Abstract
Recent developments in our understanding of vitamin D show that it plays a significant role in immunological health, uniquely occupying both an anti-microbial and immunoregulatory niche. Vitamin D deficiency is widespread amongst renal transplant recipients (RTRs), thus providing one patho-mechanism that may influence the achievement of a successful degree of immunosuppression. It may also influence the development of the infectious, cardiovascular and neoplastic complications seen in RTRs. This review examines the biological roles of vitamin D in the immune system of relevance to renal transplantation (RTx) and evaluates whether vitamin D repletion may be relevant in determining immunologically-related clinical outcomes in RTRs, (including graft survival, cardiovascular disease and cancer). While there are plausible biological and epidemiological reasons to undertake vitamin D repletion in RTRs, there are few randomized-controlled trials in this area. Based on the available literature, we cannot at present categorically make the case for routine measurement and repletion of vitamin D in clinical practice but we do suggest that this is an area in urgent need of further randomized controlled level evidence.
Keywords: vitamin D, renal transplantation, cardiovascular disease, cancer, immune system, transplant rejection
Introduction
The biology of vitamin D (VitD) is highly topical at present, with significant research being carried out in the contexts of cardiovascular, autoimmune and allergic conditions, chronic kidney disease (CKD) and cancer (1). A recent systematic review of prospective observational studies showed that VitD deficiency (definitions of VitD status are given in Table 1) is a significant determinant of all-cause mortality in patients with CKD(2). Renal transplant recipients (RTRs) have a high prevalence of VitD deficiency versus controls(3). This arises for several reasons, including the mild-to-moderate degree of renal functional impairment which characterizes most allografts (causing loss of renal tubular CYP27B1), raised serum concentrations of fibroblast growth factor (FGF-23)(4), immunosuppressive drugs inducing VitD catabolism(5) and medically-advised sun-avoidance behavior (see below). FGF-23 actively inhibits VitD through suppression of CYP27B1, reducing 1-alpha-hydroxylation of 25-hydroxyvitaminD (25(OH)D3) and induction of CYP24A1, which enhances calcitriol and 25(OH)D3 degradation(6) (Figure 1). The natural history of 25(OH)D3 and 1,25(OH)2D3 in incident RTRs has been reviewed elsewhere(7); while the skeletal, renal and gastro-intestinal effects of VitD on calcium and phosphate homeostasis are well known, with VitD deficiency linked to increased risk of post-renal transplantation (RTx) bone mineral loss and fractures(8), VitD is also recognized to exert effects on both the innate and adaptive immune systems. In so doing, VitD status in RTRs can affect immunologically-driven post-transplant outcomes, notably allograft rejection, transplant function and development of de novo post-transplant malignancies. This mini-review examines the immunological effects of VitD that are of relevance to RTx and evaluates existing clinical evidence for VitD measurement and repletion in this cohort.
Table 1.
Current definitions of vitamin D status based on 25(OH)D levels
| Definition | Equivalent 25(OH)D3 serum level (UK) | Equivalent 25(OH)D3 serum level (US) | Notes |
|---|---|---|---|
| Vitamin D toxic | >375 nmol/l | >150 ng/l | (69) |
| Vitamin D sufficient | >75 nmol/l | >30 ng/ml | |
| Vitamin D insufficient | 50–75 nmol/l | 20–30 ng/ml | |
| Vitamin D deficient | <50 nmol/l | <20 ng/ml | Recent increase in threshold from <11 ng/ml has led to an estimated increase in prevalence from 2-14% (70) |
Figure 1. Effects of vitamin D on mineral biology.
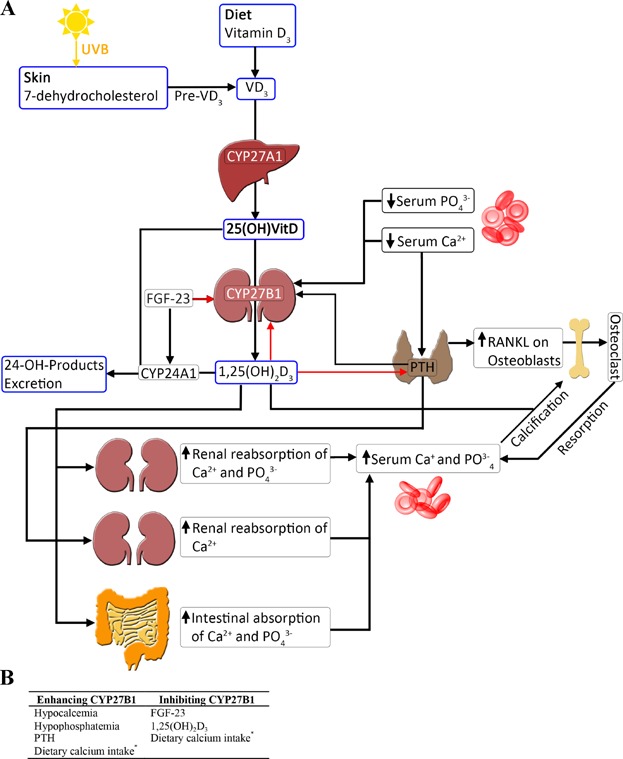
(A) schematic showing biogenesis of vitamin D. Vitamin D3 derived from either the diet or UVB irradiation in the skin is metabolized to 25-hydroxyvitaminD (25(OH)D3) in the liver through an enzymatic reaction catalyzed by CYP27A1. 25(OH)D3 is subsequently metabolized to the active form 1,25-dihydroxyvitaminD (1,25(OH)2D3) in the kidneys by CYP27B1. Both 25(OH)D3 and 1,25(OH)2D3 are converted by CYP24A1 to 24 hydroxylated products and excreted. CYP27B1 is tightly regulated: a drop in serum calcium levels is detected by the parathyroid gland and results in secretion of parathyroid hormone (PTH). Both PTH and reduced serum calcium and phosphate concentration directly stimulate CYP27B1 activity, and thus increased 1,25(OH)2D3 production. 1,25(OH)2D3, in a negative feedback loop, down-regulates its own production through inhibiting CYP27B1 activity as well as PTH production. 1,25(OH)2D3 has multiple systemic effects which ultimately result in restoration of serum calcium levels, as well as re-calcification of bones. FGF-23 is produced by osteocytes and decreases circulating concentrations of 1,25(OH)2D3, through induction of CYP24A1 and suppression of CYP27B1. In the schematic, black arrows represent induction, red arrows represent inhibition. (B) factors controlling CYP27B1 activity. * a low calcium diet reduces extra-renal CYP27B1, particularly in the colon, and enhances renal CYP27B1.
Immunological Effects of Vitamin D Relevant to RTx (Figure 2)
Figure 2. Biological functions of Vitamin D in the immune system and their potential relevance to transplantation.
