Abstract
The cells that initiate and propagate cancer are important therapeutic targets. However, the progression from cells of origin to tumor-propagating cells is poorly defined for most human cancers. Mouse models indicate that both basal and luminal cells can initiate prostate cancer, while studies with human prostate tissue have demonstrated a role for basal cells in transformation. Our recent study provides evidence that a common cell of origin can produce alternative variants of human epithelial cancer. Our findings also reveal that the cell of origin that initiates cancer is not continuously required to maintain and propagate the disease. Importantly, the cells responsible for initiating human prostate cancer can have a distinct cellular phenotype from the cells needed to maintain it.
Keywords: prostate cancer, tumor-propagating cells, epithelial cells, basal-like cells
Introduction
Since the cell-types driving cancer are important therapeutic targets, it is essential to determine whether the same cell population that initiates cancer is responsible for maintaining tumor progression. Understanding fundamental mechanisms of human cancer biology, including (1) whether different histological variants of cancer arise from common or distinct target cells and (2) as tumors progress whether the cell-type of origin is continually required to maintain the disease, are critical for developing new effective therapeutic strategies against cancer.
Cell of origin for prostate cancer
The “cell of origin” or “target cell” refers to the normal cell-type in an adult tissue or organ that acquires the first tumor promoting characteristics [1, 2]. To investigate the contribution of distinct cell populations to the initiation and progression of human prostate cancer, our group developed a human tissue-regeneration assay [3–5]. This assay utilizes defined genetic alterations to initiate human prostate cancer in a purified subset of primary human prostate epithelial cells [3–5]. A combination of oncogenes commonly found altered in prostate cancer is introduced into primary human epithelial cells via lentiviral transduction. This assay allows for the isolation of distinct cell types from primary benign human tissues such as basal or luminal cells based on surface marker expression [3–5]. Using this approach we have previously demonstrated that primary human basal cells can serve as efficient target cells for prostate cancer initiation [4, 5]. In mouse models of prostate cancer both basal and luminal cells can serve as cells of origin for prostate cancer [6–8]. Several groups have utilized genetically engineered mice in which PTEN is deleted specifically in the basal or luminal cells using lineage-specific promotes to express the Cre recombinase. These studies demonstrate that basal and luminal cells can both initiate prostate cancer, with variations in aggressiveness of the resulting cancer depending on the mouse genetic background and incidence of Pten deletion by the basal or luminal lineage promoters used [6–8]. Recent studies also suggest that the microenvironment may influence the susceptibility of distinct prostate epithelial cell populations to cancer initiation [9, 10].
Tumor heterogeneity
Tumor heterogeneity poses a significant challenge to cancer treatment as distinct histological variants respond to treatments differently. Two models have been previously postulated that may explain the origins of tumor heterogeneity [1, 11]. First, different histological variants of cancer may arise from distinct target cells in the normal tissue, each giving rise to different tumor phenotypes [1, 11]. Alternatively, distinct genetic alterations may take place in a common target cell that is capable of multi-lineage differentiation or may change its phenotype over time as the tumor evolves to generate multiple histological variants of cancer [1, 11].
To investigate if histologically distinct phenotypes of human prostate cancer arise from common or distinct cells of origin, we introduced defined oncogenes found commonly altered in prostate cancer such as Myc and myristoylated AKT (myrAKT) into primary human basal cells via lentiviral transduction [5]. The recovered tumors contained features of both adenocarcinoma and a rarely observed histological variant of prostate cancer, squamous cell carcinoma, with each variant characterized by activation of distinct signaling pathways [5]. Although squamous cell carcinoma is not commonly found in clinical settings, it is associated with aggressive disease and resistance to androgen ablation, chemotherapy and radiation [12]. One of the advantages of the tissue recombination assay is that the oncogenes are introduced in primary human cells via lentiviral transduction which allows clonality analysis based on identity of lentiviral integration sites within the genome. Therefore, the adenocarcinoma and squamous tumor phenotypes allow the opportunity to determine the origins of such heterogeneity. To address if histological variants arise from the same target cell or different cells, we performed laser capture microdissection of adjacent adenocarcinoma or squamous cell carcinoma regions. Lentiviral integration site analysis revealed that different histological variants of prostate cancer shared integration sites indicating they share a clonal origin [5]. These results demonstrate that distinct histological phenotypes of human cancer can be clonally-derived from a common cell of origin.
