larson et al. (2002) found that a longer duration of illness was also associated with smaller left inferior prefrontal gray volumes. This latter finding is consistent with observations by Brambilla et al. (2001) who noted increased age in middle-aged bipolar subjects was associated with smaller gray matter volumes, and suggesting that subjects with bipolar disorder may show increased gray matter loss as they age or as a consequence of recurrent affective episodes (see also Moorhead et al., 2007). Our finding of decreased frontal volumes in older adults with bipolar disorder compared with non-psychiatric ill group may support findings that not only is specific regional gray matter volume loss associated with bipolar disorder, but also that gray matter loss may be more apparent in later life.
There are several limitations to our study. First, the data is derived from a regional parcellation of the brain and does not measure specific structures. Therefore, these results should be viewed as providing guidance for future, more specific analyses as well as support for current studies testing the anterior limbic network hypothesis. Second, the hypotheses are exploratory, and hence, no adjustment is made for multiple testing to correction for type 2 error. If a correction was made for the number of tests conducted, some of the results will no longer be significant. Finally, though we were able to control for gender and age (and subjects were screened for dementia or substance abuse), other factors could also influence volumetric brain matter changes. Future studies will need to further elucidate the role of medication effects, duration of illness, number/type of episodes, medical illness or compliance with treatment.
Our observation of decreased gray matter volume in the inferior frontal areas of the brain suggest that these areas may play a primary role in the mood and cognitive symptoms of bipolar disorder, and complements previous structural neuroimaging studies that suggest an underlying neuropathology in the anterior limbic network. Further, these data raise the possibility that gray matter volume loss may accelerate in certain regions of bipolar subjects as they age. Additional studies examining the effect of illness course and duration, medication status, and cognitive functioning in older adults with bipolar disorder may further clarify these findings.
KEY POINTS.
Structural MRI studies have identified regional volumetric changes in the frontal and temporal gray matter that have been associated with bipolar disorder.
Aging is also associated with gray matter volume loss, particularly in the frontal and temporal lobes.
In late life bipolar disorder, gray matter volume deficits are found in the inferior frontal regions independent of age.
The areas of the brain most affected by volume changes appear to be structures associated with the putative anterior limbic network.
Acknowledgments
The authors thank Ms. Denise F. Messer, M.A. for the parcellation volume measurements. This study was supported by NIMH grants #MH57027 and #MH68848, and NARSAD Junior Investigator Award.
Footnotes
CONFLICT OF INTEREST
There are no conflicts of interest by any author for this study.
References
- Altshuler LL, Conrad A, Hauser P, et al. Reduction of temporal lobe volume in bipolar disorder: a preliminary report of magnetic resonance imaging (letter) Arch Gen Psychiatry. 1991;48:482–483. doi: 10.1001/archpsyc.1991.01810290094018. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Brieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Arch Gen Psychiatry. 1998;55:663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, et al. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Toga AW, Blanton RE, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161(1):99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Harenski K, Nicoletti M, et al. Differential effects of age on brain gray matter in bipolar patients and healthy individuals. Neuropsychobiology. 2001;43(4):242–247. doi: 10.1159/000054897. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Wilkinson WE, Parashos IA, et al. Quantitative cerebral anatomy of the aging human brain: a cross-sectional study using magnetic resonance imaging. Neurology. 1992;42:527–536. doi: 10.1212/wnl.42.3.527. [DOI] [PubMed] [Google Scholar]
- Coffman JA, Bornstein RA, Olson SC, Schwarzkopf SB, Nasrallah HA. Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biol Psychiatry. 1990;27(11):1188–1196. doi: 10.1016/0006-3223(90)90416-y. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Dekaban AS. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Dupont RM, Jernigan TL, Heindel W, et al. Magnetic resonance imaging and mood disorders. Localization of white matter and other subcortical abnormalities. Arch Gen Psychiatry. 1995;52(9):747–755. doi: 10.1001/archpsyc.1995.03950210041009. [DOI] [PubMed] [Google Scholar]
