Summary
Background
Alteplase is effective for treatment of acute ischaemic stroke but debate continues about its use after longer times since stroke onset, in older patients, and among patients who have had the least or most severe strokes. We assessed the role of these factors in affecting good stroke outcome in patients given alteplase.
Methods
We did a pre-specified meta-analysis of individual patient data from 6756 patients in nine randomised trials comparing alteplase with placebo or open control. We included all completed randomised phase 3 trials of intravenous alteplase for treatment of acute ischaemic stroke for which data were available. Retrospective checks confirmed that no eligible trials had been omitted. We defined a good stroke outcome as no significant disability at 3–6 months, defined by a modified Rankin Score of 0 or 1. Additional outcomes included symptomatic intracranial haemorrhage (defined by type 2 parenchymal haemorrhage within 7 days and, separately, by the SITS-MOST definition of parenchymal type 2 haemorrhage within 36 h), fatal intracranial haemorrhage within 7 days, and 90-day mortality.
Findings
Alteplase increased the odds of a good stroke outcome, with earlier treatment associated with bigger proportional benefit. Treatment within 3·0 h resulted in a good outcome for 259 (32·9%) of 787 patients who received alteplase versus 176 (23·1%) of 762 who received control (OR 1·75, 95% CI 1·35–2·27); delay of greater than 3·0 h, up to 4·5 h, resulted in good outcome for 485 (35·3%) of 1375 versus 432 (30·1%) of 1437 (OR 1·26, 95% CI 1·05–1·51); and delay of more than 4·5 h resulted in good outcome for 401 (32·6%) of 1229 versus 357 (30·6%) of 1166 (OR 1·15, 95% CI 0·95–1·40). Proportional treatment benefits were similar irrespective of age or stroke severity. Alteplase significantly increased the odds of symptomatic intracranial haemorrhage (type 2 parenchymal haemorrhage definition 231 [6·8%] of 3391 vs 44 [1·3%] of 3365, OR 5·55, 95% CI 4·01–7·70, p<0·0001; SITS-MOST definition 124 [3·7%] vs 19 [0·6%], OR 6·67, 95% CI 4·11–10·84, p<0·0001) and of fatal intracranial haemorrhage within 7 days (91 [2·7%] vs 13 [0·4%]; OR 7·14, 95% CI 3·98–12·79, p<0·0001). The relative increase in fatal intracranial haemorrhage from alteplase was similar irrespective of treatment delay, age, or stroke severity, but the absolute excess risk attributable to alteplase was bigger among patients who had more severe strokes. There was no excess in other early causes of death and no significant effect on later causes of death. Consequently, mortality at 90 days was 608 (17·9%) in the alteplase group versus 556 (16·5%) in the control group (hazard ratio 1·11, 95% CI 0·99–1·25, p=0·07). Taken together, therefore, despite an average absolute increased risk of early death from intracranial haemorrhage of about 2%, by 3–6 months this risk was offset by an average absolute increase in disability-free survival of about 10% for patients treated within 3·0 h and about 5% for patients treated after 3·0 h, up to 4·5 h.
Interpretation
Irrespective of age or stroke severity, and despite an increased risk of fatal intracranial haemorrhage during the first few days after treatment, alteplase significantly improves the overall odds of a good stroke outcome when delivered within 4·5 h of stroke onset, with earlier treatment associated with bigger proportional benefits.
Funding
UK Medical Research Council, British Heart Foundation, University of Glasgow, University of Edinburgh.
Introduction
Intravenous alteplase (recombinant tissue plasminogen activator) is approved for the treatment of acute ischaemic stroke. Previous analyses of pooled data from randomised trials concluded that alteplase is beneficial when administered to some patients within 4·5 h, but that the magnitude of benefit diminishes with increasing treatment delay.1, 2 However, uncertainties remain about the balance of benefit and risk when alteplase is given later after onset of symptoms, to older patients, or to patients with very severe or mild strokes. Present guidance3, 4, 5, 6 and marketing authorisation7, 8 from Europe and elsewhere recommends the routine use of alteplase within 4·5 h of stroke onset but, in the USA, the Food and Drug Administration has approved the use of alteplase only within 3 h of stroke onset.9 Marketing authorisation for Europe7 and Australia,8 but not for the USA9 or Japan, cautions against the use of alteplase for severe and mild stroke. Marketing of alteplase in some European countries is also restricted to patients younger than 80 years7 (despite clinical guidelines based on observational studies that recommends its use in older patients3, 10, 11, 12), whereas no such age restriction applies in many other countries, including the USA.
IST-313—designed to resolve some of these uncertainties—included 3035 patients randomly assigned to alteplase or control up to 6 h after the onset of stroke. The principal investigators from IST-3 and other trials of alteplase13, 14, 15, 16, 17, 18, 19 agreed to make their individual-patient data available for analysis. The chief goal of this analysis was to explore the extent to which treatment delay affected the effect of alteplase and to establish whether age or stroke severity affected treatment effects. These analyses assessing potential effect modification are only possible with individual patient data. Key secondary aims included estimating the effect of alteplase on symptomatic intracranial haemorrhage and on 90-day mortality.
Methods
Study design and inclusion criteria
We established a collaboration to undertake this meta-analysis of individual patient data. We included all completed randomised phase 3 trials of intravenous alteplase for treatment of acute ischaemic stroke for which data were available. Retrospective checks confirmed that no eligible trials had been omitted. These checks included reference to a previous systematic review,20 an updated review of the Cochrane Stroke Group's Specialised Register of Trials, and enquiry among collaborators and the manufacturer of alteplase (Boehringer Ingelheim, Ingelheim, Germany). Individual patient data were sought from eligible trials. Before accessing the combined dataset, the collaboration agreed on a statistical analysis plan.21 The study protocol is available online.
