Abstract
Recurrent aphthous stomatitis (RAS) is a well-known oral disease with unclear etiopathogenesis for which symptomatic therapy is only available. This kind of study aimed to highlight the main points that the general practitioners should be taken in their consideration. We have collected our data from PubMed line from 1972 to 2011. Our criteria included the papers that refer to the general predisposing factors, and the general treatment of RAS. Some papers which indicated to the specific details related to RAS that needed a consultant or specialist in Oral Medicine have not included. There is no clear guideline of the etiology, diagnosis, and management of RAS; therefore, the majority of the general practitioners refer most of the cases to appropriate specialist.
Keywords: Aphthous stomatitis, etiological factors, major aphthous stomatitis
Introduction
Recurrent aphthous stomatitis (RAS) is considered as the most common oral mucosal lesion. These present as recurrent, multiple, small, or ovoid ulcers, having yellow floors and are surrounded by erythematous haloes, present first in childhood or adolescence.1 Aphthous ulcers affect up to 25% of the general population, and 3-month recurrence rates are as high as 50%.1 It is more common in female. Aphthous ulcers increase by increasing age and minor aphthous ulcers are 80% of suffered patient.1 Aphthous ulcers were reported from 5 to 66% among different nations.1 The cause of aphthous ulcers is unknown, and therefore many factors are still implicated in the disease including hormonal changes, trauma, drugs, food hypersensitivity, nutritional deficiency, stress, and tobacco.
Clinical Features
There are three clinical presentations of RAS: Minor RAS, major RAS, and herpetiform ulceration.2
Minor RAS: It is the most common form of RAS and approximately 85% of patients have lesions of this type.3 Minor aphthous can involve the non-keratinized mucosa of the oral cavity (the labial and buccal mucosa, the floor of the mouth and the ventral or lateral surface of the tongue).4 Moreover, the ulcers are usually concentrated in the anterior part of the mouth. The ulcers are superficial, usually <1 cm in diameter, their size is approximately 4-5 mm in diameter. The classification of minor RAS does not depend on the dimensions of the lesions alone, but on a number of other clinical features such number of ulcers from 1 to 5.7-9 Ulcer shape varies somewhat according to the location of the lesion, more rounded on the labial or buccal mucosa (Figure 1) and elongated in the buccal sulcus. Minor aphthae does not result in scarring despite years of recurrent ulceration and tend to heal within 10-14 days.5,6
Figure 1.
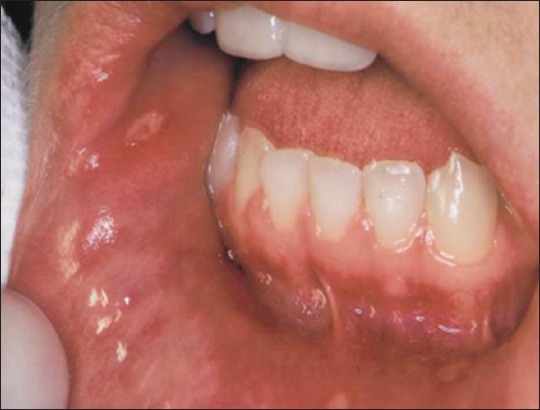
Minor aphthous ulceration.
Major RAS
Major RAS is less common than minor RAS lesions (approximately 10-15% of all RAS). These lesions are similar in appearance to those of minor RAS; however, they are larger than 10 mm in diameter, are deeper, often scarred, and can last for weeks to months (Figure 2). These lesions have a predilection for lips, tongue, soft palate, and the palatal fauces and cause significant pain and dysphagia. They are frequently found in patients infected with human immunodeficiency virus (HIV).7,8
Figure 2.
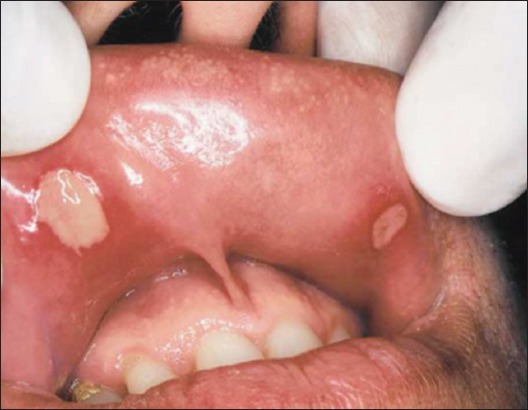
Major aphthous ulceration.
