Abstract
New multifunctional, degradable, polymeric biomaterial systems would provide versatile platforms to address cell and tissue needs in both in vitro and in vivo environments. While protein-based composites or alloys are the building blocks of biological organisms, similar systems have not been largely exploited to dates to generate ad hoc biomaterials able to control and direct biological functions, by recapitulating their inherent structural and mechanical complexities. Therefore, we have recently proposed silk-tropoelastin material platforms able to conjugate a mechanically robust and durable protein, silk, to a highly flexible and biologically active protein, tropoelastin. This review focuses on the elucidation of the interactions between silk and tropoelastin in order to control material structure, properties, and ultimately functions. In addition, an approach is provided for novel material designs to provide tools to control biological outcomes via surface roughness, elasticity, and net charge for neuronal and mesenchymal stem cell-based tissue engineering.
1. Introduction
Multicomponent polymeric systems allow the engineering of materials with novel and complementary properties [1]. Combining multiple elements offers an efficient means of optimizing preexisting properties of the individual polymer components, while broadening final utility. The human body consists of a variety of protein-protein composites, which determine the structure and function in highly organized tissues, such as bone and cartilage. Multifunctional, degradable, polymeric biomaterial systems are powerful systems, which can be tailored to specific cell and tissue needs both in vitro and in vivo. For example, material systems that can provide mechanically robust and durable platforms incorporating highly flexible and dynamic elements would allow control over biological functions by mimicking the elasticity of tissue structures. Several protein-based composite materials have been recently developed for biomedical applications, in forms of particles, fibers, films, foams, and hydrogels. In particular, collagen has been added to elastin in form of thin films [2] and microfibers [3], to knitted silk scaffold for tendon regeneration [4], and to electrospun silk mat for dura mater replacement [5] in order to improve mechanical and biological properties. Despite the excellent biological features, reconstituted collagen constructs do not offer biologically relevant mechanical strength and long-term stability.
The combination of a highly elastic, dynamic and biologically active structural protein, tropoelastin, with a highly versatile, tough, and durable protein, silk, can generate novel multifunctional composite systems. In the context of natural polymers, tropoelastin offers a wide range of advantages by providing biologically relevant mechanical features together with improved cellular interactions and tissue regeneration [6-8]. In comparison, silk from Bombyx mori silkworms is extensively used in the biomedical and material fields, due to exquisite integration of mechanical properties, relatively slow degradation, and versatile processability into a variety of material formats for multifaceted applications [9]. However, elastin-based biomaterials suffer from inadequate mechanical strength and require chemical cross-linking to achieve structural integrity, thus limiting their clinical applications in tissue regeneration [10, 11]. To overcome this limitation, tropoelastin has been previously combined with silk-derived peptides through genetic engineering [12, 13]. These silk-elastin block (SELPs) copolymers provide versatility in chemistry and control of functional properties. However, the cost-benefit ratio of recombinant silk-elastin copolymer production is high relative to the needs for large scale implementation. Therefore, alternative strategies have been sought to easily combine tropoelastin and silk in aqueous solution, by physical blending, a more traditional approach to polymer composites albeit with relatively limited previous exploration and applicability to the needs in the fields of biomaterials and regenerative medicine. Polymers with good miscibility can be combined in blends, with improved physical and chemical properties, which are strongly influenced by the phase behavior of the blend or by the interactions between the components [1]. These proteins encompass a range of biomaterial needs; tropoelastin provides highly flexible and dynamic structural features, while silk offers mechanical toughness and controllable degradation. Additionally, silk stabilizes the tropoelastin, eliminating the need for chemical cross-linkers in these silk-tropoelastin systems.
The goal of this review is to provide an overview of these new silk-tropoelastin biomaterials; including the mechanistic interactions between the two biopolymers that give rise to the unique features of the alloys, together with the investigation of cell responses mediated by the mechanical, charge density and morphological features of silk-tropoelastin biomaterials for mesenchymal and neuronal lineages [14-16]. Recent findings have contributed to the understanding and control over the interactions of these two protein components at the molecular-level, analogous to the extracellular matrix mode of assembly where the level of interactions is modulated by controlling interfaces among biopolymers components (e.g. glycosaminoglycans, collagen fibers) [17]. We also see the approaches developed here as a path forward for other polymeric combinations that can yield new and useful structural and functional features for biomaterials and regenerative medicine, along with other material fields.
