Summary
Background
Gene-based warfarin dosing algorithms have largely been developed in homogeneous populations, and their generalizability has not been established.
Objectives
We sought to assess the performance of published algorithms in a racially diverse and multiethnic sample, and determine if additional clinical variables or genetic variants associated with dose could enhance algorithm performance.
Patients and methods
In 145 compliant patients on warfarin with a goal international normalized ratio (INR) of 2–3, stable, therapeutic doses were compared with predicted doses using 12 reported algorithms that incorporated CYP2C9 and VKORC1 variants. Additional covariates tested with each model included race, concurrent medications, medications known to interact with warfarin and previously described CYP4F2, CALU and GGCX variants.
Results
The mean patient age was 67 ± 14 years; 90 (62%) were male. Eighty-two (57%) were Caucasian, 28 (19%) African-American, 20 (14%) Hispanic and 15 (10%) Asian. The median warfarin dose was 35 mg per week (interquartile range 23–53 mg per week). Gene-based dosing algorithms explained 37–55% of the variation in warfarin dose requirements. Neither the addition of race, number of concurrent medications nor the number of concurrent medications interacting with warfarin enhanced algorithm performance. Similarly, consideration of CYP4F2, CALU or GGCX variant genotypes did not improve algorithms.
Conclusions
Existing gene-based dosing algorithms explained between approximately one-third and one-half of the variability in warfarin dose requirements in this racially and ethnically diverse cohort. Additional clinical and recently described genetic variants associated with warfarin dose did not enhance prediction in our patient population.
Keywords: CYP2C9, CYP4F2, pharmacogenetics, prediction, VKORC1, warfarin
Warfarin is a highly effective oral anticoagulant that is increasingly prescribed in the United States [1]. Its narrow therapeutic window is a significant drawback limiting its use [2]. Indeed, a substantial proportion of adverse drug reactions are attributed to warfarin [3]. Therapeutic warfarin dose requirements are affected by numerous factors, including age, weight, height, comorbid medical conditions, concurrent medications, diet and compliance [4].
Common polymorphic variants and rare coding region mutations in two genes have been shown to significantly alter warfarin dose requirements. One of these, CYP2C9, encodes a cytochrome P450 enzyme responsible for the majority of warfarin clearance [5]. The other, VKORC1, encodes a vitamin K epoxide reductase subunit, the direct target of warfarin that is essential for the regeneration of reduced vitamin K during synthesis of several coagulation factors [6].
These discoveries have prompted calls for testing individuals for variants in these genes prior to initiating warfarin therapy [7], leading to several gene-based dosing algorithms aimed at improving efficacy and reducing adverse effects that occur during traditional anticoagulant therapy guided by clinical variables. Warfarin dose requirements often vary between races [8-10], yet published algorithms were developed largely in racially homogenous populations and their generalizability has not been established.
We therefore sought to assess the performance of published gene-based algorithms in a racially diverse, multiethnic population. Furthermore, we sought to determine if the consideration of race or ethnicity, or genotypes for several additional nucleotide variants previously associated with altered warfarin dose requirements [11-17], affect the performance of these algorithms.
Methods
Study sample
Between July 2007 and June 2009, adult patients taking warfarin and managed at an institutional anticoagulation clinic either at The Mount Sinai Hospital or Elmhurst Hospital in New York City, or referred to the study investigators, were assessed for enrollment. Subjects were included in the analysis if the target international normalized ratio (INR) was between 2.0 and 3.0 and at least two consecutive laboratory measurements with INR measurements separated by at least 1 week were within the therapeutic range. Thus, the study sample was comprised of patients with established warfarin dose requirements. Prior to participation, all subjects provided written informed consent approved by the institutional review board governing research involving human subjects.
Study protocol
Subjects underwent standardized interviews for collection of relevant demographic and clinical characteristics and history of adverse events during anticoagulant therapy. A four-item binary compliance questionnaire validated for the use of antihypertensive therapy was adapted for this study and administered to all subjects (Table S1) [18]. Doses of warfarin were abstracted from a computerized database or medical records. The average therapeutic dose was calculated as the mean weekly dose of warfarin during the past 2 weeks in which two INR values at least a week apart were within the goal range. When the average therapeutic dose was not available, the patient’s reported weekly dose was substituted.
Race or ethnicity was assessed by self-report and classified as Caucasian, African-American, non-African-American Hispanic, Asian or other. Concurrent medications were classified as inhibitors or potentiators of warfarin if they were listed as having established or probable, and major or moderate interactions with warfarin according to Micromedex® [19].
Genotyping
In total, we genotyped 23 variant alleles in genes previously associated with warfarin dosing. The CYP2C9 allele designations refer to those defined by the Cytochrome P450 Allele Nomenclature Committee (http://www.cypalleles.ki.se). Genotyping of six CYP2C9 alleles (*1, *2, *3, *4, *5, *6) and seven VKORC1 nucleotide variants (g.-1639G > A, g.85G > T [p.V29L], g.121G > T [p.A41S], g.134T > C [p.V45A], g.172A > G [p.R58G], g.1331G > A [p.V66M], g.3487T > G [p.L128R]) was performed using the Tag-It™ Mutation Detection Kit (Luminex Molecular Diagnostics, Toronto, ON, Canada) according to the manufacturer’s instructions. The CYP2C9 and VKORC1 genotypes were determined using Tag-It™ Data Analysis Software (Luminex Molecular Diagnostics) and the wild-type CYP2C9*1 allele was assigned in the absence of other detectable variant alleles. CYP2C9*11 and the VKORC1 (Accession Number AY587020) g.-4931T > C (381T > C), g.1173C > T (6484C > T), g.2255C > T (7566C > T) and g.3730G > A (9041G > A) alleles were genotyped as previously described [15,20]. Additionally, the VKORC1 g.106G > T (p.D36Y) and g.698C > T (6009C > T), CYP4F2*3 (c.1297G > A; p.V433M) and g.7222002G > A (rs2189784), CALU (c.11G > A; p.R4Q) and GGCX (g.64593573C > G; rs11676382) variants were analyzed [11-14,17,21,22]. VKORC1 p.D36Y and g.698C > T were genotyped as previously described [15] and CYP4F2 (*3 and rs2189784), CALU and GGCX by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) using the primers listed in Table S2 and PvuII, BssSI, HpyCH4V and HindIII (New England Biolabs, Ipswich, MA, USA), respectively. All digested PCR products were visualized by agarose gel electrophoresis and respective control samples were confirmed by bidirectional sequencing using amplification primers and subsequently used as rotating positive controls.