Herpetiform ulceration
Herpetiform ulceration constitutes only 5-10% of all RAS cases.3 A resemblance between this term with herpes simplex virus (HSV) infection exists. Herpetiform ulcers are small (1-2 mm) and multiple ulcers (5-100) may be present at the same time (Figure 3). Although any non-keratinized oral mucosa may be involved, characteristically the affected sites are the lateral margins and ventral surface of the tongue and the floor of the mouth.9 Individual ulcers are grey and without a delineating erythematous border, making them difficult to visualize. These ulcers have small size, and cause painful and may make eating and speaking difficult. A single crop of ulcers may last for approximately 7-14 days, and the period of remission between attacks is variable. Herpetiform ulcers may coalesce to form larger confluent areas of ulcer, usually with marked erythema. The patients affected are predominantly female and generally those ulcers have a later age onset than the other types of RAS.4
Figure 3.
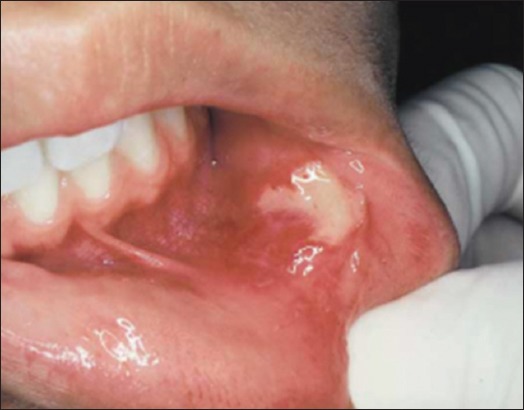
Herpetiform ulceration.
Predisposing and Environmental Factors
Hormonal changes
McCullough et al.,10 reported that female patients with RAS relate the onset of their oral ulceration to their menstrual cycle, pregnancy, and dysmenorrhea. It has been reported that the RAS usually improved during pregnancy,11 also RAS may be affected by the sex steroids.12
Trauma
RAS patients often report aphthous ulcers at sites of trauma, particularly due to toothbrushing, or the site of a local anesthetic injection and dental treatment.13,14
Drugs
Boulinguez et al.,15 reported that there is association between the use of some drugs such as (sodium hypochlorite – piroxicam – phenobarbital – phenindione - niflumic acid – nicorandil - gold salts - captopril) and RAS. Furthermore, the use of other drugs such as non-steroidal anti-inflammatory drugs (NSAIDs, e.g., pro-propionic acid, phenylacetic acid, and diclofenac) can stimulate the formation the oral ulcers very similar to RAS.16
Food hypersensitivity
Some foods such as chocolate, coffee, peanuts, cereals, almonds, strawberries, cheese, tomatoes, and wheat flour (containing gluten) may be implicated in some patients.17,18 Besu et al.,19 reported that there is a strong association between high levels of serum anti-cow’s milk proteins immunoglobulin A (IgA), IgG and IgE antibodies and clinical manifestations of recurrent aphthous ulcers.
Nutritional deficiency states
Nutritional markers associated with anemias (iron, serum ferritin) have been reported to be twice as common in RAS patients as in controls and up to 20% of RAS patients may have a nutritional deficiency.20 Nolan et al.,21 found that 28.2% of patients with RAS had deficiencies of vitamins B1, B2, and or B6. They indicated that those patients could benefit from vitamin replacement therapy.
Stress
Gallo et al.,22 reported that there was a higher level of psychological stress among RAS group patients when compared to the control group. Although the majority of researches have been unable to validate the concept that stress plays an important role in the development of RAS, the literature indicates that stress may play a role in the development of RAS.
Tobacco
Tobacco use is a risk factor for oral cancer, oral mucosal lesions and periodontal disease. The incidence of RAS was found to be lower in smokers than in non-smokers and clinical observation suggests that some smokers experience an increase in mouth ulcers upon stopping smoking.23 Patients who stop smoking often complain of RAS (mouth). A feature of interest is that RAS are infrequently seen in patients who smoke tobacco. The main explanation is that tobacco may increase keratinization of the oral mucosa, which in turn may render the mucosa less susceptible to ulceration.24 Hill et al.,25 describe a case of complex aphthosis which began within weeks of stopping smoking. After failing to respond to conventional agents, the patient was successfully treated with nicotine lozenges. They recommend considering the use nicotine replacement therapy when conventional management has failed, particularly in ex-smokers.
All the predisposing and environmental factors mentioned above can be investigated and diagnosed from the general practitioners but the other environmental factors such as (infection factors (for both bacterial agents and viral agents), serology of RAS, and the systemic disease associated with RAS (celiac disease, Behcet’s disease), and HIV associated with RAS should refer to the appropriate specialists because it is very difficult for general practitioners to diagnose and manage such those disease.