2. Core components
Silk fibroin
Silks are a class of protein polymers spun into fibers by some Lepidoptera larvae, including insects, silkworms, and spiders [18]. The most utilized silks are from the domesticated silkworm, Bombyx mori, and secondarily from spiders, such as Araneus diadematus [19, 20]. Silk is a fibrous protein characterized by a highly repetitive primary sequence that determines significant homogeneity in protein secondary structure (β-sheets for most silks). The B. mori silk fibers consist of two proteins-a hydrophobic silk fibroin (often referred to as 'silk') at the core of the fiber and hydrophilic sericins, a set of 'glue-like' proteins that surround and stabilize the silk fibroin core [21, 22]. Silk fibroin consists of a ~26 kDa light chain and a ~370 kDa heavy chain linked by a disulfide bond. The sequence repeats in the fibroin heavy chain include alanine-glycine repetitions with serine or tyrosine, with typical GAGAGS, GAGAGY and GAGAGVGY sequences [23].These large hydrophobic domains allow for tight packing of stacked sheets of hydrogen bonded anti-parallel chains of the protein [24, 25]. These features are also responsible in part for the robust mechanical properties of silk materials, the relatively low water content of the native fibers, the slow degradation of silk in vivo and the ability to exploit these physical cross-links (beta sheets) and avoid the need for chemical crosslinking to stabilize silk materials.
The presence of large hydrophobic domains, alternating with smaller hydrophilic regions, determine the protein assembly and provides the basis for the exceptional mechanical properties of silk fibers (Figure 1A) [26]. The modulation of silk secondary structure from amorphous to beta-sheet crystalline structure can be induced in vitro with various methodologies, including methanol treatment, heat and aqueous vapour exposures, lowering pH, vortex or sonication processes [27]. Silk fibres display remarkable strength and toughness (i.e. in the case of Bombyx mori silk, strength of 500 MPa and elastic modulus of 5-12 GPa) [9, 28, 29], which in the case of spider silks translate to mechanical performance comparable with synthetic fibers such as Kevlar [30]. Due to its mechanical properties and high versatility, this family of natural proteins offers a valuable set of features as biomaterial platform.
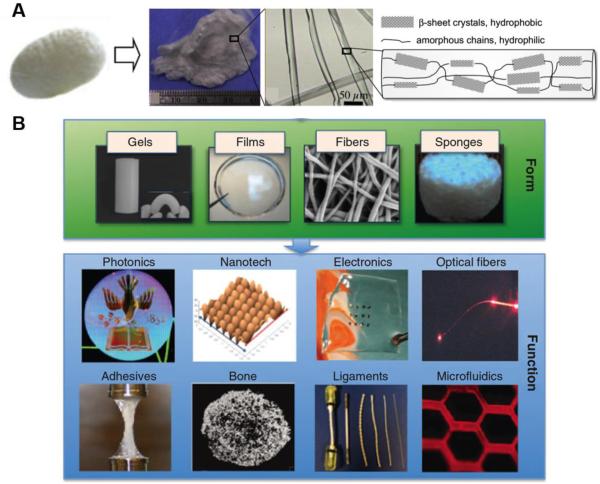
Figure 1. Silk structure and material platforms.
A) Silk fibroin is extracted from Bombyx mori cocoons through an alkaline process. Silk fibers consist of repetitive β-sheet structures (hydrophobic domains) alternated with amorphous chains (hydrophilic regions). Aqueous silk solution can then be processed in a wide range of forms, as hydrogels, fibers, and sponges. The properties of these forms can be adapted to generate multi-functional material platforms. Adapted from [26] [9].
Unlike many other protein and polymer system, silk can be processed under aqueous conditions and without the use of chemical cross-linkers to stabilize the materials. Silk has been processed into a range of material formats, including scaffold, sponges, films, hydrogels, fibers and coatings, offering many new biomaterials, tissue engineering scaffolds and drug release systems, as well as bio-optics and bio-electronics platforms (Figure 1B) [9]. Silk is compatible with multiple sterilization regimes, including autoclaving, ethylene oxide, plasma, gamma irradiation and dry heat [27] and is well tolerated in the body and does not elicit a significant immune response. The ability to control beta-sheet content in silk-based constructs allows tunable proteolytic degradation of silk-based devices for biomedical application, ranging from hours to years depending on the beta-sheet content [31, 32].