Statistical analysis
Concordance between calculated average therapeutic warfarin dose and self-reported weekly dose was tested using Spearman’s rank-sum correlation coefficient. Average therapeutic warfarin doses were compared with predicted dose requirements using 12 previously reported multiple linear regression algorithms accounting for clinical variables and variants in CYP2C9 and VKORC1 [23-34]. Identified CYP2C9 variants that were not included in respective algorithms (e.g. CYP2C9*5) were designated as *1, in keeping with the original genotype classification utilized during derivation of these algorithms. Predicted weekly doses were generated from each algorithm.
All analyses were performed with R v2.9.2 [35]. Associations between average therapeutic dose and CYP2C9 and VKORC1 genotypes, race and concurrent medications were assessed using the Kruskal–Wallis, Wilcoxon rank-sum or Spearman’s rank-sum methods as appropriate for continuous variables. The associations between categorical variables were evaluated using Fisher’s exact test. Algorithm-derived gene-based warfarin doses were evaluated for association with average therapeutic warfarin dose using the global F-test. The proportion of variance explained by each algorithm (R2) was calculated. As the R2 increases with an increasing number of variables in a given linear regression model irrespective of whether additional variables significantly explain variance in the outcome, we compared different algorithms with one another using the adjusted R2 statistic, which adjusts for the number of variables in the model, allowing comparison of models with different numbers of terms.
where n = number of subjects and k = number of predictors. Race and concurrent medications were added as covariates to regression models that did not already include these variables to determine their incremental contribution to algorithm performance. Other added covariates included treatment with any concomitant warfarin inhibitors or potentiators, or the additive genetic effects of genotyped VKORC1 variants not included in the respective algorithm, CYP4F2, CALU or GGCX variant genotypes. Covariates were added separately and assessed for their additive contribution to algorithm performance by calculating the change in the adjusted R2 statistic, where increases indicated enhanced predictive ability, null values indicated no enhancement and negative values indicated worse prediction. The associations between added covariates and warfarin dose requirements, adjusted for other variables in the algorithms, were assessed by partial F-tests. All P-values are two-sided, with significance thresholds set at 0.05. The study has 80% statistical power to detect the partial R2 of 0.08.
Results
Of the 156 subjects enrolled, compliance information for one subject was unavailable, and 10 subjects answered affirmatively to at least two questions on the compliance assessment; these subjects were therefore not included in the primary analysis. Of the remaining 145 subjects, the mean age was 67 ± 14 years; 90 (62%) were male (Table 1). Eighty-two subjects (57%) were Caucasian, 28 (19%) African-American, 20 (14%) Hispanic and 15 (10%) Asian. The most frequent indications for anticoagulation were atrial fibrillation in 106 patients (73%) and venous thromboembolism in 21 patients (15%).
Table 1.
Subject characteristics
| Variable | |
|---|---|
| Age (years) | 67 ± 14 |
| Male | 90 (62) |
| Race | |
| Caucasian | 82 (57) |
| African-American | 28 (19) |
| Hispanic | 20 (14) |
| Asian | 15 (10) |
| Body mass index (kg m−2) | 29 ± 7 |
| Smoker | 6 (4) |
| Primary indication for anticoagulation | |
| Atrial fibrillation | 106 (73) |
| DVT or PE | 21 (15) |
| Other | 18 (12) |
| Number of concurrent medications | 5.7 ± 2.9 |
| Number of concurrent medications that interact with warfarin | |
| 0 | 46 (32) |
| 1 | 56 (39) |
| ≥ 2 | 43 (30) |
| Mean therapeutic warfarin dose (mg per week) | 40 ± 26 |
| Median therapeutic warfarin dose (mg per week) | 35 (23, 53) |
| Non-compliance score | |
| 0 | 132 (91) |
| 1 | 13 (9) |
DVT, deep vein thrombosis; PE, pulmonary embolism.
Total number of subjects = 145. Data presented as mean ± SD, median (interquartile range), or n (%).
The median therapeutic warfarin dose was 35 mg per week (interquartile range 23–53 mg per week) and was highly correlated with the self-reported weekly warfarin dose (Spearman’s r = 0.91, P < 0.0001). On average, subjects were taking 5.7 ± 2.9 concurrent medications. Fifty-six subjects (39%) were taking one and 43 (30%) at least two medications that potentially interact with warfarin. The five most frequent medications potentially interacting with warfarin were simvastatin (n = 25), levothyroxine (n = 18), acetaminophen (n = 17), amiodarone (n = 7) and esomeprazole (n = 10). Five subjects reported clinically significant bleeding events during warfarin treatment, two requiring transfusions of blood products. One of these bleeding events occurred within the first year of therapy.
