Abstract
Background:
The major hazard to the orthodontic tooth movement is the friction developing at the bracket wire interface. In the past, there have been various attempts to reduce this friction. We believe that coating the commercially available orthodontic wires with nanoparticles can result in a successful reduction of this friction. The objective of this study is to develop a novel method of coating orthodontic archwires with nanoparticles.
Materials and Methods:
Stainless steel (Ormco, CA, USA), titanium molybdenum alloy (Ormco, CA, USA) and nickel-titanium (G and H Wire Company, USA) orthodontic wires with a rectangular cross-section dimension of 0.019”× 0.025”, were selected. The wires were later coated with a uniform and smooth nanoparticle film using 100 ml nanocremics. The coating procedure described in this article is a sol-gel thin film dip coating method.
Results:
The coating procedure was verified by comparing the surface topography of nanocoated archwires with the commercially available archwires in an environmental scanning electron microscope (ESEM). The ESEM images prove that the surface topography of the coated wires was found to be smoother with less surface deteriorations as compared to the commercially available wires.
Conclusion:
Commercially available orthodontic wires can be successfully coated using a novel method of sol-gel thin film dip coating method.
Keywords: Nanocremics, nickel-titanium, stainless steel, titanium molybdenum alloy
Introduction
In the past few decades, a number of wire alloys with a wide spectrum of mechanical properties have been introduced, adding versatility to orthodontic treatment. Selecting the appropriate archwire requires a thorough knowledge of the biomechanical properties of it. In the past, there have been attempts to improve the mechanical properties of orthodontic wires using different types of alloys and surface treatment of wires. These attempts were successful to some extent. Coating the orthodontic wires with nanoparticles is one such attempt that is still under research. In the current study, we shall discuss the novel method of coating orthodontic wires with nanoparticles.1
The nanoparticle used in our study is nanocremics (Figure 1a) which is commercially available nanoparticle coating precursor solution. This was the solution of choice in our study as it was readily available commercially in India. Secondly the laboratory procedure for coating of the same could be easily carried out in India. The nanocremics solution coating procedure described here is the sol-gel thin film dip coating method. In order to evaluate the adherence of the coating on to the orthodontic wire, the coated orthodontic wires were analyzed by scanning electron microscope (SEM).1,2
Figure 1.

(a) Nanocremics – commercially available nanoparticle coating precursor solution. (b) Commercially available orthodontic wires inserted into the bathtub containing nanoparticle solution. (c) Wires kept in a hanger where they are further painted with nanoparticle solution. (d) Wires were then air dried with the help of drier.
Materials and Methods
Commercially available straight length orthodontic wires of stainless steel (SS), nickel-titanium (NiTi) and titanium molybdenum alloy (TMA) were first cleaned under running water to discard any dust particles which will interfere with our coating procedure.
The orthodontic wires were inserted into the bathtub containing nanoparticle solution for 30 min (Figure 1b).
Later the wires were removed from the tub and kept in a hanger where they further painted with nanoparticle solution (Figure 1c).
The wires were then air dried with the help of drier for 2 min (Figure 1d).
The wire with the hanger was then placed in the hot air oven at 160°C for 3 min. In this way, the commercially available orthodontic wires were coated with nanoparticles (Figure 2a-c).
Figure 2.

(a-c) The wire with the hanger were then placed in the hot air oven at 160°C.
Results
The coated wires were analyzed by a SEM (Figure 3) to check for the surface topography.
Figure 3.

Scanning electron microscope machine.
SEM was used to assess the surface topography of the archwires. The SEM used for our study was based on the technology of environmental SEM (ESEM).
The ESEM gives us microscopic images of both wet and dry sample specimen. With the help of ESEM, we can examine the specimen in a faster and easy way. Another advantage of ESEM is that there is no need to modify the surface of the original specimen. In order to procure the ESEM images of coated and uncoated archwires; 20 mm length of straight archwires were taken. They were cleaned with distilled water to remove any precipitates. Later the specimen were mounted in a machine holder and observed under field-emission ESEM. The images were recorded at ×500 magnification. Figure 4a-f shows SEM pictures of the uncoated and coated (straight) wire with the nanoparticles with ×500 magnification. The SEM study reveals that the orthodontic wires coated with nanoparticles has fewer surface irregularities than the commercially available uncoated wires. Thus, the surface topography of the coated wires was found to be smoother with less surface deteriorations as compared to the uncoated wires.