Hereditary predisposition
Family history may have a role in the formation of RAS, and reports of cases within the same family are present in 24-46% of the times.6,7 Furthermore, ulcers tend to occur earlier and more severe in presentation than those without family history in patients with family history of RAS.6,7
Immunological features of RAS
Numerous associations of human leukocyte antigen (HLA) and RAS antigen have been reported in the medical literature. The association between the disease and HLAB12 was described by some authors Lehner et al. and Malmström et al.,6,7 However, it was not confirmed by other authors.9 In groups of patients of different ethnical origin, a significant association between HLA-DR2 and RAS was noticed.7
RAS pathophysiology seems to be associated with a disorder in immunomodulation.9 Lymphocytes seem to be the predominant cells in aphthoid lesions, and there was a variation in the CD4+/CD8+ ratio during its different stages - prodrome or pre-ulceration, ulceration, and healing.9
The systemic disorders that are associated with RAS
The systemic disorders that are associated with lesions clinically similar to RAS are nutritional deficiencies leading to anemias, Behcet’s syndrome, cyclic neutropenia, HIV infection, PFAPA, reactive arthritis, Sweet’s syndrome, Magic syndrome.5-7
Behçet’s disease is a multisystemic disorder characterized by oral and genital ulcers and cutaneous (erythema nodosum, pustular vasculitis), ocular (anterior or posterior uveitis), arthritic, vascular (both arterial and venous vasculitis), central nervous system (meningoencephalitis) and gastrointestinal involvement.5-7 Behçet’s disease is commonly seen around the Mediterranean Sea and along the ancient “Silk Road” in places such as Turkey, Iran, Korea, and Japan. The prevalence of the disease is reported to be 1:250 to 1:1000 in Turkey and 0.1:100,000 to 0.6:100,000 in the USA and northern Europe.5-7
Aphthous stomatitis represents a potentially debilitating disorder in HIV-infected persons, approximately 5-15% of HIV-infected patients developed aphthous stomatitis. Despite that painful aphthous oral lesions may develop in immunocompetent persons, but they typically have a more self-limited course than seen with HIV-infected persons, especially those with advanced immunosuppression. HIV-infected individuals characteristically have oral ulcers that are larger, more painful, heal more slowly, and recur more frequently in comparison with immunocompetent persons.5-7
In cyclic neutropenia circulating neutrophils decrease in number or may even be absent temporarily. The disease is characterized by periodic febrile episodes beginning during infancy and is associated with otitis, furuncles, mastoiditis, and RAS.3-7
Oral aphthous lesion may also be associated with PFAPA syndrome,5-7 reactive arthritis (Reiter’s syndrome),5-7 Sweet’s syndrome “acute febrile neutrophilic dermatosis”3-7 and Magic Syndrome.5-7
Diagnosis of RAS
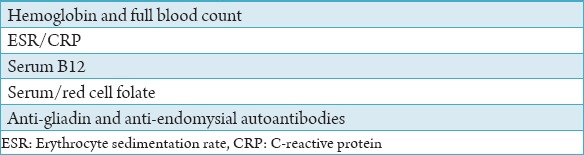
The correct diagnosis of RAS is dependent on a detailed and accurate clinical history and examination of the ulcers. The main points to be elicited in the clinical history are shown in Table 1. Furthermore, it is necessary to carry out an external examination including palpation of the cervical lymph nodes. The important features to be noted when examining a patient with oral ulceration include family history, frequency of ulceration, duration of ulceration, number of ulcers, site of ulcers (non-keratinized or keratinized), size and shape of ulcers, associated medical conditions, genital ulceration, skin problems, gastrointestinal disturbances, drug history, edge of ulcer, base of ulcer, and surrounding tissue (Tables 2 and 3). Furthermore, the investigation tests for patients with persistent RAS including hemoglobin and full blood count, erythrocyte sedimentation rate/C-reactive protein, serum B12, serum/red cell folate, anti-gliadin, and anti-endomysial autoantibodies (Table 3). Clinical assessment of an ulcer includes inspection and palpation, which complement each other. The base of the ulcer can be necrotic, granular purulent, or covered with mucus.
Table 1.
Clinical features of RAS.
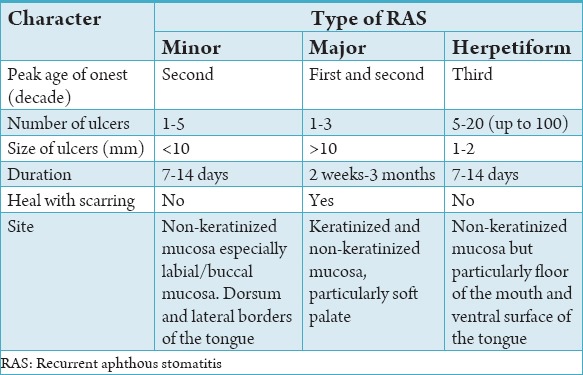
Table 2.
The important features to be noted from general practitioners.