Genotype frequencies for the remaining subjects are displayed in Table 2. Warfarin doses differed significantly across genotypes for identified CYP2C9 (P = 0.03) and VKORC1 (P < 0.001) variants (Fig. 1), with the exception of VKORC1 g.106G > T (p.D36Y, P = 0.27) and g.698C > T (P = 0.27). However, the small number of subjects with the g.106G > T (n = 5) and g.698C > T (n = 22) variants may limit meaningful comparisons between genotype groups. Notably, warfarin doses did not significantly vary according to CYP4F2*3 (P = 0.15), CYP4F2-rs2189784 (P = 0.49), CALU (P = 0.16) or GGCX (P = 0.80) genotype (Fig. 1). Although differences in warfarin dose requirements according to CYP4F2*3, CYP4F2-rs2189784, CALU and GGCX genotype have been described primarily in Caucasians, we did not identify differences when restricting analyses to this racial group (P = 0.25, 0.29, 0.23 and 0.65, respectively). Similarly, warfarin dose did not differ significantly according to race/ethnicity (P = 0.17) (Fig. 2). Genotype frequencies differed according to race/ethnicity for VKORC1 g.-4931T > C (P = 0.04), VKORC1 g.-1639G > A (P < 0.001), VKORC1 g.1173C > T (P < 0.001), VKORC1 g.2255C > T (P < 0.001) and CYP4F2*3 (P < 0.04) (Table S3). Thirty-nine subjects (27%) classified themselves as Ashkenazi Jewish, all of whom were Caucasian. Two of these individuals carried the VKORC1 g.106G > T (p.D36Y) variant.
Table 2.
Genotype frequencies
| Nucleotide variant | Genotype | Frequency (n, %) |
|---|---|---|
| CYP2C9 | *1/*1 | 105 (72) |
| *1/*2 | 21 (15) | |
| *1/*3 | 16 (11) | |
| *1/*5 | 2 (1) | |
| *2/*2 | 1 (1) | |
| VKORC1 g.-4931T > C | TT | 44 (30) |
| CT | 73 (50) | |
| CC | 28 (19) | |
| VKORC1 g.-1639G > A | GG | 68 (47) |
| GA | 56 (39) | |
| AA | 21 (15) | |
| VKORC1 g.106G > T (p.D36Y) | GG | 140 (97) |
| GT | 5 (3) | |
| TT | 0 (0) | |
| VKORC1 g.698C > T | CC | 114 (78) |
| CT | 30 (21) | |
| TT | 1 (1) | |
| VKORC1 g.1173C > T | CC | 68 (47) |
| CT | 54 (37) | |
| TT | 23 (16) | |
| VKORC1 g.1331G > A (p.V66M) | GG | 144 (99) |
| GA | 1 (1) | |
| AA | 0 (0) | |
| VKORC1 g.2255C > T | CC | 57 (39) |
| CT | 63 (43) | |
| TT | 25 (17) | |
| VKORC1 g.3730G > A | GG | 48 (33) |
| GA | 75 (52) | |
| AA | 22 (15) | |
| CYP4F2*3 (p.V433M) | GG | 80 (55) |
| GA | 53 (37) | |
| AA | 12 (8) | |
| CYP4F2-rs2189784 | GG | 57 (39) |
| GA | 67 (46) | |
| AA | 21 (15) | |
| CALU c.11G > A (p.R4Q) | GG | 95 (65) |
| GA | 43 (30) | |
| AA | 7 (5) | |
| GGCX-rs11676382 | CC | 135 (93) |
| GC | 9 (6) | |
| GG | 1 (1) |
Genotypes for compliant subjects are displayed (n = 145). No CYP2C9*4, *6, *11 or VKORC1 g.85G > T, g.121G > T, g.134T > C, g.172A > G, or g.3487T > G variants were identified.
Fig. 1.
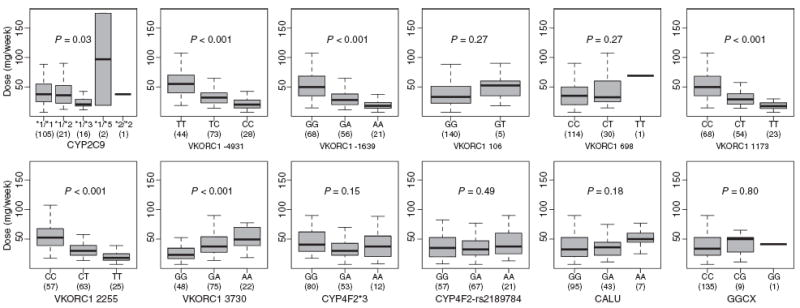
Therapeutic warfarin dose according to CYP2C9, VKORC1, CYP4F2, CALU and GGCX genotype. Weekly therapeutic warfarin doses were compared across genotypes. The boxes display the interquartile range of warfarin doses. Whiskers represent observed doses beyond this range with values less than or equal to 1.5 times the interquartile range. The median dose is indicated by the solid black bar. The number of individuals with each genotype is displayed in parenthesis.
Fig. 2.

Therapeutic warfarin dose according to race. Weekly therapeutic warfarin doses were compared across races. The boxes display the inter-quartile range of warfarin doses. Whiskers represent observed doses beyond this range with values less than or equal to 1.5 times the interquartile range. The median dose is indicated by the solid black bar.
The number of concurrent medications was inversely associated with the average therapeutic warfarin dose (Spearman’s r = −0.25, P = 0.002). Subjects taking at least one potentiator of the anticoagulant effect (n = 88) had lower warfarin dose requirements than those taking no potentiators, although the association was not significant [(median, inter-quartile range) 33, 23–44 mg per week for those on at least one warfarin potentiator vs. 40, 23–55 mg per week for those receiving no potentiator, P = 0.19]. Only five subjects were receiving warfarin inhibitors, too few to permit meaningful comparisons.