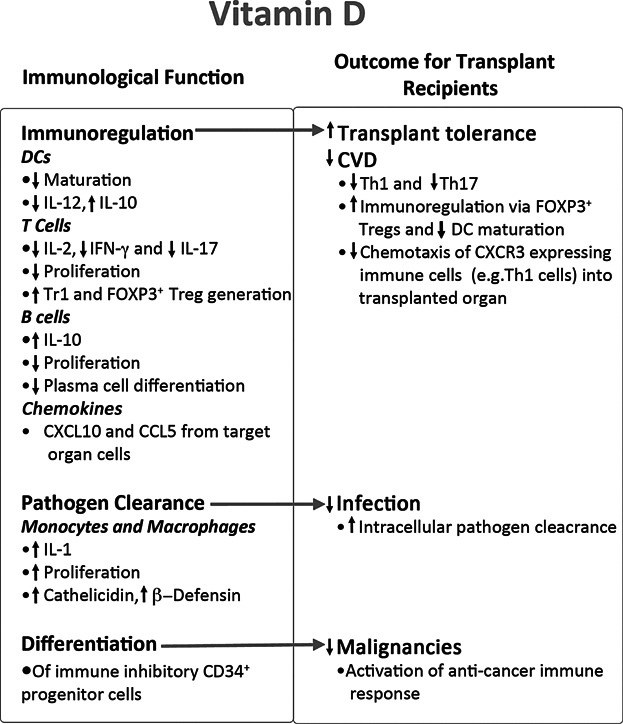
The biological impact of vitamin D on different immune parameters are shown on the left and the mechanisms by which these effects may impact on renal transplantation is indicated on the right.
The VitD receptor (VDR) is ubiquitously expressed in immune cells, including activated CD4+ and CD8+ T lymphocytes, and cells of the innate immune system, such as macrophages and dendritic cells (DCs). Immune cells not only express the VDR but many contain the machinery for producing biologically active 1,25(OH)2D3 through inducible expression of the CYP27B1 (9). These findings, along with strong epidemiological evidence linking VitD deficiency to multiple autoimmune diseases, suggest a physiological role for VitD in immune homeostasis. Experimentally, VitD metabolites, particularly 1,25(OH)2D3, have multiple effects on immune system functioning, instructing both antimicrobial and immunoregulatory functions.
Immunoregulatory actions of VitD
VitD has clear effects on immune system functioning, characterized by inhibition of proliferation(10), interleukin (IL)-2 (11) and interferon (IFN)-γ production by CD4+ T cells (12) and reduced cytotoxicity of CD8+ T cells (13). While VitD also enhances IL-4 production by CD4+ T cells, its ability to enhance regulatory T cell differentiation is particularly important. Not only does VitD induce differentiation of suppressive FOXP3+ regulatory T cells (Tregs)(14), the most critical of immunoregulatory T cells for the prevention of autoimmune diseases in humans, but also IL-10-producing FOXP3- type 1 regulatory T cells (Tr1 cells) (15) as well as IL-10 producing B cells (16). Although a definitive role for Tr1 cells or IL-10-producing B cells in transplant survival has not previously been described, FOXP3+ Treg numbers infiltrating transplanted tissues do correlate, in general, with improved outcomes (17).
The immunomodulatory effects of VitD are mediated through both direct effects on T cells and indirectly through modification of DC function (18). DCs play a central role in the initiation, magnitude and quality of the adaptive immune response and modification of their function by VitD is clearly of relevance to transplantation as both passenger and recipient DCs are critical for induction of direct and indirect alloresponses, respectively (19). VitD inhibits the maturation and antigen-presenting capacity of DCs and induces them to behave in a “tolerogenic” manner preferentially stimulating naïve T cells both in vitro and in vivo (20) to mature into FOXP3+ Tregs and Tr1 cells and enhancing the suppressive activity of these regulatory T cells (21). Inhibition of DC-derived IL-12 production by VitD is also of great relevance as IL-12 is a central mediator in Th1 differentiation, a cell population intimately associated with transplant rejection.
VitD also regulates chemokine-chemokine receptor interactions, key steps in migration of inflammatory cells to sites of allograft rejection (22). The CXCL10-CXCR3 axis is particularly important in transplant rejection, with levels of CXCL10 being associated with rejection in human transplant recipients(23). CXCL10 is secreted by immune cells as well as resident cells of tissues and organs (23) and recruits multiple immune cells, including T-cells, NK cells, macrophages and DCs through engagement of CXCR3. Thus, CXCL10 plays a role in the initiation and maintenance of Th1 alloresponses (24). VitD decreases CXCL10 secretion by tubular epithelial cells, thus inhibiting immune cell infiltration of renal transplants and potentially protecting against allograft rejection (25).
Antimicrobial actions
Monocyte activation with interferon (IFN)-γ or LPS results in up-regulation of both CYP27B1 as well as the VDR(26) Autocrine engagement of the VDR results in production of natural anti-microbial peptides, such as cathelicidin (CAP) and β-defensin 4 (DEFB4)(27), enhancing innate immune clearance of pathogen. Production of CAP is further increased by the presence of pro-inflammatory IL-17, synergising to remove inciting pathogens. Likewise, active (1,25(OH)2D3) VitD can be stimulatory to other innate immune cells, such as monocytes and macrophages, promoting proliferation and secretion of highly inflammatory IL-1(11).
How can these immunological functions impact on transplant outcomes?
The balance between regulatory and inflammatory immune components is a key determinant of graft outcomes, resolution of chronic infections and responsiveness to neo-antigens such as cancerous cells. From an immunological perspective, the dual functions of VitD (antimicrobial vs immunoregulatory) appear counterintuitive; however, these functions are context- and time-dependent and carefully regulated, with the balance between the two in any given situation, dictating outcome. By modulating adaptive immune responses and down-regulating DC proliferation, maturation and antigen presentation capacity, VitD can ameliorate the risk of transplant rejection. Additional mechanisms, including regulation of chemokines responsible for leukocyte infiltration and down-regulating renal TGF-β1 production (which has pro-fibrotic activity), may also inhibit the evolution of rejection in RTx(28). The ability of VitD to inhibit cell growth, promote apoptosis, alter cell adhesion and inhibit metastasis and angiogenesis is of great relevance to the risk of cancer development in RTRs (see below), as is the ability of VitD to induce differentiation of immune inhibitory CD34+ progenitor cells (observed in higher amounts in some cancers)(29). These potential protective roles of VitD are supported by multiple empirical observations.
