Abstract
Neprilysin (NEP) is a key cell surface peptidase in the maintenance of airway homeostasis and the development of pulmonary disorders. However, little information is available about the effect of particulate matter (PM) on airway NEP. In this controlled human exposure study, changes in induced sputum were measured in eleven subjects at baseline, overshot (OS) mucking, and diesel exhaust (DE) exposure days. Neither OS condition nor DE exposure was found to induce significant changes in total protein, but DE induced significant increases in cell numbers of macrophages and epithelium. Moreover, significant increases in soluble NEP were observed following OS mining dust particulates (0.43 ± 0.06, p = 0.023) and DE exposure (0.30 ± 0.04, p = 0.035) when compared with the baseline control (0.40 ± 0.03), with 42% and 31% average net increase, respectively. Pearson’s correlation analyses indicated that sputum NEP activity were significantly associated with personal exposure product [Elemental carbon concentration, mg/m3 X time, min. (C X T)]. Data suggest that the changes in NEP activity may be an early, accurate endpoint for airway epithelial injury and provided a new insight into the mechanism of the airway effects in these exposure conditions.
Keywords: Particulate matter, Diesel exhaust, Mining dust, Sputum, Neprilysin, Airways, Inflammation
Introduction
Airborne particulate matter (PM) pollution is a major risk to human health. Acute and chronic adverse effects of PM, especially association between elevated levels of ambient PM and mobility or mortality, have been demonstrated epidemiologically (Dockery et al., 1994; Samet et al., 2000; US EPA., 2002; Health Effect Institute. 2003; Nel, 2005). Studies over a broad range of geographical areas indicate that with each 10 μg/m3 ambient PM increase, the daily mortality is augmented by approximately 1-5 % (Pope et al., 2002; Schwartz et al., 2002). Small short-term increases in PM levels, especially diesel exhaust particles (DEP), have been associated with increases in symptoms of respiratory illnesses, including asthma, bronchitis and airway hyper-responsiveness (Pandya et al, 2002; Gong et al.,2003). PM exposure also causes irritation (Rudell et al., 1999; Wong et al., 2003), inflammation (Diaz-Sanchez et al., 1994; Salvi et al., 1999; Nightingale 2000), and functional impairment in the lung (Brunekreef, 1997; McCreanor et al., 2007). However, the mechanisms underlying these health effects still need to be elucidated and biomarkers of effect should be adopted to better address these health risks.
Neprilysin (NEP, also known as neutral endopeptidase, enkephalinase, and CALLA) is a key cell surface peptidase which plays an important role in the maintenance of homeostasis and in the development of many disorders including asthma, chronic obstructive pulmonary disease (COPD), and lung cancer (Borson, 1991; Di Maria et al., 1998; D’Adamio et al., 1989; Djokic et al., 1989). NEP is abundantly expressed on airway epithelial cells, and is also presented in airway smooth muscle cells, submucosal gland cells, and fibroblasts in the lung (Baraniuk et al., 1995). NEP is also present on immune-inflammatory cells, such as macrophages and neutrophils. Its substrates include neurokinins, cytokines, endothelins, angiotensin-II, bombesin, gastrin-releasing peptide, atrial natriuretic peptide, enkephalins, insulin-B chain, and the chemotactic peptide N-formyl-Met-Leu-Phe. These substrates play important roles in numerous physiological and pathophysiological processes, including inflammatory processes (Bozic, 1996; Lotz et al.,1988), airway hyperresponsiveness (Wu & Lee, 1999; Lilly et al., 1994), and carcinogenesis (Papandreou et al., 1998; Usmani et al, 2000; Suzuki et al., 2001; Tomoda et al., 2003). When NEP activity is inhibited, its substrates are less rapidly inactivated and accumulate in the tissue, thus contributing to the exaggerated response or individual susceptibility to environmental stressors (Dusser et al., 1989). Moreover, NEP is a necessary modulator in the development of childhood asthma (Joos et al., 2000), not only because of the vulnerable nature of developmental processes, but also because their airway sensory innervations develop rapidly during early postnatal life in parallel with the developing lung (Hislop et al., 1990). These studies taken together suggest that loss or a decrease of NEP may possibly be involved in the mechanisms of PM-induced effects (Di Maria et al., 1998; Joos et al., 2000).
NEP activity is reduced by mechanical removal of the epithelium, some virus infections, and cigarette smoke. Several of the stimuli known to induce bronchoconstrictor responses in asthmatic patients have been found to decrease airway NEP activity (Di Maria et al., 1998). Little information is available about the effect of PM on airway NEP and its relevancy to PM-induced health effects. Our in vivo study has first demonstrated that NEP activity in rat lung was significantly reduced by the ambient level of diesel exhaust for three weeks (DE, Wong et al., 2003; 2007). Because of its high density of NEP expression in airway epithelium and important regulatory role, it is not surprising that reduction in NEP activity is accompanied with increases in bronchopulmonary plasma extravasation, vascular permeability, cytokine expression, as well as inflammatory/mast cell infiltration, possibly evoked by endogenous peptides after DE exposure. In this human investigation, we evaluated acute changes in airway NEP activity in human subjects following exposure either to mining dust particulates or to DE, a major source of ultrafine particles. It is hypothesized that airway tissue NEP activity is decreased in these mining exposure conditions, as indicated by increases of soluble NEP activity in induced sputum. This hypothesis was generated based on the following evidence: 1) soluble forms of NEP activity have been detected in body fluids, including BAL fluid (Van Der Velden et al., 1999). These soluble counterparts may either be derived from shedding of the entire membrane-bound enzyme or may be formed by post-translational cleavage of membrane-bound form. Therefore, induced sputum could provide an ideal, simple method of testing soluble and cellular NEP; 2) Expression of NEP varies widely in ‘normal human lung’ tissue from different individuals (Cohen et al., 1996), which could, at least in large part if not all, be attributed to environmental factors including PM exposure. 3) Furthermore, a significant decrease in NEP activity in lung tissue has been demonstrated after repeat exposure of rats to the ambient and occupational levels of DE (Wong et al., 2003).