Performance of gene-based warfarin dosing algorithms
Characteristics of the selected warfarin dosing algorithms are displayed in Tables S4 and S5. The performance of the 12 tested gene-based warfarin dosing algorithms is displayed in Tables 3 and 4. All the tested algorithms explained variation in warfarin dose requirements better than chance (P < 0.001 for each). Overall, they accounted for 37–55% of the observed variance in warfarin dose requirements.
Table 3.
Performance of gene-based warfarin dosing algorithms alone and with race or number of concurrent medications added as covariates
| Algorithm | Original algorithm
|
Race added*
|
No. of concurrent medications added
|
|||
|---|---|---|---|---|---|---|
| R2 | Adj. R2 | Δ adj. R2 | P value race | Δ adj. R2 | P value medications | |
| Wu 2008 [31] | 0.55 | 0.50 | – | – | 0 | 0.67 |
| Gage 2008 [30] | 0.54 | 0.51 | – | – | 0 | 0.46 |
| Anderson 2007 [27] | 0.52 | 0.50 | 0.01 | 0.11 | 0.01 | 0.43 |
| Herman 2006 [34] | 0.52 | 0.50 | 0 | 0.19 | 0 | 0.36 |
| Zhu 2007 [29] | 0.51 | 0.49 | 0.01 | 0.11 | 0 | 0.34 |
| IWPC 2009 [33] | 0.50 | 0.45 | – | – | 0 | 0.80 |
| Tham 2006 [26] | 0.48 | 0.46 | −0.01 | 0.78 | 0 | 0.63 |
| Wadelius 2009 [32] | 0.46 | 0.43 | 0 | 0.26 | 0.01 | 0.96 |
| Wadelius 2005 [24] | 0.46 | 0.42 | 0.01 | 0.29 | 0 | 0.78 |
| Sconce 2005 [23] | 0.45 | 0.44 | 0 | 0.22 | −0.01 | 0.71 |
| Takahashi 2006 [25] | 0.38 | 0.35 | 0 | 0.65 | 0 | 0.87 |
| Miao 2007 [28] | 0.37 | 0.35 | −0.01 | 0.70 | 0.01 | 0.76 |
Table 4.
Performance of gene-based warfarin dosing algorithms alone and with selected VKORC1, CYP4F2 or CALU variants added as covariates
| Algorithm | Original algorithm
|
VKORC1 g.698C > T added
|
VKORC1 g.2255C > T added*
|
VKORC1 g.3730G > A added†
|
CYP4F2*3 added
|
CYP4F2-rs2189784 added
|
CALU p.R4Q added
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Adj. R2 | Δ adj. R2 | P value | Δ adj. R2 | P value | Δ adj. R2 | P value | Δ adj. R2 | P value | Δ adj. R2 | P value | Δ adj. R2 | P value | |
| Wu 2008 [31] | 0.55 | 0.50 | 0 | 0.28 | – | – | 0 | 0.30 | 0 | 0.58 | 0 | 0.78 | 0 | 0.56 |
| Gage 2008 [30] | 0.54 | 0.51 | 0 | 0.41 | 0 | 0.60 | 0 | 0.21 | 0 | 0.70 | 0 | 0.88 | 0 | 0.63 |
| Anderson 2007 [27] | 0.52 | 0.50 | −0.01 | 0.45 | −0.01 | 0.63 | 0 | 0.13 | −0.01 | 0.44 | −0.01 | 0.72 | 0 | 0.28 |
| Herman 2006 [34] | 0.52 | 0.50 | 0 | 0.41 | −0.01 | 0.92 | – | – | −0.01 | 0.54 | −0.01 | 0.83 | 0 | 0.39 |
| Zhu 2007 [29] | 0.51 | 0.49 | 0 | 0.50 | 0 | 0.40 | 0 | 0.09 | 0 | 0.37 | −0.01 | 0.63 | 0 | 0.28 |
| IWPC 2009 [33] | 0.50 | 0.45 | 0 | 0.47 | 0 | 0.71 | 0 | 0.41 | 0 | 0.99 | 0 | 0.65 | 0 | 0.99 |
| Tham 2006 [26] | 0.48 | 0.46 | 0 | 0.32 | 0.01 | 0.05 | 0 | 0.32 | −0.01 | 0.96 | −0.01 | 0.59 | 0 | 0.45 |
| Wadelius 2009 [32] | 0.46 | 0.43 | 0 | 0.42 | −0.01 | 0.91 | 0 | 0.25 | 0 | 0.65 | −0.01 | 0.93 | 0 | 0.63 |
| Wadelius 2005 [24] | 0.46 | 0.42 | −0.01 | 0.98 | 0 | 0.44 | 0 | 0.72 | 0 | 0.99 | 0 | 0.43 | 0 | 0.78 |
| Sconce 2005 [23] | 0.45 | 0.44 | −0.01 | 0.45 | −0.01 | 0.93 | 0 | 0.24 | −0.01 | 0.54 | −0.01 | 0.86 | −0.01 | 0.53 |
| Takahashi 2006 [25] | 0.38 | 0.35 | 0 | 0.55 | 0.01 | 0.21 | 0 | 0.55 | 0 | 0.99 | 0 | 0.68 | 0 | 0.82 |
| Miao 2007 [28] | 0.37 | 0.35 | −0.01 | 0.60 | 0 | 0.21 | −0.01 | 0.62 | −0.01 | 0.83 | −0.01 | 0.58 | −0.01 | 0.78 |
Contribution of additional covariates to performance of gene-based warfarin dosing algorithms
To determine if race or the number of concurrent medications improved performance, these covariates were added separately to each algorithm that lacked these variables. Adjustment for race increased the proportion of variance in observed therapeutic warfarin doses explained by each algorithm by up to 3%, although the resulting change in the adjusted R2 statistic ranged only from −0.01 to 0.01, demonstrating that the inclusion of race resulted in essentially no enhancement in the predictive value for each algorithm achieved (Table 3). Furthermore, race was not significantly associated with warfarin dose after controlling for the other variables in each algorithm.