Figure 4.
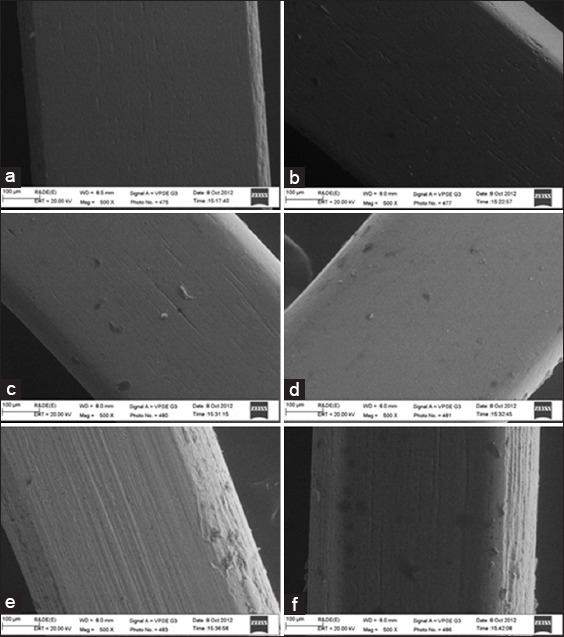
(a) Scanning electron microscope (SEM) image for commercially available uncoated stainless steel (SS) wire (0.019” × 0.025”). (b) SEM image for nanoparticle coated SS wire (0.019” × 0.025”). (c) SEM image for commercially available uncoated nickel-titanium (NiTi) wire (0.019” × 0.025”). (d) SEM image for nanoparticle coated NiTi wire (0.019” × 0.025”). (e) SEM image for commercially available uncoated titanium molybdenum alloy (TMA) wire (0.019” × 0.025”). (f) SEM image for nanoparticle coated TMA wire (0.019”× 0.025”).
Discussion
The main motto of this study is to develop the novel method of coating the orthodontic archwires with nanoparticles. Once the surface treatment of wire is done thoroughly with this novel method; in the next series of articles we will be discussing about the physical properties of this nanocoated wires and how is it beneficial to us for clinical use.
One of the most important benefits of coating the archwires with nanoparticles would be the significant reduction in the frictional resistance at the bracket wire interface.
In orthodontics, many studies have evaluated factors that influence frictional resistance between the bracket and archwire using experimental testing models that included one and three brackets.3-6 These studies have shown that the most important factors involved in the determination of the level of friction are: The bracket and wire material, the surface condition of the archwire and bracket slot, the wire section, the torque at the wire-bracket interface, the type and force of ligation, use of self-ligating bracket, inter bracket distance, and oral functions.7-9
Consequently, the above factors are important when considering the clinical application of sliding mechanics, as they could influence friction. Such a reduction in friction can help shorten overall treatment time, especially in patients undergoing extractions where tooth translation is achieved by sliding mechanics.3
Frictional forces measured in this study were comparable in magnitude and range with those reported by other investigators, great care was taken to ensure the methodology was comparable with previously published work.10
Our study is an extension of the research by Redlich et al. on orthodontic SS wires coated with nanoparticles. These nanoparticles consist of inorganic fullerene impregnated in the electroless nickel phosphorus film. It was found in this study that the nanocoating of archwires reduces the friction considerably. Additional tests were carried out to test the biocompatibility of the nanoparticles.1
In the research study, they have used inorganic fullerene-like tungsten disulfide (IF-WS2) nanoparticles. The inorganic fullerene-like tungsten disulfide (IF-WS2) nanoparticles were described first in 1992. It was shown that under certain reducing and sulfidizing conditions, at elevated temperatures, tungsten oxide (WO3) nanoparticles could form nested WS2 fullerene-like nanostructures creating layers that resemble an onion or a nanotube. These nanoparticles are constructed of multiple layers that can be represented as a sandwich of covalently bonded S-W-S moieties within the plane. The layers are weakly connected through van der Waals forces only. The size of these nanoparticles ranges from 20 to 200 nm depending on the WO3 precursor size. It is known that WS2 and MoS2 particles with a layered structure (platelets) provide good lubricity. Synthesis of fullerene-like WS2 nanoparticles has allowed remarkable improvement of friction and wear properties under different contact conditions. Low friction and wear are associated with the penetration of solid lubricant WS2 nanoparticles into the interface between rubbed surfaces. As the load between the bodies increases, the nanoparticles gradually deform and exfoliate, leaving nanoplatelets of the sandwiched material to coat the asperities at the interface. The weak forces, between the thin sheets of the exfoliated nanoparticles, allow a very low shear force sliding motion between the two contacting bodies.