Tropoelastin
Tropoelastin is a monomeric precursor of the polymer elastin, a major component of the elastic fiber. Elastic fiber, consisting of the elastin core wrapped in a sheath of microfibrils, provides the elasticity necessary for repetitive and reversible deformation of a range of mammalian tissues, including lung, blood vessels, skin, tendons and ligaments [33, 34]. In addition to the unique mechanical properties, elastin plays an important role in cell signaling in tissues [35]. Elastogenesis in the human body occurs predominantly during late fetal and early neonatal stages and as a result of the extreme durability of elastin (half life of ~70 years), there is very little elastin turnover in healthy tissues [36]. The critical role of elastin in healthy tissue functions is confirmed in the severity of conditions where elastin is damaged or missing, both induced (e.g. aortic aneurysm, emphysema, burn injuries) and genetic (e.g. supravalvular aortic stenosis, cutis laxa) [37].
Elastin's capacity for efficient elasticity and a unique cell signaling role stems from the properties of its precursor tropoelastin. Recombinant DNA technology and subsequent expression of full length tropoelastin protein in an E. coli system has allowed an improved understanding of the structure and function of tropoelastin, which has helped to translate the protein toward utility in biomaterial systems [38]. Tropoelastin is a ~60 kDa protein (depending on the splice variant) encoded by a single copy gene located in the 7q12.2 region in humans [36, 39]. It is a 20 nm long asymmetric molecule with a gradual coil along the long spring like axis of the molecules, accounting for its elasticity and a foot region encompassing the cell interactive C-terminus (Figure 2A) [40]. Single tropoelastin molecule has the capacity to extend ~8 times its resting length and return to its original state following deformation, acting as a near prefect spring with minimal energy loss. The Young's modulus of a single tropoelastin molecule is ~3 kPa (determined by atomic force microscopy) making it significantly more elastic than other ECM and elastic fiber molecules [40, 41]. Tropoelastin is characterized by alternating hydrophilic and hydrophobic domains encoded by separate exons (Figure 2B) [42, 43]. The hydrophilic domains, rich in Lys and Ala, are involved in tropoelastin cross-linking to elastin, while the hydrophobic domains, rich in Val, Pro, Ala and Gly, are implicated in monomer association in solution, confer elasticity and contain cell interacting domains [42].
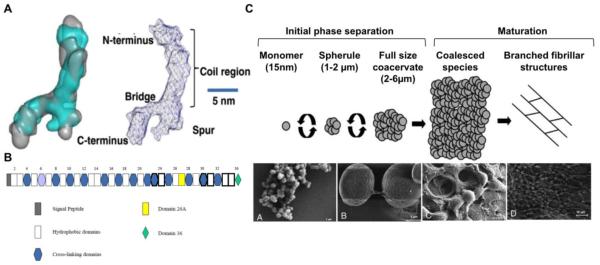
Figure 2. Tropoelastin structure and assembly.
(A) Tropoelastin molecule structure obtained by superimposing filtered average small angle x-ray scattering (SAXS) and small angle neutron scattering (SANS) models (left) and labeled diagram of the model for full-length tropoelastin showing proposed locations of the N-terminus, the spur region containing exons 20–24, and the C-terminus (Scale bar, 5 nm). Adapted from[40]. (B) Human tropoelastin domain map, showing all exons. Exons subject to alternative splicing are outlined in bold. Exon 26A is rarely expressed in human tropoelastin. Domain 36 is uniquely designated because of its distinct structural features. Adapted from [42]. (C) Stages of in vitro tropoelastin self-assembly. The initial phase separation is a reversible process characterized by the formation of 1–2 μm spherules which progressively grow into ~6 μm assemblies. The second maturation stage involves irreversible aggregation of full-sized coacervates into larger species which may display branched fibrillar structures. Scanning electron micrograph of tropoelastin spherules (A), full-sized coacervates (B), and coalesced species (C–D). Adapted from [46, 47].