The incorporation of the number of concurrent medications did not improve the performance of the algorithms, with changes in the adjusted R2 statistic ranging from −0.01 to 0.01 (Table 3). The association between the number of concurrent medications and warfarin dose was not significant after controlling for the other variables in each of the algorithms. Furthermore, stratification of concurrent medications as warfarin potentiators or inhibitors did not improve the performance of the models.
The addition of VKORC1 g.698C > T, g.2255C > T or g.3730G > A genotypes to algorithms that did not already include these covariates did not enhance their performance, nor did inclusion of CYP4F2 or CALU genotype (Table 4). The inclusion of VKORC1 p.D36Y or GGCX genotype also did not alter algorithm performance, although data are not displayed owing to the small number of subjects with these variants (n = 5 and n = 10, respectively).
The results of the analyses were essentially unaffected by the inclusion of the 11 individuals excluded from the primary analysis owing to non-compliance or missing compliance information (total n = 156).
Discussion
Published gene-based warfarin dosing algorithms explained between approximately one-third and one-half of the observed variance in warfarin dose requirements in this group of 145 highly compliant patients on stable anticoagulant therapy. Our independent validation of 12 dosing algorithms demonstrates that, while heterogeneity in algorithm performance exists, published algorithms explain a large proportion of variance in warfarin dose requirements. In the present study, neither the addition of race nor concurrent medications to algorithms that did not already include these variables significantly enhanced performance. Similarly, neither the addition of nucleotide variants previously reported to be associated with warfarin dose that were not included in the original algorithms (i.e. VKORC1 p.D36Y, VKORC1 g.698C > T, CYP4F2*3, CYP4F2-rs2189784, CALU p.R4Q, and GGCX-rs11676382), nor the inclusion of variants included in some but not all algorithms (i.e. VKORC1 g.2255C > T and g.3730G > A) enhanced algorithm performance.
The performance of algorithms observed in the present study is reinforced by three other studies that examined fewer algorithms [31,36,37]. Few published pharmacogenetic algorithms that include CYP2C9 and VKORC1 variants have been evaluated in multiracial and multiethnic patient populations. Additional variants within the vitamin K and warfarin metabolic pathways have been identified that may influence interindividual variation in warfarin dosing, yet these associations have not been widely examined in non-Caucasian patients. Thus, we sought to assess the performance of 12 published algorithms in a racially diverse and multiethnic patient cohort from the New York metropolitan area. To our knowledge, this is the first study to thoroughly evaluate this collection of dosing algorithms and to determine if their performance was enhanced by the addition of other clinical variables or recently described pharmacogenetic variants.
While it has long been recognized that race influences warfarin dose requirements [8-10] and therefore potentially affects dose prediction, race has only recently been incorporated as a variable into algorithms proposed for clinical use [38]. The fact that the addition of race/ethnicity did not improve performance implies that its influence on warfarin dose requirements is already captured by clinical or genetic variables included in the algorithms. Alternatively, our analysis may have been underpowered to detect such an effect. Further studies will be needed to validate our findings, although a sample size of 1084 individuals would be required to achieve statistical significance based on the observed effect size. In addition, neither the number of concurrent medications nor whether these specifically interact with warfarin contributed significantly to algorithm performance, suggesting that their influence on warfarin dose requirements is also captured by existing algorithms.
Interestingly, the addition of the VKORC1 p.D36Y genotype, recently associated with warfarin doses greater than 10 mg per day among Ashkenazi Jewish individuals [12], did not enhance the performance of established algorithms, although a trend towards higher dose requirements was observed among carriers. This is probably because of the small number of carriers in our sample. Similarly, while CYP4F2*3 and CYP4F2-rs2189784 have recently been associated with warfarin dosing [13,14,21,39], the modest effect of these alleles and their lower frequency among the diverse racial and ethnic groups in our study sample may explain why they were not associated with warfarin dose. Similar factors may underlie the lack of association between warfarin dose and variants in CALU (p.R4Q) and GGCX (rs11676382), which were previously associated with higher and lower doses, respectively [16,17]. The present findings suggest that these alleles have limited ability to facilitate prediction of stable warfarin doses in a diverse patient population, after consideration of clinical variables and common variants in CYP2C9 and VKORC1 known to predict dose.
Given that variants associated with warfarin dose differ according to race, it may be most efficient in clinical practice to prioritize genotyping of variants with substantial effects if they have a high prevalence in the patient population being treated. For example, consideration of the VKORC1 p.D36Y genotype may be warranted when treating individuals of Ashkenazi Jewish descent. Whether consideration of this variant would substantially improve performance of published algorithms in a population with a high prevalence of the p.D36Y allele merits further investigation.
