Abstract
Rationale
Angiogenesis plays an important role in wound healing and tumor growth. Fucosyltransferases synthesize fucosylated glycans and may play a major role in vascular biology.
Objective
To examine the role of an alpha(1,2) fucosyltransferase (Fut2) in angiogenesis.
Methods and results
We found that Fut2 mRNA and protein expression is inducible in human dermal micro-vascular endothelial cells (HMVECs). After finding that Fut2 is inducible in HMVECs, we examined if Fut2 contributes to angiogenesis. We found that Fut2 null endothelial cell (EC) migration and tube formation were significantly less compared to wild type (wt) ECs. Angio-genesis was impaired in Fut2 null compared to wt mice in the mouse Matrigel plug and the sponge granuloma angiogenesis assays. To assess the characteristics of Fut2 null ECs in vivo, we performed Matrigel plug angiogenesis assays in wt mice using Fut2 null and wt mouse ECs. We found a significant decrease in Fut2 null EC incorporation in neoangiogenesis compared to wt ECs. ERK1/2 activation, fibroblast growth factor receptor2, and vascular endothelial growth factor expression were less in Fut2 null ECs, suggesting a possible mechanism of impaired angio-genesis when Fut2 is lacking.
Conclusions
These data suggest a novel role for Fut2 as a regulator of angiogenesis.
Keywords: Angiogenesis, Fut2, Endothelial cells, VEGF, bFGF, ERK1/2 phosphorylation
Introduction
Fucosylated glycans are synthesized by a group of enzymes, called fucosyltransferases (Futs), which add L-fucose to sugars, proteins, and lipids [1, 2]. Thirteen fucosyltransferase genes have been identified in the human genome. These fucosyltransferases are involved in the synthesis of various fucosylated glycans which have an important role in blood transfusion reactions, selectin-mediated leukocyte-endothelial adhesion, and angiogenesis [3–7].
Alpha(1,2)fucosyltransferase (Fut2) regulates the expression of the H antigen, a precursor of blood group A and B antigens. Approximately 20 % of Caucasians are nonsecretors who do not express ABO antigens in saliva, as they are homozygous for Fut2 null alleles [8, 9]. Non-secretion of ABO blood group antigens into body fluids is associated with the development of cholera and infection with meningococcus, pneumococcus, and hemophilus influenza [10, 11].
A number of studies have shown that fucosyltransferases contribute to cell growth, tumor growth, and cell proliferation. Hiller et al. [12] have shown that transfection of human colon carcinoma cells with antisense to fucosyltransferases-III or -VI impairs the proliferation and tumorigenic ability of these cells by disrupting the synthesis of sialyl Lewisx/a (sLex/a). Silencing of Fut1 and Fut2, two members of alpha(1,2)fucosyltransferase, reduces the expression of fucosylated nucleolin and inhibits bovine postcapillary venular endothelial cell (EC) growth and proliferation [13]. These studies underscore the importance of fucosyltransferases in tumor growth and proliferation.
In this study, we found that interleukin (IL)-1β and tumor necrosis factor-α (TNF-α) upregulate Fut2 expression in human dermal microvascular endothelial cells (HMVECs). Fut2 plays an important role in angiogenesis in vitro and in vivo, as there is impaired basic fibroblast growth factor (bFGF)-mediated angiogenesis in Fut2 null compared to wt mice. There was a marked decrease in fibroblast growth factor receptor2 (FGFR2) in Fut2 null ECs compared to wild type (wt) ECs when these cells were stimulated with TNF-α. In this study, we determined if there was a decrease in potent angiogenic factors in the absence of Fut2. Fut2 null ECs have less vascular endothelial cell growth factor (VEGF) expression compared to wt ECs. In addition, ERK1/2 activation was absent in Fut2 null ECs. Targeting Fut2 may be beneficial in treating angiogenesis-dependent diseases.
Materials and methods
HMVEC culture and induction of Fut2
HMVECs (1.5 × 105/well) were plated on fibronectin coated 6-well plates and cultured in EGM-2 with all growth factors. Medium was switched to EGM-2 with 0.1 % bovine serum albumin (BSA) when cells were 80 % confluent. HMVECs were stimulated with interleukin (IL–1b, 1.7 nmol/L) for various time points.
Messenger (m)RNA and complimentary (c)DNA preparation from HMVECs
mRNAs were purified using RNAeasy mini RNA isolation kits in conjunction with QIAshredders (Qiagen Inc., Valencia, CA). cDNA were prepared using a one step Platinum Supermix from Invitrogen following the manufacturer's instructions.
Reverse transcription (RT) PCR analysis for Fut2 mRNA expression in HMVECs
RT PCR was performed with cDNA using an Eppendorf Mastercycler (Westbury, NY). Primers for Fut2 were (forward) 5′-CGA CTG GAT GGA GGA GGA-3′; (reverse) 5′-GAG CTC GGA ACC AGT CCA-3′ and primers for actin were (forward) 5′-GCC ATG TAC GTT GCT ATC CA-3′; (reverse) 5′-GTC AGG CAG CTC GTA GCT CT-3′. The predicted size for Fut2 is 308 bp, and for β-actin is 428 bp.
Quantitative (q)PCR analysis for Fut2 expression in HMVECs
HMVECs were stimulated with either IL–1β (1.7 nmol/L) or TNF-α (1.7 nmol/L) for one hour followed by mRNA purification and cDNA synthesis. qPCR was performed with Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen, Carlsbad, CA) using an Eppendorf Mastercycler ep realpex thermalcycler (Eppendorf, Hamburg, Germany). For quantification, the relative abundance of each gene was normalized to β-Actin.
Isolation of mouse ECs
We performed all animal experiments with female mice (6–8 weeks old). All the animal experiments were performed in accordance with National Institutes of Health guidelines and the University of Michigan Medical School's Institutional Animal Care and Use Committee. Fut2 null mice have been well characterized [14, 15]. Some of the C57BL/6 control mice were bred in our animal facility while others were purchased from the National Cancer Institute (Bethesda, MA).
