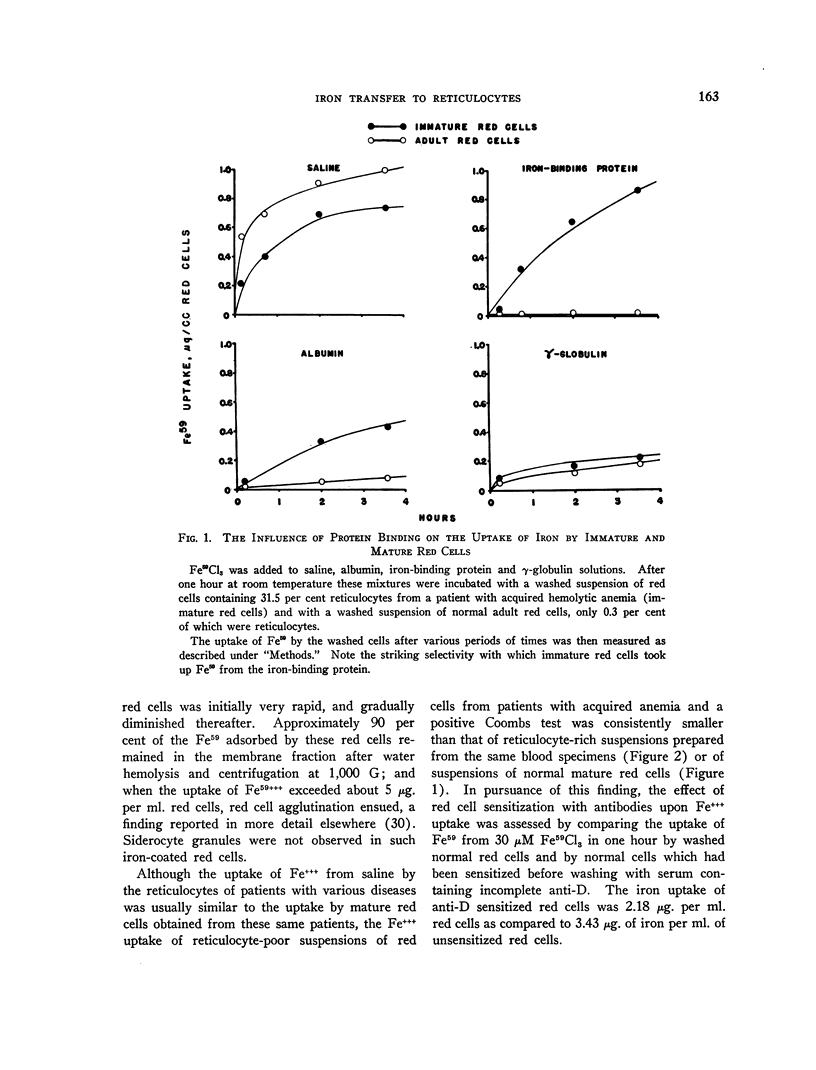
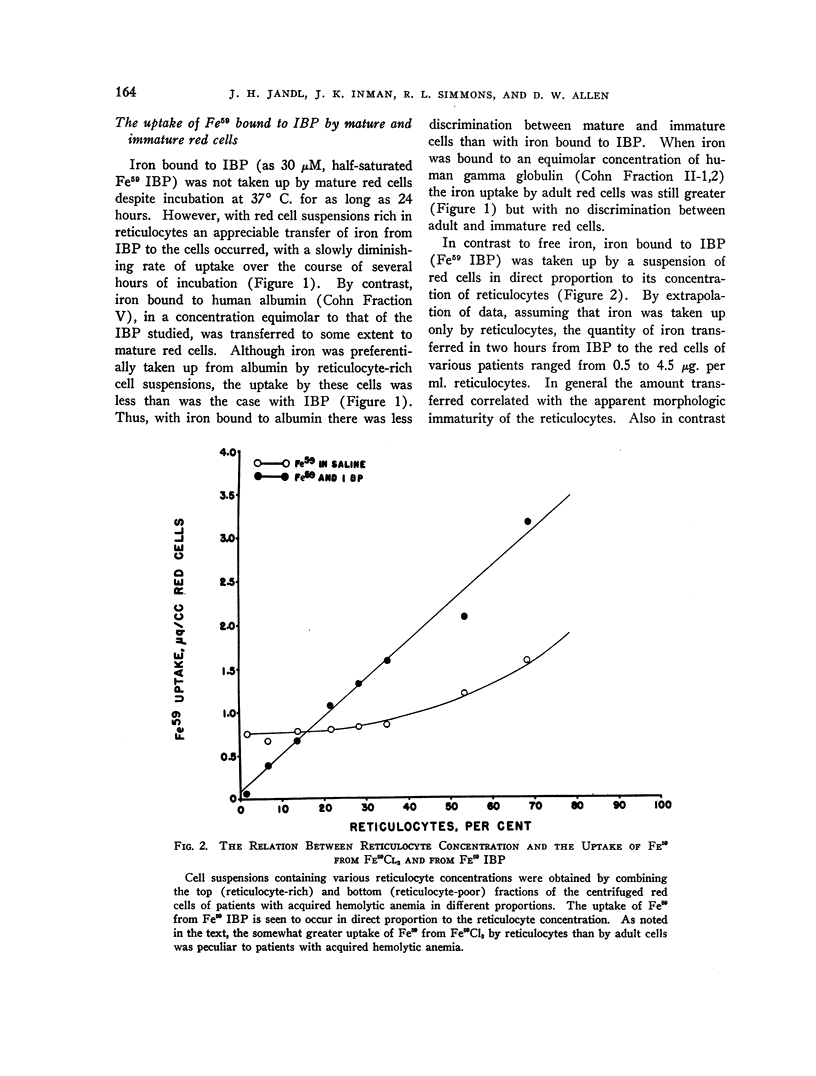
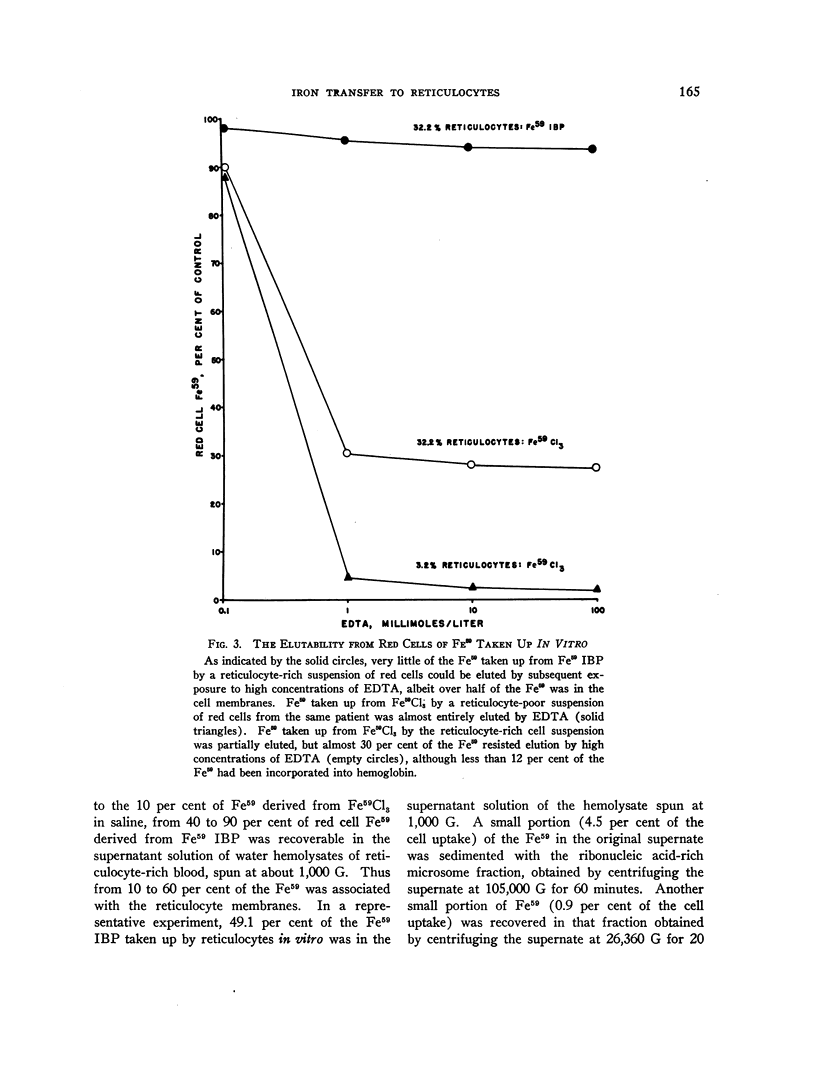
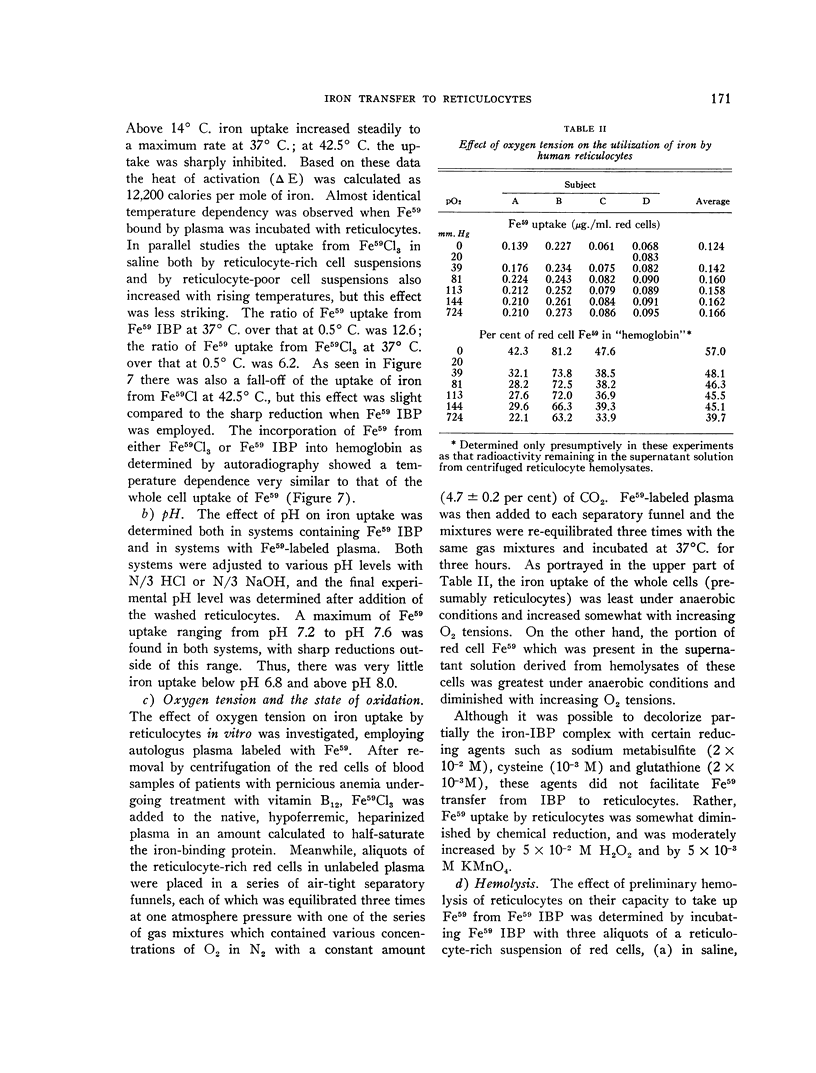
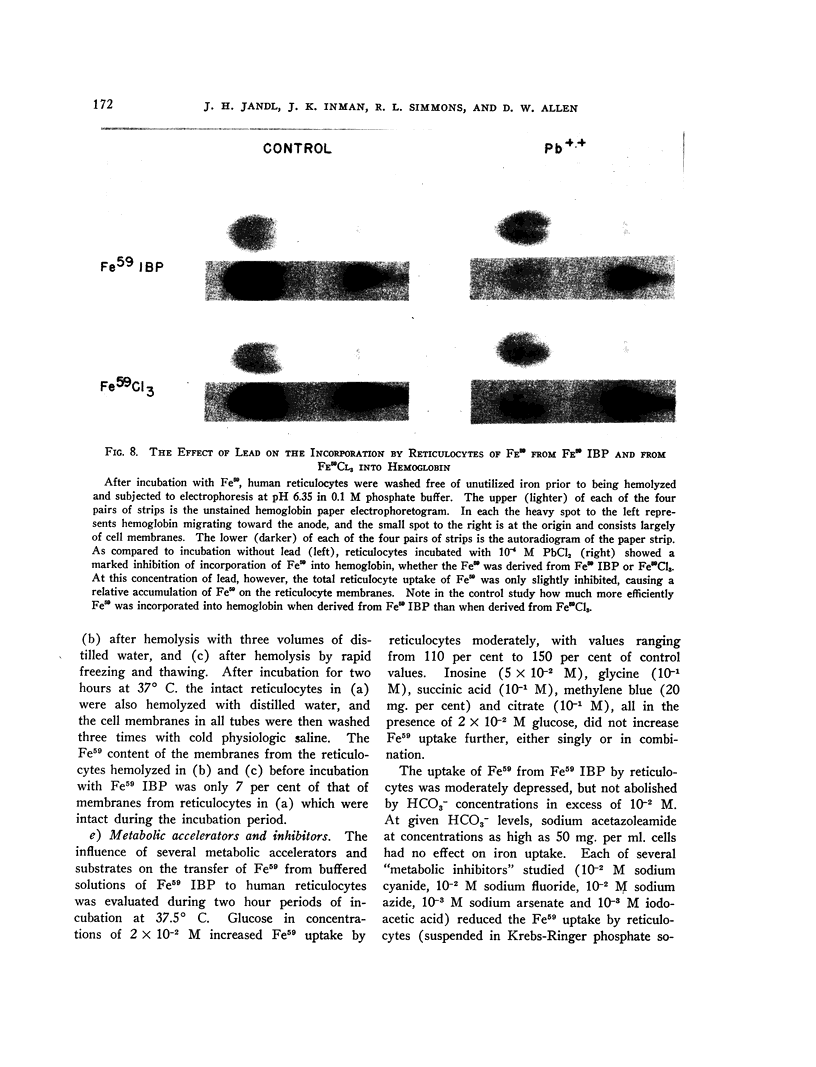
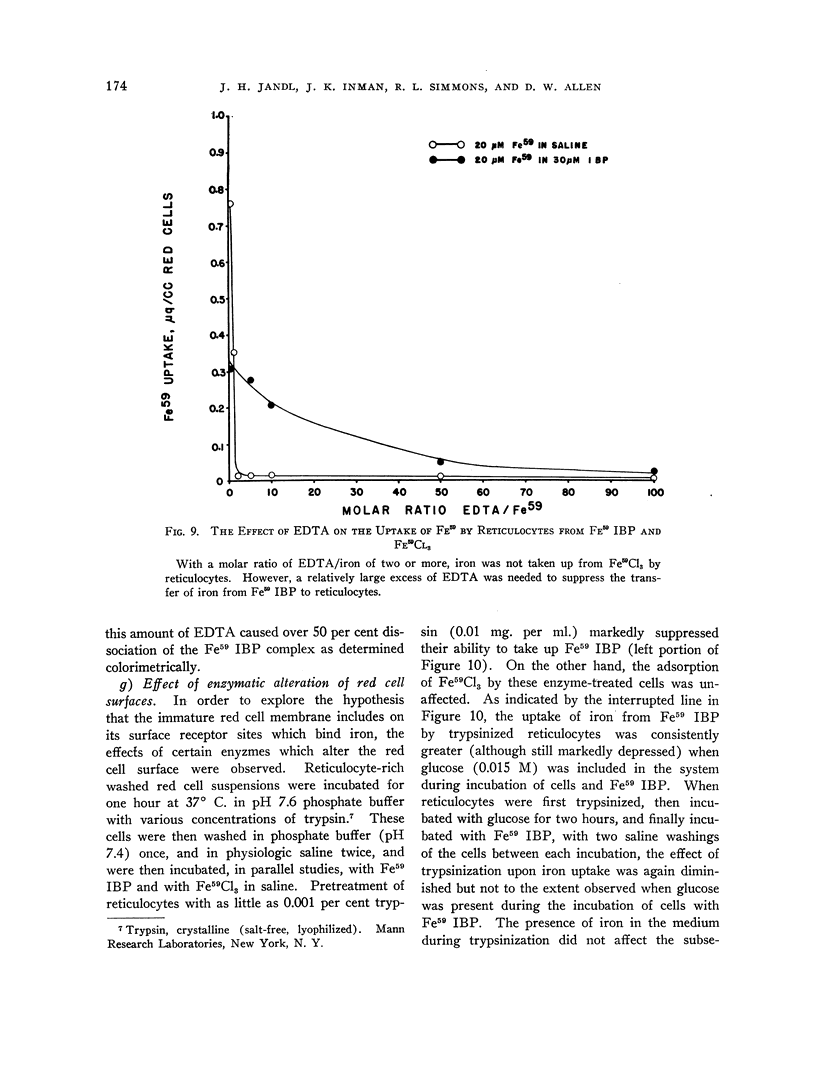
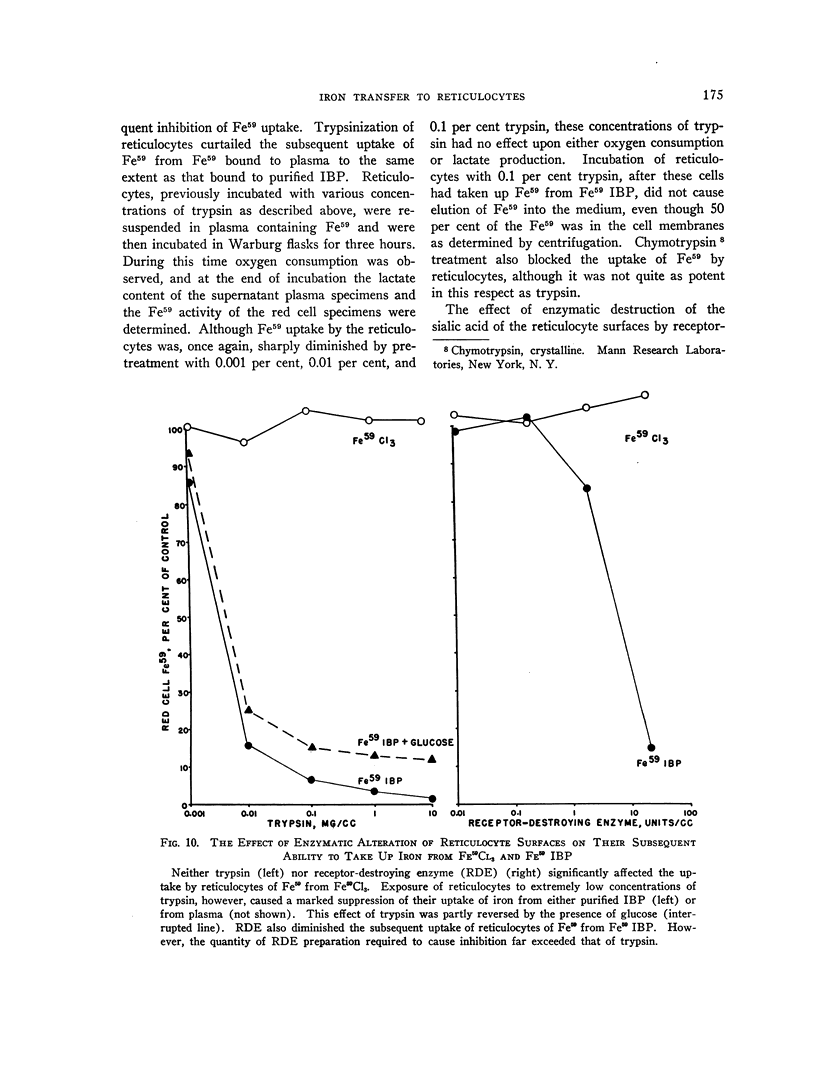
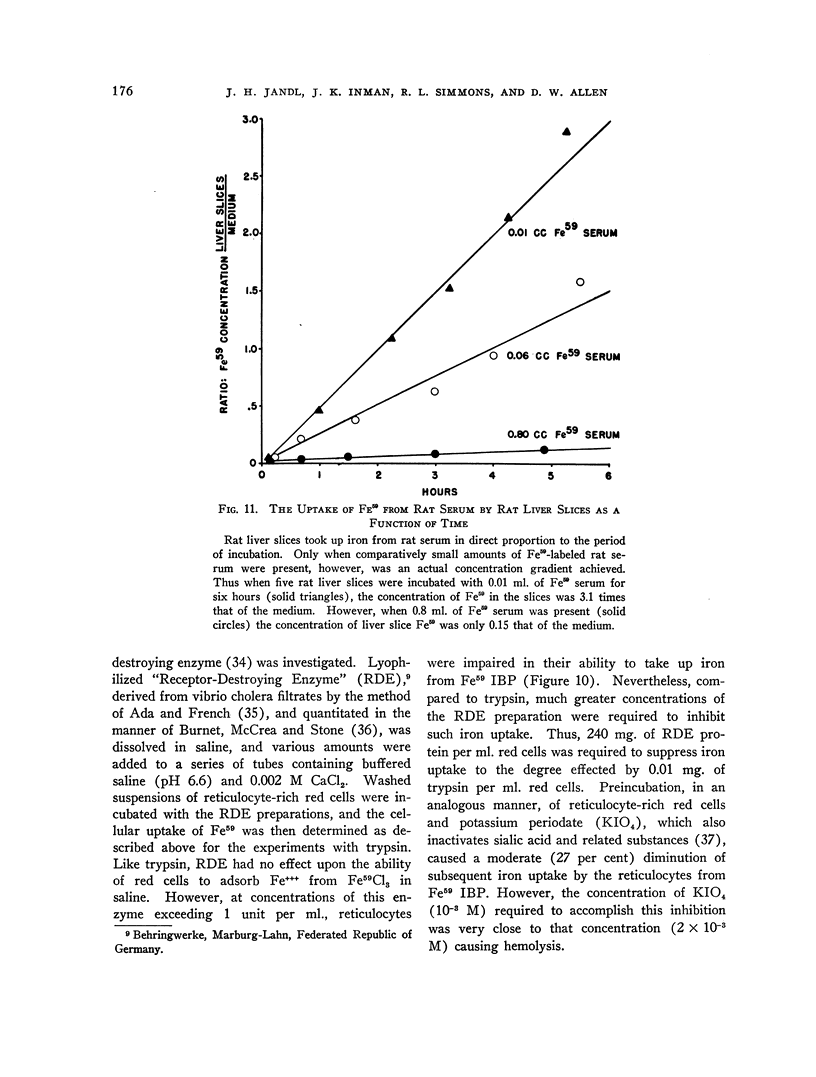
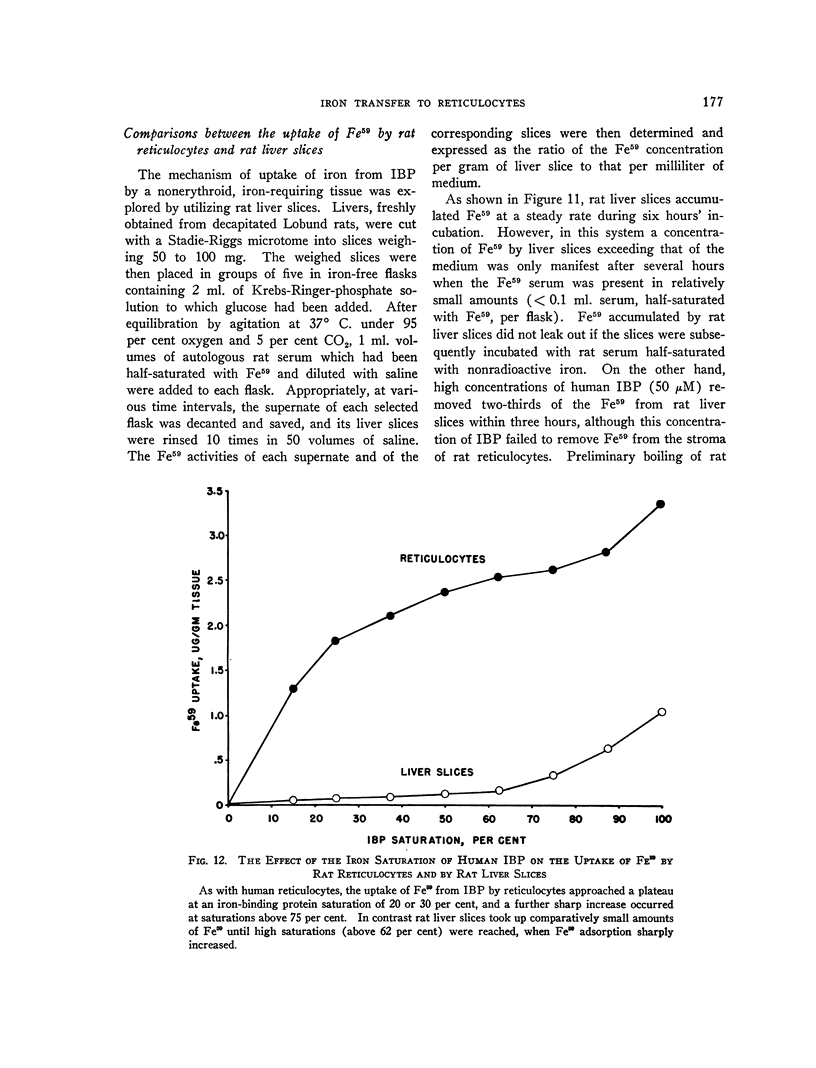
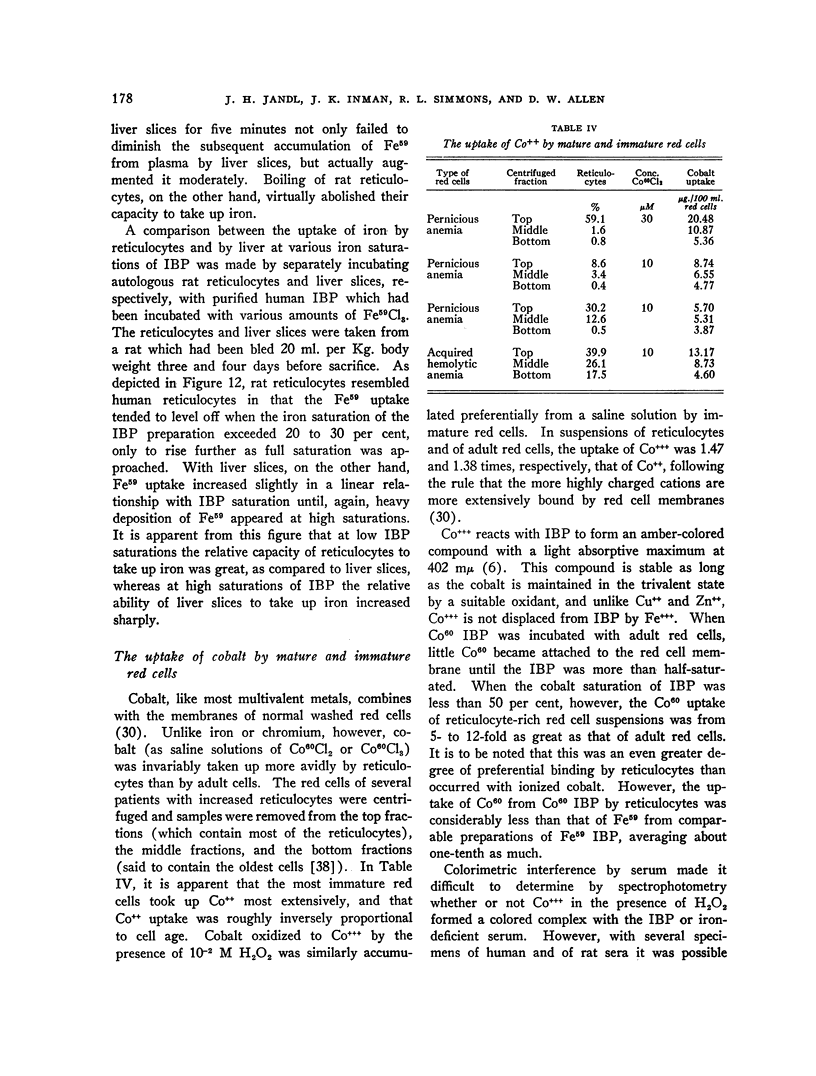
Full text
PDF

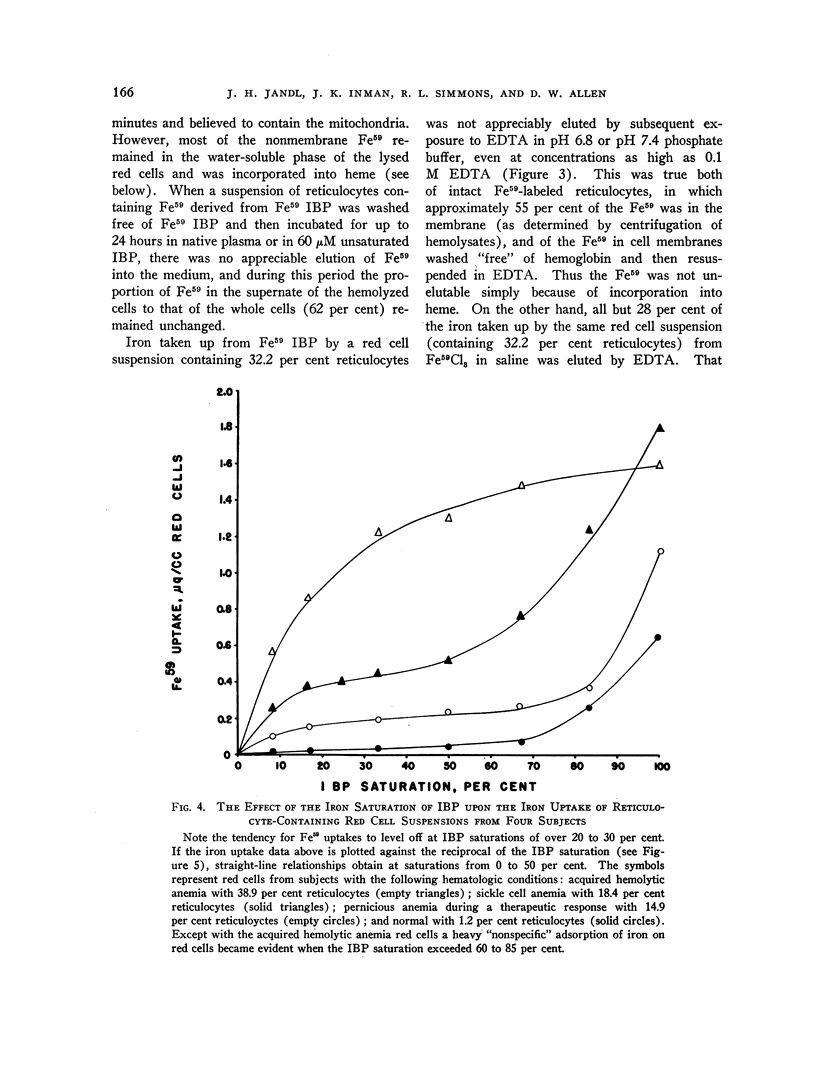
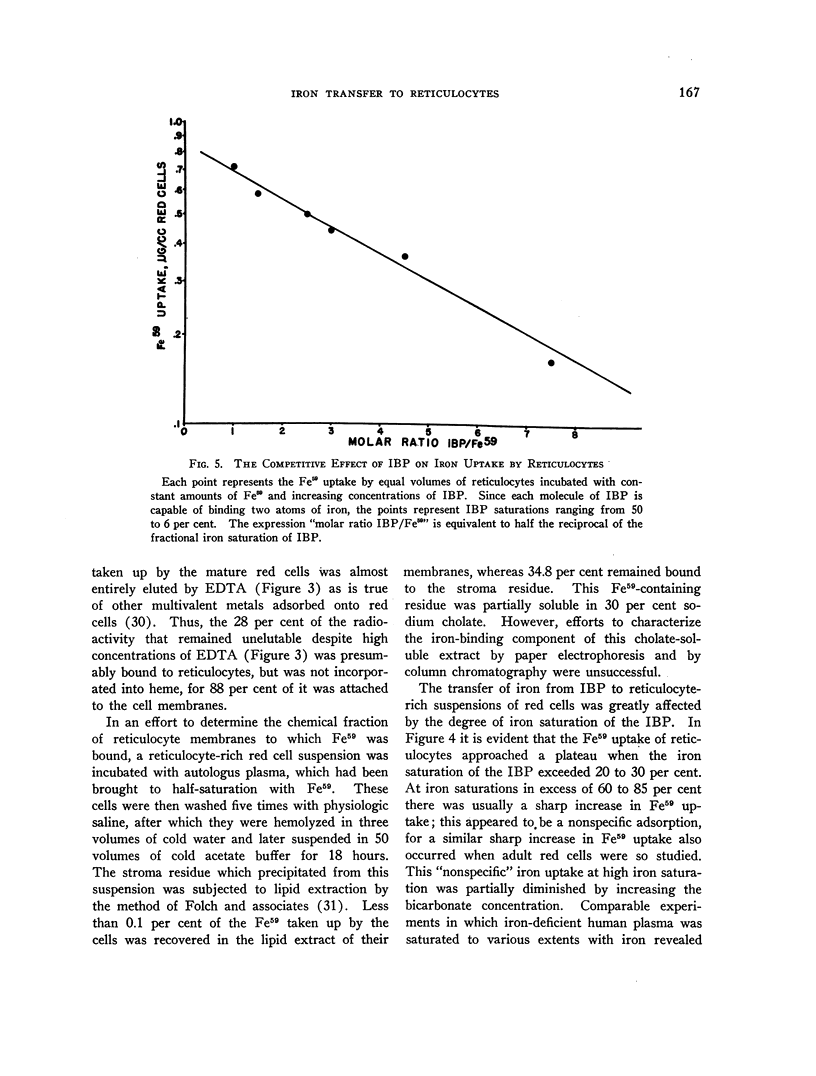
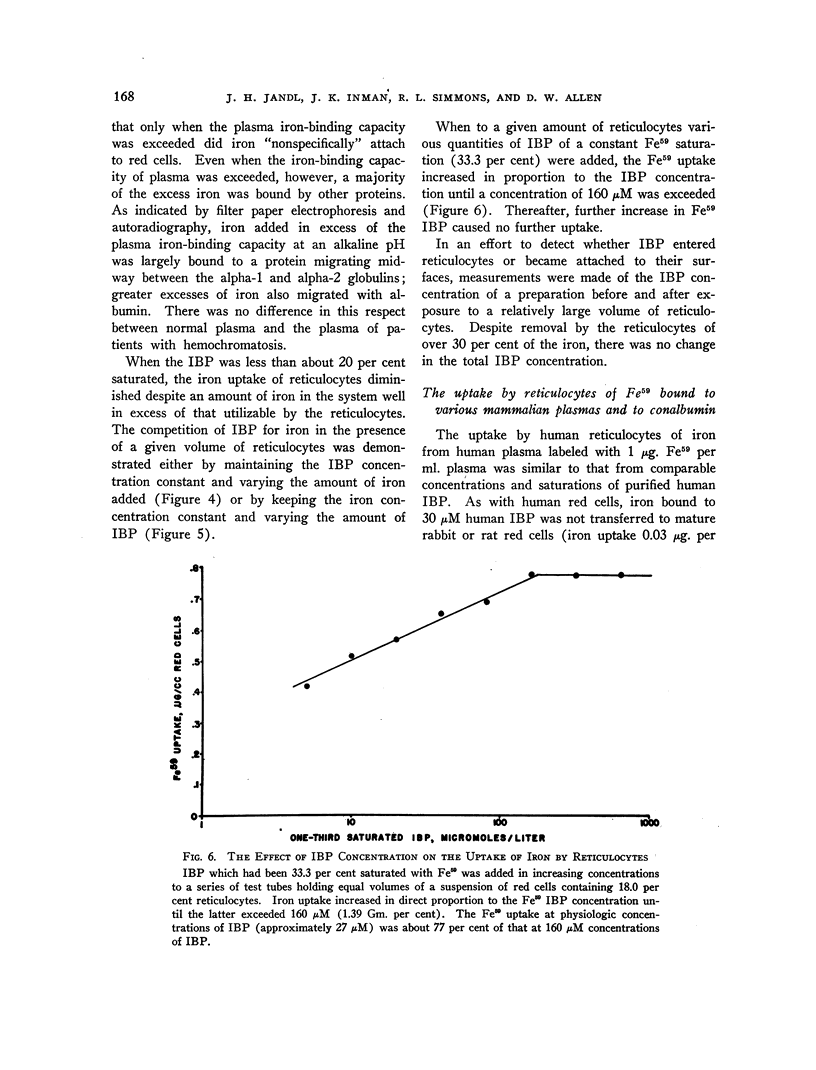

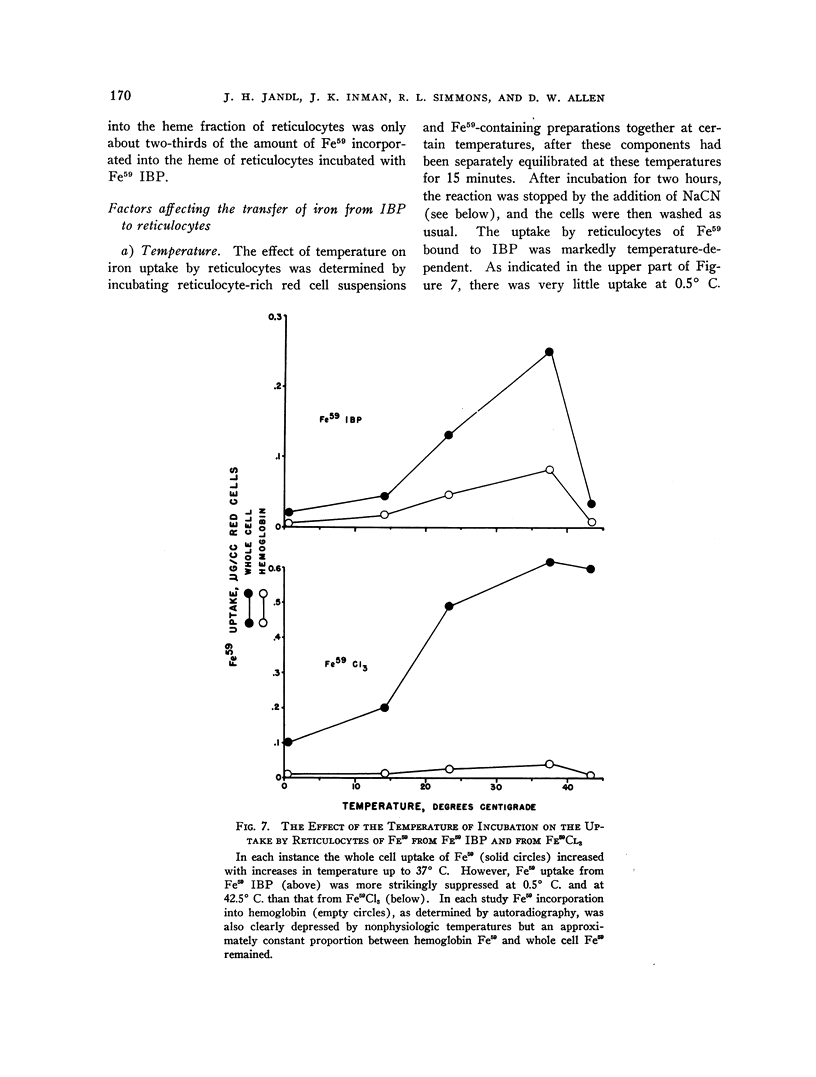








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHENBRUCKER H., CARTWRIGHT G. E., GOLDBERG A., WINTROBE M. M. Studies on the biosynthesis of heme in vitro by avian erythrocytes. Blood. 1956 Sep;11(9):821–833. [PubMed] [Google Scholar]
- BESSIS M. C., BRETON-GORIUS J. Iron particles in normal erythroblasts and normal and pathological erythrocytes. J Biophys Biochem Cytol. 1957 May 25;3(3):503–504. doi: 10.1083/jcb.3.