In addition, one African-American patient was identified in our cohort who carried the VKORC1 p.V66M (g.1331G > A) variant which is consistently associated with warfarin ‘resistance’ [40-42] and has a carrier frequency of ~1 in 100 in this racial group [20]. Our patient had a mean therapeutic dose of 165 mg per week, suggesting that although the allele is rare, its inclusion into genotyping panels, if cost-effective, may be clinically useful. Moreover, CYP2C9*5 (p.D360E) causes impaired warfarin hydroxylation [43,44] and is more prevalent among African-American and Hispanic individuals than Caucasians and Asians [20,45]. Given the fact that it is not currently included in any published warfarin dosing algorithm, the two individuals in our cohort identified as CYP2C9*1/*5 heterozygotes were treated as *1/*1 in the algorithm analysis, which probably influenced the accuracy of dose prediction. Thus, for African-American and Hispanic patients, this allele might be a candidate for inclusion in pharmacogenetic warfarin dosing algorithms.
Our findings are generalizable only to individuals with characteristics similar to those in the present study sample. We had limited power to detect differences in warfarin dose requirements among Hispanics and Asians, and we did not adjust for dietary vitamin K intake, which may alter warfarin dose requirements [4] and may theoretically differ across racial groups. Other variants that may be associated with variability in warfarin dose were not genotyped [46]. In addition, while we ruled out large effects of additionally tested factors on dose prediction, we cannot rule out smaller effects. Future studies with larger sample sizes will be needed to support or refute our findings.
In conclusion, independent validation demonstrates that published gene-based warfarin dosing algorithms account for between approximately one-third and one-half of the observed variance in warfarin dose requirements in a racially and ethnically diverse patient population. Ongoing studies, such as the Clarification of Optimal Warfarin Dosing through Genetics (COAG) trial (clinicaltrials.gov identifier NCT00839657), may help establish whether genotype-guided warfarin dosing improves control during the initiation of anticoagulation in clinical practice. Whether the inclusion of other variables that affect warfarin dose requirements can improve the performance of pharmacogenetic dosing algorithms is fertile ground for further study.
Addendum
S. A. Lubitz: Study conception and design, data analysis and interpretation, drafting and critical revision of the manuscript. S. A. Scott: Study conception and design, data analysis and interpretation, drafting and critical revision of the manuscript. E. B. Rothlauf: Data acquisition, critical revision of the manuscript. A. Agarwal: Data acquisition, critical revision of the manuscript. I. Peter: Data interpretation, critical revision of the manuscript. D. Doheny: Study conception and design, data acquisition, critical revision of the manuscript. S. van der Zee: Data interpretation, critical revision of the manuscript. M. Jaremko: Data interpretation, critical revision of the manuscript. C. Yoo: Data acquisition, critical revision of the manuscript. R.J. Desnick: Study conception and design, critical revision of the manuscript. J. L. Halperin: Study conception and design, critical revision of the manuscript.
Supplementary Material
Acknowledgments
We are grateful to J. Meller for assistance with patient recruitment. This research was supported in part by an NIH Cooperative Study grant (N268200800003C) and an NIH grant (5 M01 RR00071) from the Division of Research Resources for the Mount Sinai General Clinical Research Center. S. A. Scott and M. Jaremko were the recipients of Biochemical/Molecular Genetics Fellowships from the Genzyme Corporation.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
Additional Supporting Information may be found in the online version of this article:
Table S1. Adapted warfarin compliance questionnaire.
Table S2. Primer Sequences for PCR Amplification.
Table S3. Genotype frequencies according to race.
Table S4. Characteristics of selected gene-based warfarin dosing algorithms.
Table S5. Selected warfarin gene-based dosing algorithms.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007;167:1414–9. doi: 10.1001/archinte.167.13.1414. [DOI] [PubMed] [Google Scholar]
- 2.van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ. Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest. 2006;129:1155–66. doi: 10.1378/chest.129.5.1155. [DOI] [PubMed] [Google Scholar]
- 3.Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006;296:1858–66. doi: 10.1001/jama.296.15.1858. [DOI] [PubMed] [Google Scholar]
- 4.Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. J Am Coll Cardiol. 2003;41:1633–52. doi: 10.1016/s0735-1097(03)00416-9. [DOI] [PubMed] [Google Scholar]
- 5.Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, Blough DK, Thummel KE, Veenstra DL, Rettie AE. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285–93. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 6.Higashi MK, Veenstra DL, Kondo LM, Wittkowsky AK, Srinouanprachanh SL, Farin FM, Rettie AE. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002;287:1690–8. doi: 10.1001/jama.287.13.1690. [DOI] [PubMed] [Google Scholar]
