Abstract
Differentiated CD4+ T cells preserve plasticity under various conditions. However, the stability of Th1 cells is unclear, as is whether Th1 cells can convert into Th17 cells and thereby contribute to the generation of IFN-γ+IL-17+CD4+ T cells, the number of which correlates with severity of colitis. We investigated whether IFN-γ+Th1 cells can convert into Th17 cells under intestinal inflammation and the mechanisms involved. IFN-γThy1.1+ Th1 cells were generated by culturing naïve CD4+ T cells from IFN-γThy1.1 CBir1 TCR-Tg reporter mice, whose TCR is specific for an immunodominant microbiota antigen, CBir1 flagellin, under Th1 polarizing conditions. IFN-γThy1.1+ Th1 cells induced colitis in Rag−/− mice after adoptive transfer and converted into IL-17+Th17, but not Foxp3+Treg cells in the inflamed intestines. TGF-β and IL-6, but not IL-1β and IL-23, regulated Th1 conversion into Th17 cells. TGF-β induction of transcriptional factor Runx1 is crucial for the conversion, since silencing Runx1 by siRNA inhibited Th1 conversion into Th17 cells. Furthermore, TGF-β enhanced histone H3K9 acetylation but inhibited H3K9 trimethylation of Runx1- and RORγt-binding sites on il-17 or rorc genes in Th1 cells. We conclude that Th1 cells convert into Th17 cells under inflammatory conditions in intestines, which is possibly mediated by TGF-β induction of Runx1.
Keywords: TGF-β, Th1, Th17, Runx1, colitis
Introduction
Multiple levels of regulations control the intestinal homeostasis, including innate and adaptive mechanisms [1, 2]. Among multiple specialized lymphocyte populations within the intestinal tract, CD4+ T cells are one of the dominant cell populations of the adaptive immune system and mediate diverse host protective and homeostatic responses. However, accumulating reports have demonstrated that CD4+ T cells, once dysregulated, also play pathogenic roles and trigger chronic intestinal inflammation, resulting in Crohn’s disease and ulcerative colitis, which are collectively termed inflammatory bowel disease (IBD)[3]. CD4+ T cells are classified based on their production of distinct cytokines. Th1 cells express IFN-γ as their signature cytokine, whereas Th17 cells express IL-17, IL-21 and IL-22 [4, 5]. Both Th1 and Th17 cells have been implicated in the pathogenesis of IBD in humans and mice, and accumulating evidence indicates that Th1 and Th17 cells present in the intestines during inflammation may functionally differ from those cells under steady-state conditions [6, 7]. Whereas steady-state Th1 and Th17 cells are largely IFN-γ+- and IL-17+-single positive, respectively, a substantial number of CD4+ T cells presenting in inflamed intestines is IFN-γ+ IL-17+ double positive [6, 8, 9]. Those populations have been proposed to play pathogenic roles in IBD as well as in other autoimmune diseases [10, 11]. It has been shown that treatment with IL-1β, IL-6, and IL-23 triggers the differentiation of IFNγ+IL-17+CD4+ T cells from naïve CD4+ cells in mice [11]. Stimulation with IL-12 or TNF-α promotes the generation of IFN-γ+IL-17+CD4+ T cells from Th17 cells in humans [12, 13]. However, how IFN-γ+IL-17+CD4+ T cells are generated remains elusive. Differentiation of CD4+ T cells is heavily influenced by cytokine milieu, oxygen supply, and multiple environmental factors. In addition to TCR stimulation and co-stimulatory molecules, cytokines serve as a third signal during T-cell differentiation. IL-12 promotes the polarization of Th1 cells through induction of transcription factor T-bet, whereas TGF-β and IL-6 promote the polarization of Th17 cells through induction of transcription factor RORγt. However, growing evidence has shown that differentiated CD4+ T cells preserve the flexibility to alter phenotypes upon external stimulation, revealing the plasticity of CD4+ T cells, especially under inflammatory conditions in intestines. We and others have shown that high levels of local IL-12 and IL-23 promote the conversion of Th17 cells into Th1 cells with abrogation of IL-17 production and a parallel gain of IFN-γ production in inflamed intestines [14, 15]. Moreover, conversion of Th17 cells into Th1 cells is essential for the development of various autoimmune diseases, including EAE and type 1 diabetes [10, 11]. While the plasticity of Treg and Th17 are well-documented [16, 17], the stability of Th1 remains controversial. Some epigenetic studies revealed high stability for Th1 cells [18, 19], a finding which is supported by numerous reports of Treg and Th17 cells conversion into Th1 cells but not vice versa [15-17]. However, several recent reports of Th1 conversion into Th2, Treg and Tfh cells under various conditions argue against the absolute stability of Th1 lineage [20-22].