Relationship between the cells of origin and tumor propagating cells
The cancer stem cell model suggests the existence of cell populations within cancer that are preferentially responsible for tumor maintenance and propagation. Pioneering studies have established that some subtypes of human leukemia are hierarchically organized and that a subset of cells shares the critical properties of normal tissue stem cells: self-renewal and differentiation to generate mature cell lineages [13, 14]. These findings gave rise to the cancer stem cell concept, functionally defined as a cell that can propagate the disease into immune-compromised mice. The major clinical implication of the cancer stem cell concept is that elimination of all mature cancer cells will initially cause tumor regression, but over time, the cancer stem cells can self-renew and drive disease recurrence. Importantly, the frequency of cancer stem cell subsets varies greatly depending on the tumor genotype and site of origin and is not necessarily rare [15]. Subsequent studies showed that several regulators of growth and self-renewal including HoxA cluster transcription factors normally restricted to the hematopoietic stem cell compartment can be acquired by more mature leukemic subsets to confer cancer-propagating activity in a cell population with a distinct phenotype from hematopoietic stem cells [16]. Emerging evidence, first in breast cancer and later in a number of other epithelial cancers, suggests that solid tumors may also be maintained by tumor-propagating cancer stem-like cells [15]. But what is the relationship between the cells of origin and tumor propagating cells? Does the tumor propagating cell share the same phenotype with the cell of origin or can its appearance change over time?
Phenotypic plasticity has been demonstrated in epithelial cancers. Studies in breast cancer suggest that basal-like tumors can arise from luminal progenitor cells carrying BRCA mutations rather than basal cell as was originally predicted [17, 18]. Moreover breast cancer contains multiple tumor propagating cell-types with discrete phenotypes [19]. In other tumors the cell population responsible for initiating cancer shares the same phenotype as the cell population that maintains and propagates cancer. Murine brain tumors can be initiated in and maintained by Nestin+ neural stem/progenitor cells [20, 21]. In the mouse intestine Lgr5+ intestinal cells can initiate and maintain adenomas [22, 23]. In murine skin cancer, tumor propagating cells resemble the cells in the hair follicle bulge that can serve as target cells for transformation [24–27]. However, in most human epithelial cancers, it has not been determined whether the cell-types that give rise to cancer are also capable of maintaining advanced disease.
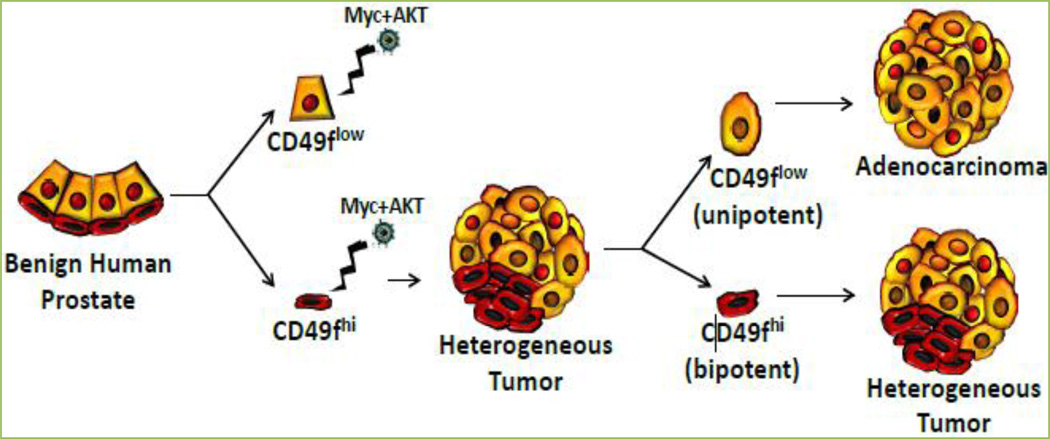
Having initiated human prostate cancer in naïve benign epithelial cells using defined genetic alterations, we asked whether the cells that initiate cancer are continually required to sustain the disease. We previously described that human prostate epithelial cells can be separated into enriched fractions of basal or luminal cells based on cell surface expression of CD49f [4, 5]. Basal-like cells express high level of CD49f (CD49fhi) and luminal-like cells are characterized by low levels of CD49f surface expression (CD49flow) [4, 5]. We found that primary tumors initiated by oncogenes Myc and myrAKT contained distinct cancer stem cell populations: basal-like tumor propagating cells (CD49fhi) with dual potential to propagate both adenocarcinoma and squamous phenotypes and luminal-like unipotent tumor propagating cells (CD49flow) that serially propagate strictly adenocarcinoma. Our new findings indicate that prostate cancer can start in basal cells and then evolve to be maintained by self-renewing cells with a different phenotype demonstrating that the stem-like component driving prostate cancer can change over time. These results indicate that human prostate adenocarcinoma can be initiated in basal cells and maintained by luminal-like cells, demonstrating that the cell of origin is not continuously required for propagation of human prostate cancer.
