Abstract
Previous studies on the neurocognitive impact of cannabis use have found working and declarative memory deficits that tend to normalize with abstinence. An unexplored aspect of cognitive function in chronic cannabis users is the ability to distinguish between veridical and illusory memories, a crucial aspect of reality monitoring that relies on adequate memory function and cognitive control. Using functional magnetic resonance imaging, we show that abstinent cannabis users have an increased susceptibility to false memories, failing to identify lure stimuli as events that never occurred. In addition to impaired performance, cannabis users display reduced activation in areas associated with memory processing within the lateral and medial temporal lobe (MTL), and in parietal and frontal brain regions involved in attention and performance monitoring. Furthermore, cannabis consumption was inversely correlated with MTL activity, suggesting that the drug is especially detrimental to the episodic aspects of memory. These findings indicate that cannabis users have an increased susceptibility to memory distortions even when abstinent and drug-free, suggesting a long-lasting compromise of memory and cognitive control mechanisms involved in reality monitoring.
Introduction
Cannabis is the most widely used recreational drug worldwide after alcohol and tobacco.1, 2 Despite changing attitudes in the perceived risks associated with this substance and decriminalization initiatives taking place in many US states and countries,1, 3 the health implications of long-term cannabis consumption are still a matter of concern.4 Regular use of cannabis has been associated with adverse health consequences, including psychiatric and neurocognitive disorders. Besides the more immediate risk of developing cannabis dependence,5 other mental disorders, such as anxiety, depression or psychosis,6, 7 and cognitive impairment have also been described.8 One recent study involving over a thousand individuals found that chronic cannabis use is associated with cognitive decline, with greater deterioration being observed in those individuals presenting a more persistent use.9 Among the various cognitive domains studied, memory is one of the most frequently identified as being negatively affected by cannabis.9, 10, 11
Impaired working and declarative memory are well-known aspects of acute intoxication.12 Cannabis preparations and delta-9-tetrahydrocannabinol, its main active principle, acutely deteriorate the ability to retain information for short periods of time,8, 13 and impair episodic memory and verbal recall.14, 15 A characteristic of cannabis consumption is that residual effects can linger for days after the most recent use.10 Typically, these deleterious effects gradually wear off and memory processes normalize after several weeks of abstinence.16, 17 However, some studies in heavy cannabis users have observed impairment persisting even months after the last consumption.9, 10 In addition to impaired performance, imaging studies in chronic cannabis users have found structural brain alterations in the hippocampus, a key area in the memory processing network. Notably, decreases in hippocampal volume showed an association with the amount of cannabis used.18, 19, 20 These structural changes may be long-lasting, as volume reductions can persist even after abstinence of 6 months.18
An unknown aspect of long-term cannabis use is its potential to disrupt memory and reality monitoring mechanisms that normally allow us to distinguish between veridical and illusory events. Avoiding memory distortions may be extremely relevant in certain contexts such as the courtroom and forensic examination, and in a more general context this ability provides us with an adequate sense of reality that guides future behavior based on past experiences. Memories of events that never occurred, or false memories, can be found in neurological and psychiatric conditions. They have been described in post-traumatic stress disorder, psychosis, dissociative disorders and in cases of confabulation or ‘honest lying' associated with confessions of uncommitted crimes, among others.21 However, in a more subtle form, false memories are also a common occurrence in everyday life in healthy individuals22 and show an increase with age.23 Susceptibility to this phenomenon probably has a neural basis, as it has been linked to individual differences in white matter microstructure.24 False memories can be induced in laboratory conditions using experimental procedures such as the Deese-Roediger-McDermott paradigm.25 In this task, participants study a list of words that are later presented together with semantically unrelated new words and semantically related new words (lures).25 Lures induce the illusion of a false memory where participants mistakenly claim that the new stimulus has been encountered previously. The correct identification of lures as previously unseen stimuli is more cognitively demanding than that of unrelated novel stimuli, the former leading to greater activation of medial temporal lobe (MTL), parietal and frontal brain regions.26 In the present study we tested susceptibility to false memories in a group of abstinent heavy cannabis users and their matched controls using the Deese-Roediger-McDermott paradigm in association with functional magnetic resonance imaging (fMRI; see online methods).