Outcomes
Our a-priori primary measure of treatment efficacy was the proportion of patients who had a good stroke outcome, defined by a modified Rankin score (mRS) of 0–1 (ie, symptom-free or residual symptoms with no loss of activity) at 3–6 months. 3-month assessments were to be used if available, but for IST-3 we used a 6-month assessment because no 3-month assessment was done. We mapped the Oxford Handicap Scale outcome assessment used in IST-3 to equivalent mRS categories.
Key secondary outcomes were fatal intracranial haemorrhage within 7 days, any symptomatic intracranial haemorrhage, and 90-day mortality (separated by cause where possible). For a full list of secondary outcomes, see the pre-specified statistical analysis plan.21 Symptomatic intracranial haemorrhage was defined in two ways: parenchymal haemorrhage of type 2 within 7 days16 and the SITS-MOST study22 definition of type 2 parenchymal haemorrhage within 36 h. For IST-3, type 2 parenchymal haemorrhage within 7 days was approximated by significant brain parenchymal haemorrhage local or remote from the infarct, or significant haemorrhagic transformation of an infarct on brain imaging within 7 days. The SITS-MOST definition was approximated in IST-3 as evidence within 24 h of clinically significant deterioration or death together with evidence of either significant brain parenchymal haemorrhage (local or distant from the infarct) or significant haemorrhagic transformation of an infarct on brain imaging which, in the judgment of the adjudication panel, was likely to have worsened mass effect or contributed to the burden of brain damage.
Statistical analysis
A full description of the analyses is provided in the pre-specified statistical analysis plan.21 Briefly, we used logistic regression, stratified by trial, to model the common linear dependence of the log odds of a particular outcome on allocation to alteplase, treatment delay (a linear variable), age (a linear variable), baseline stroke severity (National Institutes of Health Stroke Scale [NIHSS] score, modelled by both linear and quadratic terms), and interactions between allocation to alteplase and each of these other baseline covariates. (The odds ratio estimates presented here are of course more extreme than corresponding risk ratio estimates would be, but have the advantage that they may be more generalisable among patients with differing likelihood of a good stroke outcome.) We cross-checked individual data against previous publications. We imputed missing data with prespecified rules.21 We assessed the extent to which treatment delay, age, and stroke severity modified (individually or jointly) the proportional effects of treatment by assessing the statistical significance of the relevant treatment interaction terms using likelihood ratio tests (ie, through comparison of minus twice the log-likelihood statistic between appropriate nested models). Other prespecified analyses included assessment of treatment effects separately according to categories of treatment delay that relate to present licence issues and previous trial time limits (≤3·0 h, >3·0≤4·5 h, >4·5 h), age (≤80 years, >80 years), and baseline stroke severity (NIHSS score 0–4, 5–10, 11–15, 16–21, ≥22). If we had data for the timing of events (eg, mortality within 90 days), we estimated average relative risks with the hazard ratios (HRs) from analogous Cox proportional hazards regression models, with a test of the proportionality assumption provided by examination of the time-dependency of the Schoenfeld partial residuals.23 All estimates of treatment effect compared patients allocated alteplase with patients not allocated alteplase, and all treatment effect size estimates are provided with their 95% CIs (calculated when necessary with bias-corrected and accelerated-corrected bootstrap techniques24) with p values less than 0·05 considered statistically significant. We did the analyses with SAS (version 9·3) and R (version 2·11·1).
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data and responsibility for the decision to submit for publication.
Results
We obtained data for 6756 participants from nine trials (eight trials involving 3721 participants randomly assigned to alteplase versus placebo and one trial13 involving 3035 participants assigned to alteplase versus open control; table 1); 3391 patients were given alteplase and 3365 were given control. Individual data were not available for five trials25, 26, 27, 28, 29 involving 270 participants. 6602 (98%) of 6756 participants had complete baseline information for treatment delay, age, and stroke severity. None of our results changed substantially according to the choice of imputation for missing data, including exclusion of those with missing data (data not shown). On average, patients in IST-3 were 11 years older than were patients in the eight previous trials and received treatment 20 min later, but they had the same mean baseline stroke severity (appendix p 1). Strong relationships existed between patient age, treatment delay, and stroke severity in all trials (appendix p 4). Patients with severe strokes were treated earlier and—particularly in IST-3—older patients had more severe strokes. In IST-3, but not previous trials, patients treated earlier also tended to be older than were patients treated later. Although 588 (19%) of 3035 participants in IST-3 treated within 4·5 h of onset of stroke were aged 80 years or younger, most of these (372 [63%]) were enrolled before revised European guidance in January, 2009, recommending the routine use of alteplase for this group.4 Baseline characteristics were well balanced by treatment allocation for the pooled data (appendix p 2).
Table 1.