ECs were isolated from Fut2 null and wt mouse lungs according to a modified method of Marelli-Berg as specified by Miltenyi (Miltenyi Biotech) [16]. Mouse lungs were digested enzymatically as described [17]. For each assay performed with mouse ECs, we harvested fresh ECs from mouse lungs (6-8 mice/group).
EC chemotaxis
Mouse EC chemotaxis was performed in 48-well modified Boyden chambers (NeuroProbe, CabinJohn, MD) using Fut2 null and wt mouse ECs [18, 19]. Test substances included bFGF (30 nmol/L, R&D Systems, Minneapolis, MN) and phosphate buffered saline (PBS, negative control).
Matrigel in vitro tube formation assay with Fut2 null and wt mouse ECs
We performed EC tube formation on growth factor reduced (GFR) Matrigel using Fut2 null and wt ECs harvested from mouse lungs, and compared the differences in EC tube formation in response to bFGF (30 nmol/L) [18, 19]. The number of tubes formed was quantitated by an observer blinded to the experimental groups.
Matrigel plug angiogenesis in vivo assay
To test the effect of Fut2 in angiogenesis in vivo, we performed Matrigel plug assays employing Fut2 null and wt mice [18, 19]. Each mouse was given a subcutaneous injection of sterile GFR Matrigel (500 μL/injection) with a 27-gauge needle. bFGF (30 nmol/L) was used as a stimulus. After 7 days, the mice were euthanized, Matrigel plugs were dissected out, and then analyzed for hemoglobin content.
Hemoglobin determination in the Matrigel plugs
Hemoglobin measurement was performed using hemoglobin standards from Sigma [18, 19]. Hemoglobin concentration is a reflection of the number of blood vessels in the plugs.
Immunofluorescence to detect angiogenesis in the Matrigel plugs
Some of the Matrigel plugs harvested from wt and Fut2 null mice were embedded in OCT, sectioned, and immunofluorescence was performed using rabbit anti-mouse vWF antibody.
Sponge granuloma in vivo angiogenesis assay
To evaluate the angiogenic role of Fut2 in inflammatory conditions, we performed the mouse sponge granuloma angiogenesis assay, an inflammatory model of angiogenesis, in Fut2 null and wt mice [19]. Sponges were extracted after 7 days, homogenized, and assayed for hemoglobin.
Matrigel plug angiogenesis assays using wt or Fut2 null ECs
Fut2 null and wt mouse ECs were dye-tagged with red PKH26 (Sigma Aldrich) and each mouse was injected with GFR Matrigel (500 μL/injection) mixed with one million dye-tagged wt or Fut2 null ECs. bFGF (30 nmol/L) was used as a stimulus. Plugs were harvested after 10 days, embedded in OCT, and cryosections were quantitated by an observer blinded to the experimental group for the incorporation of red dye-tagged wt or Fut2 null ECs.
Western blots for Fut2, FGFR2, and ERK1/2 phosphorylation
Fut2 null and wt mouse ECs were stimulated with TNF-α (1.7 nmol/L) or IL–1β (1.7 nmol/L) for 24 h. Cell lysates were collected and Western blots were performed using mouse anti-human Fut2 antibody (Santa Cruz), rat anti-mouse FGFR2 antibody (R&D Systems)[18], or rabbit anti-human phospho-ERK1/2 antibody (Cell Signaling).
qPCR analysis for VEGF expression in mouse ECs
qPCR was performed to measure bFGF and VEGF using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen, Carlsbad, CA) with Fut2 null and wt mouse ECs after stimulating with IL-1β or TNF-α for 1 h. Primers used for mouse bFGF were (forward) 5′-CACCAGGCCACTT CAAGGA-3′, and (reverse) 5′-GATGGATGCGCAGGAA GAA-3′ [20], while primers for VEGF were (forward) 5′-C AGGCTGCTGTAACGATGAA-3′, and (reverse) 5′-CAG GAATCCCAGAAACAACC-3′ [21]. For quantification, the relative abundance of each gene was normalized to β-Actin.
Collecting conditioned medium from wt and Fut2 null mouse ECs
Mouse ECs were stimulated with IL-1β (1.7 nmol/L) or TNF-α (1.7 nmol/L) for 24 h, conditioned medium collected, and enzyme linked immunosorbent assays (ELISAs) were performed for VEGF and bFGF.
Statistical analysis
For statistical evaluation of all experiments, Student's t tests were performed. Stars indicate significantly different values (*p < 0.05).
Results
IL-1β increases Fut2 mRNA expression in HMVECs
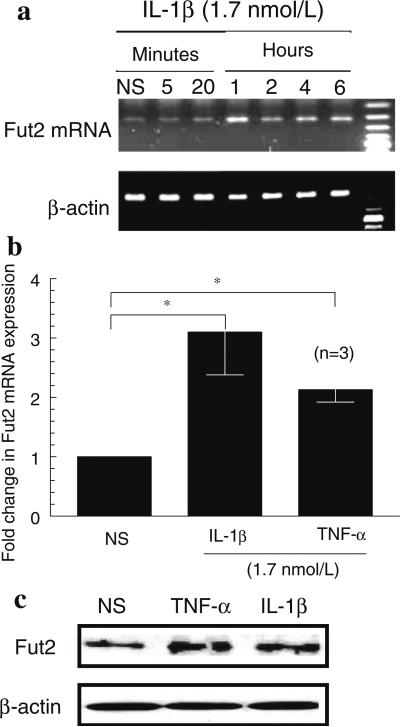
We performed RT PCR to detect Fut2 mRNA expression and found that Fut2 mRNA is inducible in HMVECs. Fut2 mRNA expression in HMVECs was increased by IL-1β in a time-dependent manner with a maximal increase between 1 and 6 h. We did not find an increase in Fut2 mRNA expression at 12 or 24 h (data not shown) (Fig. 1a).