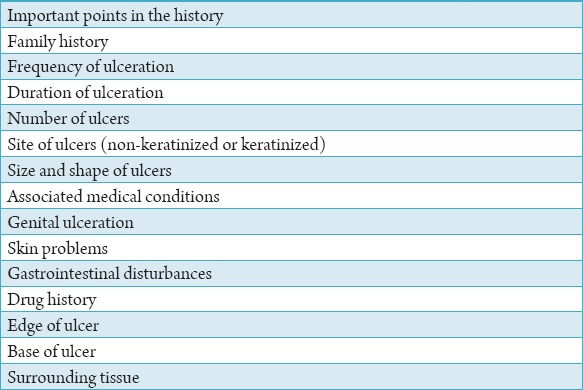
Table 3.
The investigation tests for patients with persistent recurrent aphthous stomatitis.
The consistency of the base (soft, firm, or hard) and fixation to underlying structures can be evaluated by palpation. The edges of the ulcer can be straight or irregular and may feel hard in contrast to the surrounding tissue. This is the characteristic induration, associated with neoplastic infiltration. Another feature of a carcinoma is its rolled border. The tissue surrounding the ulcer may be white, speckled, erythematous, or normal in appearance. Patients with persistent RAS should have follow-up for an underlying hematinic disorders. This includes a full blood count and measurement of inflammatory markers and hematinic (serum ferritin, serum B12, serum and red cell folate). Screening for deficiencies of vitamin B complexes or zinc deficiencies is not routinely carried out, but may be indicated in certain groups of patients. RAS associated with a systemic condition should be referred to the appropriate specialist for further investigations. If there is any suspicion of coeliac disease, either due to patient’s history or evidence of malabsorption on routine testing, then serological testing for appropriate IgA autoantibodies should be carried out and patient is referred to a gastroenterologist for endoscopy and biopsy of the small intestine.
Differential diagnosis
The diagnosis of RAS is typically established from the history and clinical presentation. However, it is important to differentiate aphthous ulcers from other stomatologie mucocutaneous diseases that have ulcerative manifestations. Usually, these conditions can be differentiated from RAS by the location of the lesion or the presence of an additional symptom. HSV infections may have similar-appearing lesions; however, primary HSV infections present with a diffuse gingival erythema and fever preceding oral mucosal vesicles and ulcers. Furthermore, recurrent HSV lesions are found primarily on attached keratinized mucosa, such as the hard palate or gingiva.26 Ulcers of RAS are not preceded by fever or vesicles, and they occur almost exclusively on movable oral mucosa, such as the buccal and labial mucosa, tongue, and soft palate.27 Recurrent aphthous lesions can be differentiated from varicella zoster virus (VZV) infections (shingles) based on clinical presentation (VZV lesions have a unilateral extraoral and intraoral distribution pattern following the trigeminal nerve) and symptoms (VZV infections have a prodrome of pain and burning prior to lesion eruption). Less common oral viral infections, such as herpangina and hand-foot-and-mouth disease, should also be included in the differential diagnosis of RAS when initial symptoms occur.28 However, Coxsaekie virus-related oral ulcers present with other symptoms, such as a low-grade fever or malaise, and will resolve within 1-2 weeks. Erythema multiforme presents with painful oral ulcers, but, unlike RAS, erythema multiforme lesions occur on both attached and movable mucosa and usually involve crusting of the lips with skin macules and papules.29 Approximately, two thirds of patients with oral lichen planus show ulcerative lesions, which primarily occur on the buccal mucosa.30 However, secondary sites on the gingiva and hard palate will distinguish oral lichen planus frotii RAS. In addition, oral lichen planus is not always painful, whereas pain is usually the chief complaint in RAS. Vesiculobullous oral lesions that tend to rupture within hours of the occurrence, resulting in painful erosions or ulcérations, are characteristic of cicatricial pemphigoid and pemphigus vulgaris.31,32 These lesions may occur on both attached and unattached oral mucosa, and a biopsy will reveal a characteristic histomorphometric pattern.