Baseline characteristics of patients in participating trials
| NINDS A | NINDS B | ECASS I | ECASS II | ATLANTIS A | ATLANTIS B | ECASS III | EPITHET | IST-3 | TOTAL | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number randomised | 291 | 333 | 620 | 800 | 142 | 613 | 821 | 101 | 3035 | 6756 | |
| Treatment delay (hours) | 2·0 (0·6) | 2·0 (0·6) | 4·4 (1·1) | 4·3 (1·1) | 4·3 (1·1) | 4·4 (0·8) | 4·0 (0·4) | 4·9 (0·8) | 4·2 (1·2) | 4·0 (1·2) | |
| ≤3·0 | 290 (>99%) | 333 (100%) | 87 (14%) | 158 (20%) | 22 (15%) | 39 (6%) | .. | .. | 620 (20%) | 1549 (23%) | |
| >3·0≤4·5 | 1 (<1%) | .. | 233 (38%) | 265 (33%) | 53 (37%) | 249 (41%) | 788 (96%) | 31 (31%) | 1148 (38%) | 2768 (41%) | |
| >4·5 | .. | .. | 295 (48%) | 370 (46%) | 67 (47%) | 321 (52%) | 6 (1%) | 69 (68%) | 1266 (42%) | 2394 (35%) | |
| Missing | .. | .. | 5 (1%) | 7 (1%) | .. | 4 (1%) | 27 (3%) | 1 (1%) | 1 (<1%) | 45 (1%) | |
| Age (years) | 66 (11) | 68 (12) | 65 (12) | 66 (11) | 66 (13) | 66 (11) | 65 (12) | 72 (13) | 77 (12) | 71 (13) | |
| ≤80 | 279 (96%) | 289 (87%) | 615 (>99%) | 792 (99%) | 142 (100%) | 608 (>99%) | 805 (98%) | 76 (75%) | 1418 (47%) | 5024 (74%) | |
| >80 | 12 (4%) | 44 (13%) | 5 (1%) | 8 (1%) | .. | 3 (<1%) | 15 (2%) | 25 (25%) | 1617 (53%) | 1729 (26%) | |
| Missing | .. | .. | .. | .. | .. | 2 (<1%) | 1 (<1%) | .. | .. | 3 (<1%) | |
| Stroke severity (NIHSS) | 14 (7) | 15 (7) | 12 (6) | 12 (6) | 13 (7) | 11 (6) | 10 (5) | 13 (6) | 12 (7) | 12 (7) | |
| 0–4 | 16 (5%) | 13 (4%) | 34 (5%) | 47 (6%) | 10 (7%) | 47 (8%) | 98 (12%) | 1 (1%) | 400 (13%) | 666 (10%) | |
| 5–10 | 78 (27%) | 98 (29%) | 189 (30%) | 339 (42%) | 57 (40%) | 279 (46%) | 389 (47%) | 40 (40%) | 1064 (35%) | 2533 (37%) | |
| 11–15 | 68 (23%) | 63 (19%) | 183 (30%) | 232 (29%) | 28 (20%) | 128 (21%) | 163 (20%) | 22 (22%) | 601 (20%) | 1488 (22%) | |
| 16–21 | 76 (26%) | 78 (23%) | 146 (24%) | 113 (14%) | 25 (18%) | 106 (17%) | 142 (17%) | 29 (29%) | 618 (20%) | 1333 (20%) | |
| ≥22 | 45 (15%) | 74 (22%) | 28 (5%) | 43 (5%) | 20 (14%) | 33 (5%) | 18 (2%) | 9 (9%) | 352 (12%) | 622 (9%) | |
| Missing | 8 (3%) | 7 (2%) | 40 (6%) | 26 (3%) | 2 (1%) | 20 (3%) | 11 (1%) | .. | * | 114 (2%) | |
| Female | 120 (41%) | 142 (43%) | 231 (37%) | 331 (41%) | 45 (32%) | 250 (41%) | 325 (40%) | 43 (43%) | 1570 (52%) | 3057 (45%) | |
| History of hypertension | 188 (65%) | 220 (66%) | 258 (42%) | 412 (52%) | 87 (61%) | 364 (59%) | 514 (63%) | 71 (70%) | 1954 (64%) | 4068 (60%) | |
| History of stroke | 49 (17%) | 34 (10%) | 83 (13%) | 158 (20%) | 31 (22%) | 89 (15%) | 89 (11%) | 11 (11%) | 699 (23%) | 1243 (18%) | |
| History of diabetes mellitus | 64 (22%) | 67 (20%) | 81 (13%) | 169 (21%) | 27 (19%) | 130 (21%) | 129 (16%) | 23 (23%) | 388 (13%) | 1078 (16%) | |
| History of atrial fibrillation | 55 (19%) | 60 (18%) | 113 (18%) | 188 (24%) | 37 (26%) | 97 (16%) | 108 (13%) | 42 (42%) | 914 (30%) | 1614 (24%) | |
| Aspirin use | 78 (27%) | 93 (28%) | 87 (14%) | 196 (25%) | 59 (42%) | 211 (34%) | 201 (24%) | 30 (30%) | 1306 (43%) | 2261 (33%) | |
| Weight (kg) | 78 (17) | 78 (19) | 74 (12) | 75 (14) | 80 (20) | 79 (18) | 78 (15) | 75 (19) | 72 (15) | 75 (16) | |
| Systolic blood pressure (mmHg) | 154 (21) | 152 (21) | 154 (23) | 152 (21) | 152 (24) | 152 (21) | 153 (21) | 148 (19) | 155 (24) | 154 (22) | |
| Diastolic blood pressure (mmHg) | 85 (13) | 85 (14) | 87 (13) | 84 (13) | 81 (14) | 82 (14) | 84 (14) | 78 (13) | 82 (15) | 83 (14) | |
Categorical data presented as n (%), continuous data presented as mean (SD). NINDS=National Institute of Neurological Disorders and Stroke; ECASS=European Cooperative Acute Stroke Study; ATLANTIS=Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke; EPITHET=Echoplanar Imaging Thrombolytic Evaluation Trial; IST=International Stroke Trial.