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEUTLER E. Iron enzymes in iron deficiency. I. Cytochrome c. Am J Med Sci. 1957 Nov;234(5):517–527. doi: 10.1097/00000441-195711000-00003. [DOI] [PubMed] [Google Scholar]
- BORSOOK H., ABRAMS A., LOWY P. H. Fructose-amino acids in liver: stimuli of amino acid incorporation in vitro. J Biol Chem. 1955 Jul;215(1):111–124. [PubMed] [Google Scholar]
- BORUN E. R., FIGUEROA W. G., PERRY S. M. The distribution of Fe59 tagged human erythrocytes in centrifuged specimens as a function of cell age. J Clin Invest. 1957 May;36(5):676–679. doi: 10.1172/JCI103468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOTHWELL T. H., HURTADO A. V., DONOHUE D. M., FINCH C. A. Erythrokinetics. IV. The plasma iron turnover as a measure of erythropoiesis. Blood. 1957 May;12(5):409–427. [PubMed] [Google Scholar]
- DOWLING J. T., FREINKEL N., INGBAR S. H. The influence of extracellular thyroxine-binding protein upon the accumulation of thyroxine by tissue slices. J Clin Invest. 1957 Jan;36(1 Pt 1):25–37. doi: 10.1172/JCI103406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWLING J. T., FREINKEL N., INGBAR S. H. Thyroxine-binding by sera of pregnant women, newborn infants, and women with spontaneous abortion. J Clin Invest. 1956 Nov;35(11):1263–1276. doi: 10.1172/JCI103381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS G. H., BRANDT C. S., THACKER E. J. Factors influencing the uptake of iron by blood and by bone marrow cells in vitro. Science. 1954 Jan 15;119(3081):94–95. doi: 10.1126/science.119.3081.94. [DOI] [PubMed] [Google Scholar]
- ERIKSEN L. Lead intoxication. I. The effect of lead on the in vitro biosynthesis of heme and free erythrocyte porphyrins. Scand J Clin Lab Invest. 1955;7(1):80–85. doi: 10.3109/00365515509134101. [DOI] [PubMed] [Google Scholar]
- FARRANT J. L. An electron microscopic study of ferritin. Biochim Biophys Acta. 1954 Apr;13(4):569–576. doi: 10.1016/0006-3002(54)90376-5. [DOI] [PubMed] [Google Scholar]