Fig. 1.
a Fut2 mRNA expression in HMVECs: IL-1β induced significantly more Fut2 mRNA expression compared to nonstimulated (NS) HMVECs. Fut2 mRNA expression was normalized to β-actin. This is one of the representative of three assays. b qPCR to examine Fut2 mRNA expression in HMVECs: IL-1β and TNF-α induced more than twofolds increase in Fut2 expression in HMVECs. c Western blots to show Fut2 protein expression in HMVECs: IL-1β and TNF-α markedly increased Fut2 protein expression. Results represent the mean of 3 individual experiments ± SEM. n = the number of experiments
IL-1β and TNF-α induce Fut2 mRNA and protein expression in HMVECs
To confirm the results obtained by RT PCR, we performed qPCR. We found that IL-1β or TNF-α stimulated Fut2 expression significantly more compared to nonstimulated HMVECs. Fut2 expression was increased twofold and threefold by TNF-α and IL-1β, respectively (Fig. 1b). The protein expression of Fut2 in HMVECs was also increased by TNF-α and IL-1β as shown in Fig. 1c.
Confirmation of EC purity in mouse lung preparations
Before using these ECs in angiogenesis assays, cells were immunostained for EC markers. Cells retained the morphological features of ECs and immunostained for vWF (Fig. 2a) and CD31 (data not shown).
Fig. 2.
a Staining of mouse ECs with vWF factor: Green immunofluorescence staining shows mouse ECs harvested from Fut2 null mouse lungs. The top panel is vWF staining while the lower panel is isotype IgG control. Nuclei of ECs stained with DAPI are blue. b Mouse EC chemotaxis: EC chemotaxis was performed with the ECs harvested from Fut2 null and wt mouse lungs. We used 6–8 mice/group. ECs from Fut2 null mice had less migration compared to wt ECs in response to bFGF (p < 0.05). Results are expressed as mean ± SEM. Three high power fields (hpf) (×400) were counted in each replicate well. *p < 0.05 was considered significant. n = number of replicates for each test group
Fut2 null ECs migrate less than wt ECs in response to bFGF
To investigate the role of Fut2 in angiogenesis, we performed EC chemotaxis assays, a facet of angiogenesis, with Fut2 null and wt mouse ECs. Fut2 null ECs had significantly (p < 0.05) reduced migration compared to wt ECs in response to bFGF, suggesting that Fut2 plays an important role in EC chemotaxis (Fig. 2b). There was a twofold decrease in Fut2 null EC migration.
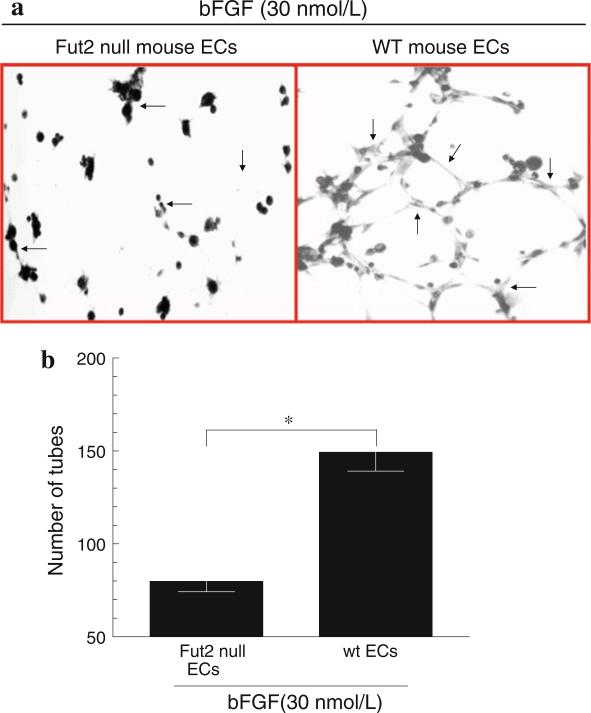
Fut2 is important in bFGF-induced EC tube formation
After finding that Fut2 null ECs migrate less in response to bFGF, we examined the importance of Fut2 in EC capillary morphogenesis. We performed Matrigel tube formation assays using Fut2 null and wt mouse ECs. Fut2 null ECs formed significantly less tubes on Matrigel compared to wt ECs when stimulated with bFGF, suggesting that Fut2 is involved in angiogenesis in vitro (Fig. 3a, b).
Fig. 3.
a Matrigel tube formation assay: A representative Matrigel tube formation assay using Fut2 null or wt mouse ECs. Fut2 null mouse ECs formed less tubes compared to wt mouse ECs in response to bFGF (30 nM). Photomicrographs were taken at 40× magnification. Arrows indicate mouse EC tube formation. b Graph for EC tube formation: Fut2 null ECs formed a twofold decrease in tube formation on GFR Matrigel as compared to wt ECs (p < 0.05). bFGF (30 nmol/L) was used as a stimulus. Results represent mean ± SEM and *p < 0.05 was considered significant. n = number of experiments for each test group
Matrigel plugs obtained from Fut2 null mice have less angiogenesis in vivo
After finding that Fut2 mediates two aspects of angiogenesis in vitro; EC migration and EC tube formation, we determined if Fut2 also regulates angiogenesis in vivo. We performed mouse Matrigel plug angiogenesis assays by employing Fut2 null and wt mice. Plugs harvested from Fut2 null mice were pale yellow, whereas plugs harvested from wt mice were red colored due to increased angio-genesis (Fig. 4a).
Fig. 4.
a Mouse Matrigel plug angiogenesis assay: We show Matrigel plugs harvested from Fut2 null and wt mice on day 7. Arrows indicate blood vessels grown in response to bFGF (30 nmol/L). Plugs obtained from wt mice were red due to exuberant blood vessel growth compared to plugs from Fut2 null mice. b Hemoglobin determination in the Matrigel plugs: Fut2 null mice have significantly less hemoglobin compared to wt mice (p < 0.05). Results are expressed as mean ± SEM. *p < 0.05 was considered significant. n = number of animals in each group. c vWF staining of plug cryosections: Immunofluorescence of plug cryosections shows green blood vessels stained for vWF. Arrows indicate more green fluorescent blood vessels in the plugs harvested from wt mice. Figure 4c shows one of the representative sections from each group. d Quantification of blood vessels in the Matrigel plugs: Plugs stained with vWF were quantitated by an observer blinded to the experimental group. Plug cryosections obtained from Fut2 null mice had significantly reduced blood vessels compared to wt plugs. Graph represents as mean ± SEM. *p < 0.05 was considered significant. n = number of mice in each group. e Mouse sponge granuloma angiogenesis assays: Fut2 null mice had ~ threefold less hemoglobin, a measure of angiogenesis, compared to wt mice. Results represent mean ± SEM and *p < 0.05 was considered significant. n = number of the mice per group
Fut2 null mice have decreased hemoglobin in the Matrigel plug angiogenesis assays
Hemoglobin was measured in the Matrigel plugs harvested from Fut2 null and wt mice. Angiogenesis was significantly reduced in Fut2 null compared to wt mice in the Matrigel plug angiogenesis assays in response to bFGF (30 nmol/L). We found more than a twofold decrease in hemoglobin in Fut2 null compared to wt mice, pointing out the importance of Fut2 in angiogenesis (Fig. 4b).