Materials and methods
Ethics
The study was approved by the Ethics Committee of Sant Pau Hospital and all participants gave their written consent to participate.
Participants
We recruited a group of 16 heavy cannabis users not seeking or having a history of treatment for their cannabis consumption. We defined heavy cannabis use as daily use for at least the last 2 years. The recruited sample had never been diagnosed with a psychiatric or neurological condition including alcohol or other drug abuse. Cannabis users were matched to a cannabis-naive (<50 occasions of cannabis use in their lifetime) group of healthy controls, free of psychiatric or neurological conditions. Fourteen controls had used cannabis <10 times and only two had used it between 10 and 50 times. To rule out a history of psychiatric and neurological disorders, users and controls were interviewed by a clinical psychologist. The two groups were matched taking into account the following socio-demographic variables: sex, age, years of education, verbal intelligence and fluid intelligence. Verbal intelligence was assessed using a Spanish version of the NART,27 known as TAP–‘Test de Acentuación de Palabras' (‘word accentuation test').28 Fluid intelligence was assessed using a computerized version of the Matrix Reasoning from the Wechsler Adult Intelligence Scale-III.29 Detailed socio-demographic data for each group is provided as Supplementary information.
Cannabis users had taken the drug an average of around 42 000 times (range: 4 000–246 375) times. The average number of years of use was 21 (3–39). The average number of daily cannabis cigarettes smoked was 5 (1–24) and the average age of initial use was 17 (12–20) years. We did not exclude tobacco smokers from the study and they were not instructed to abstain from tobacco during the study. Ten participants in the cannabis group and four in the control group were currently using tobacco. Participants abstained from cannabis use for at least 4 weeks prior to testing. Urine samples were taken during the 4-week period and immediately before the experimental session. All participants tested negative for cannabis, alcohol, benzodiazepines, amphetamines, opiates and cocaine on their day of participation.
Memory paradigm
The memory paradigm consisted in a modified version of the Deese-Roediger-McDermott paradigm25 and included a study phase and a testing phase (see Supplementary information). Both phases were conducted with the participant in the MRI scanner. Stimuli were presented using goggles and behavioral responses were recorded by button press using a magnet-compatible response pad.
The study phase comprised 20 lists of four words. Prior to the presentation of the four words comprising a list, the name of that list was announced on the screen. Of the 20 lists, fifteen comprised four semantically related Spanish words and the other 5 lists comprised 3 semantically related words plus a catch word. Catch words were semantically unrelated to the list announced and were used to control for the participant's attention during the task. A total of 80 stimuli were presented during the study phase: 75 legitimate words plus 5 catch words. Participants were requested to indicate by button press whether the presented word belonged to the announced list. The order of presentation of the 20 word lists was randomized between participants. The study phase lasted 11 min.
Approximately 15 min after completion of the study phase, the test phase was conducted and lasted 14 min. Participants were presented with the 75 legitimate words shown during the study phase plus 40 semantically unrelated new words and 40 semantically related new words (lures, see stimuli tables in the Supplementary information file). Stimuli were presented in semi-random order with the restriction that the same type of stimulus (old, new or lure) was not presented more than twice in succession. We used a rapid presentation event-related design. Stimulus duration was 500 ms. The stimulus onset asynchrony was on average 5.125 s and it was jittered between 4 s and 10 s. The order and timing of events were optimized using the Optseq2 software (http://surfer.nmr.mgh.harvard.edu/optseq/). Participants were required to judge whether a word had been presented in the study phase and make an old vs new decision by button press. The task had the following outcomes: (1) a studied word was correctly classified as old or ‘hit' (true memory recognition); (2) a studied word was incorrectly classified as new or ‘miss' (3) a non-studied word was correctly classified as new or ‘correct rejection of new word' (4) a non-studied word was incorrectly classified as old or ‘false alarm' (5) a lure was correctly classified as new or ‘false memory rejection' and (6) a lure was incorrectly classified as old or ‘false recognition'.