In IST-3, 244 patients had their baseline NIHSS score predicted from other measurements recorded at their baseline assessment. Ignoring these patients, the numbers of IST-3 patients in each category of baseline NIHSS score above would be 385, 972, 531, 559 and 344 respectively.
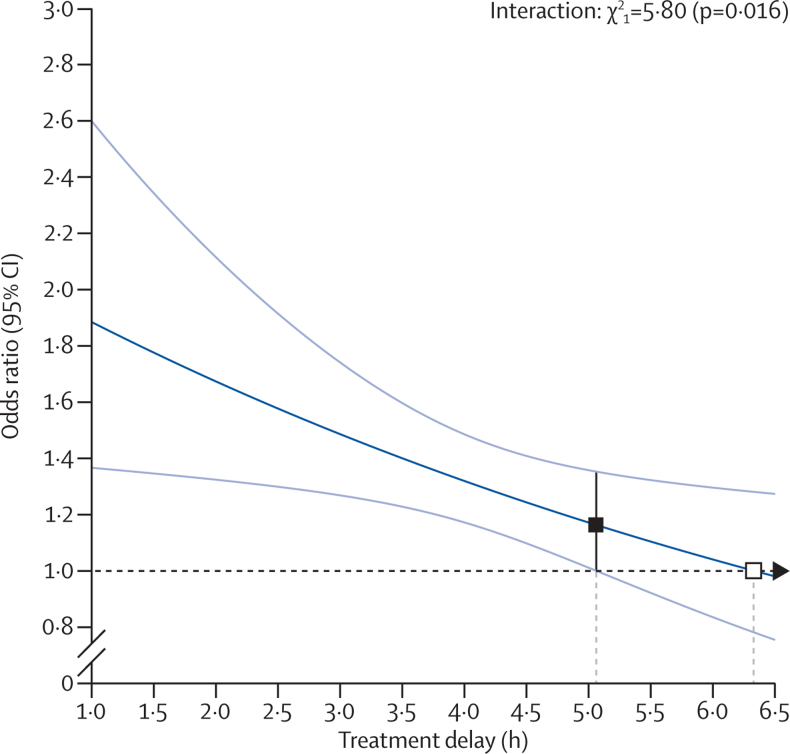
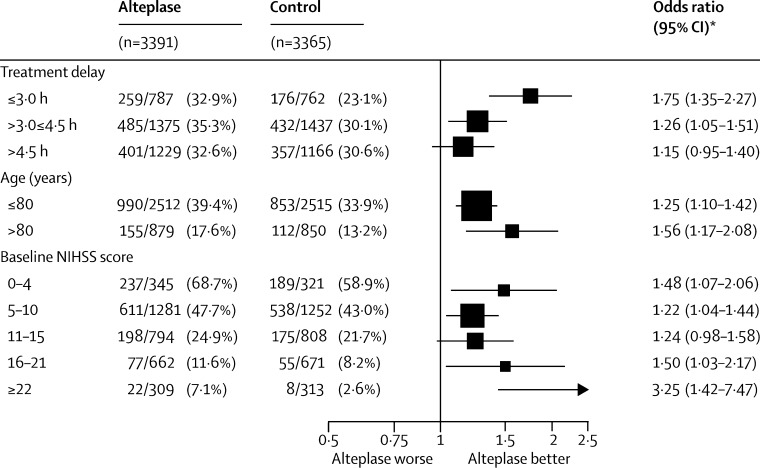
Overall, 2110 (31%) of 6756 patients achieved a good stroke outcome (mRS of 0 or 1) at 3–6 months (appendix p 3). Alteplase significantly increased the odds of a good outcome, with earlier treatment resulting in significantly greater proportional benefit (p=0·016 for trend of increasing proportional benefit with earlier treatment; figure 1). We estimated the time at which alteplase has no effect to be 6·3 h (95% CI 5·0–13·8) and the time at which the lower 95% CI for the estimated treatment benefit first crossed 1·0 to be 5·1 h (figure 1). When estimated in the three predefined subgroups of treatment delay, alteplase significantly increased the odds of a good outcome when given within 3·0 h (OR 1·75, 95% CI 1·35–2·27; p<0·0001) or after 3·0 h up to 4·5 h (OR 1·26, 95% CI 1·05–1·51, p=0·0132), but not after 4·5 h (OR 1·15, 95% CI 0·95–1·40; p=0·15; figure 2). The effect of alteplase on a good outcome was chiefly driven by treatment delay; after controlling for treatment delay, neither age nor severity of stroke contributed significant additional predictive value (appendix p 5). After allowing for differences explained by treatment delay, the effect of alteplase on a good outcome reported in IST-3 was consistent with that reported in the eight previous trials (p for inconsistency=0·92).
Figure 1.
Effect of timing of alteplase treatment on good stroke outcome (mRS 0–1)
The solid line is the best linear fit between the log odds ratio for a good stroke outcome for patients given alteplase compared with those given control (vertical axis) and treatment delay (horizontal axis; pinteraction=0·016). Estimates are derived from a regression model in which alteplase, time to treatment, age, and stroke severity (handled in a quadratic manner) are included as main effects but the only treatment interaction included is with time to treatment. Only 198 patients (159 from IST–3) had a time from stroke onset to treatment of more than 6 h. The white box shows the point at which the estimated treatment effect crosses 1. The black box shows the point at which the lower 95% CI for the estimated treatment effect first crosses 1·0. mRS=modified Rankin Scale.