Treatment of RAS
The etiology of RAS is still unknown. There is no agreement in the treatment of RAS therefore, many therapies have been tried, few have been subjected to double-blind randomized controlled. The aim of the treatment of RAS is to decrease symptoms; reduce ulcer number and size; increase disease-free periods. The treatment approach should be determined by disease severity (pain), the patient’s medical history, the frequency of flare-ups and the patient’s ability to tolerate the medication. Some patients have RAS episodes lasting for only a few days, occurring only a few times a year, those need palliative therapy for pain and maintain the good oral hygiene. Drug therapy is considered for patients who experience multiple episodes of RAS each mouth and/or present with symptoms of severe pain and difficulty in eating. The general practitioners should determine the possible nutritional deficiencies or allergies causing the onset of the disease before initiating the application of the medications for RAS. Kozlak et al.,33 have suggested that consuming sufficient amounts of vitamin B12 and folate may be a useful strategy to reduce the number and/or duration of RAS episodes. The traditional treatment of RAS is included glucocorticoids and antimicrobial therapy. These medications have been applied as topical pastes, mouthrinses, intralesional injections and systemically by the oral route. A topical anesthetic such as 2% viscous lidocaine hydrochloride is used to palliate the pain.34
Topical agents
Several pastes and gels can be used to coat the surface of the ulcers and to form a protective barrier against secondary infection and further mechanical irritation. The topical agents are the first option of the treatment of RAS. Patient should apply a small amount of gel or cream after rinsing, and avoid eating or drinking for 30 min. This can be repeated 3 or 4 times daily.35
Mouthwashes
Tetracycline is an antibiotic mouthwash. It reduces the ulcer size, duration, and pain because of the ability of that one to block collagenase activity.34 Chlorhexidine gluconate is an antibiotic agent may decrease the number of ulcer days.35 Chlorhexidine can cause brown staining of the teeth and tongue.
Topical gels, creams, and ointments
Topical medications are washes away from the target area; therefore, it is better to use different kinds of adhesive vehicles in combination with the drug. Topical corticosteroids may limit the inflammatory process associated with the formation of aphthae. Those medications can act on the lymphocytes and alter the response of effector cells to precipitants of immunopathogenesis (e.g., trauma and food allergies). Al-Na’mah et al.,36 have concluded that the novel dexamucobase was found to be equally effective in treating oral aphthous ulceration, with some advantages, as the widely used preparation Kenalog in Orabase. Meng et al.,37 have indicated that amlexanox oral adhesive pellicles are as effective and safe as amlexanox oral adhesive tablets in the treatment of minor RAS for this Chinese cohort. However, pellicles seem to be more comfortable to use when compared with the dosage form of tablets. Therefore, in clinical practice, amlexanox oral adhesive pellicles may be a better choice for RAS patients. Some topical glucocorticoids such as fluocinonide and clobetasol may be preferable when used alone or mixed with orabase.34
Systemic medications
It is indicated for severe and constantly recurring ulcerations, the topical management is not effective in this cases. Pakfetrat et al.,38 have conducted a double-blind randomized clinical trial to compare colchicine versus prednisolone (immunomodulant agents) in RAS and reported that low dose prednisolone and colchicine were both effective in treating RAS. Given that the two therapies had similar efficacy, yet colchicine was associated with more side effects. de Abreu et al.,39 reported that clofazimine should be considered for the treatment of RAS. Moreover, Weckx et al.,40 reported that levamisole is not effective in the prophylactic treatment of RAS. Kaya et al.,41 documented that levamisole which is the first member of a potential new class of immunologically active, possibly thyromimetic compounds. It appears to regulate cell-mediated immune reactions by restoring effector functions of peripheral T-lymphocytes and phagocytes and by stimulating precursor T-lymphocytes to differentiate into mature cells. It shares these effects with a number of other immune regulators.41
Diclofenac, a NSAIDs, reduces duration of pain by inhibiting the production of cyclooxygenase enzyme and preventing the arachidonic acid converting to other compounds like prostaglandins. Seemingly, diclofenac can act as sodium channel blocker which is mediated by topical analgesic.33,34
The drug pentoxifylline, a non-selective phosphodiesterase inhibitor with hemorheological properties, has many potential uses.40,42 It has been demonstrated to inhibit irritant and contact hypersensitivity,43 and has been used in the treatment of rheumatoid arthritis, multiple sclerosis, and other immune-mediated conditions. It inhibits particularly tumor necrosis factor-α production44 and possibly the production of some other T-helper cell 1 and proinflammatory cytokines, such as interleukin-1β,45,46 that are thought to be important in the RAS disease process.
In severe cases of RAS, immunosuppressive, and anti-inflammatory drugs have shown varying degrees of success. Drugs commonly used include corticosteroids, dapsone, colchicine, thalidomide.47
Dapsone seems to inhibit the migration of polymorphonuclear leukocytes by inhibiting lysosomal enzyme activity and interfering with the cellular response to chemotactic stimuli.48
Laser therapy (high power and low power) have been used for RAS and reported as case studies and clinical trial studies. In some studies low-level laser therapy had efficacy like or better than topical steroids.49
Chavan et al.,50 have indicated that treatment often includes the use of a chlorhexidine mouthwash (without alcohol base) and a short course of topical corticosteroids as soon as the ulcers appear.
It is recommended that the continuous education training of the general practitioners will increase their knowledge to diagnose and manage RAS because that disease is a very common seen in the dental clinic and the general practitioners should be familiar to deal with simple types of RAS such as minor, major, and herpetiform.