Functional magnetic imaging protocol
Data were acquired in a 3-Tesla Siemens Magnetom Trio Scanner. Structural images of the brain were obtained by means of a T1-weighted MPRAGE sequence: 256 × 256 matrix; 240 1-mm sagittal slices. Functional images were obtained using an echo-planar-imaging sequence. The pulse-sequence parameters were as follows: time to repeat=2000 ms; time to echo=29 ms; flip angle=80° matrix=128 × 128; slice thickness=4 mm. Each volume comprised 36 transversal slices (2 × 2 × 4 mm voxel). A total of 412 volumes were acquired during the test phase.
Preprocessing of imaging data
fMRI data were analyzed using the SPM8 software. Raw echo-planar-imaging images were slice time and motion corrected. Echo-planar-imaging images were then co-registered to each individual's structural T1 image. T1 images were normalized to the T1 Montreal Neurologic Institute template and the obtained parameters were used to transform the echo-planar-imaging images into Montreal Neurologic Institute space. Normalized images were subjected to high-pass temporal filtering (128 s or 0.008 Hz) and to spatial smoothing using an 8 mm Gaussian filter.
Statistical analysis
A first-level analysis was performed for each individual using a design matrix that included the following predictors: ‘hit', ‘miss', ‘correct rejection of new word', ‘false alarm', ‘false memory rejection', ‘false recognition'. Motion correction parameters and the temporal and hemodynamic response function dispersion derivatives were introduced in the model as covariates. The contrast of interest ‘false memory rejection'>‘correct rejection of new word' was calculated for each participant.
The second level analysis involved a between-groups (cannabis and controls) comparison using an independent-samples t-test for the ‘false memory rejection'>‘correct rejection of new word' contrast. Both the controls>cannabis and cannabis>controls contrasts were calculated. We considered clusters to be significantly different between groups for P-values <0.001 uncorrected and a spatial extension of 10 contiguous voxels.
To assess for correlations between activation values and drug-use variables, mean fMRI parameter values for the different statistically significant clusters (region of interest) were calculated for each individual. The voxels included in the calculations for each cluster were those showing P-values <0.001 uncorrected.
Results
Behavior
The analysis of behavioral data obtained in the study phase did not detect differences between groups regarding their degree of attention. Thus, the number of correctly identified catch trials, expressed as mean±s.d., was 4.00±0.63 for the controls and 4.18±0.75 for the cannabis users t(30)=−0.76, P>0.1.
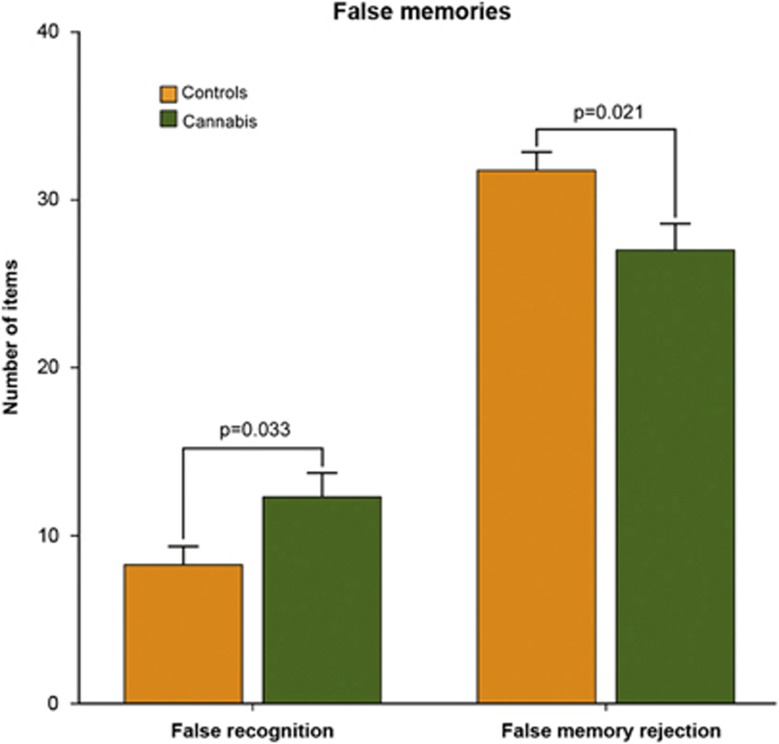