- Harvey I, Persaud R, Ron MA, Baker G, Murray RM. Volumetric MRI measurements in bipolars compared with schizophrenics and healthy controls. Psychol Med. 1994;24(3):689–699. doi: 10.1017/s0033291700027847. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Roversi F, Pallanti S, et al. Fronto-thalamostriatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biol Psychiatry. 2005;57(7):733–742. doi: 10.1016/j.biopsych.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, Shenton ME, Salisbury DF, et al. Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry. 1999;156(7):1091–1093. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22(4):581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jones LD, Payne ME, Messer DF, et al. Temporal lobe volume in bipolar disorder: relationship with diagnosis and antipsychotic medication use. J Affect Disord. 2008 Aug 6; doi: 10.1016/j.jad.2008.07.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Geddes JR, Ettinger U, Williams SCR, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65(9):1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52(2):93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- McDonald C, Bullmore E, Sham P, et al. Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry. 2005;186:369–377. doi: 10.1192/bjp.186.5.369. [DOI] [PubMed] [Google Scholar]
- Moorhead TW, McKirdy J, Sussmann JE, et al. Progressive gray matter loss in patients with bipolar disorder. Biol Psychiatry. 2007;62(8):894–900. doi: 10.1016/j.biopsych.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Milham MP, Bain EE, et al. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage. 2006;30(2):485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Payne ME, Fetzer DL, MacFall JR, Provenzale JM, Byrum CE, Krishnan KR. Development of a semi-automated method for quantification of MRI gray and white matter lesions in geriatric subjects. Psychiatry Res. 2002;15(1–2):63–77. doi: 10.1016/s0925-4927(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Barta PE, Powers RE, et al. Ziskind-Somerfeld Research Award1996. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry. 1997;41(1):1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62:433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23(8):3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch SC, Lewine RJ, Kalin NH, et al. Limbic-hypothalamic-pituitary-adrenal axis activity and ventricular-to-brain ratio studies in affective illness and schizophrenia. Neuropsychopharmacology. 1992;6:95–100. [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Selective preservation and degeneration within the prefrontal cortex in aging and Alzheimer disease. Arch Neurol. 2001;58:1403–1408. doi: 10.1001/archneur.58.9.1403. [DOI] [PubMed] [Google Scholar]
- Sassi RB, Brambilla P, Hatch JP, et al. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol Psychiatry. 2004;56(7):467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM, Zimmerman ME, DelBello MP, Keck PE, Jr, Hawkins JM. Frontosubcortical neuroanatomy and the continuous performance test in mania. Am J Psychiatry. 1999;156(1):139–141. doi: 10.1176/ajp.156.1.139. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Harris GJ, Tien AY, et al. Decreased regional cortical gray matter volume in schizophrenia. Am J Psychiatry. 1994;51(6):842–848. doi: 10.1176/ajp.151.6.842. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello M, Adler CMKM, Sax KW. Neuroimaging in bipolar disorder. Bipolar Disord. 2000;2:148–164. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56(3):254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Wilson DR, Tohen M, Woods BT, Douglass AW, Stoll AL. Structural brain abnormalities in first-episode mania. Biol Psychiatry. 1993;33(8–9):602–609. doi: 10.1016/0006-3223(93)90098-x. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex. 2004;14(9):966–973. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Visser PJ, van Boxtel MP, Jolles J. The relation between global and limbic brain volumes on MRI and cognitive performance in healthy individuals across the age range. Neurobiol Aging. 2000;21:569–576. doi: 10.1016/s0197-4580(00)00133-0. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Seeman MV, Bury A, Langevin R, Wortzman G, Katz R. Deficits in gray matter volume are present in schizophrenia but not bipolar disorder. Schizophr Res. 1997;26(2–3):85–92. doi: 10.1016/s0920-9964(97)00042-x. [DOI] [PubMed] [Google Scholar]