In this study, we investigated the role of microbiota Ag-specific Th1 cells in colitis induction and the stability of Th1 cells under inflammatory conditions in intestines by using IFN-γThy1.1 CBir1 TCR transgenic reporter mice, whose TCRs are specific for an intestinal microbiota antigen, CBir1 flagellin. To this end, we demonstrated that adoptive transfer of purified CBir1-specific IFN-γ+Th1 cells to Rag−/− mice induced colitis and that these Th1 cells can convert into IL-17+ Th17, but not Foxp3+ Treg, cells in the inflamed intestines. TGF-β and IL-6, but not IL-1β and IL-23, regulated Th1 conversion into Th17 cells. Further, TGF-β induction of transcriptional factor Runx1 was essential for the conversion, in that silencing Runx1 by siRNA inhibited Th1 conversion into Th17 cells.
Results
IL-17+IFN-γ−, IL-17−IFN-γ+, and IL-17+IFN-γ+ CD4+ T cells are present in inflamed intestines
We have previously established a microbiota Ag-specific T cell-mediated animal model of colitis by adoptive transfer of CD4+ T cells from CBir1 TCR-transgenic (CBir1 Tg) mice, which are specific for an immunodominant bacterial commensal flagellin, into Rag−/− mice [23]. To examine the phenotypes of CD4+ T cells presenting in the inflamed intestines, we isolated CBir1 Tg CD4+ T cells and transferred them through i.v. route into Rag−/− mice for colitis development. We also injected PBS or OTII CD4+ T cells into Rag−/− mice to serve as negative controls. At 4 weeks post-adoptive transfer, the Rag−/− recipient mice were sacrificed, and the histopathology of the small intestine, large intestine, and cecum determined. As shown previously, no Rag−/− mice receiving PBS developed colitis; however, the Rag−/− recipient mice that received CBir1 T cells developed severe colitis (Fig. 1A and B). In contrast, the Rag−/− recipient mice that received OTII T cells, which are specific for a model antigen, Ovalbumin, not presenting an intestinal lumen, did not develop severe colitis (data not shown). The small intestines of these recipient mice did not show any inflammation (data not shown). IL-17−IFN-γ+ Th1 and IL-17+IFN-γ− Th17 cells, as well as significant proportions of IL-17+IFN-γ+ double-positive CD4+ T cells, were present in the lamina propria of inflamed intestines (Fig. 1C). Of note, the levels of IL-4+ Th2 cells were undetected in the inflamed intestinal lamina propria (data not shown). Collectively, these data indicated that microbiota Ag-specific T cell are able to induce colitis and Th1, Th17 cells, and IL-17+IFN-γ+ double-positive CD4+ T cells, are present in the colon during colitis.
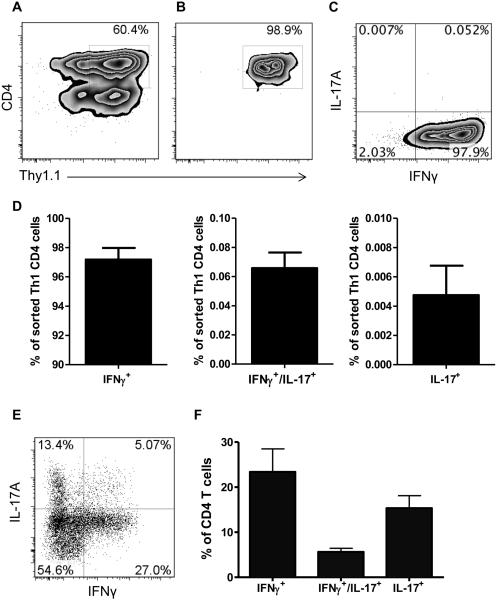
Figure 1. IL-17+, IFN-γ+, and IL-17+IFN-γ+ CD4+ cells are found in the lamina propria of colitic mice.
Naïve splenic CD4+ T cells from CBir1 Tg mice were i.v. injected into Rag−/− mice. Four weeks post T-cell transfer, the severity of colonic inflammation was assessed by histological analysis after hemytoxlin and eosin staining. (A) Histopathology of colon (original magnification, 20×). (B) Pathological scores of accessed colons. Bars show mean +/− SEM of 4 mice from a single experiment representative of 4 experiments. (C) Flow cytometry of CD4+ cells isolated from colon lamina propria of Rag−/− recipient measured for expression of IFN-γ and IL-17. One representative plot out of 4 experiments is shown.