In an effort to test this hypothesis, we found that soluble NEP activity in sputum of subjects significantly increased, indicating loss of airway NEP activity following acute mining dust particulate or DE exposure. Changes in NEP activity may be through the epthelial membrane injury, possiblly being independent of pre-inflammary response of cytokins reported in our previous publication (Burgess et al., 2007).
Methods
Experimental design
The current study was approved by the University of Arizona (UA) Institutional Review Board. The study methods are described in greater detail in a previous publication (Burgess et al., 2007). Informed consent was obtained from all subjects volunteering to participate in the study. Mining students undergoing undergraduate and graduate training in mining engineering at UA were eligible for participation if they were 18 year old or greater. Students, who were current or previous smokers, had existing lung diseases, and who were taking inhaled steroids, were excluded. Eleven subjects, 10 males and one female, ranging in age from 19-33 (mean 23.7 ± 4.3 years), completed the study (Table 1). Seven (64%) of the subjects described themselves as White, 2 (18%) as Hispanic, and one each as Asian and other. None of the subjects were current asthmatics, and no subjects reported taking anti-inflammatory medications. At baseline and before exposure experiments, none of the subjects reported having had a cold, flu, allergies or respiratory symptoms within the previous six days. These subjects had no records having exposed to significant high emission exhaust or room dust, drilling mist, and other particulate sources within one month. The study was carried out at the San Xavier Mining Laboratory, a research and training faculty devoted to occupational health and safety in the mining and underground construction industries, operated under the auspices of the UA College of Engineering, in collaboration with the UA College of Public Health. Mine access and ventilation are designed to simulate underground conditions found in an actual production facility. Respiratory protection was not worn during the experimental process. Changes of cell numbers by type, protein, NEP activity in collected sputum of subjects were evaluated following mining dust particulate and DE exposure that characteristics two major health risks during mucking (removal of ore) operations.
Table 1.
Characteristics of Subjects and Exposure Conditions
| Subjects | Age (years) | Gender | Race Codesa | Exposure Conditions (mg/m3) |
|||
|---|---|---|---|---|---|---|---|
| Baselineb | OS | DE | Time (min.) | ||||
| 01 | 26 | M | 1 | 0.01 | 0.46 | 1.20 | 60 |
| 02 | 33 | M | 4 | 0.01 | 0.00 | 0.64 | 66 |
| 03 | 19 | M | 1 | 0.01 | 1.52 | 1.80 | 56 |
| 04 | 21 | M | 1 | 0.01 | – | 0.75 | 134 |
| 05 | 22 | M | 1 | 0.01 | 1.20 | 0.50 | 134 |
| 06 | 23 | M | 1 | 0.01 | – | 0.38 | 119 |
| 07 | 21 | F | 1 | 0.01 | 0.00 | 0.32 | 85 |
| 08 | 19 | M | 1 | 0.01 | 0.09 | 0.12 | 85 |
| 09 | 30 | M | 2 | 0.01 | 0.00 | 0.38 | 93 |
| 10 | 26 | M | 3 | 0.01 | 0.00 | 0.15 | 66 |
| 11 | 22 | M | 2 | 0.01 | 0.03 | 0.09 | 90 |
Race codes: 1, White; 2, Hispanic; 3, Asian; 4, Others;
Two samples collected over a period of 66-68 minutes as baseline particulate background for all of subjects;
-Subject had no personal particulate exposure data due to handling.
Exposure condition
Two different exposure conditions were used in this study:
1) Mining dust particulate exposure was characterized following overshot (OS) mucking process with a pneumatic (no emission) Eimco 12B OS Mucker. Experiments were conducted in 3 m × 3 m drifts and a 15 hp axial auxiliary fan provided ventilation for the heading. Personal particulate exposure was performed during OS mucking, using SKC aluminum cyclones in the subjects’ breathing zone attached to sampling pumps (SKC AirChek 2000, Eighty Four, PA). The pumps were calibrated at 2.5 SL/min, with an expected 50% cut size of 4.0 μm. Pre- and post-sampling flow rates of the pumps were within 95%. Particulate sample measurements were collected on preweighed PVC filters and analyzed by gravimetric analysis (Cahn 21 Automatic Electrobalance, Ventron Corporation, CA). Gravimetric sampling results were corrected for changes in weight of field blanks collected each sampling day. Particles concentration during OS mucking indicated a mean of 500 ± 770 μg/m3 and a range of 0 to 1,520 μg/m3. Sampling times averaged 110 minutes (range 81-199 minutes).