- 7.FDA Approves Updated Warfarin (Coumadin) Prescribing Information. 2007 [Google Scholar]
- 8.Absher RK, Moore ME, Parker MH. Patient-specific factors predictive of warfarin dosage requirements. Ann Pharmacother. 2002;36:1512–7. doi: 10.1345/aph.1C025. [DOI] [PubMed] [Google Scholar]
- 9.Blann A, Hewitt J, Siddiqui F, Bareford D. Racial background is a determinant of average warfarin dose required to maintain the INR between 2.0 and 3.0. Br J Haematol. 1999;107:207–9. doi: 10.1046/j.1365-2141.1999.01672.x. [DOI] [PubMed] [Google Scholar]
- 10.Dang MT, Hambleton J, Kayser SR. The influence of ethnicity on warfarin dosage requirement. Ann Pharmacother. 2005;39:1008–12. doi: 10.1345/aph.1E566. [DOI] [PubMed] [Google Scholar]
- 11.Borgiani P, Ciccacci C, Forte V, Sirianni E, Novelli L, Bramanti P, Novelli G. CYP4F2 genetic variant (rs2108622) significantly contributes to warfarin dosing variability in the Italian population. Pharmacogenomics. 2009;10:261–6. doi: 10.2217/14622416.10.2.261. [DOI] [PubMed] [Google Scholar]
- 12.Loebstein R, Dvoskin I, Halkin H, Vecsler M, Lubetsky A, Rechavi G, Amariglio N, Cohen Y, Ken-Dror G, Almog S, Gak E. A coding VKORC1 Asp36Tyr polymorphism predisposes to warfarin resistance. Blood. 2007;109:2477–80. doi: 10.1182/blood-2006-08-038984. [DOI] [PubMed] [Google Scholar]
- 13.Caldwell MD, Awad T, Johnson JA, Gage BF, Falkowski M, Gardina P, Hubbard J, Turpaz Y, Langaee TY, Eby C, King CR, Brower A, Schmelzer JR, Glurich I, Vidaillet HJ, Yale SH, Qi Zhang K, Berg RL, Burmester JK. CYP4F2 genetic variant alters required warfarin dose. Blood. 2008;111:4106–12. doi: 10.1182/blood-2007-11-122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi F, McGinnis R, Bourgeois S, Barnes C, Eriksson N, Soranzo N, Whittaker P, Ranganath V, Kumanduri V, McLaren W, Holm L, Lindh J, Rane A, Wadelius M, Deloukas P. A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet. 2009;5:e1000433. doi: 10.1371/journal.pgen.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott SA, Edelmann L, Kornreich R, Desnick RJ. Warfarin pharmacogenetics: CYP2C9 and VKORC1 genotypes predict different sensitivity and resistance frequencies in the Ashkenazi and Sephardi Jewish populations. Am J Hum Genet. 2008;82:495–500. doi: 10.1016/j.ajhg.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vecsler M, Loebstein R, Almog S, Kurnik D, Goldman B, Halkin H, Gak E. Combined genetic profiles of components and regulators of the vitamin K-dependent gamma-carboxylation system affect individual sensitivity to warfarin. Thromb Haemost. 2006;95:205–11. doi: 10.1267/THRO06020205. [DOI] [PubMed] [Google Scholar]
- 17.Rieder MJ, Reiner AP, Rettie AE. Gamma-glutamyl carboxylase (GGCX) tagSNPs have limited utility for predicting warfarin maintenance dose. J Thromb Haemost. 2007;5:2227–34. doi: 10.1111/j.1538-7836.2007.02744.x. [DOI] [PubMed] [Google Scholar]
- 18.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Micromedex Healthcare Series. Greenwood Village, Colo: Thomson Healthcare; DRUGDEX® System [Internet database] [Google Scholar]
- 20.Scott SA, Jaremko M, Lubitz SA, Kornreich R, Halperin JL, Desnick RJ. CYP2C9*8 is Prevalent in African-Americans: Implications for Pharmacogenetic Dosing. Pharmacogenomics. 2009;10:1243–55. doi: 10.2217/pgs.09.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang JE, Jorgensen AL, Alfirevic A, Williamson PR, Toh CH, Park BK, Pirmohamed M. Effects of CYP4F2 genetic polymorphisms and haplotypes on clinical outcomes in patients initiated on warfarin therapy. Pharmacogenet Genomics. 2009;19:781–9. doi: 10.1097/FPC.0b013e3283311347. [DOI] [PubMed] [Google Scholar]
- 22.Osman A, Enstrom C, Arbring K, Soderkvist P, Lindahl TL. Main haplotypes and mutational analysis of vitamin K epoxide reductase (VKORC1) in a Swedish population: a retrospective analysis of case records. J Thromb Haemost. 2006;4:1723–9. doi: 10.1111/j.1538-7836.2006.02039.x. [DOI] [PubMed] [Google Scholar]
- 23.Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP, Wood P, Kesteven P, Daly AK, Kamali F. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005;106:2329–33. doi: 10.1182/blood-2005-03-1108. [DOI] [PubMed] [Google Scholar]
- 24.Wadelius M, Chen LY, Downes K, Ghori J, Hunt S, Eriksson N, Wallerman O, Melhus H, Wadelius C, Bentley D, Deloukas P. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J. 2005;5:262–70. doi: 10.1038/sj.tpj.6500313. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi H, Wilkinson GR, Nutescu EA, Morita T, Ritchie MD, Scordo MG, Pengo V, Barban M, Padrini R, Ieiri I, Otsubo K, Kashima T, Kimura S, Kijima S, Echizen H. Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese, Caucasians and African-Americans. Pharmacogenet Genomics. 2006;16:101–10. doi: 10.1097/01.fpc.0000184955.08453.a8. [DOI] [PubMed] [Google Scholar]
- 26.Tham LS, Goh BC, Nafziger A, Guo JY, Wang LZ, Soong R, Lee SC. A warfarin-dosing model in Asians that uses single-nucleotide polymorphisms in vitamin K epoxide reductase complex and cytochrome P450 2C9. Clin Pharmacol Ther. 2006;80:346–55. doi: 10.1016/j.clpt.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JL, Horne BD, Stevens SM, Grove AS, Barton S, Nicholas ZP, Kahn SF, May HT, Samuelson KM, Muhlestein JB, Carlquist JF. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007;116:2563–70. doi: 10.1161/CIRCULATIONAHA.107.737312. [DOI] [PubMed] [Google Scholar]