- FOLCH J., ASCOLI I., LEES M., MEATH J. A., LeBARON N. Preparation of lipide extracts from brain tissue. J Biol Chem. 1951 Aug;191(2):833–841. [PubMed] [Google Scholar]
- GITLIN D., JANEWAY C. A., FARR L. E. Studies on the metabolism of plasma proteins in the nephrotic syndrome. I. Albumin, gamma-globulin and iron-binding globulin. J Clin Invest. 1956 Jan;35(1):44–56. doi: 10.1172/JCI103251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTSCHALK A. Neuraminic acid; the functional group of some biologically active mucoproteins. Yale J Biol Med. 1956 Apr;28(5):525–537. [PMC free article] [PubMed] [Google Scholar]
- GRANICK S. Iron metabolism. Bull N Y Acad Med. 1954 Feb;30(2):81–105. [PMC free article] [PubMed] [Google Scholar]
- HOFMANN E. C., RAPOPORT S. Der Einfluss der Hämolyse auf die Atmung von Reticulocyten. Biochem Z. 1955;326(7):499–506. [PubMed] [Google Scholar]
- JANDL J. H., SIMMONS R. L. The agglutination and sensitization of red cells by metallic cations: interactions between multivalent metals and the red-cell membrane. Br J Haematol. 1957 Jan;3(1):19–38. doi: 10.1111/j.1365-2141.1957.tb05768.x. [DOI] [PubMed] [Google Scholar]