Experimental evidence from animal models shows that survival of allografts of bone marrow, heart, kidney, liver, pancreatic islets, skin and small intestine are significantly prolonged by administration of VitD and its analogues(30), with increased resistance to opportunistic infections(31), supporting the assertion that immunomodulation by VitD is a determining factor of outcomes. Additionally a small (9 donors and 9 transplant recipients) prospective study in which donors received calcitriol therapy, which was then continued in the recipients, showed an expansion of CD4+CD25+ Tregs in the calcitriol-treated group(32). Another small prospective study treating 24 transplant recipients with calcitriol observed decreased co-stimulatory molecule expression (HLA-DR, CD28, CD86 and CD40) on white blood cells. Together these studies provide evidence of the immunomodulatory properties of Vit D receptor agonists (VDRAs – active vitamin D compounds, such as calcitriol and paricalcitol) after transplantation. VDRAs could thus be used as potentially immunomodulatory agents in RTx. Calcitriol analogues, such as paricalcitol, which could exert immunomodulatory activity with a lower risk of causing hypercalcemia, have been developed for clinical use for secondary hyperparathyroidism (33),(34).
Vitamin D Repletion Studies in RTx
Given plausible biological links between VitD and the pathophysiology of diseases endemic in the RTR population, the clinical evidence for VitD repletion in RTRs is reviewed here, excluding those predominantly focusing on skeletal outcomes, which are reviewed elsewhere(35). It should be noted that important clinical safety data for VitD repletion can be found in 3 separate comprehensive Cochrane reviews of bone disease in non-dialysis, dialysis and RTx(8),(36) where adverse effects of VitD repletion were described only in the minority of studies (4/16 studies in CKD, 8/60 studies of dialysis and 0/23 studies in RTx) suggesting it is generally a well-tolerated and safe therapy. However, higher repletion doses than those used in these studies are needed to bring serum levels significantly above 30 ng/ml (75 nmol/L).
Vitamin D and allograft outcomes (Table 2)
Table 2.
Clinical studies of the correlation between vitamin D and allograft function
| Study | Design | Study population and use of Vitamin D | Outcome and notes |
|---|---|---|---|
| Observational studies | |||
| Falkiewicz et al, 2009 (38) | Prospective study of adult transplant recipients (n = 90) with measured 1,25(OH)2D3 on day 3, month 1, 6, 12, 18 and 24 post-transplant | Patients were followed-up for 24 months. | All patients had received alfalcalcidol as part of routine care pre-transplant. Despite this, severe 1,25(OH)2D3 deficiency was present in 83% on day 3. In only 50% the concentration rose to normal levels during follow-up. |
| The effect of 1,25(OH)2D3 levels on outcomes (incidence of acute rejection, graft function, de novo malignancy and cardiovascular events) was analyzed. | The incidence of delayed graft function was higher in those with 1,25(OH)2D3 deficiency. There was a negative correlation between initial and 1 month 1,25(OH)2D3 levels and graft function during follow-up. Those with 1,25(OH)2D3 deficiency had poorer outcomes (death from cardiovascular events, acute rejection episodes, graft loss and cancer). | ||
| Wesseling-Perry et al 2011 (41) | Prospective analysis of pediatric transplant recipients with stable transplant function at recruitment (n = 68) | Associative study analyzing link between mineral ion abnormalities and GFR/acute rejection over a 2 year follow-up period. Measurement of 25(OH)D3, 1,25(OH)2D3 and FGF-23 was made at mean ± s.d. 4.9 ± 0.5 years post-transplant and correlated with transplant outcomes over the next 2 years. | 4 patients were lost to follow-up, so only 64 were included in the analysis. |
| VitD levels do not, but FGF-23 levels do, correlate with number of episodes of acute rejection and decline in eGFR over 2 year follow-up. | |||
| Kim et al 2012 (39) | Observational study of adult transplant recipients (n = 106) with known VitD levels prior to transplantation. | Measurement of 25(OH)D3 pre and post-transplantation with exclusion of osteoporotic patients. Patients were followed up every 6 months for 36 months. | Pre-transplant VitD deficiency was identified in multiple logistic regression analysis as a significant independent risk factor for decline in eGFR over 36 months post-transplantation. |
| Bienaimé et al 2013 (40) | Prospective cohort study of adult transplant recipients (n = 634) with measured 25(OH)D3 levels at 3 months post-transplant. | Measured 25(OH)D3 levels at 3-months post transplantation were correlated with clinical variables over a median follow-up of 48.6 months. | 19 patients were lost to follow-up and 30 had lost their graft, 28 had died with a functioning graft |
| There was no association between 3 month VitD levels and either graft loss or death during the follow-up period. | |||
| 25(OH)D3 level at 3 months was an independent predictor of mGFR and progression of IF/TA at 12 months. | |||
| Interventional studies | |||
| Tanaci et al 2003 (42) | Retrospective cohort analysis of adult patients (n = 92) treated, or not, with VitD | Outcomes of 43 transplant recipients in whom VitD was prescribed for clinically detectable osteoporosis (500 ng daily calcitriol) were compared to 49 patients without osteoporosis and not receiving VitD | 8 patients in the treatment arm were excluded from analysis due to non-compliance with treatment. |
| The group treated with VitD had more acute rejection episodes before treatment that control group; after treatment rejection episodes between treatment and control groups were the same. There was no difference in mean graft survivals between the two groups. | |||
| Follow-up ranged from 3-28 months | |||
| Uyar et al 2006 (44) | Retrospective interventional study of adult transplant recipients (n =110) treated, or not, with VitD | Outcomes of transplant recipients treated (n = 59) or not (n = 51) with calcitriol were compared. Calcitriol (dose not stated) was initiated at mean (±s.d) 24 ± 19.1 months post-transplantation. | By 3rd year of follow-up, patients given calcitriol had significantly lower creatinine and required fewer steroid pulses. |
| There was no difference in the number of acute rejection episodes. | |||
| Courbebaisse et al 2011 (45) | Retrospective cohort analysis of adult transplant recipients (n = 64) treated, or not, with VitD | 49 patients with serum 25(OH)D3 levels below 30ng/mL received cholecalciferol 100,000IU fortnightly from months 4 to 6, then every 2 months until 12 months post-transplantation. They were compared to 47 historical control patients with 25(OH)D3 levels below 30 ng/mL that had not received cholecalciferol. In the final analysis, due to exclusion criteria, only n = 32 patients in each group were analyzed. | Due to exclusion criteria, data from only 68% of the initial cohort were analyzed (n = 32 in each group). |
| There was no difference between the two groups in renal function (mGFR), proteinuria nor epithelial phenotypic changes by 12 months. Urinary PIIINP/Creatinine ratio, a surrogate marker of renal fibrosis, was no different between the two groups. Banff scoring for renal fibrosis (IF/TA) was also no different between the two groups. | |||
| Özdemir et al 2011 (43) | Retrospective cohort analysis of adult transplant recipients (n = 102) treated, or not, with VitD | 102 patients who had undergone transplant renal biopsy were studied. 40 had received calcitriol (dose not given) for 12 months from mean ± s.d 18 ± 6 months post-transplant. They were compared to 62 that had not had calcitriol | Calcitriol-treated patients experienced fewer episodes of acute rejection. On renal biopsy they had significantly lower tubular and interstitial HLA-DR expression and less peritubular capillary destruction than control subjects. This was reflected by better 5-year graft survival in calcitriol-treated patients and a multiple logistic regression model in which calcitriol-treatment had an independent (beneficial) effect on graft survival. |
VitD, Vitamin D; 25(OH)D3, 25-hydroxyvitamin D; 1,25(OH)2D3, 1,25-Dihroxyvitamin D; IU, international units; mGFR, measured (iohexol clearance) glomerular filtration rate; PIIINP, procollagen III aminoterminal propeptide; IF/TA, interstitial fiobrosis/tubular atrophy.