Plugs harvested from Fut2 null mice have less angiogenesis compared to wt plugs
To visualize the effect of bFGF-mediated angiogenesis in Fut2 null and wt mice, some of the plugs were embedded in OCT, sectioned, and immunofluorescence was performed using anti-vWF antibody, an EC marker. Plugs harvested from Fut2 null mice had significantly reduced blood vessels compared to wt plugs (Fig. 4c, d).
Fut2 null mice have less angiogenesis in the inflammatory sponge granuloma model
There was a significant decrease in hemoglobin, a direct correlate of neovascularization, in the sponges harvested from Fut2 null mice compared to the sponges from wt mice. A ~ threefold decrease in angiogenesis was found in Fut2 null sponges in comparison with wt mouse sponges (p < 0.05, Fig. 4e).
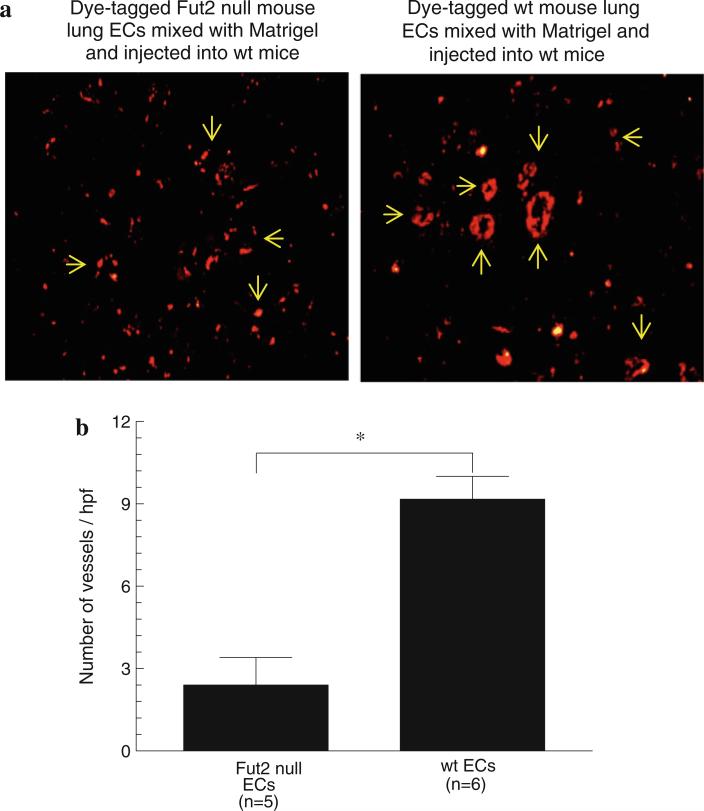
Fut2 null ECs are less involved in angiogenesis compared to wt mouse ECs
We performed a unique in vivo assay using dye-tagged Fut2 null and wt mouse ECs. We mixed either Fut2 null or wt ECs with Matrigel and performed Matrigel plug angiogenesis assays in wt mice. We found a fourfold decrease in incorporation of Fut2 null ECs in blood vessels in response to bFGF compared to wt ECs, pointing out that Fut2 is essential for EC incorporation into blood vessels (Fig. 5a, b).
Fig. 5.
a Mouse Matrigel plug angiogenesis with dye-tagged Fut2 null or wt ECs: Fut2 null ECs form less blood vessels in the Matrigel plugs when injected into wt mice. Arrows indicate the formation of blood vessels by wt and Fut2 null ECs. There are more scattered Fut2 null ECs compared to wt ECs. b Dye-tagged wt ECs form significantly more tubes compared to Fut2 null ECs. Results represent mean ± SEM and *p < 0.05 was considered significant. n = number of the mice per group
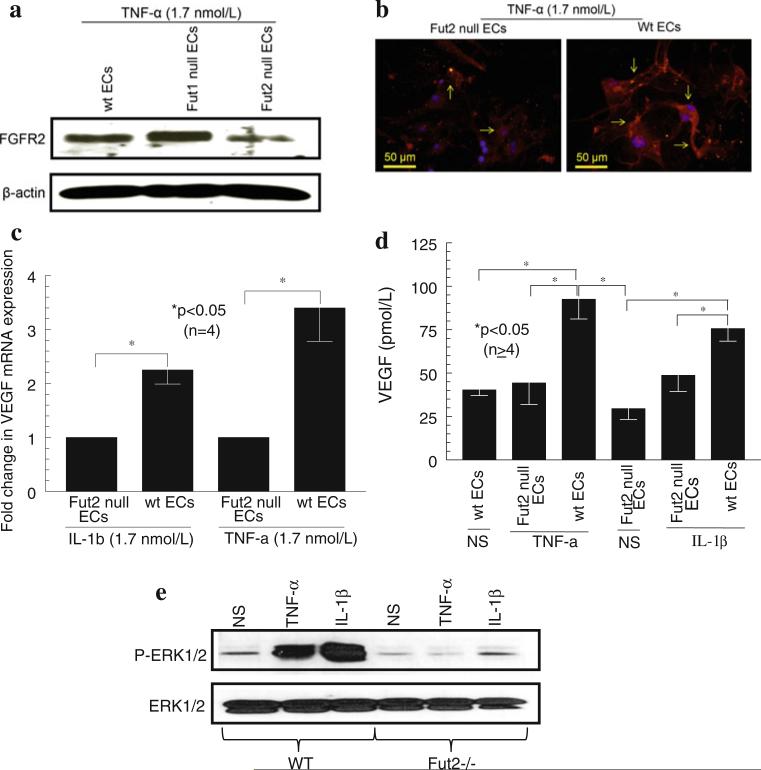
FGFR2 is markedly decreased in Fut2 null ECs
After finding a defect in angiogenesis in Fut2 null mice, we investigated the mechanism of impaired angiogenesis when Fut2 is lacking. We found a marked decrease in FGFR2 in Fut2 null ECs compared to wt ECs when these cells were stimulated with TNF-α. This indicates that the decrease in FGFR2 may account for some of the impaired angiogenesis in Fut2 null mice (Fig. 6a, b).