The analysis of behavioral data in the test phase showed no differences between groups in the number of correctly recognized studied words (true memory recognition; mean±s.d.: cannabis users, 64±6; controls, 65±6; t(30)=0.4, P>0.1) or in the number of correctly rejected new words (correct rejection of new words: cannabis users, 37±3; controls, 39±0.7; t(30)=1.9, P=0.076). No differences were found either in the time (in milliseconds) taken to correctly recognize studied words (cannabis users, 1185±199; controls, 1089±195; t(30)=−1.36, P>0.1), or to correctly reject new words (cannabis users, 1200±345; controls, 1043±196; t(30)=−1.58, P>0.1). However, as shown in Figure 1, cannabis users showed significantly more false memories. A two-way analysis of variance, with outcome (false recognition vs false memory rejection) as within-subjects factor and participant group (cannabis vs controls) as between-subject factors, showed a significant interaction (F(1,30)=5.60, P=0.025). Lure words were falsely recognized as studied words more often (false recognition; cannabis users, 12±6; controls, 8±4; t(30)=−2.24, P=0.033), and were rejected less often (false memory rejection; cannabis users, 27±6; controls, 32±4; t(30)=2.46, P=0.021).
Figure 1.
Behavioral data. The graphs show performance results in the memory task. Cannabis users performed significantly worse than controls, showing increased false recognition and decreased false memory rejection. Error bars denote one s.d. of mean.
fMRI
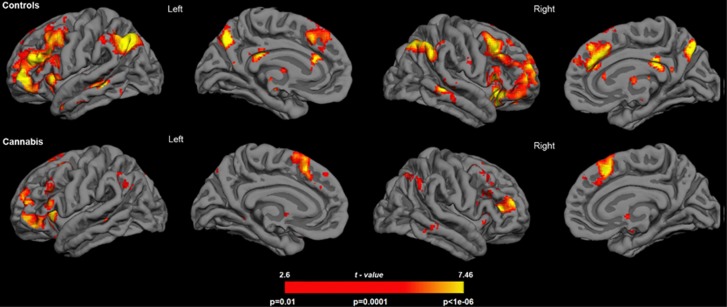
Imaging data were analyzed specifically looking for differences between groups in the pattern of blood oxygenation level dependent (BOLD) response associated with the correct rejection of lures or false memory rejection as compared with the correct rejection of new words. Figure 2 shows the mentioned contrast separately for each of the two participant groups. Note the larger extension and lower P-values of active voxels in the control group.
Figure 2.
Rendering of fMRI results for each participant group. The statistical maps show the results of the voxel-wise comparison ‘false memory rejection'>‘correct rejection of new word'. For depiction purposes results are shown at P=0.01.
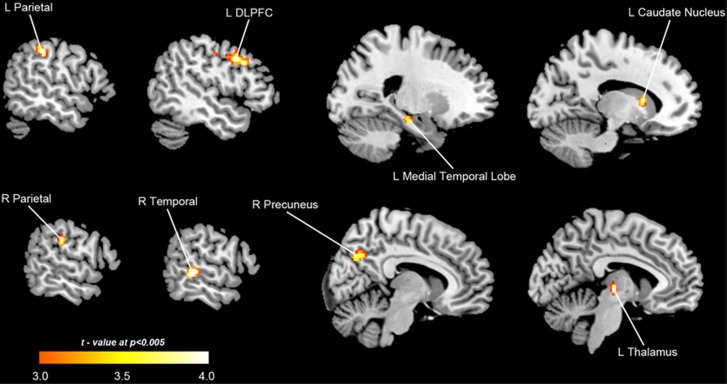
Figure 3 and Table 1 show the results of the between-groups comparison. Control participants showed higher activation for the contrast false memory rejection>correct rejection of new words in parietal, prefrontal, temporal and subcortical structures. All these structures have previously been found to be involved in the correct identification of false relative to new semantic stimuli.26 Greater behavioral efficacy in the control group was thus associated with greater brain activity for the rejection of lures than for the rejection of new unrelated words.
Figure 3.