Conclusion
RAS remains a common oral mucosal disorder in most communities of the world; its precise etiology remains unclear. No precise trigger has ever been demonstrated, and there is no conclusive evidence for a genetic predisposition to RAS in most patients. Lesions arise as a consequence of immunologically mediated cytotoxicity of epithelial cells. There is no safe therapy to ensure no recurrence of ulcers. There have been few studies conclusively prove that any agent, apart from anti-inflammatory agents, can reduce the frequency or severity of RAS more than can placebo.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Barrons RW. Treatment strategies for recurrent oral aphthous ulcers. Am J Health Syst Pharm. 2001;58(1):41–50. [PubMed] [Google Scholar]
- 2.Stanley HR. Aphthous lesions. Oral Surg Oral Med Oral Pathol. 1972;33(3):40716. doi: 10.1016/0030-4220(72)90470-7. [DOI] [PubMed] [Google Scholar]
- 3.Rogers RS., 3rd Recurrent aphthous stomatitis: Clinical characteristics and associated systemic disorders. Semin Cutan Med Surg. 1997;16(4):278–83. doi: 10.1016/s1085-5629(97)80017-x. [DOI] [PubMed] [Google Scholar]
- 4.Scully C, Porter S. Recurrent aphthous stomatitis: Current concepts of etiology, pathogenesis and management. J Oral Pathol Med. 1989;18(1):21–7. doi: 10.1111/j.1600-0714.1989.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 5.Sircus W, Church R, Kelleher J. Recurrent aphthous ulceration of the mouth;a study of the natural history, aetiology, and treatment. Q J Med. 1957;26(102):235–49. [PubMed] [Google Scholar]
- 6.Akintoye SO, Greenberg MS. Recurrent aphthous stomatitis. Dent Clin North Am. 2005;49(1):31–47. doi: 10.1016/j.cden.2004.08.001. vii. [DOI] [PubMed] [Google Scholar]
- 7.Natah SS, Konttinen YT, Enattah NS, Ashammakhi N, Sharkey KA, Häyrinen-Immonen R. Recurrent aphthous ulcers today: A review of the growing knowledge. Int J Oral Maxillofac Surg. 2004;33(3):221–34. doi: 10.1006/ijom.2002.0446. [DOI] [PubMed] [Google Scholar]
- 8.Boldo A. Major recurrent aphthous ulceration: Case report and review of the literature. Conn Med. 2008;72(5):271–3. [PubMed] [Google Scholar]
- 9.Shashy RG, Ridley MB. Aphthous ulcers: A difficult clinical entity. Am J Otolaryngol. 2000;21(6):389–93. doi: 10.1053/ajot.2000.18872. [DOI] [PubMed] [Google Scholar]
- 10.McCullough MJ, Abdel-Hafeth S, Scully C. Recurrent aphthous stomatitis revisited;clinical features, associations, and new association with infant feeding practices? J Oral Pathol Med. 2007;36(10):615–20. doi: 10.1111/j.1600-0714.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- 11.Dolby AE. Recurrent Mikulicz’s oral apthae. Their relationship to the menstrual cycle. Br Dent J. 1968;124(8):359–60. [PubMed] [Google Scholar]
- 12.Bishop PM, Harris PW, Trafford JA. Oestrogen treatment of recurrent aphthous mouth ulcers. Lancet. 1967;1(7504):1345–7. doi: 10.1016/s0140-6736(67)91760-6. [DOI] [PubMed] [Google Scholar]
- 13.Kvam E, Gjerdet NR, Bondevik O. Traumatic ulcers and pain during orthodontic treatment. Community Dent Oral Epidemiol. 1987;15(2):104–7. doi: 10.1111/j.1600-0528.1987.tb00493.x. [DOI] [PubMed] [Google Scholar]
- 14.Wray D, Graykowski EA, Notkins AL. Role of mucosal injury in initiating recurrent aphthous stomatitis. Br Med J (Clin Res Ed) 1981;283(6306):1569–70. doi: 10.1136/bmj.283.6306.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boulinguez S, Cornée-Leplat I, Bouyssou-Gauthier ML, Bedane C, Bonnetblanc JM. Analysis of the literature about drug-induced aphthous ulcers. Ann Dermatol Venereol. 2000;127(2):155–8. [PubMed] [Google Scholar]