Tropoelastin assembly into elastic fibers occurs through a multistep process involving: (i) tropoelastin synthesis by elastogenic cells (such as smooth muscle cells, endothelial cells, fibroblasts) and secretion into the extracellular space, (ii) self-association of tropoelastin molecules in a process known as coacervation, (iii) deposition of coacervated tropoelastin on microfibrillar components and (iv) lysyl oxidase-mediated cross-linking to form the insoluble elastic fiber [42, 44, 45]. Tropoelastin's intrinsic capacity for self assembly is demonstrated by its ability to coacervate in vitro under physiological temperature and salt conditions in the absence of cellular components [46]. This process involves the association of tropoelastin molecules upon reaching the transition temperature in a reversible phase separation stage. At this point, tropoelastin associates into 1-2 µm spherules and 2-6 µm assemblies, followed by a maturation stage where tropoelastin coacervates undergo irreversible aggregation and formation of fibrillar structures (Figure 2C) [46, 47]. This process is exploited in the formation of synthetic human elastin hydrogels, where the final structure is stabilized with a chemical cross-linker to form highly elastic, hydrated, porous constructs that support cellular interactions [48].
Cell responses to elastin are diverse and include cell migration and chemotaxis (monocytes, neutrophils, fibroblasts), cell adhesion and proliferation (fibroblasts, endothelial cells) and promotion or inhibition of cell differentiation (keratinocytes and dermal fibroblasts respectively). These responses are mediated through the elastin binding protein (interacts with GXXPG sequences in the hydrophobic domains of tropoelastin), integrins especially αvβ3 which interacts with the GRKRK sequence at the C-terminus of tropoelastin) and through interactions of glycosaminoglycans on the cell surface near the C-terminus region [35].
Full length recombinant human tropoelastin has been increasingly explored in the biomaterials field as a surface coating for blood interacting devices (e.g. vascular grafts and stents) as well as for scaffolding platforms for regenerative medicine, especially for vascular and skin systems. Tropoelastin has been formed into a number of materials including a range of porous hydrogel formats, electrospun mats and tubes and composite materials in combinations with polycaprolactone, collagen and silk [35].
3. Controlling silk-tropoelastin biomaterial functions
The extracellular matrices of tissues are populated by large families of hydrated proteins and glycosaminoglycans, resulting in biological materials that impact mechanics, cell interactions, regeneration and repair, and many other cell- and tissue-related processes [49, 50]. Two main proteins that dominate many tissues and modulate their mechanical behavior include elastin and collagens, which confer elasticity and stiffness, respectively. For example, blood vessels contain elastin and collagen ranging from a 3:2 ratio in large arteries to 1:2 ratio in small tributaries [51], while stiffer matrices such as in articular cartilage contain a higher percentage of collagen (>25%) and traces of elastin (<5%) [52, 53]. This protein composite approach in natural tissues suggests direct utility towards biomaterial designs, where composition, chemistry, and structural organization directly impact functions. The use of collagen in such composite systems is limited due to inadequate mechanical properties with regenerated collagens, rapid in vivo degradation, and loss of structural integrity arising from cell-mediated remodeling [54]. Further, the generation of adequate amounts of recombinant human collagens remains problematic due to a combination of the complex post-translational requirements combined with the challenging in vitro recapitulation of the self-assembly and structural hierarchy features of collagens. Finally, residual biological contaminants in collagen preparations and the need for chemical cross-linking to improve stability, with the consequent negative effects on biological signaling are added concerns. Therefore, silk has been explored in the lieu of collagen, as it has been previously demonstrated to be a robust, slowly degrading biomaterial with good biocompatibility, and has been FDA-approved for some medical devices which streamlines the clinical application of future silk-derived materials [24]. Analogous to human tissues, where extracellular matrices are comprised of proteins organized in composite formats to control mechanical and biological functions, a protein-based composite biomaterials platform based on silk-tropoelastin systems provides a broader spectrum of materials properties, including a range of compliance, degradation and dynamic features. However, a key issue that had to be addressed was the requirement for strong molecular recognition between the two protein components (silk and tropoelastin) in order to attain an adequate range of functional features for the composite materials, analogous to what is found in vivo with the combination of collagen and tropoelastin.