2) DE exposure was characterized from a diesel-powered 1984 Jarvis Clark JS-220 load-haul-dump (LHD) vehicle with a two cubic yard bucket and an 82 HP Deutz F6L-912W diesel engine fitted with a catalytic converter. Experiments conducted using the LHD were employed in a conventional 4 m × 4 m tunnel decline and employed the same type of ventilation as that of OS mucking. During baseline and DE exposure, two samples, as particulate background, collected over a period of 66-68 minutes demonstrated concentrations of less than10 μg/m3. Diesel exhaust particles (DEP) were collected on precleaned 37mm open-face quartz fiber filters (SKC, Eighty Four, PA) with MSA personal sampling pumps (Escort Elf, Pittsburgh, PA) and analyzed for elemental carbon according to NIOSH method 5040 by the Wisconsin State Hygiene Laboratory (Madison, WI). Personal exposure to DE, as measured by elemental carbon (N = 11) averaged 538 ± 512 (range 91-1800) μg/m3 (Table 1). Exposure times averaged 89 (range 56-134) minutes. Nitrogen dioxide and carbon monoxide concentrations were assessed with a MSA multi-gas detector (Mine Safety Appliance Company, Pittsburgh, PA) during a single mucking shift using the diesel powered LHD. For a single experimental shift monitored for 60 minutes, peak concentrations for NO2 and CO respectively were 1.5 ppm and 22 ppm.
All subjects underwent three evaluations, including a baseline non-exposure day, an OS (non-DE) mucking day, and an LHD (DE exposure) mucking day. These exposure days for all but two subjects per exposure (three day for OS exposure, four days for DE) were at least one week apart. First sputum induction and a health history and exposure questionnaire were completed on non-exposure days. On mucking days, groups of 1-3 subjects first completed an interim health history and mucked for a 1-2 hour period, depending on individual available non-class period, using either OS or an LHD unit. One hour following cessation of mucking, the subjects completed sputum induction. One hour post-exposure test time was chosen to consider the timelines of both the acute response of airway and diffusion of soluble NEP activity. Mucking using the LHD and OS mucker involve low levels of physical activity, since the energy required for moving rock is provided by the piece of equipment used, not the operation. However, the contribution of low level physical exertion to the changes seen in airway NEP activity could not be determined in the current study design and is therefore a limitation that should be further examined in future studies
Sputum induction and treatment
Induced sputum was collected using DeVilbiss Ultra-Neb 99HD ultrasonic nebulizers (Somerset, PA) filled with 3% saline set on maximum output Sputum samples were diluted with 10% Sputolysin (Calbiochem, San Diego, CA) in phosphate buffered saline with penicillin-streptomycin and 0.5% bovine serum albumin. Supernatant was removed by centrifugation and frozen to −80 °C for later analysis of NEP activity. The cellular pellet was reconstituted in 1 ml of the phosphate buffered saline in order to perform total cell counts with the use of a hemocytometer and Trypan Blue stain (Sigma Chemical CO, St. Louis). A portion of the cell pellet was cytocentrifuged (Shandon Cytospin, ThermoShandon, Pittsburgh, PA) onto a microscope slide and stained with Diff-Quik® (Dade Behring AG, Switzerland) for cell number analysis. Protein concentration was determined using a Coomassie Plus Protein Assay (Pierce, Rockford, IL) using BSA as a standard.
NEP enzyme activity measurement
Cell-free NEP activity in sputum was measured spectrophotometrically by a coupled assay as described previously (Wong et al., 2004). Briefly, five μl of cell-free extract were incubated with 1 mM succinyl-Ala-Ala-Phe-p-nitroanilide (Suc-Ala-Ala-Phe-pNA) (Bachem Bioscience Inc., King of Prussia, PA) as a substrate in 0.1 M Tris-HCl (pH 7.6) and 1 μl (0.14 units/μl) of porcine kidney aminopeptidase N (Sigma, St. Louis, MO). The reaction (total volume: 250 μl) was measured in duplicate in a 96-well microtiter plate. In this coupled activity assay, NEP cleaves Suc-Ala-Ala-Phe-pNA between Ala and Phe, yielding Phe-pNA. AP-N subsequently cleaves Phe-pNA, generating pNA as the final product. The increase in specific absorbance at 405 nm (as a result of the accumulation of free p-nitroaniline) was determined after 30 min incubated at 37 °C using a plate reader (BIO-TEK Instruments, Winooski, Vermont). Cell-free (substrate alone or substrate with AP-N) and substrate-free blanks were run in parallel. Protein concentration was determined by using a Coomassie Plus Protein Assay (Pierce, Rockford, IL) with BSA as a standard.
Statistical Analysis
Statistical analyses were performed using SPSS version 15 (Chicago, Illinois). ANOVA and/or t-test was used to compare means of sputum NEP activity, total protein, and cell profile among groups which were normalized as appropriate if not following a Gaussian distribution. Additionally, Pearson’s correlation coefficient was calculated to determine whether there is a linear relationship either between personal exposure and NEP activity or betwwen NEP activity and cell types. Data were expressed as mean ± standard error of the mean (SEM) and p < 0.05 will be considered to be significant (2-tailed).
Results
Cell profile
Exposure of subjects to OS or DE induced an increased trend of inflammatory cells (Table 2). For DE exposure, there was a significant increase in total cell number in sputum. The increase in cell type was macrophages, but not neutrophils and lymphocytes following DE exposure. However, the changes in all of these measurements did not reach a statistical significant level after OS exposure, possibly due to limited sample size. Data suggests that DE exposure resulted in macrophage-related pre-inflammatory or inflammatory response in airways.