Given the immunomodulatory effects of VitD, it has been hypothesized that reduced serum 25(OH)D3 concentrations are associated with poorer graft outcomes. Reduced serum 25(OH)D3 concentrations in RTRs is commonplace(37). Three out of four observational studies published to date draw a direct link between VitD levels and allograft outcomes (summarized in Table 2). Notably, in an observational study of 90 Polish RTRs, 25(OH)D3 deficiency at time of transplantation was significantly associated with delayed graft functioning and an increased risk of acute rejection episodes over a two year follow-up period (38). This would be clinically highly significant as both of these are known risk factors for graft fibrosis and impaired allograft function. The other two observational studies showed an association between 25(OH)D3 levels at time of transplantation and renal function over a 2–4 year follow-up period (39),(40). The more recent, a study of 634 patients, demonstrated an association between low serum 25(OH)D3 at 3 months post-transplantation and increased risk of interstitial fibrosis/tubular atrophy on 12 month transplant biopsies at, but not with mortality. The fourth observational study is not directly comparable to the first three as it was carried out in a pediatric cohort with stable graft function some time (mean ± s.d. 4.9 ± 0.5 years) after transplantation (41). Given the low event rate (only 6 patients out of 64 had a decrease in GFR of ≥50% and there were only 14 acute rejection episodes), this was an underpowered study to determine the effects of VitD on long-term transplant function.
Interventional studies of VitD supplementation in the context of renal transplantation have also yielded conflicting data, most likely attributable to difference in patient selection, control group selection, time since transplantation, VitD repletion regimen and formulation of VitD. These caveats mean that it is difficult to directly compare study cohorts and to formulate an ideal repletion strategy. While supplementation post-transplant with calcitriol was associated in 3 studies with either reduced numbers of acute rejection episodes (42),(43), better transplant function(44) and improved graft survival(43) a smaller interventional study, using choecalciferol in the first year post-transplantation, gave conflicting results(45). There are significant difficulties in conducting clinical VitD research, which are elaborated below, but these trials can be individually critiqued. The dataset of Tanaci et al(42) is a retrospective small series with baseline imbalances between osteoporotic and non-osteoporotic cohorts; the study of Özdemir et al(44) does not disclose the calcitriol dosing regime and has a surprisingly high late rejection rate in the control group while Courbebaisse et al(45) was not a randomized prospective study and the repletion strategy only achieved a mean 25(OH)D3 concentration of 31.8 ± 7.1 ng/mL, arguably below the nephroprotective threshold. Some of the discrepancy between studies may also be explained by the lack of a contemporary control population in the latter study.
In conclusion, there is an association between serum VitD concentrations and allograft outcomes, however the evidence for causality has yet to be tested in an RCT.
Vitamin D and Cancer (Table 3)
Table 3.
Clinical studies of the correlation between vitamin D and malignancies
| Study | Design | Use of Vitamin D | Results |
|---|---|---|---|
| Observational studies | |||
| Ducloux et al 2008 (47) | Retrospective cohort analysis of adult kidney transplant recipients (n = 363) with known pre-transplant 25(OH)D3 levels | Pre-transplant 25(OH)D3 levels were correlated with risk of development of post-transplant cancers, with respect for other known risk factors, over a 3-year follow-up period | 32 cancers were observed, more frequently in those with VitD deficiency and insufficiency. |
| Low VitD level was identified as an independent risk factor for development of post-transplant cancer over 3 years of follow-up (hazard ratio 1.12, for each 1 ng/ml decline in 25(OH)D3). | |||
| Marcen et al 2012 (48) | Observational prospective study of adult kidney transplant recipients recruited post-transplantation (n = 389) | 25(OH)D3 levels measured at 3, 6 and 12 months post-transplant were correlated with cardiovascular events and new malignancies. | 331 patients were analyzed as those that had lost their grafts within the first 12 months post-transplantation were excluded. |
| Over a 10 year follow-up, no difference was observed between cumulative incidence of malignancy in patients with normal VitD level, VitD insufficiency or VitD deficiency (21.3% vs 22.7% vs 16.7% cumulative incidence, respectively). | |||
| Interventional studies | |||
| Obi et al 2012(49) | Prospective cohort analysis of adult Japanese kidney transplant recipients recruited 1 year post-transplantation (n = 218), with 25(OH)D3 levels measured at recruitment | Patient exposure to VDRAs (calcitriol and alfacalcidol) and baseline 25(OH)D3 was correlated with development of malignancies | 92 patients had received AVDs at recruitment. |
| During median follow-up of 2.9 years, 5 AVD (2.1 per 100 patient years) users and 11 non-AVD users (3.5 per 100 patient years) developed malignancies. Although there was no correlation between 25(OH)D3 level and risk of malignancy, AVD users were at lower risk of developing malignancy by Cox proportional hazard regression (hazard ratio 0.21; 95% CI 0.07–0.65). | |||
25(OH)D3, 25-hydroxyvitamin D; VitD, Vitamin D; VDRAs, Vitamin D receptor agonists; CI, confidence interval.
RTRs are at a 3–5 fold increased risk of developing malignancies compared to the general population and an inverse correlation between general population serum 25(OH)D3 concentrations and the risk of solid organ malignancies (especially breast and colorectal cancer) is observed epidemiologically (46).
Limited observational epidemiological data exist analyzing VitD status and de novo malignancies in RTRs(47),(48). The shorter of the two studies(47), with a 3 year follow-up period, describes a significant increase in malignancy risk with VitD deficiency, with a hazard ratio of 1.12 for every 1 ng/mL decline in 25(OH)D3. However, a longer follow-up study with the same number of patients found no association over a 10 year follow-up period between VitD levels and risk of de novo malignancy (48). Further work is needed to establish whether these results can be explained by risk segregation with cancer type, particularly viral-related cancers. A single interventional repletion study exists in the literature (49) describing a decreased post-transplantation malignancy risk associated with VDRA supplementation (calcitriol and alfacalcidol). This study needs to be assessed with the caveat that the overall “event rate” was exceedingly small (2.1 and 3.5 de novo malignancies per 100 patient years in VitD-treated and untreated subjects, respectively).