Mechanistic interactions between tropoelastin and silk
In the context of protein-protein composites, the miscibility between the two protein components is critical to define the level of protein interactions, and consequently the functions of the final materials. The nature and extent of interactions between the constituents determine the presence of a homogenous macrophase within the protein blend [55]. Experimentally, support for protein miscibility in protein mixtures is the presence of a single glass transition (Tg), which is expected to be between the Tg of the individual components [56]. The thermal behavior of silk-tropoelastin films was studied at different protein content ratios with temperature modulated differential scanning calorimetry [14]. The Tg increased proportionally to the increase in tropoelastin content, from 178°C for pure silk to 190°C in the case of pure tropoelastin. The presence of a single glass transition peak for each silk-tropoelastin blend demonstrated that a homogenous single macrophase was formed for each composition. The experimental evidence showed that silk and tropoelastin were suitable protein polymer candidates for the development of a fully miscible system [14].
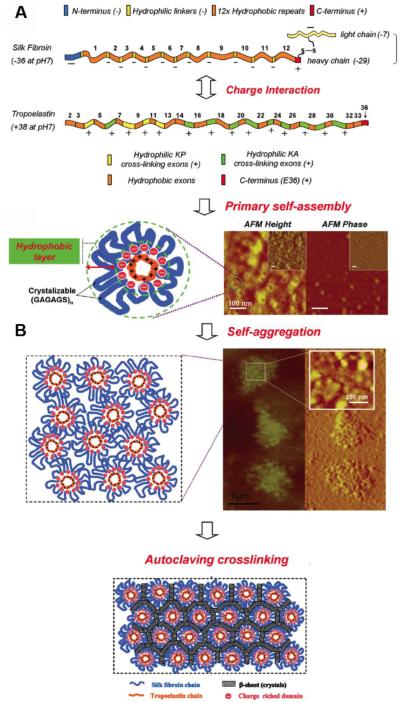
The mechanisms of interaction between silk and human tropoelastin have been elucidated and a model for silk-tropoelastin materials has been reported [15]. Based on bioinformatics analysis, both proteins are characterized by periodic hydrophobic-hydrophilic domains, where charge-rich repeating units are the hydrophilic regions, positively (+38) and negatively (−36) charged in case of human tropoelastin and silk fibroin, respectively (Figure 3A) [28, 57, 58]. Therefore, silk-tropoelastin biomaterials spontaneously form insoluble stable aggregates, because of their charge-driven strong physical interactions in solution, while the subsequent formation of beta-sheet crystal regions (via the hydrophobic domains present in both proteins) stabilize their conformation in the solid state [15]. In particular, we hypothesized that these different interactions were responsible for the protein aggregation. The initial assembly of tropoelastin and silk in solution is determined by the attraction of opposite charges between the charged amino acid side chains, where the short tropoelastin chains represent the core of a micelle aggregate and the silk long chains act as a shell to minimize the energy of the system in an aqueous environment [15]. In addition, the hydrophobic-hydrophilic interactions stabilize the primary silk-tropoelastin aggregates in a multi-micelle organization, supported by atomic force microscopy measurements [59]. Subsequently the formation of hydrogen-bonded beta-sheet crystalline structures result in silk-tropoelastin physical cross-linking by exposure to water vapor or via thermal treatment, such as by autoclaving [31, 60] (Figure 3B).
Figure 3. Silk-tropoelastin interactions model.
a) Bioinformatics analysis of silk fibroin and human tropoelastin chains. Both proteins characterized by periodic hydrophobic-hydrophilic domains, where the length of tropoelastin single chain is shorter (60kDa) than that of silk (approximately 420 kDa). Considering the net charges of both proteins derived from the charge-rich amino acid side groups, each tropoelastin unit (+38) can attract and neutralize nearly one silk single chain (−36) at pH 7 in aqueous environment. b) Primary assembly, micelle-mode aggregation, and multi-micelle cross-linking model of silk-tropoelastin biomaterials. Adapted from [15].