Table 2.
Sputum cell profiles in subjectsa exposed to mining dust or diesel exhaust
| Cell Numbersb |
|||
|---|---|---|---|
| Cell types | Baseline | OSc | DEd |
| Inflammatory cells (X106/ml) | |||
| Total | 7.98 ± 1.68 | 12.53 ± 3.28 | 20.29 ± 7.65* |
| Macrophages | 6.53 ± 1.32 | 10.00 ± 2.61 | 16.10 ± 5.57* |
| Neutrophils | 1.29 ± 0.49 | 2.23 ± 0.68 | 4.05 ± 2.32 |
| Lymphocytes | 0.15 ± 0.05 | 0.22 ± 0.07 | 0.21 ± 0.08 |
| Epithelial cells (X103/ml) | 2.80 ± 0.823 | 9.34 ± 5.27* | 12.43 ± 7.98* |
Eleven healthy subjects underwent three evaluations, at least one week apart, at a non-exposure (baseline) day, a dust, non-DE, exposure (OS) day, and a DE exposure (DE) day;
Cell number analysis was performed with Diff-Quik® (Dade Behring AG, Switzerland) on a microscope slide;
Mining particles sample were collected on pre-weighed PVC filters and analyzed by gravimetric analysis;
DE exposure levels were measured by elemental carbon according to NIOSH method 5040;
p < 0.05 when compared to Baseline (N = 11).
Moreover, sputum epithelia were significantly increased by 3.34-fold and 4.44-fold following exposure of subjects to OS and DE, respectively (Table 2). Obviously both exposure conditions resulted in acute airway epithelial shedding.
Total Protein and NEP activity
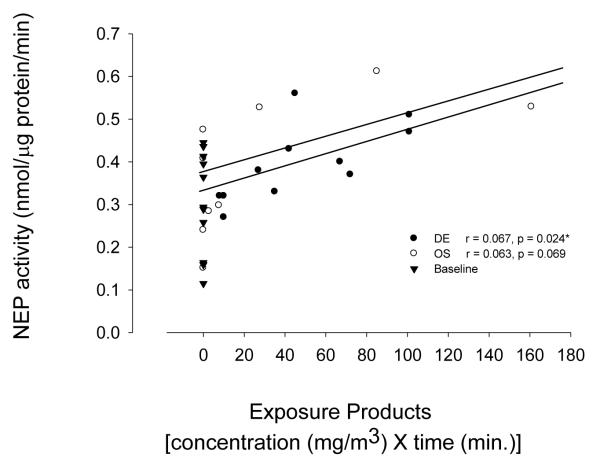
Neither OS exposure (0.89 ± 0.07, mean ± SEM) nor DE exposure (0.88 ± 0.08) was found to induce a significant change in total sputum protein when compared with baseline control (0.82 ± 0.09) (Table 3). Analysis of soluble NEP activity in sputum was done for all 11 healthy nonsmoking volunteers before exposure as baseline. The result showed a range of values from 0.115 to 0.445, averaging 0.303 ± 0.036 (mean ± SEM) nmol/μg protein/minute. Individual increases in NEP activity were observed to be 72.7% (8/11) following OS exposure and 81.8% (9/11) following DE exposure (Table 3). When compared with baseline control, the average net increases in OS and DE exposure groups were 42% and 31%, respectively, with maximum changes 0.53 and 0.26 nmol/μg protein/minute. The changes indicated that a decrease or loss of airway NEP activity occurred after acute exposure of subjects to either ~500 μg/m3 concentrations of μg/m3 mining dust particulates (OS) or ~538 μg/m3 elemental carbon of DE (including particles background <10 μg/m3). To further determine the effects of OS and DE on soluble NEP activity in sputum, an effort were made with Pearson’s correlation analyses. The result indicated that individual sputum NEP activity after DE exposure, but not baseline exposure, were significantly associated with products of exposure concentration X exposure time (Figure 1). However, the association degree between OS exposure and NEP activity was not intensify enough to induce a significant change statistically in current sample size.
Table 3.
Induced sputum protein and Neprilysin (NEP)
| Total protein (μg/μl)a |
NEP (nmol/μg protein/min)b |
|||||
|---|---|---|---|---|---|---|
| Baseline | OS | DE | Baseline | OS | DE | |
| 1.05 | 0.95 | 1.07 | 0.364 | 0.527 | 0.369 | |
| 0.66 | 0.96 | 0.99 | 0.445 | 0.475 | 0.431 | |
| 1.00 | 0.90 | 0.97 | 0.436 | 0.612 | 0.507 | |
| 1.27 | 1.30 | 1.21 | 0.258 | 0.380 | 0.465 | |
| 0.41 | 0.63 | 0.84 | 0.395 | 0.529 | 0.399 | |
| 0.56 | 0.62 | 0.43 | 0.294 | 0.824 | 0.558 | |
| 0.82 | 1.08 | 1.13 | 0.413 | 0.408 | 0.272 | |
| 1.16 | 0.84 | 0.79 | 0.159 | 0.298 | 0.382 | |
| 0.74 | 0.95 | 0.92 | 0.289 | 0.240 | 0.332 | |
| 0.94 | 1.09 | 1.00 | 0.164 | 0.151 | 0.324 | |
| 0.41 | 0.47 | 0.32 | 0.115 | 0.284 | 0.320 | |
|
| ||||||
| Mean | 0.82 | 0.89 | 0.88 | 0.303 | 0.430 | 0.396 |
| SEM | 0.09 | 0.07 | 0.08 | 0.036 | 0.058 | 0.026 |
| p-values* | 0.24 | 0.42 | 0.023 | 0.035 | ||
Protein concentration was determined using a Coomassie Plus Protein Assay using BSA as a standard.