IFN-γ+ Th1 cells convert into IL-17-producing Th17 cells but not Foxp3+ Treg cells
We previously demonstrated that IL-17+ Th17 cells could convert to IFN-γ+ Th1 cells through IL-17 induction of mucosal innate IL-12 and IL-23 production in inflamed intestines [6], which could at least partially explain the presence of IL-17+IFN-γ+ T cells. However, as recent reports demonstrated the conversion of Th1 cells into Th2, Tregs, and Tfh under various conditions [20-22], we investigated whether IFN-γ+ Th1 cells could also convert into IL-17+ Th17 cells, and thus contribute to the generation of IL-17+IFN-γ+ T cells under inflammatory conditions. Taking advantage of IFN-γThy1.1 CBir1 Tg reporter mice [15], we generated Th1 cells under standard Th1 polarizing conditions for 4-5 days and then sorted them by using a FACS sorter. We were able to sort IFN-γThy1.1+ CD4+ Th1 cells with a purity of 98.9% (Fig. 2A and B), which expressed IFN-γ but not IL-17 (Fig. 2C and D). We then transferred the sorted CBir1 Tg IFN-γThy1.1+ Th1 cells to Rag−/− mice. At 8 weeks post Th1-cell transfer, the recipient mice developed mild colitis (data not shown). When we determined the phenotypes of CD4+ T cells isolated from the intestinal lamina propria, most Th1 cells were found to have lost expression of IFN-γ (over 65%). Although there was no Foxp3 expression by those adoptively transferred Th1 cells, which is consistent with our previous reports [15], significant portions of CD4+ T cells expressed IL-17 (Fig. 2E and F), including both IL-17 single-positive and IL-17+IFN-γ+ double-positive T cells. Collectively, these data indicated that conversion of Th1 cells into Th17, but not Treg, cells occurred under inflammatory conditions in the intestines.
Figure 2. IFN-γ-producing Th1 cells convert into IL-17-producing Th17 cells in inflamed colon.
Splenic CD4+ T cells from IFN-γThy1.1 CBir1 TCR Tg reporter mice were cultured under classical Th1 differentiation conditions with mIL-12/anti-IL-4 for 4 days. IFN-γThy1.1+CD4+ double-positive cells were sorted. (A) Gating strategy for sorting of IFN-γThy1.1+CD4+ cells. Representative flow cytometry plot of splenic T cells before sorting, stained for CD4 and Thy1.1. (B-D) After sorting. (B) Representative flow cytometry plot of IFN-γThy1.1+CD4+ cells. (C) Representative flow cytometry plot of IL-17 and IFN-γ. (D) Bar charts of cytokine expression from pool of 2 experiments. (E) Sorted CBir1 Tg IFN-γThy1.1+ CD4+ cells were transferred into Rag−/− mice. 8 weeks post-transfer, lamina propria CD4+ cells of Rag−/− recipient mice were measured for expression of IFN-γ and IL-17 by flow cytometry. One representative plot out of 2 experiments is shown. (F) Bar charts of cytokine expression of lamina propria CD4+ T cells 8 weeks post-adoptive to Rag−/− mice from pool of 2 experiments.
TGF-β, IL-6 and IL-2 differentially regulate the conversion of Th1 cells into Th17 cells
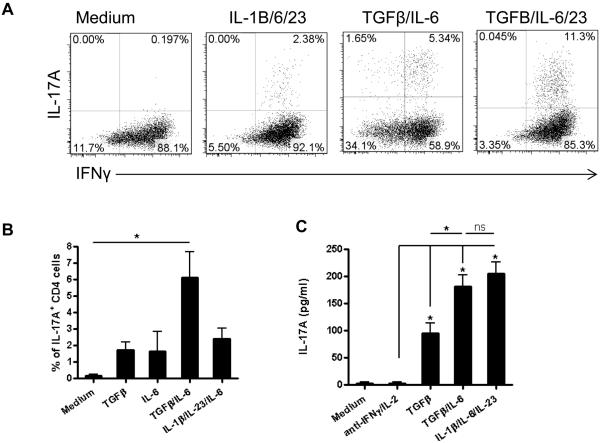
Among cytokines present at an elevated level in inflamed intestines of colitic mice, Th17-skewing cytokines, TGF-β, IL-6, IL-1β and IL-23 [24], could potentially induce Th1 conversion into Th17 cells, whereas IL-2 would antagonize Th17 responses. IL-2 not only affects T cell proliferation, but also regulates T cell differentiation by differentially regulating key cytokine receptors, IL-12Rβ2 and IL-6R, and by activating STAT5, which competes with STAT3 for the IL-17 promoter binding site, resulting in the enhancement of Th1 but the inhibition of Th17 cell differentiation [25-27]. There is evidence that the consumption of IL-2 in the microenvironment can enhance Th17-cell induction [28]. Hence, we then investigated which cytokines promoted Th1 conversion into Th17 cells. In conjunction with the neutralization of IL-2 and IFN-γ, we treated differentiated and sorted CBir1 Tg IFN-γThy1.1+ Th1 cells with various cytokines, including TGF-β/IL-6/IL-23 and IL-1β/IL-6/IL-23. We examined the expression of IFN-γ and IL-17 in day 5 cultures with flow cytometry analysis. Neutralization of IL-2 and IFN-γ alone did not induce Th1 cell expression of IL-17. While IL-1β/IL-6/IL-23 only induced Th1 conversion into Th17 cells at a minimal level, TGF-β and IL-6 greatly induced Th1 cells to express IL-17 but not Foxp3. Expression of IL-17 was further increased with TGF-β/IL-6/IL-23 (Fig. 3A and Supporting Information Fig. 1). TGF-β or IL-6 alone only exerted a minimal effect to Th17 cell conversion (Fig. 3B and C). Treatment with IL-1β or IL-23 alone did not stimulate IL-17 production (data not shown).
Figure 3. TGF-β and IL-6 stimulate Th1 cells to convert into IL-17-producing Th17 cells.