Due to the increased risk of skin malignancies with immunosuppression (particularly squamous cell carcinomas), there has been longstanding advice to RTRs to avoid solar UV exposure. In RTRs, regular application of SPF-50 sunscreen is associated with fewer skin lesions over a 2-year period, but also a lower mean concentration of 25-OH VitD levels (mean value 53 ng/ml versus 60 ng/ml)(50). Higher levels of VitD are similarly associated with an increased risk of cancer, explained by greater UV exposure conferring increased disease risk(51). These data demonstrate the difficulties of drawing conclusions using only epidemiological studies.
Other Key Effects of Vitamin D in RTRs
VitD status contributes significantly to skeletal health. A Cochrane review(8) in 2007 concluded that from 24 trials (1,299 patients) no individual intervention (bisphosphonates, vitamin D sterol or calcitonin) was associated with reduced fracture risk in RTRs compared with placebo, but by combining results for all active interventions against placebo it could be demonstrated that any treatment of bone disease was associated with reduced risk of fracture (RR 0.51, 95% CI 0.27–0.99). Bisphosphonates (any route), VitD sterol, and calcitonin all increased lumbar spine bone mineral density. Bisphosphonates and VitD also had a beneficial effect on the bone mineral density at the femoral neck. This represents the “classical” VitD therapeutic paradigm and is reviewed in depth elsewhere(35).
Cardiovascular disease (CVD) is the commonest cause of death in RTRs, with chronic inflammation a key etiological factor. As well as epidemiological data showing a link between low serum VitD concentrations and predisposition to cardiovascular events, meta-analyses have shown that oral VitD treatment contributes to improved all-cause mortality through an associated reduction of deaths from cardiovascular events(52). However a recent systematic analysis showed that the quality of current trial data is inadequate to draw conclusions about the relationship between VitD status and mortality from CVD in the general population(53). Further discussion of the role of VitD in CVD is beyond the scope of this review but has been reviewed elsewhere(54).
Issues in Vitamin D Research
There are several caveats that cloud the interpretation of clinical VitD research data. First, reliably assessing VitD status and activity is itself a challenge(55). Measurement of serum 25(OH)D3 concentration is widely used because this species has a 3–4 week half-life, whereas the biologically most active vitamin D species - 1,25(OH)2D3 - has a life-life of only hours. 25(OH)D3 is an indirect test as it does not measure the most active vitamin D species and does not accurately predict VitD concentrations in tissues. The biological function of VitD can also be modulated by polymorphisms in VitD binding protein and the VDR, which are not accounted for in currently available trials. This is relevant because up to 3% of the human genome can be influenced by VitD(9), including steroid sensitivity(56)). Additionally there remains controversy over the accuracy of different VitD assays. Standardization of assays has recently been improved but not resolved(57). Second, as there is no consensus on what should constitute repletion in interventional trials, seasonal (UVB-driven) effects on study cohorts' serum VitD concentrations are important and relevant to patients with CKD, on dialysis or after renal transplantation(58).
Third, the species and route of administration of VitD treatment used in interventional studies is confounding. There are 6–8 different possible forms of ViD, including ergocalciferol, cholecalciferol, calcidiol, calcitriol, 1-alfacalcidol and paricalcitol, with almost no head to head studies comparing them in RTRs. These have different affinities for the VDR, potencies, biological activities, and side-effect profiles - for a detailed discussion see(59). VitD can raise serum creatinine, either due to an effect on the renin-angiotensin-aldosterone system or direct alteration in tubular handling of creatinine(60). Further variables include the route (oral, intramuscular and intravenous – the latter confers greater bioavailability) and frequency of administration, whether daily, weekly or monthly(61).
Although there is a high prevalence of VitD insufficiency in transplantation, there is no consensus dosing strategy for VitD repletion. One study showed that 100,000 IU of cholecalciferol fornightly for 2 months (equivalent to 6,600 IU/day) corrected 25(OH)D3 insufficiency in RTRs and significantly decreased serum PTH without side effects. This study also highlighted that 100,000 IU of cholecalciferol every other month from months 6 to 12 post-transplant (the “maintenance period”) was insufficient to maintain serum 25(OH)D3 levels above 30 ng/ml in about half of the patients studied(69), consistent with a previous report(62). The authors pharmacokinetically simulated an optimal dosing regimen to maintain 25(OH)D3 concentrations between 30 and 80 ng/ml (100,000 IU six times fortnightly, then 100,000 IU monthly until the end of the first year)(63), but this proposal remains to be tested prospectively.
Lastly, and most importantly, the optimum marker denoting biological VitD repletion has yet to be determined. Although biochemical markers (principally PTH and alkaline phosphatase) have traditionally been used to monitor repletion, the reliability and clinical relevance of PTH levels to infer changes in 25(OH)D3 levels in RTRs has been called into question. In a cohort study of 419 renal transplant recipients, 25(OH)D3, eGFR and serum phosphate combined only accounted for 19% of the variance in PTH levels, indicating that VitD supplementation alone is likely to have only a limited effect on PTH levels (64). Bone mineral density, graft and patient survival are all relevant, additional, parameters/biomarkers for consideration.
Future Directions
Although tentative associations have been made between VitD repletion and improvement of clinical outcomes in RTRs, this review highlights deficiencies in our current knowledge that need to be addressed. Table 4 lists three actively recruiting VitD repletion trials, evaluating a range of primary endpoints. Encouragingly, there is focus on allograft function, cardiovascular outcomes and de novo malignancy.
Table 4.