Cell-free NEP activity in induced sputum was measured spectrophotometrically;
p-values based on a two-tailed, paired sample t-test (N = 11).
Figure 1.
Correlations of soluble neprilysin (NEP) activity in subject sputum (N =11) with mining dust particulates (OS) and diesel exhaust (DE) exposure [concentrations, mg/m3 X time, min. (C X T)]. DE exposure was not corrected for pre-exposure levels in NEP activity (Data not shown). Cell-free NEP activity in induced sputum was measured spectrophotometrically. Personal particulate exposure was performed during OS mucking, using SKC aluminum cyclones in the subjects’ breathing zone attached to sampling pumps (SKC AirChek 2000, Eighty Four, PA). DE was collected on precleaned 37mm open-face quartz fiber filters (SKC, Eighty Four, PA) with MSA personal sampling pumps (Escort Elf, Pittsburgh, PA) and analyzed for elemental carbon according to NIOSH method 5040 by the Wisconsin State Hygiene Laboratory (Madison, WI). *Statistically significant.
Associations of sputum NEP activity with cell types
To further analyze the cellular sources effect of NEP activity, Pearson’s correlation analyses was carried out. Data showed that sputum NEP activity was not significantly associated with changes in total cells number and cell types in sputum, including macrophages, neutrophils, and epithelial cells (Data not shown).
Discussion
Mining workers in underground frequently face two major occupational health risks, one from mining dust particulates and another from diesel exhaust. To characterize their acute respiratory effects, we conducted the current controlled human exposure study in the San Xavier Mining Laboratory, a research and training faculty devoted to occupational health and safety in the mining and underground construction industries. Our previous publication has been shown that acute changes in sputum IL-10 following exposure of human volunteers to DE, while other sputum measurements including IL-1β, IL-4, IL-6, IL-8, TNF-a, 8-OHdG did not significantly change under OS and DE exposure conditions due to sampling time and limited sample size (Burgess et al., 2007). Also, there were no significant changes in cross-shift spirometry, as measured by FEV1 (3.81 ± 0.90 vs. 3.75 ± 0.90, p = 0.367 for OS exposure; 4.03 ± 0.99 vs. 3.89 ± 1.01, p = 0.08 for DE exposure) and FVC(L) (4.93 ± 1.16 vs. 4.84 ± 1.25, p = 0.307 for OS exposure; 5.16 ± 1.25 vs. 5.00 ± 1.34, p = 0.178 for DE exposure).
In this paper, we report that there is acute loss of NEP activity from airway tissue after exposure of these subjects to either mining dust particulates or DE, as indicated by increases of soluble NEP activity in sputum. This finding was similar to the results of our previous animal study, showing that NEP activities were consistently reduced in rat lungs exposed to the ambient (35.3 μg/ m3 particles) and occupational (669.3 μg/ m3 particles) levels of DE for three weeks (Wong et al., 2003). Although the relevancy and the mechanism underlying this effect remain to be determined, a decrease or loss of NEP activity in airways may be an early and important endpoint for these exposures. Obviously, changes in NEP activity occurred before current known preinflammatory cytokine biomarkers (such as IL-6 and IL-8), oxidative indicator (8-OHdG), and spirometry measurements. More importantly, its change may be mechanistically linked to many adverse effects in the lung, being observed in our previous animal studies (Wong et al., 2003: Witten et al., 2005).
The decrease or loss of NEP activity in airways could change its substrates-induced response pattern and degree, consequently resulting in inflammatory response. It is well known that NEP effectively controls the bioavailability of peptide mediators released via sensory nerve terminals or immuno-inflammatory cells. A critical factor that governs tissue response for NEP is its distribution density and co-location with the peptide receptors on cell membrane. When a decrease or loss of NEP activity from airway tissue, accumulating peptides rapidly diffuse into tissue, leading to an abnormal neuron-immune communication (Dusser et al., 1989; Wu & Lee, 1999). The resulting effects of these peptides, especially from sensory fibers, are characterized by obvious vasodilatation, increased postcapillary venule permeability, inflammatory cell influx, and mucus secretion, collectively termed as neurogenic inflammation. Therefore, it is possible that the exposure-induced change in NEP activity in this study may indirectly be associated with an increase in inflammatory cells through its substrate mechanisms above (Lilly et al., 1994; Lu et al., 1996; 1997).
Moreover, soluble NEP (especially extracellular domain) might possibly enter into the blood system with loss of airway epithelial barrier integrity, leading to systemic effects. This speculation is supported by several previous studies that increased NEP activities in serum were observed from underground miners exposed to coal dust particles (Soleilhac et al., 1996) or patients with pulmonary inflammatory diseases, such as sarcoidosis and adult respiratory distress syndrome (Johnson et al., 1985; Almenoff et al.,1986). Increased NEP activities in serum may reflect local tissue damage with subsequent shedding of membrane-bound enzymes and an abnormal release from the airways in response to lung injury (Soleilhac et al., 1996). In this study, significant increases of soluble NEP activity following exposures suggest that membrane-bound NEP in airways was modified or impaired structurally and functionally. Therefore, it is reasonable to consider serum NEP as a potential biomarker in future studies if the systemic contribution of NEP was characterized in future studies.