Figure 2.
Effect of alteplase on good stroke outcome (mRS 0–1), by treatment delay, age, and stroke severity
*For each of the three baseline characteristics, estimates were derived from a single logistic regression model stratified by trial, which enables separate estimation of the OR for each subgroup after adjustment for the other two baseline characteristics (but not for possible interactions with those characteristics). mRS=modified Rankin Scale.
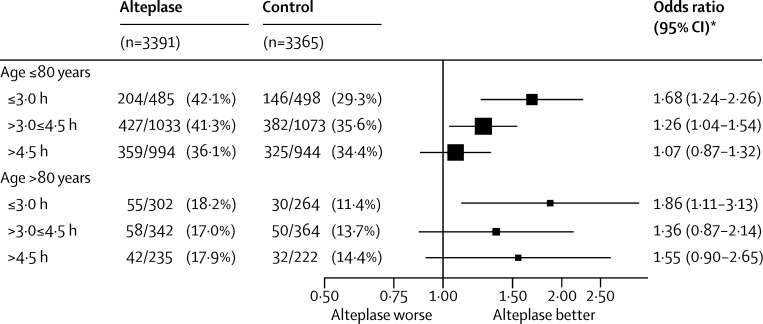
Age did not change the effect of alteplase on odds of a good outcome (p=0·53; appendix p 5). The effect of alteplase treatment was similar for patients aged 80 years or younger (mean treatment delay 4·1 h; 990 [39%] vs 853 [34%]; OR 1·25, 95% CI 1·10–1·42, p<0·0001) and for those older than 80 years (mean treatment delay 3·7 h; 155 [18%] vs 112 [13%]; OR 1·56, 95% CI 1·17–2·08, p=0·0023; figure 2). We found no evidence that old age shortened the period during which alteplase could effectively be given (p=0·08, in the direction of lengthening not shortening the period; figure 3). Nor did we find clear evidence that stroke severity modified the effect of alteplase (p=0·06). In particular, there was no evidence that alteplase was less effective for patients who had had the least or most severe strokes (figure 2, appendix p 5), reinforcing findings from one of the individual trials.30
Figure 3.
Effect of alteplase on a good stroke outcome (mRS 0–1) by age, with different treatment delays
Effect of age on the interaction between treatment delay and treatment effect p=0·08 (ie, not significant but, if anything, in the direction of it lengthening, not shortening, the period during which alteplase is effective in older people). *All six estimates derived from a single stratified logistic regression model that enables the odds ratio to be estimated separately for each group (also adjusted for baseline National Institutes of Health Stroke Scale score). mRS=modified Rankin Scale.
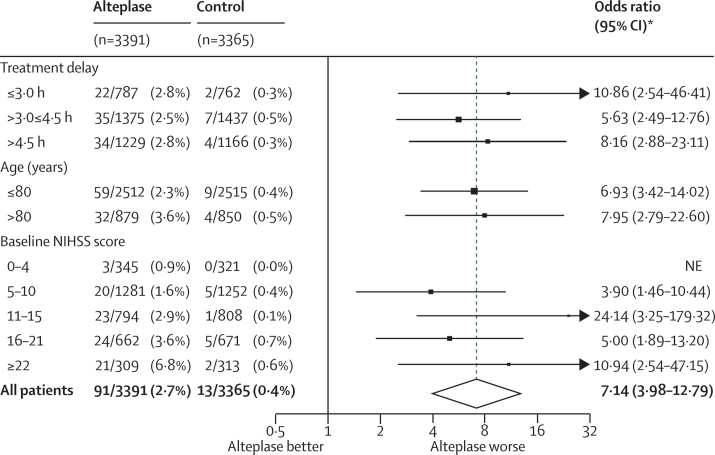
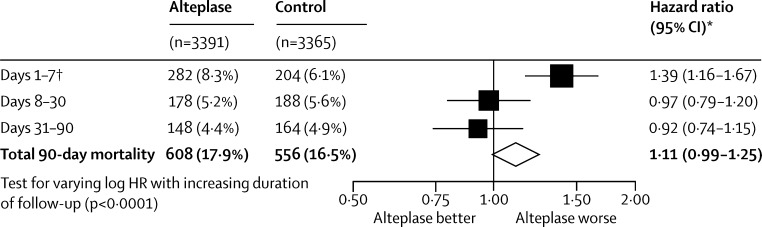
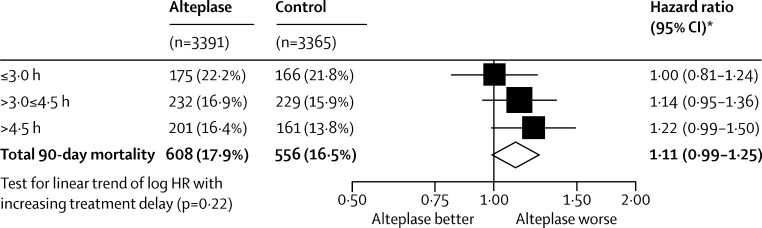
Alteplase increased the likelihood of symptomatic intracranial haemorrhage. Type 2 parenchymal haemorrhage within 7 days occurred in 231 (6·8%) of 3391 patients assigned alteplase versus 44 (1·3%) of 3365 assigned control (OR 5·55, 95% CI 4·01–7·70; p<0·0001) and SITS-MOST criteria type 2 parenchymal haemorrhage within 36 h occurred in 124 (3·7%) of 3391 versus 19 (0·6%) of 3365 (OR 6·67, 95% CI 4·11–10·84; p<0·0001). Fatal type 2 parenchymal haemorrhage within 7 days occurred in 91 (2·7%) patients assigned alteplase versus 13 (0·4%) assigned control (OR 7·14, 95% CI 3·98–12·79; p<0·0001). The proportional increase in risk of fatal intracranial haemorrhage was much the same, irrespective of treatment delay, age, or stroke severity (pinteraction>0·7 for all), but the absolute excess risk increased with increasing stroke severity (figure 4). Alteplase did not increase the risk of other early causes of death (ie, those other than intracranial haemorrhage), and had no significant effect on later causes of death (figure 5). Consequently, the early excess mortality caused by intracranial haemorrhage did not translate into a significant excess of overall mortality at 90 days (608 [17·9%] vs 556 [16·5%], HR 1·11 (95% CI 0·99–1·25); p=0·07; figure 5). The trend towards a larger relative increase in 90-day mortality with greater treatment delay was not statistically significant (ptrend=0·22; figure 6), although the statistical power to detect any true trend was limited by the number of deaths. The effects of alteplase on symptomatic intracranial haemorrhage, fatal intracranial haemorrhage, and 90-day mortality reported in IST-3 were consistent with those reported in the eight previous trials (all p values for inconsistency >0·1).