Mechanical performance of silk-tropoelastin biomaterials
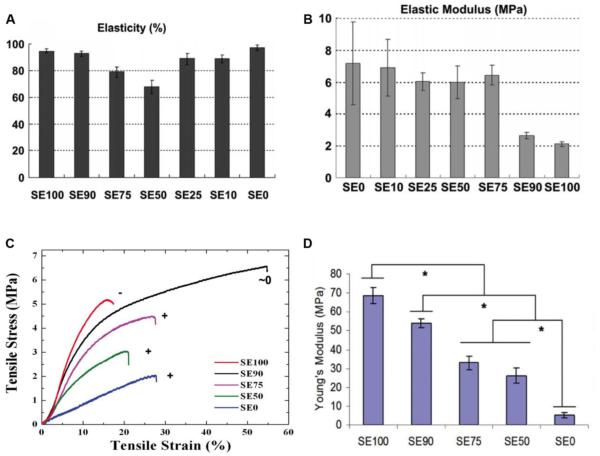
Composite systems allow the design of materials with optimized mechanical properties by adjusting the proportions of the different components. In the biomaterial field, the combination of a highly elastic material, as tropoelastin, together with a mechanically tough component, silk, would be beneficial in the production of mechanically tunable protein-based systems. The surface mechanical properties of silk-tropoelastin films was controlled by varying the composition of the initial blend [14]. The elasticity of the material was calculated as resilience, defined as the energy released after removal of the applied stress divided by the total energy. The local elasticity of silk-tropoelastin films at different protein ratios was characterized with nano-indentation measurements in comparison to pure tropoelastin and silk control films [14]. Pure tropoelastin showed the highest resilience (97%), while the silk control was significantly lower (94%) (Figure 4A). In general, the prevalence of both tropoelastin and silk components maintained high resilience, greater than 85%, which is significantly higher than that of traditional elastic synthetic rubbers (e.g. polybutadiene) [61]. The value of the elastic modulus was extrapolated based on the Hertz model from the measure of the resilience (Figure 4B) [62, 63]. The effect of tropoelastin was dominant on the film protein system elastic properties up to 75 wt%, and the silk component started to dominate the mechanical response at 90 wt%, where the elastic modulus was significantly reduced [14].
Figure 4. Mechanical performance of silk-tropoelastin protein system.
Local elasticity (resilience, A) and elastic modulus (B) of silk-tropoelastin films measured by AFM nano-indentation. Adapted from [14] Representative stress/strain behavior for autoclaved silk-tropoelastin films (C) and evaluation of Young’s modulus (D). Adapted from [15].
The bulk mechanical properties of silk-tropoelastin films, stabilized by beta-sheet crystal networks achieved through autoclaving, were evaluated at 37°C at physiological salt concentration [15]. Representative stress/strain behavior of silk-tropoelastin films at different ratios (Figure 4C) showed increased strain at failure and reduced slope in the linear region with increased tropoelastin content. The mechanical function of tropoelastin within the protein system mimicked the role of elastin in biological tissues, such as skin and vasculature, where the inherent elasticity allows for the recoil of the initial unloaded configuration. The modulus of elasticity significantly decreased with increased tropoelastin content (Figure 4D), as was previously shown in the case of water vapor annealed silk-tropoelastin films [16]. Furthermore, the stability of a neutral charge protein system (silk : tropoelastin ratio of 90:10) in an aqueous environment was evident on the mechanical performance of the material, which displayed significantly higher strain at failure in comparison to all other samples [15].
4. Silk-tropoelastin biomaterials: platform to control cell responses
Stem cell and neuron cell types need a large variety of environmental signals to maintain their phenotype or to direct their fate towards a specific lineage. Recent data have shown that the culture substrate is not an inert, solid-state environment, as multiple physical mechanisms (i.e. substrate elasticity and net charge) affect cell phenotype and responses as a reflection of culture conditions.
Mechanical and morphological properties: tools to control stem cell behavior
External physical stimuli include the geometry at the micro- and nanoscale, elasticity, and external mechanical stimuli transferred from the substrate to cells [64]. In particular, soft matrices (elastic modulus = ~1 kPa) have shown promise toward the stimulation of mesenchymal stem cells towards a neuronal-like phenotype. Increasing the substrate stiffness determined a shift to a myogenic cell fate, whereas the stiffest matrices (elastic modulus ~30-100 kPa) can induce osteogenic differentiation, due to mechanical similarities with collagenous bone [65, 66]. In addition, the substrate elasticity of human tropoelastin has been previously shown to promote the retention of the undifferentiated state of human hematopoietic stem cells [67]. Therefore, silk-tropoelastin protein systems were proposed as a tool to control myogenic differentiation and human bone marrow stem cell (hMSC) fate through substrate elasticity and morphology.