The cellular origin and mechanism of soluble NEP activity in sputum of exposed people are multiple. In this case, airway epithelial cells could be major source of soluble NEP activity found in sputum due to exposure-induced membrane damage. The reasons for this speculation are 1) Epithelial cells not only abundantly express NEP that was directly targeted by inhaled particles and/or gases; 2) NEP structurally is a cell-surface metalloprotease with a large extracellular domain (700 amino acids), which contains six potential N-glycosylation sites and the pentapeptide consensus sequence (His-Glu-[Ile, Leu, Met]-X-His) of zinc-binding metalloproteases, in which the two histidines coordinate zinc and the glutamic acid. Obviously, its large body of extracellular domain with the catalytic sequence not only illustrates its critical structural basis to rapidly cleave substrates, but also to possibly be highly susceptible to toxic insults. Therefore, a most likely mechanism is the shedding process of affected epithelial cells in airways after exposure, with membrane-bound proteins being released with portions of plasmic membrane or as proteolipid aggregates. In addition, inflammatory cells, especially affected neutrophils and macrophages during degranulation after exposure, might be another possible resource of soluble NEP activity in sputum. However, we did not find in this study that the increase in sputum NEP activity was correlated with changes of either neutrophils or macrophages numbers following both exposure conditions. Increases in cell numbers of total, macrophages, and epithelium just occurred in DE exposure, but not OS exposure, suggesting the changes in NEP activity seems to be independent of increased inflammatory or epithelial cells. Taken together, we speculate that increase in sputum NEP activities of subject may be attributable more to non-shedding epithelial cells after airway injury induced by exposure conditions.
This study provides a novel endpoint and insight into a mechanism for the airway adverse effects in mining conditions. We realize that it dose not suffice to actually determine if lower exposures at realistic enviromental concentrations have an impact on NEP activity. Also, it unlikely that this human study alone will suffice to experimentally test whether exposure to realistic levels of PM cause a decrease in NEP activity that has functional consequences for airway biology due to limited sample size. In this regard, we are conducting the animal study to establish how the expression pattern of NEP is changed in tissues and BAL fluid with NEP null mice. We hope that soluble NEP activity in sputum could serve as potential early biomarker to identify population risk if these results are further confirmed by in vivo animal and larger human population of investigations at the ambient exposure level.
Supplementary Material
Acknowledgements
This study was supported by research contract of Health Effects Institute (HEI)/U.S. EPA (to S.S.W.), training grant of CDC/NIOSH and NIEHS (to J.L.B.), and center grant number ES06694. The contents are solely the responsibility of the authors and do not necessarily represent the official views of HEI/U.S. EPA or CDC/NIOSH.
Abbreviations
- NEP
Neprilysin
- DE
diesel exhaust
- DEP
Diesel exhaust particle
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- IL
Interleukin
- PM
particulate matter
- LHD
load-haul-dump
- OS
Overshot
- SEM
standard error of the mean
- COPD
chronic obstructive pulmonary disease
- UA
University of Arizona
- 8-OHdG
8-hydroxy-2′-deoxyguanosine
References
- Almenoff J, Skovron ML, Teirstein AS. Thermolysin-like serum metalloendopeptidase. A new marker for active sarcoidosis that complements serum angiotensin-converting enzyme. Ann. NY. Acad. Sci. 1986;465:738–743. doi: 10.1111/j.1749-6632.1986.tb18553.x. [DOI] [PubMed] [Google Scholar]
- Baraniuk JN, Ohkubo K, Kwon OJ, Mak J, Ali M, Davies R, Twort C, Kaliner M, Letarte M, Barnes PJ. Localization of neutral endopeptidase (NEP) mRNA in human bronchi. Eur. Respi.r J. 1995;8:1458–1464. [PubMed] [Google Scholar]
- Borson DB. Roles of neutral endopeptidase in airways. Am. J. Physiol. 1991;260:L212–L225. doi: 10.1152/ajplung.1991.260.4.L212. [DOI] [PubMed] [Google Scholar]
- Bozic CR, Lu B, Höpken UE, Gerard C, Gerard NP. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Janssen NA, de Hartog J, Harssema H, Knape M, van Vliet P. Air pollution from truck traffic and lung function in children living near motorways. Epidemiology. 1997;8:298–303. doi: 10.1097/00001648-199705000-00012. [DOI] [PubMed] [Google Scholar]
- Burgess JL, Fleming JE, Mulenga EM, Josyula A, Hysong TA, Joggerst PJ, Kurzius-Spencer M, Miller HB. Acute changes in sputum IL-10 following underground exposure to diesel exhaust. Clinical Toxicology. 2007;45:255–260. doi: 10.1080/15563650601072142. [DOI] [PubMed] [Google Scholar]