(A) IFN-γThy1.1+ CBir1 Tg CD4+ cells were cultured with splenic APCs and different cytokines in the presence of anti-IL-2 and anti-IFN-γ antibodies for 5 days. IL-17 and IFN-γ expression was determined by flow cytometry. (B) The percentage of IL-17A+ CD4+ cells was quantified and shown as mean +/− SD of three experiments. (C) The total IL-17 production in day 5 culture supernatants was detected by an ELISA and shown as mean +/− SD of three experiments. *p < 0.05, One-way ANOVA with Tukey’s t test.
TGF-β and IL-6 regulate the expression of Rorα, Rorc and Runx1 in Th1 cells
We then investigated the mechanisms involved in the TGF-β/IL-6 promotion of IL-17 production in Th1 cells. It has been shown that RORγt, RORα, Runx1, and Batf can regulate IL-17 expression and Th17 cell differentiation [4, 29]. To determine whether TGF-β and IL-6 regulate expression of those transcription factors in Th1 cells, we treated CBir1 Tg IFN-γThy1.1+ Th1 cells with TGF-β, IL-6, or both in conjunction with neutralizing antibodies against IL-2 and IFN-γ for 4 days, and determined gene expression by real-time PCR. We found that TGF-β promoted Th1 cell expression of Rorc, Rorα, and Runx1. IL-6 promoted expression of Rorc and Rorα at a lower level than that with TGF-β. However, IL-6 did not affect Runx1 expression. In addition, IL-6 further enhanced TGF-β-induced expression of Rorc but not of Rorα and Runx1 (Fig. 4A-C). TGF-β and IL-6, either alone or in combination, did not affect Batf expression in Th1 cells (Fig. 4D).
Figure 4. TGF-β dominates the induction of Th17-associated genes in Th1 cells.
IFN-γThy1.1+ CBir1 Tg CD4+ cells were cultured with splenic APCs with TGF-β, IL-6 or TGF-β/IL-6, and anti-IL-2 and anti-IFN-γ antibodies for 4 days. The expression of (A) rorc (B) rorα (C) runx1 and (D) Batf was detected by RT-PCR. Data are shown as mean +/− SEM of 3 experiments, normalized to gapdh expression. *p < 0.05, One-way ANOVA with Tukey’s t test.
TGF-β-induced Runx1 mediates Th1 conversion into Th17 cells
It has been shown that Runx1 induces transactivation of the rorc promoter and promotes IL-17 production [30]. We found that treatment with TGF-β induced the transcription of Runx1 in IFN-γThy1.1+ Th1 cells as early as 24 hr post treatment (data not shown). To determine the role of TGF-β-induced Runx1 in Th1 conversion into Th17 cells, we used Runx1 siRNA to knock down Runx1 expression in IFN-γThy1.1+ Th1 cells, and then treated the cells with TGF-β and anti-IL-2/anti-IFN-γ antibodies. We examined via real-time PCR whether Runx1 knockdown would influence the expression of genes associated with Th17 cells. We were able to knockdown 50% of Runx1 expression in Th1 cells after transfection with Runx1 siRNA (Fig. 5A). Knockdown of Runx1 greatly decreased the expression of IL-17, RORγt and RORα (Fig. 5B-D), indicating that TGF-β-induced Runx1 plays an essential role in Th1 conversion into Th17 cells.
Figure 5. TGF-β-induced Runx1 plays a crucial role in Th1 conversion into Th17 cells.
IFN-γThy1.1+ CBir1 Tg CD4+ cells were transfected with Runx1 siRNA or control siRNA, allowed to rest for 24 h and stimulated with anti-CD3/anti-CD28 with TGF-β and anti-IL-2/anti-IFN-γ for 2 days. (A) siRNA knockdown efficiency was confirmed by RT-PCR of runx1 at 24 h post transfection. The fold change in runx1 expression is shown as mean +/− SEM of 2 samples from a representative of two experiments. Values are normalized to gapdh expression. (B, D) The expression of (B) il-17, (C) rorc and (D) rorα was detected by RT-PCR. Results are shown as mean +/− SEM of 2 samples from a representative of two experiments, and normalized to gapdh expression. *p < 0.05, Student’s t-test.
TGF-β and IL-6 increase the accessibility of Runx1 binding sites in promoters of rorc and il-17a
The consensus Runx1- and RORγt-binding sites in human and mouse il-17 loci were reported previously [31]. The Runx1 binding site, TGTGGT, is located at a promoter region, ranging from −2 kb to the start codon. RORγt-binding sites, AGGTCA and TGACCT, are located in both the promoter region and a distal upstream CNS-5 enhancer region. On the other hand, potential Runx1-binding regions but not the exact Runx1-binding sites in the mouse rorc gene have been described previously [32]. From there, we further narrowed down and identified three Runx1-binding sites in the rorc locus by aligning human and mouse rorc loci. Three Runx1-binding sites containing a perfect consensus binding sequence for Runx1, TGTGGT, were identified in human and mouse rorc loci (Supplemental Fig. 2). It indicates that, in both mouse and human rorc gene, the binding of Runx1 protein to regulatory regions of the rorc gene possibly influences the transcription of rorc gene.