Group differences between controls and cannabis users. The images show the results of the voxel-wise independent-samples t-test controls>cannabis users for the contrast ‘false memory rejection'>‘correct rejection of new word'. The brain regions depicted showed significantly higher activation in the control group as compared with the cannabis using group at P=0.001 uncorrected. No significant results were obtained for the contrast cannabis users>controls. For depiction purposes results are shown at P=0.005.
Table 1. Areas of increased BOLD response in controls relative to cannabis users for the contrast: ‘false memory rejection'>‘correct rejection of new word'.
| Brain region | Lateralization | BA | MNI (x, y, z) | Maximum t | n voxels |
|---|---|---|---|---|---|
| Temporal cortex | Right | 22 | 60, −34, 2 | 5.14 | 64 |
| Dorsolateral prefrontal | Left | 9 | −52, 16, 34 | 5.06 | 76 |
| Red nucleus/thalamus | Left | − | −6, −18, −2 | 4.76 | 24 |
| Parietal cortex | Left | 40 | -56, −32, 42 | 4.66 | 71 |
| Parietal cortex | Right | 40 | 60, −26, 28 | 4.03 | 15 |
| Caudate | Left | − | −14, 12, 10 | 3.90 | 20 |
| Medial temporal lobe | Left | 35/28 | −22, −22, −16 | 3.87 | 17 |
| Precuneus | Right | 7 | 10, −70, 32 | 3.82 | 23 |
Abbreviations: BA, Brodmann area; BOLD, blood oxygenation level dependent; MNI, coordinates in Montreal Neurological Institute stereotactic space; t, t-value (df=30).
Correlation analysis
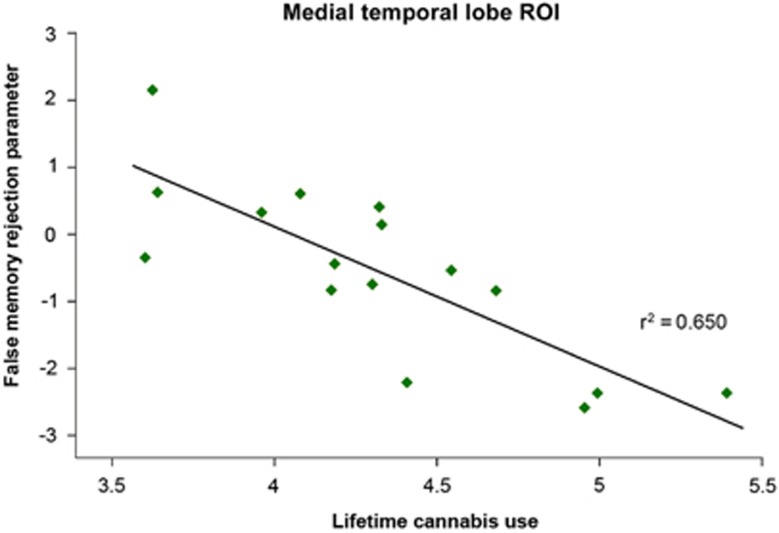
To look for potential associations between the pattern of brain activation and history of cannabis use, we defined regions of interest for each of the statistically significant areas identified in the between-groups comparison. The parameters (beta values) associated with false memory rejection in each region of interest were extracted only for the cannabis group, and their values were correlated with drug-use data: lifetime cannabis consumption, years of use and amount of cannabis used daily. As shown in Figure 4, a significant negative correlation (r=−0.806, r2=0.650, P<0.001) was found between activity in the MTL regions of interest and lifetime cannabis use (log value of the estimated number of cannabis cigarettes smoked).
Figure 4.
Correlation between MTL activity and cannabis exposure. The scatter plot shows the relationship between the individual statistical parameters in the MTL (beta values) associated with the 'false memory rejection' condition and lifetime cannabis use (log of estimated total number of cannabis cigarettes).