Further studies will need to be conducted to evaluate pathways that regulate the self-renewal capacity of prostate tumor propagating cells in vivo. Given that the stem-like populations driving prostate cancer initiation and progression have distinct phenotypes, the major clinical challenge will be finding strategies to inhibit common growth promoting and survival mechanisms in order to effectively eliminate all tumor-initiating and propagating cells regardless of phenotype.
Figure 1. Cell populations with distinct phenotypes can initiate and propagate human prostate cancer.
Model of transformation of epithelial subsets (basal-CD49fhi and luminal-CD49flow) with Myc and myrAKT resulting in tumors from the basal fraction but not from the luminal population. CD49fhi cells isolated from mixed tumors (containing both adenocarcinoma and squamous features) could propagate mixed tumors, while CD49floK18+ luminal-like tumor cells propagate strictly adenocarcinoma in the absence of CD49fhi or K14+ p63+ basal-like cells.
Acknowledgements
A.S.G. and T.S. are both supported by awards from the Prostate Cancer Foundation and Department of Defense Prostate Cancer Research Program.
References
- 1.Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. http://dx.doi.org/10.1038/nature09781 PMid: 21248838. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein AS, Stoyanova T, Witte ON. Primitive origins of prostate cancer: in vivo evidence for prostate-regenerating cells and prostate cancer-initiating cells. Mol Oncol. 2010;4:385–396. doi: 10.1016/j.molonc.2010.06.009. http://dx.doi.org/10.1016/j.molonc.2010.06.009 PMid: 20688584 PMCid:PMC2939195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein AS, Drake JM, Burnes DL, Finley DS, Zhang H, Reiter RE, et al. Purification and direct transformation of epithelial progenitor cells from primary human prostate. Nat Protoc. 2011;6:656–667. doi: 10.1038/nprot.2011.317. http://dx.doi.org/10.1038/nprot.2011.317 PMid: 21527922 PMCid:PMC3092477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–571. doi: 10.1126/science.1189992. http://dx.doi.org/10.1126/science.1189992 PMid: 20671189 PMCid:PMC2917982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoyanova T, Cooper AR, Drake JM, Liu X, Armstrong AJ, Pienta KJ, et al. Prostate cancer originating in basal cells progresses to adenocarcinoma propagated by luminal-like cells. Proc Natl Acad Sci U.S.A. 2013;110:20111–20116. doi: 10.1073/pnas.1320565110. http://dx.doi.org/10.1073/pnas.1320565110 PMid: 24282295 PMCid:PMC3864278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu TL, Huang YF, You LR, Chao NC, Su FY, Chang JL, et al. Conditionally ablated Pten in prostate basal cells promotes basal-to-luminal differentiation and causes invasive prostate cancer in mice. Am J Pathol. 2013;182:975–991. doi: 10.1016/j.ajpath.2012.11.025. http://dx.doi.org/10.1016/j.ajpath.2012.11.025 PMid: 23313138. [DOI] [PubMed] [Google Scholar]
- 7.Wang ZA, Mitrofanova A, Bergren SK, Abate-Shen C, Cardiff RD, Califano A, et al. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nat Cell Biol. 2013;15:274–283. doi: 10.1038/ncb2697. http://dx.doi.org/10.1038/ncb2697 PMid: 23434823 PMCid:PMC3743266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi N, Zhang B, Zhang L, Ittmann M, Xin L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer cell. 2012;21:253–265. doi: 10.1016/j.ccr.2012.01.005. http://dx.doi.org/10.1016/j.ccr.2012.01.005 PMid: 22340597 PMCid:PMC3285423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein AS, Witte ON. Does the microenvironment influence the cell types of origin for prostate cancer? Genes Dev. 2013;27:1539–1544. doi: 10.1101/gad.222380.113. http://dx.doi.org/10.1101/gad.222380.113 PMid: 23873937 PMCid:PMC3731542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon O-J, Zhang L, Ittmann M, Xin L. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc Natl Acad Sci U.S.A. 2014;111:E592–E600. doi: 10.1073/pnas.1318157111. http://dx.doi.org/10.1073/pnas.1318157111 PMid: 24367088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. http://dx.doi.org/10.1038/nature12624 PMid: 24048065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphrey PA. Histological variants of prostatic carcinoma and their significance. Histopathology. 2012;60:59–74. doi: 10.1111/j.1365-2559.2011.04039.x. http://dx.doi.org/10.1111/j.1365-2559.2011.04039.x PMid: 22212078. [DOI] [PubMed] [Google Scholar]
- 13.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. http://dx.doi.org/10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 14.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. http://dx.doi.org/10.1038/367645a0 PMid: 7509044. [DOI] [PubMed] [Google Scholar]
- 15.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev. 2008;8:755–768. doi: 10.1038/nrc2499. http://dx.doi.org/10.1038/nrc2499 PMid: 18784658. [DOI] [PubMed] [Google Scholar]
- 16.Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. http://dx.doi.org/10.1038/nature04980 PMid: 16862118. [DOI] [PubMed] [Google Scholar]
- 17.Molyneux G, Geyer FC, Magnay FA, McCarthy A, Kendrick H, Natrajan R, et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell stem cell. 2010;7:403–417. doi: 10.1016/j.stem.2010.07.010. http://dx.doi.org/10.1016/j.stem.2010.07.010 PMid: 20804975. [DOI] [PubMed] [Google Scholar]
- 18.Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–913. doi: 10.1038/nm.2000. http://dx.doi.org/10.1038/nm.2000 PMid: 19648928. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Villadsen R, Sorlie T, Fogh L, Gronlund SZ, Fridriksdottir AJ, et al. Tumor initiating but differentiated luminal-like breast cancer cells are highly invasive in the absence of basal-like activity. Proc Natl Acad Sci U.S.A. 2012;109:6124–6129. doi: 10.1073/pnas.1203203109. http://dx.doi.org/10.1073/pnas.1203203109 PMid: 22454501 PMCid:PMC3341000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006. http://dx.doi.org/10.1016/j.ccr.2008.12.006 PMid: 19111880 PMCid:PMC2650425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Li Y, Yu T-S, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. http://dx.doi.org/10.1038/nature11287 PMid: 22854781 PMCid:PMC3427400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. http://dx.doi.org/10.1038/nature07602 PMid: 19092804. [DOI] [PubMed] [Google Scholar]
- 23.Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JS, van de Wetering M, et al. Lineage Tracing Reveals Lgr5+ Stem Cell Activity in Mouse Intestinal Adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. http://dx.doi.org/10.1126/science.1224676 PMid: 22855427. [DOI] [PubMed] [Google Scholar]
- 24.Lapouge G, Youssef KK, Vokaer B, Achouri Y, Michaux C, Sotiropoulou PA, et al. Identifying the cellular origin of squamous skin tumors. Proc Natl Acad Sci U.S.A. 2011;108:7431–7436. doi: 10.1073/pnas.1012720108. http://dx.doi.org/10.1073/pnas.1012720108 PMid: 21502497 PMCid:PMC3088632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White AC, Tran K, Khuu J, Dang C, Cui Y, Binder SW, et al. Defining the origins of Ras/p53-mediated squamous cell carcinoma. Proc Natl Acad Sci U.S.A. 2011;108:7425–7430. doi: 10.1073/pnas.1012670108. http://dx.doi.org/10.1073/pnas.1012670108 PMid: 21502519 PMCid:PMC3088581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. http://dx.doi.org/10.1038/nature10525 PMid: 22012397. [DOI] [PubMed] [Google Scholar]
- 27.Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. http://dx.doi.org/10.1038/nature06835 PMid: 18385740. [DOI] [PubMed] [Google Scholar]