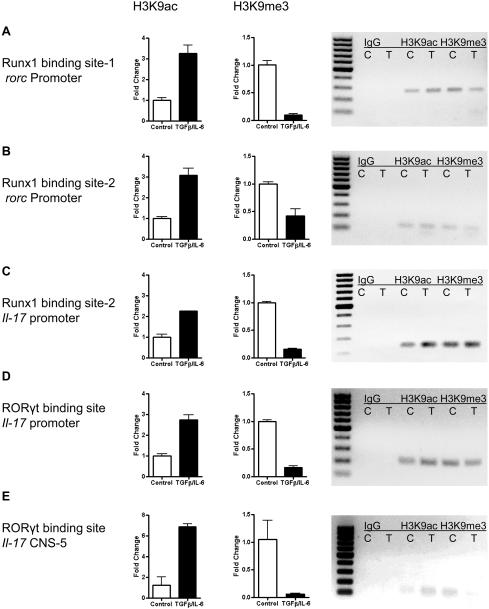
Histone modification markers are widely used to investigate the plasticity of CD4+ T cell differentiation [33, 34]. To examine the effect of TGF-β on accessibility of Runx1- and RORγt-binding sites located at mouse rorc and il-17 genes in Th1 cells, we performed a ChIP assay by using antibodies against H3K9ac and H3K9me3. The acetylation on the 9th lysine of H3 histone (H3K9) indicates an accessible state of the transcriptional factor binding sites, while the trimethylation on the same amino acid indicates a silent state of the binding sites. We found that in IFN-γThy1.1+ Th1 cells treated with TGF-β/IL-6, the levels of H3K9 acetylation in Runx1-binding sites of the rorc promoter were increased, whereas the levels of H3K9 trimethylation in these binding sites were decreased compared to those in control Th1 cells (Fig. 6A and B). Consistently, treatment with TGF-β/IL-6 increased the levels of H3K9 acetylation at the Runx1- and RORγt-binding sites in the il-17 promoter, whereas the levels of H3K9 trimethylation in these binding sites were decreased compared to the levels in control Th1 cells (Fig. 6C and D). The levels of H3K9 acetylation in RORγt-binding site in il-17a CNS-5 were also increased while the levels of H3K9 trimethylation were decreased in TGF-β/IL-6 treated Th1 cells. Taken together, these data indicate that TGF-β/IL-6 treatments enhance the accessibility of Runx1- binding sites in mouse promoters of il-17 and rorc and also of the RORγt- binding site in the il-17 promoter, thereby sequentially increasing IL-17 production in Th1 cells.
Figure 6. Treatment of Th1 cells with TGF-β and IL-6 increases the accessibility of RORγt and Runx1 binding sites.
IFN-γThy1.1+ CBir1 Tg CD4+ cells were cultured with splenic APCs with TGF-β/IL-6 and anti-IL-2/anti-IFN-γ for 24 h. Cellular DNA and proteins were fixed, sheared and immunoprecipitated with antibodies against H3K9ac, H3K9me3, or control IgG. The accessibility of multiple Runx1- and RORγt-binding sites on il-17, and rorc genes were analyzed by RT-PCR. Equal volumes of RT-PCR products were subjected to electrophoresis. (A) Runx1-binding site-1 on rorc promoter. (B) Runx1-binding site-2 on rorc promoter. (C) Runx1-binding site on il-17 promoter. (D) RORγt-binding site on il-17 promoter. (E) RORγt-binding site on il-17 CNS-5 enhancer. . Bars indicate fold changes of precipitated DNA after immunoprecipitation with H3K9ac Ab (left) and H3K9me3 Ab (right) of the indicated promoter binding sites and are shown as mean +/− SEM of two experiments. Precipitation from control without TGF-β/IL-6 was set to 1. Normalized on gapdh. Images on the right show representative gel electrophoresis of RT-PCR products, igG, isotype control, C, unstimulated, T, TGF-β/IL-6
Discussion
It is increasingly clear that both Th1 and Th17 cells reactive to commensal bacterial Ags are effector T cells driving colitis development both in experimental colitis and human IBD [35, 36]. With the progression of colitis, not only IFNγ+single-positive and IL-17+single- positive CD4+ T cells, but also IFNγ+ IL-17+ double-positive CD4+ T cells, accumulate in the inflamed intestines. We previously demonstrated that the severity of disease correlated with the presence of IFNγ+ IL-17+ double-positive CD4+ T cells in the lamina propria [6]. High levels of IFNγ+ IL-17+ CD4+ T cells are also present in the inflamed lesions from patients with IBD in contrast to their absence in the steady state [9], suggesting that such cells contribute to the pathogenesis of colitis. However, how these IFNγ+ IL-17+ CD4+ T cells are generated in the inflamed tissues is not fully defined. IL-12 is able to polarize human Th17 cells towards Th1 phenotype [12]. More recently, TNF-α has also been shown to have the same effect [13]. We and others have shown previously that commensal bacterial Ag-specific, IL-17-producing Th17 cells can convert into IFNγ-producing Th1 cells in the intestines under inflammatory conditions, mediated by local innate cell production of IL-12 and IL-23 [6, 14], indicating the generation of the pathogenic IFNγ+ IL-17+ CD4+ T cells from Th17 cells. Our current study further demonstrated that commensal bacterial Ag-specific, IFNγ-producing Th1 cells can also convert into IL-17-producing Th17 cells during intestinal inflammation, thereby providing a new mechanism driving the generation of the pathogenic IFNγ+ IL-17+ CD4+ T cells during colitis development.