Trials currently recruiting for Vitamin D in RTRs
| Trial, Location | Design | Primary endpoints |
|---|---|---|
| VITA-D, Vienna (65) | Phase 3 placebo-controlled trial. 200 kidney transplant recipients with 25(OH)D3 <50 ng/ml will be randomized 5 days post-transplant to either placebo or VitD (6800 IU daily for one year). | 1-year MDRD eGFR, number of infections, CRP, number of acute rejection episodes, bone mineral density (DEXA scans within the first 4 weeks, then at 5 and 12 months post-transplant). |
| VITALE, Paris (66) | Phase 4 placebo-controlled trial, comparing high (100 000 IU fortnightly then monthly) versus low (12 000 IU fortnightly then monthly) dose VitD over two year follow-up to patients 12–48 months post-transplant, with stable renal function over the previous 3 months, and VitD insufficiency (25(OH)D3 <30 ng/mL) at recruitment. n = 320 patients in each group. | De novo development of diabetes, cardiovascular complications, de novo cancer, patient death |
| CANDLE-KIT, Osaka (67) | Phase 4 open-label trial VitD supplementation and anemia correction (|with Mircera®) over 2 year follow-up. 246 patients will be recruited who are at least 12 months post-transplant, with eGFR ranging from 15 to 60 ml/min. Inclusion criteria will not include VitD levels but patients must have Hb <10.5 g/dl without iron deficiency. They will be randomized to low Hb (≥9.5 and <10.5 g/dl) with no VitD, low Hb (≥9.5 and <10.5 g/dl) with VitD (1000 IU per day), high Hb (≥12.5 and <13.5 g/dl) without VitD or high Hb (≥12.5 and <13.5 g/dl) with VitD (1000 IU per day). Outcomes will be followed-up for 2 years. | Change in MDRD eGFR over 2 years of follow-up. |
All three trials currently recruiting will use cholecalciferol as the vitamin D (VitD) formulation. 25(OH)D3, 25-hydroxyvitamin D; MDRD, modification of diet in renal disease; Hb, hemoglobin.
The VITA-D trial (65) is a randomized, placebo-controlled double-blind study of 200 transplant recipients with follow-up duration of one-year, with entry criteria being 25(OH)D3 serum concentration of <50 nmol/l. Incidence of acute rejection episodes, number and severity of infections (as measured by CRP) and GFR will be monitored. VITA-D is primarily aimed at evaluating short-term outcomes as only newly transplanted patients are being recruited and will be the first trial to report on VitD supplementation in de novo RTRs. The VITALE trial (66) will evaluate the differential effect of low and high dose cholecalciferol supplementation. 640 Patients ranging from 12 to 48 months post-transplantation will be recruited to capture medium-term outcomes, particularly the development of new cancers and CVD. Although better powered than VITA-D, follow-up is still short at 24 months, in comparison with epidemiological literature in general. CANDLE-KIT(67) will recruit 246 RTRs, of at least one year post-transplantation, and randomize them to receive no additional treatment or combinations of cholecalciferol and an erythropoiesis stimulating agent. Transplant function over a two-year follow-up period will be the primary outcome measure of this trial. Interestingly, entry criteria for this trial do not include baseline VitD insufficiency/deficiency.
Conclusion
Research concerning the benefits of VitD supplementation in RTR is clearly still evolving. While there is consistent epidemiological evidence suggesting an association between replete VitD status and improved clinical outcomes in RTRs, particularly skeletal outcomes (bone mineral density and fractures), we lack compelling evidence at the moment that measurement and repletion of VitD is mandatory for RTRs. The KDIGO guidelines recommend the use of VitD in RTRs for the prevention and treatment of transplant bone disease(68), but as yet a hard case for VitD repletion to optimize immunomodulation in RTRs has not been made. Given recent developments in our understanding of its molecular properties, VitD probably has a multifaceted role, which cannot be appreciated by examining hard clinical endpoints such as mortality. Future work is urgently needed to translate molecular biology into clinical outcomes.
Acknowledgments
This work was funded by grants from the Medical Research Council (GL), the Wellcome Trust (BA), the British Heart Foundation (GL, BA, DJG), Kidney Patients' Association and Guy's and St Thomas' Charity (GL, BA, DJG). The research was also funded by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The authors acknowledge the support of the MRC Centre for Transplantation.
Glossary
- CKD
chronic kidney disease
- RTx
renal transplant
- RTRs
renal transplant recipients
- PTH
parathyroid hormone
- NODAT
new onset diabetes after transplantation
- CVD
cardiovascular disease
Disclosure
The authors of this manuscript have no conflict of interest to disclose as set out by the American Journal of Transplantation.
References
- 1.Pludowski P, Holick MF, Pilz S, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013;12:976–989. doi: 10.1016/j.autrev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am. J. Kidney Dis. 2011;58:374–382. doi: 10.1053/j.ajkd.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Dm S, Cc M. Prevalence of 25 (OH) vitamin D (calcidiol) deficiency at time of renal transplantation: a prospective study. Clin. Transplant. 2007;25:683–688. doi: 10.1111/j.1399-0012.2007.00696.x. [DOI] [PubMed] [Google Scholar]
- 4.Baia LC, Humalda JK, Vervloet MG, Navis G, Bakker SJL, de Borst MH. Fibroblast growth factor 23 and cardiovascular mortality after kidney transplantation. Clin. J. Am. Soc. Nephrol. 2013;8:1968–1978. doi: 10.2215/CJN.01880213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyal O, Aharon M, Safadi R, Elhalel MD. Serum vitamin D levels in kidney transplant recipients: the importance of an immunosuppression regimen and sun exposure. Isr. Med. Assoc. J. 2013;15:628–633. [PubMed] [Google Scholar]
- 6.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 7.Evenepoel P. Recovery versus persistence of disordered mineral metabolism in kidney transplant recipients. Semin. Nephrol. 2013;33:191–203. doi: 10.1016/j.semnephrol.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Palmer SC, McGregor DO, Strippoli GF. Interventions for preventing bone disease in kidney transplant recipients. Cochrane database Syst. Rev. 2007:CD005015. doi: 10.1002/14651858.CD005015.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr. Rev. 2008;29:726–76. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol) J. Clin. Invest. 1984;74:1451–1455. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhalla AK, Amento EP, Krane SM. Differential effects of 1,25-dihydroxyvitamin D3 on human lymphocytes and monocyte/macrophages: inhibition of interleukin-2 and augmentation of interleukin-1 production. Cell. Immunol. 1986;98:311–322. doi: 10.1016/0008-8749(86)90291-1. [DOI] [PubMed] [Google Scholar]