- Cohen AJ, Bunn PA, Franklin W, Magill-Solc C, Hartmann C, Helfrich B, Gilman L, Folkvord J, Helm K, Miller YE. Neutral endopeptidase: variable expression in human lung, inactivation in lung cancer, and modulation of peptide-induced calcium flux. Cancer Res. 1996;56:831–839. [PubMed] [Google Scholar]
- Cohen AJ, Franklin WA, Magill C, Sorenson J, Miller YE. Low neutral endopeptidase levels in bronchoalveolar lavage fluid of lung cancer patients. Am. J. Respir. Crit Care. Med. 1999;159:907–910. doi: 10.1164/ajrccm.159.3.9806062. [DOI] [PubMed] [Google Scholar]
- D’Adamio L, Shipp MA, Masteller EL, Reinherz EL. Organization of the gene encoding common acute lymphoblastic leukemia antigen (neutral endopeptidase 24.11): multiple miniexons and separate 5′ untranslated regions. Proc. Natl. Acad. Sci. U. S. A. 1989;86:7103–7107. doi: 10.1073/pnas.86.18.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maria GU, Bellofiore S, Geppetti P. Regulation of airway neurogenic inflammation by neutral endopeptidase. Eur. Respir. J. 1998;12:1454–1462. doi: 10.1183/09031936.98.12061454. [DOI] [PubMed] [Google Scholar]
- Diaz-Sanchez D, Dotson AR, Takenaka H, Saxon A. Diesel exhaust particles induce local IgE production in vivo and alter the pattern of IgE messenger RNA isoforms. J. Clin. Invest. 1994;94:1417–1425. doi: 10.1172/JCI117478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djokic TD, Nadel JA, Dusser DJ, Sekizawa K, Graf PD, Borson DB. Inhibitors of neutral endopeptidase potentiate electrically and capsaicin-induced noncholinergic contraction in guinea pig bronchi. J. Pharmacol. Exp. Ther. 1989;248:7–11. [PubMed] [Google Scholar]
- Dockery DW, Pope CA. Acute respiratory effects of particulate air pollution. Annu. Rev. Public Health. 1994;15:107–132. doi: 10.1146/annurev.pu.15.050194.000543. [DOI] [PubMed] [Google Scholar]
- Dusser DJ, Djokic TD, Borson DB, Nadel JA. Cigarette smoke induces bronchoconstrictor hyperresponsiveness to substance P and inactivates airway neutral endopeptidase in the guinea pig. Possible role of free radicals. J. Clin. Invest. 1989;84:900–906. doi: 10.1172/JCI114251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency (US) Health Assessment Document for Diesel Engine Exhaust. EPA/600/8-90/057F. National Center for Environmental Assessment, Office of Research and Development; Washington DC: 2002. [Google Scholar]
- Gong H, Jr., Sioutas C, Linn WS. Controlled exposures of healthy and asthmatic volunteers to concentrated ambient particles in metropolitan Los Angeles. Res. Rep. Health Eff. Inst. 2003;118:1–36. [PubMed] [Google Scholar]
- Health Effect Institute . Communication 10. Health Effect Institute; Boston, MA: 2003. Improving estimation of diesel and other emissions for epidemiological studies. [Google Scholar]
- Hislop AA, Wharton J, Allen KM, Polak JM, Haworth SG. Immunohistochemical localization of peptide-containing nerves in human airways: Age-related changes. Am. J. Respir. Cell Mol. Biol. 1990;3:191–198. [PubMed] [Google Scholar]
- Johnson AR, Coalson JJ, Ashton J, Larumbide M, Erdos EG. Neutral endopeptidase in serum samples from patients with adult respiratory distress syndrome. Comparison with angiotensin-converting enzyme. Am. Rev. Respir. Dis. 1985;132:1262–1267. doi: 10.1164/arrd.1985.132.6.1262. [DOI] [PubMed] [Google Scholar]
- Joos GF, Germonpre PR, Pauwels RA. Role of tachykinins in asthma. Allergy. 2000;55:321–337. doi: 10.1034/j.1398-9995.2000.00112.x. [DOI] [PubMed] [Google Scholar]
- Lilly CM, Kobzik L, Hall AE, Drazen JM. Effects of chronic airway inflammation on the activity and enzymatic inactivation of neuropeptides in guinea pig lungs. J. Clin. Invest. 1994;93:2667–2674. doi: 10.1172/JCI117280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M, Vaughan JH, Carson DA. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241:1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- Lu B, Figini M, Emanueli C, Geppetti P, Grady EF, Gerard NP, Ansell J, Payan DG, Gerard C, Bunnett N. The control of microvascular permeability and blood pressure by neutral endopeptidase. Nat. Med. 1997;8:904–907. doi: 10.1038/nm0897-904. [DOI] [PubMed] [Google Scholar]
- Lu B, Gerard NP, Kolakowski LF, Jr., Finco O, Carroll MC, Gerard C. Neutral endopeptidase modulates septic shock. Ann. N. Y. Acad. Sci. 1996;780:156–163. doi: 10.1111/j.1749-6632.1996.tb15119.x. [DOI] [PubMed] [Google Scholar]
- McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L, Harrington R, Svartengren M, Han IK, Ohman-Strickland P, Chung KF, Zhang J. Respiratory effects of exposure to diesel traffic in persons with asthma. N. Engl. J. Med. 2007;357:2348–2358. doi: 10.1056/NEJMoa071535. [DOI] [PubMed] [Google Scholar]