The plasticity of CD4+ T cells has been investigated intensively both in mice and humans. It has been shown that under certain conditions, especially an inflammatory state, Treg cells can convert into Th1, Th17, and Tfh cells; moreover, Th17 cells can convert into Treg, Th1, and Tfh cells. Among all CD4+ T cell lineages, Th1 cells have been considered as one of the most stable subsets [17]. However, some recent reports demonstrated that Th1 cells could convert into Th2, Tfh and Treg cells in vivo under various conditions [20-22]. A conversion of IFNγ+ Th1 cells into IL-4+ Th2 cells and IFNγ+IL-4+ double-positive cells was observed when Th1 cells were adoptively transferred to a mice model infected with the gastrointestinal helminth Nippostrongylus brasiliensis [20]. In an elegant recent study, it was demonstrated that human Th1 cells can convert into Foxp3+ Treg cells in human-into-mouse xenogeneic GVHD, which was mediated by overexpression of the programmed death ligand-1 (PDL1) through inactivation of STAT1 [21]. However, it is still unclear whether Th1 cells can convert into Th17 cells. By using IFN-γThy1.1 CBir1 TCR-Tg reporter mice, whose TCR is specific for an immunodominant microbiota Ag, we investigated the stability of Th1 cells under intestinal inflammatory conditions. In inflamed intestines, transfer of purified, CBir1-specific IFN-γ+ Th1 cells induced colitis in Rag−/− mice, and IFN-γ+ Th1 cells lost IFNγ expression and converted into IL-17+-single-positive Th17 cells, as well as IFNγ+ IL-17+ CD4+ T cells, but not Foxp3+ Treg cells (Fig. 2). Interesting, TGF-β, which is present at high levels in inflamed intestinal tissues, promoted Th1 conversion into Th17 cells in vitro, and this was enhanced by IL-6. In contrast, IL-1β and IL-23, which have been shown to induce Th17 cells co-expressing T-bet and RORγt [11], did not affect Th1 conversion into Th17 cells.
RORγt is a master transcription factor driving Th17 cell differentiation and production of IL-17. Among multiple genes which regulate the expression and function of RORγt, Runx1 has been found to induce RORγt expression, influence Th17 cell differentiation by forming a complex with RORγt, and upregulating IL-17 expression by binding to its enhancer and promoter [31]. T-bet interacts with Runx1, and this interaction blocks the Runx1-mediated transactivation of Rorc, which encodes RORγt, and also blocks the Runx1- and RORγt-mediated synergetic activation of IL-17 transcription [32]. Data from our studies demonstrate that TGF-β promoted Th1 cell expression of Runx1, as well as that of Rorc, Rorα, and IL-17. Knockdown of Runx1 expression in Th1 cells significantly down-regulated the TGF-β-induced expression of Rorc, Rorα, and IL-17, thereby indicating that the TGF-β induction of Runx1 expression in Th1 cells plays a crucial role in Th1 conversion into Th17 cells. By using a ChIP assay, we further demonstrated that TGF-β increased the levels of histone H3K9 acetylation in Runx1-binding sites of promoters of rorc and il-17, as well as in the RORγt binding site in il-17 promoter. Whereas TGF-β decreased the levels of histone H3K9 trimethylation in these binding sites, indicating that TGF-β treatment may enhance the accessibility of Runx1-binding sites in mouse rorc promoter, sequentially increasing IL-17 production in Th1 cells.
In summary, our current study demonstrates for the first time that commensal bacterial, Ag-specific Th1 cells can convert into Th17 cells in intestines under inflammatory conditions. Interestingly, TGF-β plays a key role in driving Th1 conversion into Th17 cells in vitro through induction of Runx1 expression, as well as epigenetic regulation of Runx1. Thus, Th1 conversion into Th17 cells during intestinal inflammation could also contribute to the generation of pathogenic IFNγ+ IL-17+ CD4+ T cells. However, it is still unclear whether TGF-β promotes Th1 conversion into Th17 in vivo, and whether Th1 cells in a steady state can also convert into Th17 cells. Thus, it will be important to determine the relationship between mucosal IFNγ+-single-positive Th1 cells and IL-17+-single-positive Th17 cells in terms of in vivo plasticity, as well as the developmental pathways of Th1 cells present in the steady state compared to inflammatory conditions in intestine.