- 28.Miao L, Yang J, Huang C, Shen Z. Contribution of age, body weight, and CYP2C9 and VKORC1 genotype to the anticoagulant response to warfarin: proposal for a new dosing regimen in Chinese patients. Eur J Clin Pharmacol. 2007;63:1135–41. doi: 10.1007/s00228-007-0381-6. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y, Shennan M, Reynolds KK, Johnson NA, Herrnberger MR, Valdes R, Jr, Linder MW. Estimation of warfarin maintenance dose based on VKORC1 (-1639 G> A) and CYP2C9 genotypes. Clin Chem. 2007;53:1199–205. doi: 10.1373/clinchem.2006.078139. [DOI] [PubMed] [Google Scholar]
- 30.Gage B, Eby C, Johnson J, Deych E, Rieder M, Ridker P, Milligan P, Grice G, Lenzini P, Rettie A, Aquilante C, Grosso L, Marsh S, Langaee T, Farnett L, Voora D, Veenstra D, Glynn R, Barrett A, McLeod H. Use of Pharmacogenetic and Clinical Factors to Predict the Therapeutic Dose of Warfarin. Clin Pharmacol Ther. doi: 10.1038/clpt.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu AH, Wang P, Smith A, Haller C, Drake K, Linder M, Valdes R., Jr Dosing algorithm for warfarin using CYP2C9 and VKORC1 genotyping from a multi-ethnic population: comparison with other equations. Pharmacogenomics. 2008;9:169–78. doi: 10.2217/14622416.9.2.169. [DOI] [PubMed] [Google Scholar]
- 32.Wadelius M, Chen LY, Lindh JD, Eriksson N, Ghori MJ, Bumpstead S, Holm L, McGinnis R, Rane A, Deloukas P. The largest prospective warfarin-treated cohort supports genetic forecasting. Blood. 2009;113:784–92. doi: 10.1182/blood-2008-04-149070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE, Lee MT, Limdi NA, Page D, Roden DM, Wagner MJ, Caldwell MD, Johnson JA. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360:753–64. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herman D, Peternel P, Stegnar M, Breskvar K, Dolzan V. The influence of sequence variations in factor VII, gamma-glutamyl carboxylase and vitamin K epoxide reductase complex genes on warfarin dose requirement. Thromb Haemost. 2006;95:782–7. [PubMed] [Google Scholar]
- 35.R Development Core Team. R: A language and environment for statistical computing. 9.2. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 36.Schelleman H, Chen J, Chen Z, Christie J, Newcomb CW, Brensinger CM, Price M, Whitehead AS, Kealey C, Thorn CF, Samaha FF, Kimmel SE. Dosing algorithms to predict warfarin maintenance dose in Caucasians and African Americans. Clin Pharmacol Ther. 2008;84:332–9. doi: 10.1038/clpt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langley MR, Booker JK, Evans JP, McLeod HL, Weck KE. Validation of clinical testing for warfarin sensitivity: comparison of CYP2C9-VKORC1 genotyping assays and warfarin-dosing algorithms. J Mol Diagn. 2009;11:216–25. doi: 10.2353/jmoldx.2009.080123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schelleman H, Limdi NA, Kimmel SE. Ethnic differences in warfarin maintenance dose requirement and its relationship with genetics. Pharmacogenomics. 2008;9:1331–46. doi: 10.2217/14622416.9.9.1331. [DOI] [PubMed] [Google Scholar]
- 39.Cooper GM, Johnson JA, Langaee TY, Feng H, Stanaway IB, Schwarz UI, Ritchie MD, Stein CM, Roden DM, Smith JD, Veenstra DL, Rettie AE, Rieder MJ. A genome-wide scan for common genetic variants with a large influence on warfarin maintenance dose. Blood. 2008;112:1022–7. doi: 10.1182/blood-2008-01-134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrington DJ, Underwood S, Morse C, Shearer MJ, Tuddenham EG, Mumford AD. Pharmacodynamic resistance to warfarin associated with a Val66Met substitution in vitamin K epoxide reductase complex subunit 1. Thromb Haemost. 2005;93:23–6. doi: 10.1267/THRO05010023. [DOI] [PubMed] [Google Scholar]
- 41.Bodin L, Perdu J, Diry M, Horellou MH, Loriot MA. Multiple genetic alterations in vitamin K epoxide reductase complex subunit 1 gene (VKORC1) can explain the high dose requirement during oral anticoagulation in humans. J Thromb Haemost. 2008;6:1436–9. doi: 10.1111/j.1538-7836.2008.03049.x. [DOI] [PubMed] [Google Scholar]
- 42.Harrington DJ, Gorska R, Wheeler R, Davidson S, Murden S, Morse C, Shearer MJ, Mumford AD. Pharmacodynamic resistance to warfarin is associated with nucleotide substitutions in VKORC1. J Thromb Haemost. 2008;6:1663–70. doi: 10.1111/j.1538-7836.2008.03116.x. [DOI] [PubMed] [Google Scholar]
- 43.Dickmann LJ, Rettie AE, Kneller MB, Kim RB, Wood AJ, Stein CM, Wilkinson GR, Schwarz UI. Identification and functional characterization of a new CYP2C9 variant (CYP2C9*5) expressed among African Americans. Mol Pharmacol. 2001;60:382–7. doi: 10.1124/mol.60.2.382. [DOI] [PubMed] [Google Scholar]
- 44.Allabi AC, Gala JL, Horsmans Y, Babaoglu MO, Bozkurt A, Heusterspreute M, Yasar U. Functional impact of CYP2C95, CYP2C96, CYP2C98, and CYP2C911 in vivo among black Africans. Clin Pharmacol Ther. 2004;76:113–8. doi: 10.1016/j.clpt.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Limdi NA, Veenstra DL. Warfarin pharmacogenetics. Pharmacotherapy. 2008;28:1084–97. doi: 10.1592/phco.28.9.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimmel SE, Christie J, Kealey C, Chen Z, Price M, Thorn CF, Brensinger CM, Newcomb CW, Whitehead AS. Apolipoprotein E genotype and warfarin dosing among Caucasians and African Americans. Pharmacogenomics J. 2008;8:53–60. doi: 10.1038/sj.tpj.6500445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