- JENSEN W. N., ASHENBRUCKER H., CARTWRIGHT G. E., WINTROBE M. M. The uptake in vitro of radioactive iron by avian erythrocytes. J Lab Clin Med. 1953 Dec;42(6):833–846. [PubMed] [Google Scholar]
- KLENK E., LEMPFRID H. Uber die Natur der Zellreceptoren für das Influenzavirus. Hoppe Seylers Z Physiol Chem. 1957;307(2-6):278–283. [PubMed] [Google Scholar]
- KRUH J., BORSOOK H. Hemoglobin synthesis in rabbit reticulocytes in vitro. J Biol Chem. 1956 Jun;220(2):905–915. [PubMed] [Google Scholar]
- MAZUR A., BAEZ S., SHORR E. The mechanism of iron release from ferritin as related to its biological properties. J Biol Chem. 1955 Mar;213(1):147–160. [PubMed] [Google Scholar]
- NEILANDS J. B. Some aspects of microbial iron metabolism. Bacteriol Rev. 1957 Jun;21(2):101–111. doi: 10.1128/br.21.2.101-111.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAOLETTI C. Role des beta-globulines plasmatiques dans le transport du fer utilisé par les cellules érythroformatrices. C R Hebd Seances Acad Sci. 1957 Jul 17;245(3):377–380. [PubMed] [Google Scholar]