Materials and Methods
Animals and Reagents
C57Bl/6 mice were obtained from Jackson Laboratory. CBir1 TCR Tg (CBir1 Tg) mice [23] and IFN-γThy1.1 CBir1 Tg reporter mice [15] were maintained in the animal resource center at UTMB. All experiments were reviewed and approved by the Institutional Animal Care and Use Committees of UTMB. Reagents for cell cultures including HEPES, sodium pyruvate, penicillin-streptomycin, and 2-mecaptoethanol were purchased from Life Technologies (Carlsbad, CA). SMARTpool siRNAs for knockdown of murine Runx1 and non-targeting siRNA were from GE Dharmacon (Lafayette, CO). Anti-mCD3, anti-mCD28, neutralizing antibodies for IL-2 and IFN-γ, Th17 polarizing cytokines, fluorochrome-conjugated anti-mouse CD4, IL-17 and IFN-γ antibodies were from BioLegend. Live/Dead dye was from Invitrogen. ChIP-IT Express Enzymatic kit, antibodies against histone H3K9ac and histone H3K9me3 were purchased from Active Motif (Carlsbad, CA). Rabbit IgG, the negative control for immune precipitation, was obtained from Cell Signaling. Primers for real time PCR were ordered from Integrated DNA Technologies (Table. S1).
Primary T-cell culture.
Splenic naïve CD4+ CD62L+ T cells were isolated from 6-12 week-old CBir1 Tg mice by using MACS isolation kits (MACS) as previously described [37]. For Th1-cell differentiation, CBir1 Tg CD4+ CD62L+ T cells were cultured with CBir1 antigen, irradiated splenic CD4− APCs, mIL-12 (10 ng/ml) and anti-IL-4 (10 μg/ml) for 5 days. For purifying IFN-γThy1.1+ CD4+ T cells, Day 5 Th1-cell cultures were stained with anti-CD4 and anti-Thy1.1 flow antibodies and sorted with FACSAria sorter.
siRNA transfection
Sorted Th1 cells were cultured for 2 days to allow cells to reach the logarithmic growth phase optimal for transfection. Lonza nucleofector and mouse T cell nucleofector kit were used for siRNA transfection in sub-cultured Th1 cells Briefly, 1x106 sub-cultured Th1 cells were transfected with 100 nM siRNA. The cells were allowed to rest at 37°C in an incubator for 6-24 h after transfection with siRNA and cultured with anti-CD3 (1μg/ml) and anti-CD28 (2 μg/ml). Knockdown efficiency was measured at 24 h post transfection. The mRNA expression of il-17, rorc, rorα, runx1 and batf were determined by RT-PCR.
Flow cytometry
As described previously [8], cells were stimulated for 5 h with PMA (50 ng/ml) and ionomycin (750 ng/ml) and stained with fluorochrome-conjugated anti-mouse antibodies agaginst Thy1.1, CD4, Live/Dead dye, IL-17, and IFN-γ.
Real time PCR (RT-PCR)
Massager RNA from cell samples was extracted with TRIzol reagent from Applied Biosystems and reversely transcribed into cDNA by qScript reverse transcriptase from Quanta Biosciences. The gene expression of rorc, rorα, runx1, and batf were determined by using TaqMan Gene Expression Assays. All data were normalized to gapdh mRNA expression.
ELISA
ELISA was performed by using mouse IL-17(A) ELISA kit from Biolegend.
Induction of colitis
1 × 106 CD4+ T cells isolated from CBir1 Tg mice were injected i.v. into Rag−/− mice. 1 × 106 CD4+ T cells isolated from OTII mice were injected i.v. into Rag−/− mice to serve as negative controls. The Rag−/− mice which received PBS also served as negative controls. The recipient mice were sacrificed 8 weeks later. Intestinal lamina propria T cells were isolated for analysis of cytokine production by flow cytometry. The histopathology of cecum and colon was also assessed.
Preparation of lamina propria lymphocytes
As previously described [38], the intestines were sliced into short pieces and treated with EDTA to remove epithelial cells and intraepithelial lymphocytes. The rest tissues were then digested with Collagenase IV for the release of lamina propria lymphocytes. The isolated cells were further purified by discontinuous density gradient centrifugation in Percoll (Amersham Pharmacia Biotech).
Histopathologic assessment
Briefly, as previously described [39], at necropsy, cecum and colon were dissected, and Swiss rolls of each were prepared. Tissues were fixed and embedded, followed by preparation of 5 μm sections and H&E staining. Samples were read on an Eclipse 80i microscope (Nikon; Melville, NY).
ChIP assay
A ChIP-IT Express Enzymatic Kit (Active Motif, Carlsbad, CA) was used for ChIP assay. Briefly, histones were cross-linked to DNA with 1% formaldehyde and incubated for 5 min at room temperature. The DNA was sheared with an enzymatic shearing cocktail for 10 min at 37°C, and quantified by using Nanodrop. Magnetic beads and 2 μg of each Ab were used to capture chromatin. Immunoprecipitates were eluted and reverse cross-linked. For RT-PCR, 1.5 μl of the eluted DNA and 40 cycles of amplification were used and all data were normalized to input DNA. RT-PCR products were subjected to electrophoresis on 1% agarose gels, stained with ethidium bromide.