Figure 4.
Effect of alteplase on fatal intracranial haemorrhage within 7 days by treatment delay, age, and stroke severity
*For each of the three baseline characteristics, estimates were derived from a single logistic regression model stratified by trial, which enables separate estimation of the OR for each subgroup after adjustment for the other two baseline characteristics (but not possible interactions with those characteristics). The overall effect in all patients is the trial-stratified logistic regression estimate adjusted only for treatment allocation. NE=not estimable.
Figure 5.
Effect of alteplase on 90-day mortality by follow-up period
Patients can only contribute to a particular risk period if they have already survived any preceding periods. *Estimated by Cox proportional hazards regression stratified by trial (and adjusted only for treatment allocation). †Includes 91 versus 13 deaths caused by intracranial haemorrhage (with evidence of parenchymal haemorrhage type 2; figure 4) and 191 versus 191 deaths from other causes.
Figure 6.
Effect of alteplase on 90-day mortality by treatment delay
*Estimated by Cox proportional hazards regression stratified by trial (and adjusted only for treatment allocation). HR=hazard ratio.
Overall, therefore, despite an average absolute increase in risk of early death caused by intracranial haemorrhage of about 2%, by 3–6 months this was offset by an average absolute increase in disability-free survival (ie, mRS 0–1) of about 10% for patients treated within 3·0 h and about 5% for patients treated between 3·1 and 4·5 h.
Discussion
Our data provide further evidence about the extent to which treatment delay alters the beneficial effect of alteplase for acute ischaemic stroke. We provide clear evidence for improved odds of a good stroke outcome when treatment is started within 4·5 h of ischaemic stroke, with earlier treatment resulting in bigger proportional and absolute benefits. The average benefit of alteplase might even extend beyond 4·5 h for some patients. The proportional benefits were similar for patients aged older than 80 years compared with younger patients, and for patients with minor or severe strokes compared with other patients.
This average expected benefit from giving alteplase within 4·5 h occurred in spite of an average absolute increase in the early risk of fatal intracranial haemorrhage of around 2%. Since alteplase had no significant effect on other early causes of death, or on later causes of death, by 90 days this 2% excess remained but was no longer statistically significant. Longer-term follow-up data are needed to test whether the effect of alteplase on patients who survive the first week after their stroke have reduced long-term risk of death. Contrary to analyses of individual patient data done before IST-3,2 the trend towards a bigger relative risk of 90-day mortality with increasing treatment delay was not statistically significant in our analysis. However, if improvements in stroke outcome among survivors do lead to parallel improvements in mortality, then one would expect that long-term survival will be greatest among those treated earliest (ie, the group most likely to benefit from alteplase).
Our results support guidelines that recommend use of alteplase irrespective of age and up to 4·5 h after onset of stroke.3, 10, 11, 12 In the USA, marketing authorisation has not been granted for use of altplase after 3·0 h,9 while in some European countries marketing authorisation limits the use of alteplase to patients aged 80 years or younger. In the present analysis, the lower limit of the 95% CI for the time at which the proportional benefit on mRS 0–1 crossed the line of no effect was 5·0 h, with statistically significant evidence of benefit in the prespecified subset of patients with treatment delay after 3·0 h, up to 4·5 h. In addition, we found no evidence that age modified either the proportional benefits or the proportional hazards of alteplase, with clear evidence of overall benefit for mRS 0–1 among the 1729 patients aged older than 80 years at randomisation. Nor was there evidence that older age shortened the period during which such benefits were seen, according with a recent report.31
The availability of individual data for a large number of patients enabled us to make a more precise assessment of the relative effects of alteplase than has been possible previously. We also included more than 1700 patients aged older than 80 years. The number of older patients with stroke is increasing as a proportion of the general population and as a proportion of those with stroke, so our analyses provide a reliable assessment of the effects of alteplase in this group.32 Our analyses differ from previous pooled analyses of alteplase trials1, 2 by including patients from IST-3, which almost doubles the number of patients available. They also differ from previous tabular meta-analyses20 through the use of individual data, which enables direct assessment of the potential for effect modification. Our prespecified analysis plan safeguarded against the potentially inappropriate combination of data from IST-3 with those from the previous studies. In fact, the results from IST-3 were consistent with earlier trials after adjustment for the main differences in patient characteristics.