Discussion
Our results show that cannabis users had a higher susceptibility to memory illusions, as observed in certain neurologic and psychiatric populations,21 and elderly individuals.23 They further identify the functional substrate of this deficit in the hypoactivation of a series of spatially distributed brain regions participating in the network involved in semantic30 and episodic31 retrieval. The network identified fits nicely with previous studies that have shown that compared with new items, recognition of false stimuli leads to greater activation of the hippocampus and the parahippocampal gyrus, and also of the left parietal and left dorsolateral prefrontal cortices in healthy subjects.26 Although activation of MTL structures in these tasks can be directly associated with memory,32 the parietal cortex can be linked to attentional processes and the dorsolateral prefrontal cortex to monitoring issues in this context.33 It has been shown that the effective rejection of lures leads to greater activation of the dorsolateral prefrontal cortex34 and lesions at this level lead to increased false recognition in neurological patients.35 Thus, rather than a compromise of memory structures per se (that is, the hippocampus), our results point to a more diffuse impairment, which leads to a reduced capacity to deal with the retrieval and monitoring demands needed to differentiate between illusory and real events.
From a theoretical perspective, two main accounts have been put forward to explain the false memory phenomenon: the fuzzy trace theory and the activation-monitoring account. The fuzzy trace theory postulates that stimuli are encoded into two types of memory traces: a ‘verbatim' trace containing specific details and features associated with the stimulus, and a ‘gist' trace that contains more general aspects of the encoding event. False memories occur when new stimuli share certain features with past events and elicit the retrieval of the gist trace, but not the verbatim trace.36 In contrast, the activation-monitoring account37 postulates that cognitive control mechanisms need to be engaged to correctly identify and reject the highly activated lures. According to this view, false memories occur when monitoring mechanisms fail to identify the non-studied but semantically related lures.
Our findings can be interpreted in the light of the two accounts described above. The between-groups comparison of fMRI activation maps showed activity not only in distributed brain areas participating in semantic30 and episodic31 retrieval, but also in cognitive control, as suggested by the significant dorsolateral prefrontal clusters identified.38, 39 The greater activation found for the control group in the medial and lateral temporal cortices suggests access to both the semantic (lateral) and episodic (medial) features of the studied stimuli. Using the terminology of the fuzzy trace account, controls would take advantage of both the verbatim and gist traces when deciding to reject a false memory. On the contrary, the inverse correlation found between lifetime cannabis use and the BOLD response in the MTL suggests that chronic exposure to cannabis may be especially detrimental to the brain structure providing the episodic or gist features to stored information. Cannabis users may have been left more dependent on the verbatim features of stimuli to decide whether a given word was a legitimate memory or not. Paradoxically, the greater activation of gist-related information in the control group compared with the cannabis group might have made them more vulnerable to false memories. Concurrent retrieval of item-based (verbatim) and context-based (gist) information in the control group might elicit conflict and require the engagement of cognitive control mechanisms, explaining the increased frontal activation observed in the controls. Thus, a more efficient conflict- or activation-monitoring, as signaled by increased dorsolateral prefrontal activity, may have led to the final outcome of better performance in the control group.
Further evidence of MTL and prefrontal impairment by cannabis is provided by magnetic resonance spectroscopy studies. Using this technique, researchers have found detrimental neurometabolic changes in these brain areas. For instance, Silveri and colleagues have reported decreased myo-inositol/creatine levels in the MTL and thalamus of users.40, 41 Hermann et al.42 have found reduced N-acetyl-aspartate/total creatine ratios in the dorsolateral prefrontal of recreational users, and Cowan et al.43 have found analogous decreases in Brodmann area 45 in the inferior frontal gyrus. Considering that analogous neurometabolic changes can be observed in older individuals44 and that reality monitoring deficits increase with age,45 we speculate that chronic cannabis use could aggravate the memory deficits associated with the normal ageing process.
Our findings extend previous knowledge on the impact of cannabis use on memory12 and executive function.8 Although there are contradictory results regarding the normalization of memory in the long term,9, 10, 16 impairment has been associated with the intensity of cannabis use, with heavy users showing deficits in various memory functions.46 Interestingly, many neuroimaging studies implementing simple memory tasks have failed to find differences in performance between heavy cannabis users and controls.12 Our findings suggest that impairment may be more subtle and affect more complex cognitive processes, like those involved in the Deese-Roediger-McDermott paradigm.