- 12.Rigby WF, Yirinec B, Oldershaw RL, Fanger MW. Comparison of the effects of 1,25-dihydroxyvitamin D3 on T lymphocyte subpopulations. Eur. J. Immunol. 1987;17:563–566. doi: 10.1002/eji.1830170420. [DOI] [PubMed] [Google Scholar]
- 13.Meehan MA, Kerman RH, Lemire JM. 1,25-Dihydroxyvitamin D3 enhances the generation of nonspecific suppressor cells while inhibiting the induction of cytotoxic cells in a human MLR. Cell. Immunol. 1992;140:400–409. doi: 10.1016/0008-8749(92)90206-5. [DOI] [PubMed] [Google Scholar]
- 14.Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Curr. Allergy Asthma Rep. 2011;11:29–36. doi: 10.1007/s11882-010-0161-8. [DOI] [PubMed] [Google Scholar]
- 15.Povoleri GAM, Scottà C, Nova-Lamperti EA, John S, Lombardi G, Afzali B. Thymic versus induced regulatory T cells - who regulates the regulators. Front. Immunol. 2013;4:169. doi: 10.3389/fimmu.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heine G, Niesner U, Chang H-D, et al. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur. J. Immunol. 2008;38:2210–228. doi: 10.1002/eji.200838216. [DOI] [PubMed] [Google Scholar]
- 17.Zuber J, Grimbert P, Blancho G, et al. Prognostic significance of graft Foxp3 expression in renal transplant recipients: a critical review and attempt to reconcile discrepancies. Nephrol. Dial. Transplant. 2013;28:1100–1111. doi: 10.1093/ndt/gfs570. [DOI] [PubMed] [Google Scholar]
- 18.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afzali B, Lombardi G, Lechler RI. Pathways of major histocompatibility complex allorecognition. Curr. Opin. Organ Transplant. 2008;13:438–444. doi: 10.1097/MOT.0b013e328309ee31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farias AS, Spagnol GS, Bordeaux-Rego P, et al. Vitamin D3 induces IDO(+) tolerogenic DCs and enhances Treg, reducing the severity of EAE. CNS Neurosci. Ther. 2013;19:269–277. doi: 10.1111/cns.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakayamada S, Takahashi H, Kanno Y, O'Shea JJ. Helper T cell diversity and plasticity. Curr. Opin. Immunol. 2012;24:297–302. doi: 10.1016/j.coi.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segerer S, Cui Y, Eitner F, et al. Expression of chemokines and chemokine receptors during human renal transplant rejection. Am. J. Kidney Dis. 2001;37:518–531. [PubMed] [Google Scholar]
- 23.Romagnani P, Crescioli C. CXCL10: a candidate biomarker in transplantation. Clin. Chim. Acta. 2012;413:1364–1373. doi: 10.1016/j.cca.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Aksoy MO, Yang Y, Ji R, et al. CXCR3 surface expression in human airway epithelial cells: cell cycle dependence and effect on cell proliferation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L909–18. doi: 10.1152/ajplung.00430.2005. [DOI] [PubMed] [Google Scholar]
- 25.Cockwell P. Chemoattraction of T cells expressing CCR5, CXCR3 and CX3CR1 by proximal tubular epithelial cell chemokines. Nephrol. Dial. Transplant. 2002;17:734–744. doi: 10.1093/ndt/17.5.734. [DOI] [PubMed] [Google Scholar]
- 26.Fabri M, Stenger S, Shin D-M, et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci. Transl. Med. 2011;3:104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang T-T, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004;173:2909–212. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 28.Adorini L, Amuchastegui S, Daniel KC. Prevention of chronic allograft rejection by Vitamin D receptor agonists. Immunol. Lett. 2005;100:34–41. doi: 10.1016/j.imlet.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Young M, Day T. Immune Regulatory Activity of Vitamin D3 in Head and Neck Cancer. Cancers (Basel) 2013;5:1072–1085. doi: 10.3390/cancers5031072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown AJ, Slatopolsky E. Vitamin D analogs: therapeutic applications and mechanisms for selectivity. Mol. Aspects Med. 2008;29:433–452. doi: 10.1016/j.mam.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Cantorna MT, Hullett DA, Redaelli C, et al. 1,25-Dihydroxyvitamin D3 prolongs graft survival without compromising host resistance to infection or bone mineral density. Transplantation. 1998;66:828–831. doi: 10.1097/00007890-199810150-00003. [DOI] [PubMed] [Google Scholar]
- 32.Ardalan MR, Maljaei H, Shoja MM, et al. Calcitriol started in the donor, expands the population of CD4+ CD25+ T cells in renal transplant recipients. Transplant. Proc. 2007;39:951–953. doi: 10.1016/j.transproceed.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Van Etten E, Mathieu C. Immunoregulation by 1, 25-dihydroxyvitamin D3: basic concepts. J. Steroid Biochem. Mol. Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin. J. Am. Soc. Nephrol. 2011;6:913–921. doi: 10.2215/CJN.06040710. [DOI] [PubMed] [Google Scholar]
- 35.Kalantar-Zadeh K, Molnar MZ, Kovesdy CP, Mucsi I, Bunnapradist S. Management of mineral and bone disorder after kidney transplantation. Curr. Opin. Nephrol. Hypertens. 2012;21:389–403. doi: 10.1097/MNH.0b013e3283546ee0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, S G. Vitamin D compounds for people with chronic kidney disease not requiring dialysis (Review) Cochrane Database Syst. Rev. 2009 doi: 10.1002/14651858.CD008175. [DOI] [PubMed] [Google Scholar]
- 37.Sadlier DM, Magee CC. Prevalence of 25(OH) vitamin D (calcidiol) deficiency at time of renal transplantation: a prospective study. Clin. Transplant. 2007;21:683–688. doi: 10.1111/j.1399-0012.2007.00696.x. [DOI] [PubMed] [Google Scholar]
- 38.Falkiewicz K, Boratynska M, Speichert-Bidzińska B, et al. 1,25-dihydroxyvitamin D deficiency predicts poorer outcome after renal transplantation. Transplant. Proc. 2009;41:3002–3005. doi: 10.1016/j.transproceed.2009.07.087. [DOI] [PubMed] [Google Scholar]
- 39.Kim H, Kang S-W, Yoo T-H, et al. The impact of pretransplant 25-hydroxy vitamin D deficiency on subsequent graft function: an observational study. BMC Nephrol. 2012;13:22. doi: 10.1186/1471-2369-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bienaimé F, Girard D, Anglicheau D, et al. Vitamin d status and outcomes after renal transplantation. J. Am. Soc. Nephrol. 2013;24:831–41. doi: 10.1681/ASN.2012060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wesseling-Perry K, Tsai EW, Ettenger RB, Jüppner H, Salusky IB. Mineral abnormalities and long-term graft function in pediatric renal transplant recipients: a role for FGF-23. Nephrol. Dial. Transplant. 2011;26:3779–3784. doi: 10.1093/ndt/gfr126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaci N, Karakose H, Guvener N, Tutuncu N, Colak T, Haberal M. Influence of 1,25-dihydroxyvitamin D3 as an immunomodulator in renal transplant recipients: a retrospective cohort study. Transplant. Proc. 2003;35:2885–2887. doi: 10.1016/j.transproceed.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Özdemir BH, Özdemir aa, Sezer S, Çolak T, Haberal M. Influence of 1,25-dihydroxyvitamin D3 on human leukocyte antigen-DR expression, macrophage infiltration, and graft survival in renal allografts. Transplant. Proc. 2011;43:500–503. doi: 10.1016/j.transproceed.2011.01.083. [DOI] [PubMed] [Google Scholar]