Statistical analysis
One-way ANOVA and unpaired, two-tailed Student’s t test were used for comparisons between groups. A p value of < 0.05 was considered statistically significant and shown as *.
Supplementary Material
Acknowledgments
This work was supported by NIH grants DK079918 and DK098370. HL is a recipient of the J.W. McLaughlin Predoctoral Fellowship, UTMB. ATC is a recipient of the J.W. McLaughlin Postdoctoral Fellowship, UTMB.
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Liu Z, Cao AT, Cong Y. Microbiota regulation of inflammatory bowel disease and colorectal cancer. Semin Cancer Biol. 2013;23:543–552. doi: 10.1016/j.semcancer.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sartor RB, Muehlbauer M. Microbial host interactions in IBD: implications for pathogenesis and therapy. Curr Gastroenterol Rep. 2007;9:497–507. doi: 10.1007/s11894-007-0066-4. [DOI] [PubMed] [Google Scholar]
- 3.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng T, Qin H, Wang L, Benveniste EN, Elson CO, Cong Y. Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. J Immunol. 2011;186:6313–6318. doi: 10.4049/jimmunol.1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shale M, Schiering C, Powrie F. CD4(+) T-cell subsets in intestinal inflammation. Immunol Rev. 2013;252:164–182. doi: 10.1111/imr.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Yao S, Dann SM, Qin H, Elson CO, Cong Y. ERK differentially regulates Th17- and Treg-cell development and contributes to the pathogenesis of colitis. Eur J Immunol. 2013;43:1716–1726. doi: 10.1002/eji.201242889. [DOI] [PubMed] [Google Scholar]
- 9.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maggi L, Cimaz R, Capone M, Santarlasci V, Querci V, Simonini G, Nencini F, et al. Brief report: etanercept inhibits the tumor necrosis factor alpha-driven shift of Th17 lymphocytes toward a nonclassic Th1 phenotype in juvenile idiopathic arthritis. Arthritis Rheumatol. 2014;66:1372–1377. doi: 10.1002/art.38355. [DOI] [PubMed] [Google Scholar]
- 14.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng T, Cao AT, Weaver CT, Elson CO, Cong Y. Interleukin-12 converts Foxp3+ regulatory T cells to interferon-gamma-producing Foxp3+ T cells that inhibit colitis. Gastroenterology. 2011;140:2031–2043. doi: 10.1053/j.gastro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 20.Panzer M, Sitte S, Wirth S, Drexler I, Sparwasser T, Voehringer D. Rapid in vivo conversion of effector T cells into Th2 cells during helminth infection. J Immunol. 2012;188:615–623. doi: 10.4049/jimmunol.1101164. [DOI] [PubMed] [Google Scholar]
- 21.Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, Foley JE, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3:111ra120. doi: 10.1126/scitranslmed.3003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu KT, Kanno Y, Cannons JL, Handon R, Bible P, Elkahloun AG, Anderson SM, et al. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity. 2011;35:622–632. doi: 10.1016/j.immuni.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 25.Liao W, Lin JX, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat Immunol. 2011;12:551–559. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Haines CJ, Gutcher I, Hochweller K, Blumenschein WM, McClanahan T, Hammerling G, et al. Foxp3(+) regulatory T cells promote T helper 17 cell development in vivo through regulation of interleukin-2. Immunity. 2011;34:409–421. doi: 10.1016/j.immuni.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou L, Littman DR. Transcriptional regulatory networks in Th17 cell differentiation. Curr Opin Immunol. 2009;21:146–152. doi: 10.1016/j.coi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, Oukka M, et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol. 2011;12:96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Shea JJ, Lahesmaa R, Vahedi G, Laurence A, Kanno Y. Genomic views of STAT function in CD4+ T helper cell differentiation. Nat Rev Immunol. 2011;11:239–250. doi: 10.1038/nri2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirahara K, Vahedi G, Ghoreschi K, Yang XP, Nakayamada S, Kanno Y, O'Shea JJ, et al. Helper T-cell differentiation and plasticity: insights from epigenetics. Immunology. 2011;134:235–245. doi: 10.1111/j.1365-2567.2011.03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 36.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 37.Qin H, Wang L, Feng T, Elson CO, Niyongere SA, Lee SJ, Reynolds SL, et al. TGF-beta promotes Th17 cell development through inhibition of SOCS3. J Immunol. 2009;183:97–105. doi: 10.4049/jimmunol.0801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cong Y, Weaver CT, Lazenby A, Elson CO. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J Immunol. 2002;169:6112–6119. doi: 10.4049/jimmunol.169.11.6112. [DOI] [PubMed] [Google Scholar]
- 39.Cao AT, Yao S, Gong B, Elson CO, Cong Y. Th17 cells upregulate poly meric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J Immunol. 2012;189:4666–4673. doi: 10.4049/jimmunol.1200955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.