A limitation of our study is the potential presence of residual THC levels in the brain in the absence of detectable levels in other biological matrices (in our case, urine). Whereas most studies in humans consider that cognitive testing after a 4-week period will assess the long-term effects of cannabis rather than its residual effects,8 a longer persistence of THC in the brain has also been observed.47 Thus, although unlikely, the presence of small amounts of THC in the body cannot be entirely ruled out.
Taken together, the present results indicate that long-term heavy cannabis users are at an increased risk of experiencing memory errors even when abstinent and drug-free. These deficits show a neural basis and suggest a subtle compromise of brain mechanisms involved in reality monitoring. Though subtle, the deficits found bear similarities with alterations observed in psychiatric and neurologic conditions and also with age-related cognitive decline. This lingering diminished ability to tell true from false may have medical, and legal implications. Future studies should address these issues and assess whether the deficits found here extend to other forms of memory distortion and reality monitoring beyond the false memory phenomenon.
Acknowledgments
This work was supported by a grant from the ‘Plan Nacional Sobre Drogas‘ of the Spanish Government. Marta Valle is supported by the ‘Fondo de Investigación Sanitaria' through grant CP04/00121 from the Spanish Ministry of Health in collaboration with Institut de Recerca de l'Hospital de la Santa Creu i Sant Pau, Barcelona. Frederic Sampedro is supported by an FPU grant from the Spanish government. The authors wish to thank José Carlos Bouso for his help during participant recruitment and data collection, and Cesar Garrido and Núria Bargalló for technical assistance.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- United Nations Office on Drugs and Crime. World Drug Report 20142014 ; http://www.unodc.org/wdr2014/ (accessed 9 Mar2014).
- Substance Abuse and Mental Health Services Administration. The NSDUH Report: Substance Use and Mental Health Estimates from the 2013 National Survey on Drug Use and Health: Overview of Findings2014 [PubMed]
- Wade L. South America. Legal highs make Uruguay a beacon for marijuana research. Science. 2014;344:1217. doi: 10.1126/science.344.6189.1217. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM. Weiss SRB. Adverse Health Effects of Marijuana Use. N Engl J Med. 2014;370:2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, Okuda M, Wang S, Grant BF, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug Alcohol Depend. 2011;115:120–130. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. BMJ. 2002;325:1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RSE, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA. 2012;109:E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: a review. Curr Drug Abuse Rev. 2008;1:81–98. doi: 10.2174/1874473710801010081. [DOI] [PubMed] [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PCL, et al. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berl) 2011;216:131–144. doi: 10.1007/s00213-011-2203-x. [DOI] [PubMed] [Google Scholar]
- Schoeler T, Bhattacharyya S. The effect of cannabis use on memory function: an update. Subst Abuse Rehabil. 2013;4:11–27. doi: 10.2147/SAR.S25869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, D'Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 2006;188:425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Curran HV, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 2002;164:61–70. doi: 10.1007/s00213-002-1169-0. [DOI] [PubMed] [Google Scholar]
- Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19–27. doi: 10.1177/0269881112460109. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Cognitive measures in long-term cannabis users. J Clin Pharmacol. 2002;42:41S–47S. doi: 10.1002/j.1552-4604.2002.tb06002.x. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, et al. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J Psychiatr Res. 2011;45:1055–1066. doi: 10.1016/j.jpsychires.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 2012;59:3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Yücel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, et al. Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. Varieties of false memory. Cogn Neuropsychol. 1999;16:197–214. [Google Scholar]