Fig. 6.
a Western blots for FGFR2: Western blots were performed with Fut2 null, Fut1 null, and wt mouse EC lysates for FGFR2. Fut2 null ECs have markedly reduced FGFR2 compared to wt and Fut1 null ECs. Blots were stripped and reprobed for β-actin to examine the equal loading of protein. One of the representative assays is shown. b Immunofluorescence to detect FGFR2 on Fut2 null and wt ECs: Fut2 null mouse ECs had markedly diminished FGFR2 on ECs when they were stimulated with TNF-α for 24 h. Arrows indicate red staining for FGFR2. The blue color is nuclei stained with DAPI. c qPCR for VEGF expression: We found a marked decrease in VEGF mRNA expression in Fut2 null ECs stimulated with IL-1β or TNF-α. n = number of experiments performed using ECs harvested from mice. d ELISAs for VEGF: Fut2 null mice have ~ twofold less VEGF secretion in comparison with wt mouse ECs (p < 0.05). Results are expressed as mean ± SEM and *p < 0.05 was considered significant. n = number of experiments. e Western blot for ERK1/2 phosphorylation: IL-1β and TNF-α stimulated ERK1/2 phosphorylation in wt ECs but not in Fut2 null ECs
Fut2 null mouse ECs have less VEGF mRNA and protein expression compared to wt mouse ECs
We found a twofold decrease in VEGF mRNA expression in Fut2 null ECs compared to wt mouse ECs, when stimulated with IL-1β (1.7 nmol/L) or TNF-α (1.7 nmol/L) for 1 h (Fig. 6c). To examine if there were differences in VEGF protein levels in Fut2 null and wt mouse ECs, mouse ECs were stimulated with IL-1β (1.7 nmol/L) or TNF-α (1.7 nmol/L) for 24 h to generate conditioned media for ELISAs. Fut2 null ECs had a twofold decreased VEGF secretion compared to wt mouse ECs (Fig. 6d). We did not find a decrease in bFGF in Fut2 null ECs compared to wt ECs at both the mRNA and protein levels (data not shown). These results suggest that lack of Fut2 may result in impaired angiogenesis due to decreased EC VEGF production.
Fut2 null mouse ECs have less ERK1/2 phosphorylation compared to wt mouse ECs
After finding a decrease in FGFR and VEGF expression in Fut2 null ECs, we examined if Fut2 is involved in phosphorylation of ERK1/2. While TNF-α and IL-1β induced significant ERK1/2 phosphorylation in wt mouse ECs, they failed to activate ERK1/2 in Fut2 null mouse ECs (Fig. 6e). The decrease in ERK1/2 phosphorylation in Fut2 null ECs may be another mechanism of impaired angiogenesis.
Discussion
Angiogenesis is vital for many physiological and pathological conditions such as, wound healing, embryogenesis, tissue repair, rheumatoid arthritis (RA), and tumor growth [22–24]. Angiogenesis is tightly regulated process which is triggered by a number of mediators such as soluble E-selectin, bFGF, VEGF, and IL-8/CXCL8 [3, 24, 25]. We and others have shown that soluble E-selectin and its ligand, sLex, are involved in capillary morphogenesis [3, 7].
There is some controversy about Fut2 expression in response to proinflammatory mediators. Some studies suggest that Fut2 is inducible while others suggest that Fut2 is not inducible by proinflammatory mediators [13, 26, 27]. To resolve this issue, we initially performed RT PCR and found that IL–1β induces Fut2 mRNA expression in HMVECs in a time dependent manner. The maximum Fut2 mRNA induced by IL–1β was at 1 h (Fig. 1). We confirmed our data by performing qPCR (Fig. 1b). We also showed that both IL-1β and TNF-α induced Fut2 expression at protein level (Fig. 1c). Our findings support the notion that Fut2 mRNA expression is inducible in ECs and human gastric cancer cells [13, 26]. Contrary to our results, Nakamura et al. [26] found that cytokines such as IL–1β and TNF-α inhibited Fut2 mRNA expression. The reason for this discrepancy may be that Nakamura et al. used human endometrial adenocarcinoma cell lines while we used normal human ECs. Our data is in agreement with various reports that fucosyltransferases can be induced by a number of inflammatory mediators [28, 29].
We and others have examined the contribution of a number of fucosyltransferases in angiogenesis, tumor growth, and proliferation, but the role of Fut2 in angio-genesis has not previously been determined [3, 5–7]. There are some disagreements regarding the role of Fut2 in tumor growth and angiogenesis. One group showed that transfection of a rat colorectal carcinoma cell line with rat Fut2 antisense ODNs decreased tumorigenicity [30, 31], while another study reported that development of colon tumors in mice is not affected in double Fut2 null and Smad3 null mice [32]. To clarify the contribution of Fut2 in angio-genesis, we employed Fut2 null ECs and Fut2 null mice in a number of angiogenesis assays.
Nguyen et al. [7] reported a role for sLex (synthesized by fucosyltransferase III) in capillary morphogenesis, indirectly suggesting a role of Futs in EC tube formation. In this study, we demonstrate that Fut2 plays an essential role in angiogenesis. We found that Fut2 null ECs showed significantly less migration in response to bFGF compared to wt ECs. When Fut2 null and wt ECs were incubated on GFR Matrigel in the presence of bFGF, Fut2 null ECs formed less tubes in comparison with wt ECs. This suggests that Fut2 regulates these two aspects of angiogenesis in vitro; EC migration and EC tube formation. A recent study from Plaumberi et al. [13] showed that silencing Fut2 or Fut1 inhibits EC proliferation and growth, indirectly supporting the contribution of Fut2 in angiogenesis and our data is in agreement with this report.