- 44.Uyar M, Sezer S, Arat Z, Elsurer R, Ozdemir FN, Haberal M. 1,25-dihydroxyvitamin D(3) therapy is protective for renal function and prevents hyperparathyroidism in renal allograft recipients. Transplant. Proc. 2006;38:2069–2073. doi: 10.1016/j.transproceed.2006.06.051. [DOI] [PubMed] [Google Scholar]
- 45.Courbebaisse M, Xu-Dubois Y-C, Thervet E, et al. Cholecalciferol supplementation does not protect against renal allograft structural and functional deterioration: a retrospective study. Transplantation. 2011;91:207–212. doi: 10.1097/TP.0b013e318200ba37. [DOI] [PubMed] [Google Scholar]
- 46.Van der Rhee H, Coebergh JW, de Vries E. Is prevention of cancer by sun exposure more than just the effect of vitamin D? A systematic review of epidemiological studies. Eur. J. Cancer. 2013;49:1422–1436. doi: 10.1016/j.ejca.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Ducloux D, Courivaud C, Bamoulid J, Kazory A, Dumoulin G, Chalopin J-M. Pretransplant serum vitamin D levels and risk of cancer after renal transplantation. Transplantation. 2008;85:1755–1759. doi: 10.1097/TP.0b013e318172cb2c. [DOI] [PubMed] [Google Scholar]
- 48.Marcén R, Jimenez S, Fernández-Rodriguez A, et al. Are low levels of 25-hydroxyvitamin D a risk factor for cardiovascular diseases or malignancies in renal transplantation. Nephrol. Dial. Transplant. 2012;27(Suppl 4):iv47–52. doi: 10.1093/ndt/gfs508. [DOI] [PubMed] [Google Scholar]
- 49.Obi Y, Ichimaru N, Hamano T, et al. Orally active vitamin d for potential chemoprevention of posttransplant malignancy. Cancer Prev. Res. 2012;5:1229–1235. doi: 10.1158/1940-6207.CAPR-12-0218. [DOI] [PubMed] [Google Scholar]
- 50.Ulrich C, Jürgensen JS, Degen A, et al. Prevention of non-melanoma skin cancer in organ transplant patients by regular use of a sunscreen: a 24 months, prospective, case-control study. Br. J. Dermatol. 2009;161:78–84. doi: 10.1111/j.1365-2133.2009.09453.x. [DOI] [PubMed] [Google Scholar]
- 51.Penny H, Frame S, Dickinson F, et al. Determinants of vitamin D status in long-term renal transplant patients. Clin. Transplant. 2012;26:E617–23. doi: 10.1111/ctr.12039. [DOI] [PubMed] [Google Scholar]
- 52.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch. Intern. Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 53.Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2011;96:1931–1942. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 54.Gunta SS, Thadhani RI, Mak RH. The effect of vitamin D status on risk factors for cardiovascular disease. Nat. Rev. Nephrol. 2013:1–11. doi: 10.1038/nrneph.2013.74. [DOI] [PubMed] [Google Scholar]
- 55.Janssen MJW, Wielders JPM, Bekker CC, et al. Multicenter comparison study of current methods to measure 25-hydroxyvitamin D in serum. Steroids. 2012;77:1366–1372. doi: 10.1016/j.steroids.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 56.Nanzer AM, Chambers ES, Ryanna K, et al. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1α,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J. Allergy Clin. Immunol. 2013;132:297–304.e3. doi: 10.1016/j.jaci.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 57.Fraser WD, Milan AM. Vitamin D assays: past and present debates, difficulties, and developments. Calcif. Tissue Int. 2013;92:118–127. doi: 10.1007/s00223-012-9693-3. [DOI] [PubMed] [Google Scholar]
- 58.Elder GJ. Vitamin D levels, bone turnover and bone mineral density show seasonal variation in patients with chronic kidney disease stage 5. Nephrology (Carlton) 2007;12:90–94. doi: 10.1111/j.1440-1797.2006.00754.x. [DOI] [PubMed] [Google Scholar]
- 59.Kalantar-Zadeh K, Kovesdy CP. Clinical outcomes with active versus nutritional vitamin D compounds in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2009;4:1529–1539. doi: 10.2215/CJN.02140309. [DOI] [PubMed] [Google Scholar]
- 60.Agarwal R, Hynson JE, Hecht TJW, Light RP, Sinha AD. Short-term vitamin D receptor activation increases serum creatinine due to increased production with no effect on the glomerular filtration rate. Kidney Int. 2011;80:1073–1079. doi: 10.1038/ki.2011.207. [DOI] [PubMed] [Google Scholar]
- 61.Leckstroem DC, Salzer J, Goldsmith DJA. The trials and tribulations of vitamin D – Time for the “sunshine” vitamin to come in out of the cold – or just more broken promises? - In press. Expert Rev. Endocrinol. Metab. 2014 doi: 10.1586/17446651.2014.908116. [DOI] [PubMed] [Google Scholar]
- 62.Wissing KM, Broeders N, Moreno-Reyes R, Gervy C, Stallenberg B, Abramowicz D. A controlled study of vitamin D3 to prevent bone loss in renal-transplant patients receiving low doses of steroids. Transplantation. 2005;79:108–115. doi: 10.1097/01.tp.0000149322.70295.a5. [DOI] [PubMed] [Google Scholar]
- 63.Courbebaisse M, Thervet E, Souberbielle JC, et al. Effects of vitamin D supplementation on the calcium-phosphate balance in renal transplant patients. Kidney Int. 2009;75:646–651. doi: 10.1038/ki.2008.549. [DOI] [PubMed] [Google Scholar]
- 64.Boudville NC, Hodsman AB. Renal function and 25-hydroxyvitamin D concentrations predict parathyroid hormone levels in renal transplant patients. Nephrol. Dial. Transplant. 2006;21:2621–2624. doi: 10.1093/ndt/gfl201. [DOI] [PubMed] [Google Scholar]
- 65.Thiem U, Heinze G, Segel R, et al. VITA-D: cholecalciferol substitution in vitamin D deficient kidney transplant recipients: a randomized, placebo-controlled study to evaluate the post-transplant outcome. Trials. 2009;10:36. doi: 10.1186/1745-6215-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thervet E. 2013. VITamine D Supplementation in RenAL Transplant Recipients - VITALE http://Clinicaltrials.gov.
- 67.Tsubakihara Y. 2013. Correcting Anemia and Native Vitamin D Supplementation in Kidney Transplant Recipients (CANDLE-KIT) http://Cinicaltrials.gov.
- 68.Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77:299–311. doi: 10.1038/ki.2009.377. [DOI] [PubMed] [Google Scholar]
- 69.Holick MF. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 70.Saintonge S, Bang H, Gerber LM. Implications of a new definition of vitamin D deficiency in a multiracial us adolescent population: the National Health and Nutrition Examination Survey III. Pediatrics. 2009;123:797–803. doi: 10.1542/peds.2008-1195. [DOI] [PubMed] [Google Scholar]