- Schacter DL. The seven sins of memory. Insights from psychology and cognitive neuroscience. Am Psychol. 1999;54:182–203. doi: 10.1037//0003-066x.54.3.182. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Bowman CR, Peterson KM. Age-related differences in the neural correlates mediating false recollection. Neurobiol Aging. 2014;35:395–407. doi: 10.1016/j.neurobiolaging.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Fuentemilla L, Càmara E, Münte TF, Krämer UM, Cunillera T, Marco-Pallarés J, et al. Individual differences in true and false memory retrieval are related to white matter brain microstructure. J Neurosci. 2009;29:8698–8703. doi: 10.1523/JNEUROSCI.5270-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB. Creating false memories: remembering words not presented in lists. J Exp Psychol Learn Mem Cogn. 1995;24:803–814. [Google Scholar]
- Cabeza R, Rao SM, Wagner AD, Mayer AR, Schacter DL. Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. Proc Natl Acad Sci USA. 2001;98:4805–4810. doi: 10.1073/pnas.081082698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE, O'Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex. 1978;14:234–244. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- Del Ser T, González-Montalvo JI, Martínez-Espinosa S, Delgado-Villapalos C, Bermejo F. Estimation of premorbid intelligence in Spanish people with the Word Accentuation Test and its application to the diagnosis of dementia. Brain Cogn. 1997;33:343–356. doi: 10.1006/brcg.1997.0877. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III (WAIS-III) The Psychological Corporation: San Antonio, TX, USA; 1981. [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon S, Moscovitch M. The nature and time-course of medial temporal lobe contributions to semantic retrieval: an fMRI study on verbal fluency. Hippocampus. 2012;22:1451–1466. doi: 10.1002/hipo.20985. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Wing EA, LaBar KS, Cabeza R. Neural similarity between encoding and retrieval is related to memory via hippocampal interactions. Cereb Cortex. 2013;23:2818–2828. doi: 10.1093/cercor/bhs258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Slotnick SD. The cognitive neuroscience of memory distortion. Neuron. 2004;44:149–160. doi: 10.1016/j.neuron.2004.08.017. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Jones TC, Petersen SE, Lageman SK, Roediger HL. Retrieval success is accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: an event-related fMRI study. J Cogn Neurosci. 2000;12:965–976. doi: 10.1162/08989290051137503. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Bindschaedler C, Harsent L, Metzler C. Pathological false alarm rates following damage to the left frontal cortex. Brain Cogn. 1996;32:14–27. doi: 10.1006/brcg.1996.0055. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF. Fuzzy-trace theory and false memory. Curr Dir Psychol Sci. 2002;11:164–169. [Google Scholar]
- Balota DA, Cortese MJ, Duchek JM, Adams D, Roediger HL, McDermott KB, et al. Veridical and false memories in healthy older adults and in dementia of the Alzheimer's type. Cogn Neuropsychol. 1999;16:361–384. [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Jensen JE, Sneider JT, Yurgelun-Todd DA, Silveri MM.Lower Left Thalamic Myo-Inositol. Levels Associated with Greater Cognitive Impulsivity in Marijuana-Dependent Young Men: Preliminary Spectroscopic Evidence at 4T J Addict Res Ther 2013. doi: : 10.4172/2155-6105.S4-009 . [DOI] [PMC free article] [PubMed]
- Silveri MM, Jensen JE, Rosso IM, Sneider JT, Yurgelun-Todd DA. Preliminary evidence for white matter metabolite differences in marijuana-dependent young men using 2D J-resolved magnetic resonance spectroscopic imaging at 4 Tesla. Psychiatry Res. 2011;191:201–211. doi: 10.1016/j.pscychresns.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Sartorius A, Welzel H, Walter S, Skopp G, Ende G, et al. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiatry. 2007;61:1281–1289. doi: 10.1016/j.biopsych.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Joers JM, Dietrich MS. N-acetylaspartate (NAA) correlates inversely with cannabis use in a frontal language processing region of neocortex in MDMA (Ecstasy) polydrug users: a 3T magnetic resonance spectroscopy study. Pharmacol Biochem Behav. 2009;92:105–110. doi: 10.1016/j.pbb.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzako H, Hashiguchi T, Sakamoto Y, Okamura H, Doi W, Takenouchi K, et al. Metabolite changes with age measured by proton magnetic resonance spectroscopy in normal subjects. Psychiatry Clin Neurosci. 1997;51:261–263. doi: 10.1111/j.1440-1819.1997.tb02595.x. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Lyle KB, Butler KM, Dornburg CC. Age-related deficits in reality monitoring of action memories. Psychol Aging. 2008;23:646–656. doi: 10.1037/a0013083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Mura P, Kintz P, Dumestre V, Raul S, Hauet T. THC can be detected in brain while absent in blood. J Anal Toxicol. 2005;29:842–843. doi: 10.1093/jat/29.8.842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.