The nanoparticle used in our study is nanocremics that is commercially available nanoparticle coating precursor solution. This was the solution of choice in our study as it was readily available commercially in India. Secondly the laboratory procedure for coating of the same could be easily carried out in India.
The WS2 nanoparticle used in the research work of Redlich et al. (2008) was coated by the following procedure. Orthodontic SS wires (Ormco, CA, USA) with a rectangular cross-section (0.019” × 0.025”) shape, were coated with a uniform and smooth Ni-P film using 100 ml solution (ENPLATE Ni-425, Enthrone Inc.). The orthodontic wires were inserted into the electroless Ni-P bath (88°C, pH 4.8, magnetic stirring) for 30 min. The plating resulted in a shiny, smooth Ni-P layer 1-2 um thick. Subsequently, the wires were submerged into another electroless solution with 200 mg IF-WS2 powder having an average particle size of 120 nm. A cationic surfactant (cetyltrimethylammonium bromide) was added to minimize the agglomeration of the nanoparticles. A short (1 min) sonication (Sonifaier 150, Branson - 30 w) was used to disperse the agglomerates and ensure the stability of the suspension. Excessive sonication time (>2 min) is known to damage the IF nanoparticles. To ensure the deposition of a uniform and relatively smooth composite Ni-P + IF film and secure its adequate adherence to the underlying archwire the following procedure was developed. First the wires were etched with HF (20%) solution. Subsequently, thin Ni coating (50 nm) was applied using e-beam evaporation. On top of this film, a pure Ni-P electroless film was deposited, and only at the final stage of the process electroless Ni-P + IF film was deposited on the etched wires. The total thickness of the coated film was 3-5 um. The wires were annealed at 400°C for 1h under nitrogen gas flow. The annealing led to the formation of a multi-crystalline Ni3P film that adhered very well to the underlying substrate.
The nanocremics solution used in our study was coated with sol-gel thin film dip coating method as discussed earlier in materials in method. In order to evaluate the adherence of the coating on to the orthodontic wire, the coated orthodontic wires were analyzed by SEM. Also, the slot of the bracket that was used for sliding the coated orthodontic wires and was checked for remnants of coated material.
On the basis of our study we found that commercially available orthodontic wires can be successfully coated using a novel method of sol-gel thin film dip coating method and that coating commercially available SS, NiTi and TMA orthodontic wires with nanoparticles improves their surface topography.
With further studies, this nanoparticle coated wires may represent a valid alternative to commercially available wires when minimal amount of friction is desired. The coatings can also be used for brackets, flexible archwires, and self-ligating systems.
We assume that the friction can further be reduced significantly by coating both the bracket as well as archwire as the corrosion factors would be eliminated this way.
Limitations of this research are that it was carried out in vitro, in a passive configuration. Tipping, torquing forces and the functional forces of the stomatognathic muscles can also affect the frictional resistance during space closure; however, these factors need to be studied.
It should be stressed out that an in vitro study can’t replicate exactly the same physiologic changes that occur in the oral cavity during an orthodontic tooth movement. Thus, another drawback of this study can be the changes in the friction at the bracket wire interface caused during various oral activities.
Preliminary toxicology tests of the IF-WS2 revealed that the material showed no apparent toxic reaction after an oral administration test in rats and no sensitization in lymph nodes following a topical dermal application.11,12 More recent inhalation tests have shown that the material has no toxic effect in rats.13 Nonetheless, the composite coating needs to be further tested. More toxicity and sensitivity tests, evaluating the effect of the nanoparticles on various body organs, and especially oral tissues are necessary. Various tests for the biocompatibility issues of nanocremics need to be undertaken.
Thirdly, since a certain amount of the nanoparticles was likely to be released from the coating, or exfoliate during motion, it was very important to examine the coated wires after sliding, to assure the adhesion properties of the nanocremics in the matrix layer. The presence of nanoparticle is a prerequisite for proper lubrication during motion.