- Nel A. Air pollution-related illness: effects of particles. Science. 2005;308:804–806. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- Nightingale JA, Maggs R, Cullinan P, Donnelly LE, Rogers DF, Kinnersley R, Chung KF, Barnes PJ, Ashmore M, Newman-Taylor A. Airway inflammation after controlled exposure to diesel exhaust particulates. Am. J. Respir. Crit. Care Med. 2000;162:161–166. doi: 10.1164/ajrccm.162.1.9908092. [DOI] [PubMed] [Google Scholar]
- Pandya RJ, Solomon G, Kinner A, Balmes JR. Diesel exhaust and asthma: hypotheses and molecular mechanisms of action. Environ. Health Perspect. 2002;110(Suppl. 1):103–12. doi: 10.1289/ehp.02110s1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou CN, Usmani B, Geng YP, Bogenrieder T, Freeman RH, Wilk S, Finstad CL, Reuter VE, Powell CT, Scheinberg D, Magill C, Scher HI, Albino AP, Nanus DM. Neutral endopeptidase 24.11 loss in metastatic human prostate cancer contributes to androgen-independent progression. Nat. Med. 1998;4:50–57. doi: 10.1038/nm0198-050. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd., Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudell B, Ledin MC, Hammarström U, Stjernberg N, Lundbäck B, Sandström T. Effects on symptoms and lung function in humans experimentally exposed to diesel exhaust. Occup. Environ. Med. 1996;53:658–662. doi: 10.1136/oem.53.10.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi S, Blomberg A, Rudell B, Kelly F, Sandström T, Holgate ST, Frew A. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am. J. Respir. Crit. Care Med. 1999;159:702–709. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- Samet JM, Zeger SL, Dominici F, Curriero F, Coursac I, Dockery DW, Schwartz J, Zanobetti A. Research Report 94. Health Effects Institute; Cambridge MA: 2000. The National Morbidity, Mortality, and Air Pollution Study, Part II: Morbidity and Mortality from Air Pollution in the United States. [PubMed] [Google Scholar]
- Schwartz J, Laden F, Zanobetti A. The concentration-response relation between PM(2.5) and daily deaths. Environ. Health Perspect. 2002;110:1025–1029. doi: 10.1289/ehp.021101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleilhac JM, Lafuma C, Porcher JM, Auburtin G, Roques BP. Characterization of a soluble form of neutral endopeptidase-24.11 (EC 3.4.24.11) in human serum: enhancement of its activity in serum of underground miners exposed to coal dust particles. Eur. J. Clin. Invest. 1996;26:1011–1017. doi: 10.1046/j.1365-2362.1996.2420580.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kikkawa F, Ino K, Nagasaka T, Tamakoshi K, Mizutani S. Imbalance between neutral endopeptidase 24.11 and endothelin-1 expression in human endometrial carcinoma. Oncology. 2001;60:258–267. doi: 10.1159/000055327. [DOI] [PubMed] [Google Scholar]
- Tomoda C, Kushima R, Takeuti E, Mukaisho K, Hattori T, Kitano H. CD10 expression is useful in the diagnosis of follicular carcinoma and follicular variant of papillary thyroid carcinoma. Thyroid. 2003;13:291–295. doi: 10.1089/105072503321582105. [DOI] [PubMed] [Google Scholar]
- Usmani BA, Shen R, Janeczko M, Papandreou CN, Lee WH, Nelson WG, Nelson JB, Nanus DM. Methylation of the neutral endopeptidase gene promoter in human prostate cancers. Clin. Cancer Res. 2000;6:1664–1670. [PubMed] [Google Scholar]
- Van Der Velden VH, Naber BA, Van Hal PT, Overbeek SE, Hoogsteden HC, Versnel MA. Peptidase activities in serum and bronchoalveolar lavage fluid from allergic asthmatics--comparison with healthy non-smokers and smokers and effects of inhaled glucocorticoids. Clin. Exp. Allergy. 1999;29:813–823. doi: 10.1046/j.1365-2222.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- Witten ML, Wong SS, Sun NN, Keith I, Kweon C-B, Foster DE, Schauer JJ. Neurogenic responses in rat lungs after nose-only exposure to diesel exhaust. Res Rep Health Eff Inst. 2005;128:1–37. [PubMed] [Google Scholar]
- Wong SS, Sun NN, Keith I, Kweon C-B, Foster DE, Schauer JJ, Witten ML. Tachykinin substance P signaling involved in diesel exhaust-induced bronchopulmonary neurogenic inflammation in rats. Arch. Toxicol. 2003;77:638–650. doi: 10.1007/s00204-003-0485-4. [DOI] [PubMed] [Google Scholar]
- Wong SS, Sun NN, Lantz RC, Witten ML. Substance P and neutral endopeptidase in development of ARDS following fire smoke inhalation. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;277:L859–L866. doi: 10.1152/ajplung.00388.2003. [DOI] [PubMed] [Google Scholar]
- Wong SS, Sun NN, Witten ML, Lantz RC, Lu B, Gerard C, Hersh LB. Diesel particulate-induced neutral endopeptidase downregulation is associated with cell proliferation. Toxicologist. 2007;96:105–106. [Google Scholar]
- Wu ZX, Lee LY. Airway hyperresponsiveness induced by chronic exposure to cigarette smoke in guinea pigs: role of tachykinins. J. Appl. Physiol. 1999;87:1621–168. doi: 10.1152/jappl.1999.87.5.1621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.