To evaluate the role of Fut2 in angiogenesis in vivo, we performed mouse Matrigel plug and inflammatory sponge granuloma angiogenesis assays using Fut2 null and wt mice. We found a significant decrease in angiogenesis in Fut2 null mice compared to wt mice, suggesting the contribution of Fut2 in angiogenesis. Our data supports the notion that Fut2 and other fucosyltransferases play an important role in tumor growth and proliferation, a process in which angiogenesis is involved [5, 7, 30, 31].
After finding impaired angiogenesis in vitro and in vivo in Fut2 null ECs, we examined the characteristics of Fut2 null ECs after injecting these cells into wt mice. To test this, we performed a novel assay in which we mixed dye- tagged Fut2 null or wt ECs with Matrigel using bFGF as a stimulus. We found a significant decrease in Fut2 null EC incorporation into blood vessels compared to wt ECs in the Matrigel plugs, suggesting that Fut2 is required for EC involvement in angiogenesis.
bFGF induces proliferation and differentiation of various cell types and is a potent angiogenic factor that mediates its angiogenic effects by binding to cell surface receptors called FGFRs [33, 34]. The interaction of FGFs and FGFRs impacts a variety of normal and pathological processes including chemotaxis, tissue development, angiogenesis, inflammation, and tumorigenesis [35]. FGFRs are glycosylated and a defect in glycosylation of FGFR2 results in craniosynostosis syndrome [36]. Manifestations of impaired bFGF-stimulated angiogenesis in vitro and in vivo prompted us to examine the expression of one of the receptors of FGF. We found that fucosylation by Fut2 is required for FGFR2 expression, as Fut2 null ECs express markedly decreased FGFR2 compared to wt ECs or Fut1 null ECs. Fut1 is another alpha(1,2) fucosyltransferase which contributes to the addition of fucose groups to a number of substrates (Fig. 6a). This implies the specificity of Fut2 in FGFR2 expression and also suggests that each fucosyltransferase is specific in its functions. Our data is in agreement with other reports suggesting that fucosylation plays an important role in the function and expression of various growth receptors, such as VEGF receptor-2, epidermal growth factor receptor, and α3β1 integrin in a variety of cell types [37–39].
VEGF is the most potent angiogenic factors and is one of the best therapeutic targets for treating cancers [24, 40]. Indeed, anti-VEGF reduces arthritis severity and joint angiogenesis in mouse collagen induced arthritis [41]. After examining impaired angiogenesis in Fut2 null mice, we assessed if angiogenic factors were decreased when Fut2 is lacking. We stimulated Fut2 null and wt ECs with IL-1β or TNF-α, and performed qPCR and ELISAs for angiogenic factors. We found a significant decrease in VEGF in Fut2 null ECs in comparison with wt ECs. Hence, we demonstrate that fucosylation of FGFR2 and VEGF by Fut2 is required for their expression. Another report has suggested that fucosylation of nucleolin by Fut2 or Fut1 is required for the expression and activity of nucleolin in bovine ECs [13]. In this study, the authors found that silencing RNA against Fut2 or Fut1 inhibits fucosylation of nucleolin and EC proliferation. Our data is consistent with the above report that fucosylation of some of the angiogenic factors and receptors is essential for their expression.
We have previously shown that the signaling intermediates such as ERK1/2 plays a crucial role in promoting angiogenesis [18]. Therefore, to further explore the mechanism of the impaired angiogenesis seen in Fut2 null ECs, we stimulated these cells with IL-1β or TNF-α and found that the ERK1/2 is not phosphorylated compared to wt ECs. This, together with the lower expression of FGFR2 and VEGF, may account for the impairment of Fut2 null ECs to form new blood vessels.
In conclusion, our data provides evidence that Fut2 is inducible in HMVECs. Fut2 is also a direct regulator of angiogenesis, as well as a mediator of FGFR2 and VEGF expression. Targeting Fut2 may provide a potential therapeutic in angiogenesis-dependent diseases such as RA and tumor growth.
Acknowledgments
We are thankful to Sivakumar Nallasivam who helped us to perform immunofluorescence with Matrigel plug sections. This study was supported by funds from the Veterans’ Administration Research Service (AEK) and the Frederick G.L. Huetwell and William D. Robinson Professorship (AEK). Additional support included funds from National Institute of Health grants AI40987 (AEK), HL58695 (AEK), AR48267 (AEK), and AR052482 (MAA).
Footnotes
Ethical Standards The experiments conducted in this manuscript comply with the current laws of the United States.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Pei-Suen Tsou, Department of Medicine, University of Michigan Medical School, Ann Arbor, MI 48109, USA.
Jeffrey H. Ruth, Department of Medicine, University of Michigan Medical School, Ann Arbor, MI 48109, USA
Phillip L. Campbell, Department of Medicine, University of Michigan Medical School, Ann Arbor, MI 48109, USA
Takeo Isozaki, Department of Medicine, University of Michigan Medical School, Ann Arbor, MI 48109, USA.
SolHee Lee, Department of Medicine, University of Michigan Medical School, Ann Arbor, MI 48109, USA.
Hubert Marotte, Department of Medicine, University of Michigan Medical School, Ann Arbor, MI 48109, USA.
Steven E. Domino, Department of Obstetrics and Gynecology, University of Michigan Medical School, Ann Arbor, MI 48109, USA
Alisa E. Koch, Department of Medicine, University of Michigan Medical School, Ann Arbor, MI 48109, USA Veteran's Administration, Ann Arbor, MI 48109, USA.