Nonetheless, the open design of IST-3 and its broader definition of significant bleeding might have inflated our estimate of the risk of parenchymal type 2 symptomatic haemorrhage. However, the number of such events associated with early neurological deterioration or death was small, limiting the potential for this to be a source of major bias. Furthermore, the overall results from IST-3 for symptomatic intracranial haemorrhage were similar to those estimated from previous trials. Patients in IST-3 were also older on average than patients in the eight previous trials. However, this difference provides one of the main strengths of our analysis—the ability to compare the effect of alteplase reliably in old and young patients. Although unknown systematic differences might also have existed between patients in IST-3 and patients in the other trials, any such characteristics would have to be strong determinants of treatment effect (rather than just predictors of risk) to produce material bias in our overall results. Future work will investigate the potential independent effect on treatment effect of a range of other characteristics, including sex, blood pressure, and baseline imaging features.
In conclusion, despite early increases in fatal intracranial haemorrhage, alteplase significantly improves the overall likelihood of a good stroke outcome at 3–6 months. The proportional benefit increases with earlier treatment and remains statistically significant up to at least 4·5 h after initial stroke symptoms, irrespective of age or stroke severity.
Acknowledgments
Acknowledgments
This collaboration is coordinated by the Clinical Trial Service Unit & Epidemiological Studies Unit at the University of Oxford, UK. The Unit receives core funding from the UK Medical Research Council and the British Heart Foundation. This work also received support from the University of Glasgow and University of Edinburgh.
Contributors
WH and EB had the original idea for this meta-analysis and implemented data definitions in 2004; KRL and EB refined the approach in 2010; CB, PS, and JW had the idea for this cycle of the meta-analysis and all authors contributed to the subsequent study protocol and statistical analysis plan. All authors contributed either to the acquisition of the original trial data or the creation of the combined dataset. JE and LB did the statistical analysis. JE and KRL wrote the first draft of the report. All authors contributed to the interpretation of the results, revision of the report, and have approved the final version of the manuscript.
Included trials
ATLANTIS A and B (Gregory Albers, James Grotta, Maarten Lansberg, Jean Marc Olivot); ECASS-1, ECASS-2, ECASS-3 (Erich Bluhmki, Werner Hacke, Markku Kaste, Kennedy Lees, Ruediger von Kummer, Danilo Toni, Nils Wahlgren); EPITHET (Stephen Davis, Geoffrey Donnan, Mark Parsons); IST-3 (Peter Sandercock, Joanna Wardlaw, Richard Lindley, Gordon Murray, Geoff Cohen, William Whiteley); NINDS A and B (Thomas Brott, James Grotta, Patrick Lyden, John Marler, Barbara Tilley).
STT Statistical Analysis Centre and Secretariat
Colin Baigent, Lisa Blackwell, Erich Bluhmki, Kelly Davies, Jonathan Emberson, Heather Halls, Lisa Holland, George Howard, Clare Mathews, Samantha Smith, Kate Wilson.
Declaration of interests
CB, LB, and JE have not accepted fees, honoraria, or paid consultancies but are involved in clinical trials of lipid-modifying treatment funded by Merck to the University of Oxford, with the University the trial sponsor in all cases. KRL has received speaker fees from and has served on the data monitoring committee of trials for Boehringer Ingelheim; his department has received research grant support from Genentech. GA has received research grant support from Lundbeck, fees for consultancy and advisory board membership from Lundbeck, Covidien, Codman, and Genentech, fees for acting as an expert witness, and owns stock in iSchemaView. EB is employed by Boehringer Ingelheim. SD has received honoraria from Boehringer Ingelheim, EVER Pharma, and Sanofi and has received fees for consultancy and advisory board membership from Boehringer Ingelheim and Sanofi. GD has received research grant support from the NHMRC (Australia) and honoraria from Pfizer and Bristol-Myers Squibb. JG has received fees for consultancy and advisory board membership from Lundbeck. RvK has received speaker fees and honoraria from Penumbra and Lundbeck. RIL has received honoraria from Boehringer Ingelheim. JMO has received speaker fees from Boehringer Ingelheim. MP has received travel support from Boehringer Ingelheim. BT has received honoraria from Pfizer. DT has received speaker fees and fees for consultancy and advisory board membership from Boehringer Ingelheim and Bayer. KT has received research grant support from the Ministry of Health, Labour, and Welfare of Japan, and speaker fees from Mitsubishi Tanabe Pharma. JW has received research grant support from the UK Medical Research Council and from Boehringer Ingelheim to the University of Edinburgh for a research scanner bought more than 10 years ago. WW has received research grant support from the UK Medical Research Council. PS has received honoraria for lectures which were paid to the department from Boehringer Ingelheim. WH has received research grant support from Boehringer Ingelheim, and speaker fees and fees for consultancy and advisory board membership from Boehringer Ingelheim. PL, TB, GC, GH, MKa, MKo, ML, GM, NW, and GJdZ declare no competing interests.
Supplementary Material
References
- 1.Hacke W, Donnan G, Fieschi C, the ATLANTIS Trials Investigators. the ECASS Trials Investigators. the NINDS rt-PA Study Group Investigators Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 2.Lees KR, Bluhmki E, von Kummer R, for the ECASS. ATLANTIS. NINDS and EPITHET rt-PA Study Group Investigators Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 3.European Stroke Organisation (ESO) Executive Committee. the ESO Writing Committee Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 4.European Stroke Organisation guidelines for stroke management: Update January 2009. Available from: http://www.eso-stroke.org/eso-stroke/education/education-guidelines.html (accessed March 3, 2014).