- 16.Healy CM, Thornhill MH. An association between recurrent oro-genital ulceration and non-steroidal anti-inflammatory drugs. J Oral Pathol Med. 1995;24(1):46–8. doi: 10.1111/j.1600-0714.1995.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 17.Hay KD, Reade PC. The use of an elimination diet in the treatment of recurrent aphthous ulceration of the oral cavity. Oral Surg Oral Med Oral Pathol. 1984;57(5):504–7. doi: 10.1016/0030-4220(84)90308-6. [DOI] [PubMed] [Google Scholar]
- 18.Eversole LR, Shopper TP, Chambers DW. Effects of suspected foodstuff challenging agents in the etiology of recurrent aphthous stomatitis. Oral Surg Oral Med Oral Pathol. 1982;54(1):33–8. doi: 10.1016/0030-4220(82)90414-5. [DOI] [PubMed] [Google Scholar]
- 19.Besu I, Jankovic L, Magdu IU, Konic-Ristic A, Raskovic S, Juranic Z. Humoral immunity to cow’s milk proteins and gliadin within the etiology of recurrent aphthous ulcers? Oral Dis. 2009;15(8):560–4. doi: 10.1111/j.1601-0825.2009.01595.x. [DOI] [PubMed] [Google Scholar]
- 20.Porter SR, Scully C, Flint S. Hematologic status in recurrent aphthous stomatitis compared with other oral disease. Oral Surg Oral Med Oral Pathol. 1988;66(1):41–4. doi: 10.1016/0030-4220(88)90064-3. [DOI] [PubMed] [Google Scholar]
- 21.Nolan A, McIntosh WB, Allam BF, Lamey PJ. Recurrent aphthous ulceration: Vitamin B1, B2 and B6 status and response to replacement therapy. J Oral Pathol Med. 1991;20(8):389–91. doi: 10.1111/j.1600-0714.1991.tb00950.x. [DOI] [PubMed] [Google Scholar]
- 22.Gallo Cde B, Mimura MA, Sugaya NN. Psychological stress and recurrent aphthous stomatitis. Clinics (Sao Paulo) 2009;64(7):645–8. doi: 10.1590/S1807-59322009000700007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirbod SM, Ahing SI. Tobacco-associated lesions of the oral cavity: Part I. Nonmalignant lesions. J Can Dent Assoc. 2000;66(5):252–6. [PubMed] [Google Scholar]
- 24.McRobbie H, Hajek P, Gillison F. The relationship between smoking cessation and mouth ulcers. Nicotine Tob Res. 2004;6(4):655–9. doi: 10.1080/14622200410001734012. [DOI] [PubMed] [Google Scholar]
- 25.Hill SC, Stavrakoglou A, Coutts IR. Nicotine replacement therapy as a treatment for complex aphthosis. J Dermatolog Treat. 2010;21(5):317–8. doi: 10.3109/09546630903271563. [DOI] [PubMed] [Google Scholar]
- 26.Mattingly G, Rodu B. Differential diagnosis of oral mucosal ulcerations. Compendium. 1993;14(2):136. [PubMed] [Google Scholar]
- 27.Rodu B, Mattingly G. Oral mucosal ulcers: Diagnosis and management. J Am Dent Assoc. 1992;123(10):83–6. doi: 10.14219/jada.archive.1992.0268. [DOI] [PubMed] [Google Scholar]
- 28.Fenton SJ, Unkel JH. Viral infections of the oral mucosa in children: A clinical review. Pract Periodontics Aesthet Dent. 1997;9(6):683–90. [PubMed] [Google Scholar]
- 29.Lozada F, Silverman S., Jr Erythema multiforme. Clinical characteristics and natural history in fifty patients. Oral Surg Oral Med Oral Pathol. 1978;46(5):628–36. doi: 10.1016/0030-4220(78)90458-9. [DOI] [PubMed] [Google Scholar]
- 30.Brown RS, Bottomley WK, Puente E, Lavigne GJ. A retrospective evaluation of 193 patients with oral lichen planus. J Oral Pathol Med. 1993;22(2):69–72. doi: 10.1111/j.1600-0714.1993.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg MA, Insler MS, Campen RB. Mucocutaneous features of autoimmune blistering diseases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84(5):517–34. doi: 10.1016/s1079-2104(97)90269-9. [DOI] [PubMed] [Google Scholar]
- 32.Terezhalmy GT, Bergfeld WF. Cicatricial pemphigoid (benign mucous membrane pemphigoid) Quintessence Int. 1998;29(7):429–37. [PubMed] [Google Scholar]
- 33.Kozlak ST, Walsh SJ, Lalla RV. Reduced dietary intake of vitamin B12 and folate in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2010;39(5):420–3. doi: 10.1111/j.1600-0714.2009.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ship JA, Chavez EM, Doerr PA, Henson BS, Sarmadi M. Recurrent aphthous stomatitis. Quintessence Int. 2000;31(2):95–112. [PubMed] [Google Scholar]