Mohammad A. Amin, Department of Medicine, University of Michigan Medical School, Ann Arbor, MI 48109, USA
References
- 1.de Vries T, Knegtel RM, Holmes EH, Macher BA. Fucosyltransferases: structure/function studies. Glycobiology. 2001;11(10):119R–128R. doi: 10.1093/glycob/11.10.119r. [DOI] [PubMed] [Google Scholar]
- 2.Oriol R, Mollicone R, Coullin P, Dalix AM, Candelier JJ. Genetic regulation of the expression of ABH and Lewis antigens in tissues. APMIS Suppl. 1992;27:28–38. [PubMed] [Google Scholar]
- 3.Koch AE, Halloran MM, Haskell CJ, Shah MR, Polverini PJ. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature. 1995;376(6540):517–519. doi: 10.1038/376517a0. doi:10.1038/376517a0. [DOI] [PubMed] [Google Scholar]
- 4.Lowe JB. Selectin ligands, leukocyte trafficking, and fucosyltransferase genes. Kidney Int. 1997;51(5):1418–1426. doi: 10.1038/ki.1997.194. [DOI] [PubMed] [Google Scholar]
- 5.Moehler TM, Sauer S, Witzel M, Andrulis M, Garcia-Vallejo JJ, Grobholz R, Willhauck-Fleckenstein M, Greiner A, Goldschmidt H, Schwartz-Albiez R. Involvement of alpha 1–2-fucosyltransferase I (FUT1) and surface-expressed Lewis(y) (CD174) in first endothelial cell–cell contacts during angiogenesis. J Cell Physiol. 2008;215(1):27–36. doi: 10.1002/jcp.21285. [DOI] [PubMed] [Google Scholar]
- 6.Amin MA, Rabquer BJ, Kumar P, Mansfield PJ, Campbell PL, Domino SE, Koch AE. A unique role for cytokine inducible fucosyltransferase 1 in angiogenesis. Arthritis Rheum. 2007;56:S583. [Google Scholar]
- 7.Nguyen M, Strubel NA, Bischoff J. A role for sialyl Lewis-X/A glycoconjugates in capillary morphogenesis. Nature. 1993;365:267–269. doi: 10.1038/365267a0. [DOI] [PubMed] [Google Scholar]
- 8.Kelly RJ, Rouquier S, Giorgi D, Lennon GG, Lowe JB. Sequence and expression of a candidate for the human Secretor blood group alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J Biol Chem. 1995;270(9):4640–4649. doi: 10.1074/jbc.270.9.4640. [DOI] [PubMed] [Google Scholar]
- 9.Rouquier S, Lowe JB, Kelly RJ, Fertitta AL, Lennon GG, Giorgi D. Molecular cloning of a human genomic region containing the H blood group alpha(1,2)fucosyltransferase gene and two H locus-related DNA restriction fragments. Isolation of a candidate for the human Secretor blood group locus. J Biol Chem. 1995;270(9):4632–4639. doi: 10.1074/jbc.270.9.4632. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell CC, Jonsdottir K, Hanson MF, Weir DM. Non-secretion of ABO blood group antigens predisposing to infection by Haemophilus influenzae. Lancet. 1986;2(8508):687. doi: 10.1016/s0140-6736(86)90193-5. [DOI] [PubMed] [Google Scholar]
- 11.Blackwell CC, Jonsdottir K, Hanson M, Todd WT, Chaudhuri AK, Mathew B, Brettle RP, Weir DM. Non-secretion of ABO antigens predisposing to infection by Neisseria meningitidis and Streptococcus pneumoniae. Lancet. 1986;2(8501):284–285. doi: 10.1016/s0140-6736(86)92103-3. [DOI] [PubMed] [Google Scholar]
- 12.Hiller KM, Mayben JP, Bendt KM, Manousos GA, Senger K, Cameron HS, Weston BW. Transfection of alpha(1,3)fucosyltransferase antisense sequences impairs the proliferative and tumorigenic ability of human colon carcinoma cells. Mol Carcinog. 2000;27(4):280–288. [PubMed] [Google Scholar]
- 13.Palumberi D, Aldi S, Ermini L, Ziche M, Finetti F, Donnini S, Rosati F. RNA-mediated gene silencing of FUT1 and FUT2 influences expression and activities of bovine and human fucosylated nucleolin and inhibits cell adhesion and proliferation. J Cell Biochem. 2010;111(1):229–238. doi: 10.1002/jcb.22692. doi:10.1002/jcb.22692. [DOI] [PubMed] [Google Scholar]
- 14.Domino SE, Zhang L, Gillespie PJ, Saunders TL, Lowe JB. Deficiency of reproductive tract alpha(1,2)fucosylated glycans and normal fertility in mice with targeted deletions of the FUT1 or FUT2 alpha(1,2)fucosyltransferase locus. Mol Cell Biol. 2001;21(24):8336–8345. doi: 10.1128/MCB.21.24.8336-8345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domino SE, Zhang L, Lowe JB. Molecular cloning, genomic mapping, and expression of two secretor blood group alpha (1,2)fucosyltransferase genes differentially regulated in mouse uterine epithelium and gastrointestinal tract. J Biol Chem. 2001;276(26):23748–23756. doi: 10.1074/jbc.M100735200. [DOI] [PubMed] [Google Scholar]
- 16.Marelli-Berg FM, Peek E, Lidington EA, Stauss HJ, Lechler RI. Isolation of endothelial cells from murine tissue. J Immunol Methods. 2000;244:205–215. doi: 10.1016/s0022-1759(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 17.Koch AE, Polverini PJ, Leibovich SJ. Stimulation of neovascularization by human rheumatoid synovial tissue macrophages. Arthritis Rheum. 1986;29(4):471–479. doi: 10.1002/art.1780290403. [DOI] [PubMed] [Google Scholar]
- 18.Amin MA, Volpert OV, Woods JM, Kumar P, Harlow LA, Koch AE. Migration inhibitory factor mediates angiogenesis via mitogen-activated protein kinase and phosphatidylinositol kinase. Circ Res. 2003;93(4):321–329. doi: 10.1161/01.RES.0000087641.56024.DA. [DOI] [PubMed] [Google Scholar]
- 19.Park CC, Morel JC, Amin MA, Connors MA, Harlow LA, Koch AE. Evidence of IL-18 as a novel angiogenic mediator. J Immunol. 2001;167(3):1644–1653. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- 20.Fricke I, Mitchell D, Petersen F, Bohle A, Bulfone-Paus S, Brandau S. Platelet factor 4 in conjunction with IL-4 directs differentiation of human monocytes into specialized antigen-presenting cells. FASEB J. 2004;18(13):1588–1590. doi: 10.1096/fj.03-1435fje. doi: 10.1096/fj.03-1435fje. [DOI] [PubMed] [Google Scholar]