- 5.Minematsu K, Toyoda K, Hirano T. Guidelines for the intravenous application of recombinant tissue-type plasminogen activator (alteplase), the second edition, October 2012: a guideline from the Japan Stroke Society. J Stroke Cerebrovasc. 2013;22:571–600. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Australian Stroke Foundation Clinical guidelines for stroke management 2010. http://strokefoundation.com.au/site/media/Clinical_Guidelines_Acute_Management_Recommendations_2010.pdf (accessed March 3, 2014).
- 7.Summary of product characteristics (alteplase) http://www.medicines.org.uk/EMC/medicine/308/SPC/Actlyse (accessed March 3, 2014).
- 8.Australian Government Department of Health. Therapeutic Goods Administration Product and Consumer Medicine Information. Actilyse. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/PICMI?OpenForm&t=&q=Actilyse&r=https://www.ebs.tga.gov.au/ (accessed March 3, 2014).
- 9.Genentech Activase (Alteplase) http://www.gene.com/download/pdf/activase_prescribing.pdf (accessed March 3, 2014).
- 10.Ford GA, Ahmed N, Azevedo E. Intravenous alteplase for stroke in those older than 80 years old. Stroke. 2010;41:2568–2574. doi: 10.1161/STROKEAHA.110.581884. [DOI] [PubMed] [Google Scholar]
- 11.Mishra NK, Ahmed N, Andersen G, the VISTA collaborators. the SITS collaborators Thrombolysis in very elderly people: controlled comparison of SITS International Stroke Thrombolysis Registry and Virtual International Stroke Trials Archive. BMJ. 2010;341:c6046. doi: 10.1136/bmj.c6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra NK, Diener HC, Lyden PD, Bluhmki E, Lees KR, the VISTA Collaborators Influence of age on outcome from thrombolysis in acute stroke: a controlled comparison in patients from the Virtual International Stroke Trials Archive (VISTA) Stroke. 2010;41:2840–2848. doi: 10.1161/STROKEAHA.110.586206. [DOI] [PubMed] [Google Scholar]
- 13.Sandercock P, Wardlaw JM, Lindley RI, the IST-3 collaborative group The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hacke W, Kaste M, Fieschi C, the European Cooperative Acute Stroke Study (ECASS) Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 15.Hacke W, Kaste M, Fieschi C. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II) Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 16.Hacke W, Kaste M, Bluhmki E, the ECASS Investigators Thrombolysis with alteplase 3 to 4·5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 17.Davis SM, Donnan GA, Parsons MW, the EPITHET investigators Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 18.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S, for the ATLANTIS Study Investigators Recombinant tissue-type plasminogen activator (alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. JAMA. 1999;282:2019–2026. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- 19.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 20.Wardlaw JM, Murray V, Berge E. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emberson J, Lees KR, Howard G, the Stroke Thrombolysis Trialists' Collaborative Group Details of a prospective protocol for a collaborative meta-analysis of individual participant data from all randomized trials of intravenous rt-PA vs. control: statistical analysis plan for the Stroke Thrombolysis Trialists' Collaborative meta-analysis. Int J Stroke. 2013;8:278–283. doi: 10.1111/ijs.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wahlgren N, Ahmed N, Dávalos A. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 23.Schoenfeld D. Partial residuals for the proportional hazards regression-model. Biometrika. 1982;69:239–241. [Google Scholar]
- 24.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. 2000;19:1141–1164. doi: 10.1002/(sici)1097-0258(20000515)19:9<1141::aid-sim479>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Haley EC, Jr, Brott TG, Sheppard GL, the TPA Bridging Study Group Pilot randomized trial of tissue plasminogen activator in acute ischemic stroke. Stroke. 1993;24:1000–1004. doi: 10.1161/01.str.24.7.1000. [DOI] [PubMed] [Google Scholar]
- 26.Mori E, Yoneda Y, Tabuchi M. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology. 1992;42:976–982. doi: 10.1212/wnl.42.5.976. [DOI] [PubMed] [Google Scholar]
- 27.Wang SY, Wang XL, Zeng H. Early intravenous thrombolysis with recombinant tissue plasminogen activator for acute cerebral infarction. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2003;15:542–545. (published in Chinese). [PubMed] [Google Scholar]
- 28.Yamaguchi T, Hayakawa T, Kiuchi H. Intravenous Tissue-plasminogen activator ameliorates the outcome of hyperacute embolic stroke. Cerebrovasc Dis. 1993;3:269–272. [Google Scholar]
- 29.Hemmen TM, Raman R, Guluma KZ, the ICTuS-L Investigators Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke. 2010;41:2265–2270. doi: 10.1161/STROKEAHA.110.592295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The NINDS t-PA Stroke Study Group Generalized efficacy of t-PA for acute stroke. Subgroup analysis of the NINDS t-PA Stroke Trial. Stroke. 1997;28:2119–2125. doi: 10.1161/01.str.28.11.2119. [DOI] [PubMed] [Google Scholar]
- 31.Frank B, Fulton RL, Lees KR, for the VISTA Collaborators The effect of time to treatment on outcome in very elderly thrombolysed stroke patients. Int J Stroke. 2014 doi: 10.1111/ijs.12249. published online March 3. [DOI] [PubMed] [Google Scholar]
- 32.Howard G, Goff DC. Population shifts and the future of stroke: forecasts of the future burden of stroke. Ann N Y Acad Sci. 2012;1268:14–20. doi: 10.1111/j.1749-6632.2012.06665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.