With further improvements in the coating method and its approval for use in the oral cavity, the problem of friction during orthodontic treatment could be minimized consequently, enhancing anchorage control, reducing duration of treatment and decreasing the risk of root resorption.
Conclusion
From the above study it can be concluded that:
Commercially available orthodontic wires can be successfully coated using a novel method of sol-gel thin film dip coating method.
Coating commercially available SS, NiTi and TMA orthodontic wires with nanoparticles improves their surface topography.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Redlich M, Katz A, Rapoport L, Wagner HD, Feldman Y, Tenne R. Improved orthodontic stainless steel wires coated with inorganic fullerene-like nanoparticles of WS(2) impregnated in electroless nickel-phosphorous film. Dent Mater. 2008;24(12):1640–6. doi: 10.1016/j.dental.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 2.Katz A, Redlich M, Rapoport L, Wagner HD, Tenne R. Selflubricating coatings containing fullerene-like WS2 nanoparticles for orthodontic wires and other possible medical applications. Tribol Lett. 2006;21:135–9. [Google Scholar]
- 3.Cacciafesta V, Sfondrini MF, Ricciardi A, Scribante A, Klersy C, Auricchio F. Evaluation of friction of stainless steel and esthetic self-ligating brackets in various bracket-archwire combinations. Am J Orthod Dentofacial Orthop. 2003;124(4):395–402. doi: 10.1016/s0889-5406(03)00504-3. [DOI] [PubMed] [Google Scholar]
- 4.Khambay B, Millett D, McHugh S. Evaluation of methods of archwire ligation on frictional resistance. Eur J Orthod. 2004;26(3):327–32. doi: 10.1093/ejo/26.3.327. [DOI] [PubMed] [Google Scholar]
- 5.Kapila S, Angolkar PV, Duncanson MG, Jr, Nanda RS. Evaluation of friction between edgewise stainless steel brackets and orthodontic wires of four alloys. Am J Orthod Dentofacial Orthop. 1990;98(2):117–26. doi: 10.1016/0889-5406(90)70005-W. [DOI] [PubMed] [Google Scholar]
- 6.Braun S, Bluestein M, Moore BK, Benson G. Friction in perspective. Am J Orthod Dentofacial Orthop. 1999;115(6):619–27. doi: 10.1016/s0889-5406(99)70286-6. [DOI] [PubMed] [Google Scholar]
- 7.Frank CA, Nikolai RJ. A comparative study of frictional resistances between orthodontic bracket and arch wire. Am J Orthod. 1980;78(6):593–609. doi: 10.1016/0002-9416(80)90199-2. [DOI] [PubMed] [Google Scholar]
- 8.Keith O, Jones SP, Davies EH. The influence of bracket material, ligation force and wear on frictional resistance of orthodontic brackets. Br J Orthod. 1993;20(2):109–15. doi: 10.1179/bjo.20.2.109. [DOI] [PubMed] [Google Scholar]
- 9.Chimenti C, Franchi L, Di Giuseppe MG, Lucci M. Friction of orthodontic elastomeric ligatures with different dimensions. Angle Orthod. 2005;75(3):421–5. doi: 10.1043/0003-3219(2005)75[421:FOOELW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Thomas S, Sherriff M, Birnie D. A comparative in vitro study of the frictional characteristics of two types of self-ligating brackets and two types of pre-adjusted edgewise brackets tied with elastomeric ligatures. Eur J Orthod. 1998;20(5):589–96. doi: 10.1093/ejo/20.5.589. [DOI] [PubMed] [Google Scholar]
- 11.Tsabari H. Israel: Harlan Biotech; 2005. Inorganic Fullerene-Like Nanospheres (IF-WS2) Acute Oral Toxicity, Acute Toxic Class Method in the Rat. Final Report. Batch No. HP6. [Google Scholar]
- 12.Haist I. Germany: BSL Disservice; 2005. Test for Sensitization (Local Lymph Node Assay–LLNA) with Inorganic Fullerene-like WS2 Nanospheres. Project No. 052052. [Google Scholar]
- 13.Moore GE. Dayton, NJ, USA: 2006. Acute Inhalation Toxicity Study in Rats–Limit Test. Product Safety Laboratories. Study No. 18503. [Google Scholar]