- 21.Jeon SH, Chae BC, Kim HA, Seo GY, Seo DW, Chun GT, Kim NS, Yie SW, Byeon WH, Eom SH, Ha KS, Kim YM, Kim PH. Mechanisms underlying TGF-beta1-induced expression of VEGF and Flk-1 in mouse macrophages and their implications for angiogenesis. J Leukoc Biol. 2007;81(2):557–566. doi: 10.1189/jlb.0806517. doi: 10.1189/jlb.0806517. [DOI] [PubMed] [Google Scholar]
- 22.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 24.Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36(2):127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 25.Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997;18(1):26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- 26.Padro M, Mejias-Luque R, Cobler L, Garrido M, Perez-Garay M, Puig S, Peracaula R, de Bolos C. Regulation of glycosyltransferases and Lewis antigens expression by IL-1beta and IL-6 in human gastric cancer cells. Glycoconj J. 2011;28(2):99–110. doi: 10.1007/s10719-011-9327-4. doi: 10.1007/s10719-011-9327-4. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura H, Jasper MJ, Hull ML, Aplin JD, Robertson SA. Macrophages regulate expression of alpha1,2 fucosyltransferase genes in human endometrial epithelial cells. Mol Hum Reprod. 2011 doi: 10.1093/molehr/gar070. doi:10.1093/molehr/gar070. [DOI] [PubMed] [Google Scholar]
- 28.Wagers AJ, Kansas GS. Potent induction of alpha(1,3)-fucosyltransferase VII in activated CD4 + T cells by TGF-beta 1 through a p38 mitogen-activated protein kinase-dependent pathway. J Immunol. 2000;165(9):5011–5016. doi: 10.4049/jimmunol.165.9.5011. [DOI] [PubMed] [Google Scholar]
- 29.Schnyder-Candrian S, Borsig L, Moser R, Berger EG. Localization of alpha 1,3-fucosyltransferase VI in Weibel-Palade bodies of human endothelial cells. Proc Natl Acad Sci U S A. 2000;97(15):8369–8374. doi: 10.1073/pnas.97.15.8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallouin F, Goupille C, Bureau V, Meflah K, Le Pendu J. Increased tumorigenicity of rat colon carcinoma cells after alpha1, 2-fucosyltransferase FTA anti-sense cDNA transfection. Int J Cancer. 1999;80(4):606–611. doi: 10.1002/(sici)1097-0215(19990209)80:4<606::aid-ijc20>3.0.co;2-m. doi:10.1002/(SICI)1097-0215(19990209)80. [DOI] [PubMed] [Google Scholar]
- 31.Labarriere N, Piau JP, Otry C, Denis M, Lustenberger P, Meflah K, Le Pendu J. H blood group antigen carried by CD44V modulates tumorigenicity of rat colon carcinoma cells. Cancer Res. 1994;54(23):6275–6281. [PubMed] [Google Scholar]
- 32.Domino SE, Karnak DM, Hurd EA. Cell surface fucosylation does not affect development of colon tumors in mice with germline Smad3 mutation. Tumour Biol. 2007;28(2):77–83. doi: 10.1159/000099153. doi:10.1159/000099153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rusnati M, Presta M. Fibroblast growth factors/fibroblast growth factor receptors as targets for the development of anti-angiogenesis strategies. Curr Pharm Des. 2007;13(20):2025–2044. doi: 10.2174/138161207781039689. [DOI] [PubMed] [Google Scholar]
- 34.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071–1121. doi: 10.1152/physrev.00038.2010. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korc M, Friesel RE. The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets. 2009;9(5):639–651. doi: 10.2174/156800909789057006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatch NE, Hudson M, Seto ML, Cunningham ML, Bothwell M. Intracellular retention, degradation, and signaling of glycosylation-deficient FGFR2 and craniosynostosis syndrome-associated FGFR2C278F. J Biol Chem. 2006;281(37):27292–27305. doi: 10.1074/jbc.M600448200. doi:10.1074/jbc.M600448200. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Itoh S, Wang X, Isaji T, Miyoshi E, Kariya Y, Miyazaki K, Kawasaki N, Taniguchi N, Gu J. Deletion of core fucosylation on alpha3beta1 integrin down-regulates its functions. J Biol Chem. 2006;281(50):38343–38350. doi: 10.1074/jbc.M608764200. doi:10.1074/jbc.M608764200. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Gu J, Ihara H, Miyoshi E, Honke K, Taniguchi N. Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. J Biol Chem. 2006;281(5):2572–2577. doi: 10.1074/jbc.M510893200. doi:10.1074/jbc.M510893200. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Fukuda T, Li W, Gao CX, Kondo A, Matsumoto A, Miyoshi E, Taniguchi N, Gu J. Requirement of Fut8 for the expression of vascular endothelial growth factor receptor-2: a new mechanism for the emphysema-like changes observed in Fut8-deficient mice. J Biochem. 2009;145(5):643–651. doi: 10.1093/jb/mvp022. doi:10.1093/jb/mvp022. [DOI] [PubMed] [Google Scholar]
- 40.Murukesh N, Dive C, Jayson GC. Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br J Cancer. 2010;102(1):8–18. doi: 10.1038/sj.bjc.6605483. doi:10.1038/sj.bjc.6605483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sone H, Kawakami Y, Sakauchi M, Nakamura Y, Takahashi A, Shimano H, Okuda Y, Segawa T, Suzuki H, Yamada N. Neutralization of vascular endothelial growth factor prevents collagen-induced arthritis and ameliorates established disease in mice. Biochem Biophys Res Commun. 2001;281(2):562–568. doi: 10.1006/bbrc.2001.4395. [DOI] [PubMed] [Google Scholar]