- 35.Casiglia JM. Recurrent aphthous stomatitis: Etiology, diagnosis, and treatment. Gen Dent. 2002;50(2):157–66. [PubMed] [Google Scholar]
- 36.Al-Na’mah ZM, Carson R, Thanoon IA. Dexamucobase: A novel treatment for oral aphthous ulceration. Quintessence Int. 2009;40(5):399–404. [PubMed] [Google Scholar]
- 37.Meng W, Dong Y, Liu J, Wang Z, Zhong X, Chen R, et al. A clinical evaluation of amlexanox oral adhesive pellicles in the treatment of recurrent aphthous stomatitis and comparison with amlexanox oral tablets: A randomized, placebo controlled, blinded, multicenter clinical trial. Trials. 2009;10:30. doi: 10.1186/1745-6215-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pakfetrat A, Mansourian A, Momen-Heravi F, Delavarian Z, Momen-Beitollahi J, Khalilzadeh O, et al. Comparison of colchicine versus prednisolone in recurrent aphthous stomatitis: A double-blind randomized clinical trial. Clin Invest Med. 2010;33(3):E189–95. doi: 10.25011/cim.v33i3.13725. [DOI] [PubMed] [Google Scholar]
- 39.de Abreu MA, Hirata CH, Pimentel DR, Weckx LL. Treatment of recurrent aphthous stomatitis with clofazimine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(5):714–21. doi: 10.1016/j.tripleo.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Weckx LL, Hirata CH, Abreu MA, Fillizolla VC, Silva OM. Levamisole does not prevent lesions of recurrent aphthous stomatitis: A double-blind placebo-controlled clinical trial. Rev Assoc Med Bras. 2009;55(2):132–8. doi: 10.1590/s0104-42302009000200014. [DOI] [PubMed] [Google Scholar]
- 41.Kaya EG, Ozbilge H, Ustundag MB, Torun YA. The effects on immune response of levamisole treatment following infection of U-937 macrophages with Candida albicans. Acta Microbiol Immunol Hung. 2011;58(4):279–88. doi: 10.1556/AMicr.58.2011.4.4. [DOI] [PubMed] [Google Scholar]
- 42.Samlaska CP, Winfield EA. Pentoxifylline. J Am Acad Dermatol. 1994;30(4):603–21. doi: 10.1016/s0190-9622(94)70069-9. [DOI] [PubMed] [Google Scholar]
- 43.Schwarz A, Krone C, Trautinger F, Aragane Y, Neuner P, Luger TA, et al. Pentoxifylline suppresses irritant and contact hypersensitivity reactions. J Invest Dermatol. 1993;101(4):549–52. doi: 10.1111/1523-1747.ep12365951. [DOI] [PubMed] [Google Scholar]
- 44.Marques LJ, Zheng L, Poulakis N, Guzman J, Costabel U. Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages. Am J Respir Crit Care Med. 1999;159(2):508–11. doi: 10.1164/ajrccm.159.2.9804085. [DOI] [PubMed] [Google Scholar]
- 45.D’Hellencourt CL, Diaw L, Cornillet P, Guenounou M. Differential regulation of TNF alpha, IL-1 beta, IL-6, IL-8, TNF beta, and IL-10 by pentoxifylline. Int J Immunopharmacol. 1996;18(12):739–48. doi: 10.1016/s0192-0561(97)85556-7. [DOI] [PubMed] [Google Scholar]
- 46.Tilg H, Eibl B, Pichl M, Gächter A, Herold M, Brankova J, et al. Immune response modulation by pentoxifylline in vitro. Transplantation. 1993;56(1):196–201. doi: 10.1097/00007890-199307000-00036. [DOI] [PubMed] [Google Scholar]
- 47.Ship JA. Recurrent aphthous stomatitis: An update. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81(2):141–7. doi: 10.1016/s1079-2104(96)80403-3. [DOI] [PubMed] [Google Scholar]
- 48.Modschiedler K, Weller M, Wörl P, von den Driesch P. Dapsone and colchicine inhibit adhesion of neutrophilic granulocytes to epidermal sections. Arch Dermatol Res. 2000;292(1):32–6. doi: 10.1007/pl00007458. [DOI] [PubMed] [Google Scholar]
- 49.Tezel A, Kara C, Balkaya V, Orbak R. An evaluation of different treatments for recurrent aphthous stomatitis and patient perceptions: Nd: YAG laser versus medication. Photomed Laser Surg. 2009;27(1):101–6. doi: 10.1089/pho.2008.2274. [DOI] [PubMed] [Google Scholar]
- 50.Chavan M, Jain H, Diwan N, Khedkar S, Shete A, Durkar S. Recurrent aphthous stomatitis: A review. J Oral Pathol Med. 2012;41(8):577–83. doi: 10.1111/j.1600-0714.2012.01134.x. [DOI] [PubMed] [Google Scholar]