- RAPOPORT S., HOFMANN E. C. Untersuchungen über den Atmugsstoffwechsel von Reticulocyten. Biochem Z. 1955;326(7):493–498. [PubMed] [Google Scholar]
- Rath C. E., Finch C. A. CHEMICAL, CLINICAL, AND IMMUNOLOGICAL STUDIES ON THE PRODUCTS OF HUMAN PLASMA FRACTIONATION. XXXVIII. SERUM IRON TRANSPORT. MEASUREMENT OF IRON-BINDING CAPACITY OF SERUM IN MAN. J Clin Invest. 1949 Jan;28(1):79–85. doi: 10.1172/JCI102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTMAN P., FISKIN R. D., BELLINGER S. B., ALEX T. The metabolism of iron by rat liver slices; the effect of chemical agents. J Biol Chem. 1956 Jun;220(2):751–757. [PubMed] [Google Scholar]
- SCHWEIGER H. G., RAPOPORT S., SCHOLZEL E. Role of non-protein nitrogen in the synthesis of haemoglobin in the reticulocyte in vitro. Nature. 1956 Jul 21;178(4525):141–142. doi: 10.1038/178141b0. [DOI] [PubMed] [Google Scholar]
- SHARPE L. M., KRISHNAN P. S., KLEIN J. R. Uptake by duck erythrocytes of iron added to blood. Arch Biochem Biophys. 1952 Feb;35(2):409–418. doi: 10.1016/s0003-9861(52)80021-9. [DOI] [PubMed] [Google Scholar]
- Schade A. L., Caroline L. An Iron-binding Component in Human Blood Plasma. Science. 1946 Oct 11;104(2702):340–341. doi: 10.1126/science.104.2702.340. [DOI] [PubMed] [Google Scholar]
- Schade A. L., Caroline L. RAW HEN EGG WHITE AND THE ROLE OF IRON IN GROWTH INHIBITION OF SHIGELLA DYSENTERIAE, STAPHYLOCOCCUS AUREUS, ESCHERICHIA COLI AND SACCHAROMYCES CEREVISIAE. Science. 1944 Jul 7;100(2584):14–15. doi: 10.1126/science.100.2584.14. [DOI] [PubMed] [Google Scholar]
- Surgenor D. M., Koechlin B. A., Strong L. E. CHEMICAL, CLINICAL, AND IMMUNOLOGICAL STUDIES ON THE PRODUCTS OF HUMAN PLASMA FRACTIONATION. XXXVII. THE METAL-COMBINING GLOBULIN OF HUMAN PLASMA. J Clin Invest. 1949 Jan;28(1):73–78. doi: 10.1172/JCI102056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS E. D. In vitro studies of erythropoiesis. II. The effect of anoxia on heme synthesis. Blood. 1955 Jun;10(6):612–615. [PubMed] [Google Scholar]
- TISHKOFF G. H., ROBSCHEIT-ROBBINS F. S., WHIPPLE G. H. Red cell stroma in dogs; variations in the stroma protein and lipid fractions related to experimental conditions. Blood. 1953 May;8(5):459–468. [PubMed] [Google Scholar]
- WARNER R. C. The metal chelate compounds of proteins. Trans N Y Acad Sci. 1954 Feb;16(4):182–188. doi: 10.1111/j.2164-0947.1954.tb01161.x. [DOI] [PubMed] [Google Scholar]
- WATSON C. J. The erythrocyte coproporphyrin; variation in respect to erythrocyte protoporphyrin and reticulocytes in certain of the anemias. AMA Arch Intern Med. 1950 Dec;86(6):797–809. doi: 10.1001/archinte.1950.00230180002001. [DOI] [PubMed] [Google Scholar]
- Walsh R. J., Thomas E. D., Chow S. K., Fluharty R. G., Finch C. A. Iron Metabolism. Heme Synthesis in Vitro by Immature Erythrocytes. Science. 1949 Oct 14;110(2859):396–398. doi: 10.1126/science.110.2859.396. [DOI] [PubMed] [Google Scholar]