The myogenic-differentiation of mouse myoblast cells was studied on water-stable silk-tropoelastin films, obtained through temperature-controlled water vapor annealing [16]. During the fabrication process, surface roughness, mechanical properties, and topographical features were controlled to selectively investigate the effect of each parameter on cell behavior. Myoblast cells were not responsive to specific topographical surface patterns, while substrate surfaces with low roughness and high stiffness positively modulated cell proliferation and differentiation. In particular, a decrease in tropoelastin content determined a significant increase in surface roughness together with significant increase in substrate stiffness, where a tropoelastin:silk ratio of 10:90 provided the best environment for proliferation and myogenic differentiation for myoblast cell culture (Figure 5A) [16]. In contrast, mesenchymal stem cells (MSCs) displayed a higher proliferation rate with greater tropoelastin content, while proliferation and osteogenic differentiation were positively affected by an increase in surface roughness and the presence of surface patterns (Figure 5B). Morphological (i.e. roughness and surface pattern) and mechanical (i.e. elastic modulus) features together with biochemical properties (i.e. tropoelastin content) of silk-tropoelastin systems effectively modulated cellular myogenic and osteogenic differentiation by varying protein ratios.
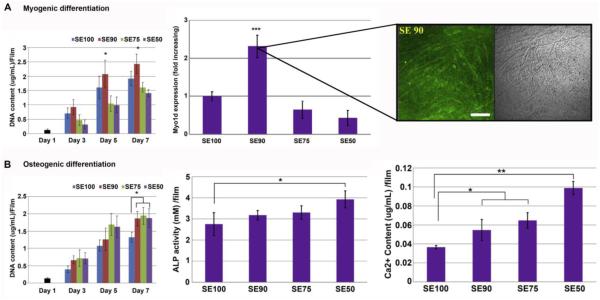
Figure 5. Modulation of myogenic and osteogenic lineages through silk-tropoelastin protein system.
A) Myoblast cell proliferation, as expression of DNA content, and transcript levels of Myo1d after 2 weeks in culture. Silk-tropoelastin 90:10 (SE90) displayed 3-fold greater myogenic expression than SE75 and SE50 (***p<0.001), as also shown by the molecular expression of Myo1d using immunohistochemical staining (Scale bar = 150 µm). B) hMSC proliferation, alkaline phosphatase (ALP) activity, and calcium content per film under osteogenic culture condition. Significant increase in ALP activity was measured for SE50 in comparison to plain silk, while greater calcium deposition was observed with increased in tropoelastin content within the films (*p<0.05 and **p<0.01). Adapted from [16].
Net charge: tool to control neuron cell behavior
Functional nerve regeneration is a complex process involving an interplay between regenerative cells and nerve conduits. Despite recent efforts to develop bioengineered nerve guides, the materials have yet to exceeded the performance of autologous nerve grafts, which remain the gold standard in peripheral nerve repair. An appropriate material for nerve repair needs to be biodegradable with cell compatible degradation products, possess tunable mechanical properties, allow controlled delivery of drugs and bioactive molecules and support extension and electrical activity of neurons. Silk-tropoelastin composite materials have the potential to satisfy these criteria and in particular offer a novel means of fine tuning the net charge of the material [68]. Electrical charge is known to play a significant role in cell functions and in particular in neurite extension and cell differentiation [69, 70]. Autoclaved silk-tropoelastin films were generated with different net charges depending on the ratio of silk to tropoelastin. Pure silk films (SE100) were negatively charged (net charge −36) and did not support the adhesion of primary embryonic rat cortical neurons (E18) [15]. The cells that did attach formed weakly adherent clusters with no neurite extensions. Addition of 10% tropoelastin (SE90) reduced the net negative charge of the films to near neutral (−3.6), which improved neuron adhesion, allowing for cell proliferation and less clustering. Increasing the net charge to +15.6 with the addition of 25% tropoelastin (SE75) generated films with a similar net charge as polylysine (+20), an in vitro gold standard for neuron growth. These films promoted good cell adhesion, neurite outgrowth and development of tightly connected neuronal networks similar to those formed on polylysine. Increasing the positive charge further by adding 50% tropoelastin (SE50, +28,5) and using pure tropoelastin films (SE0, +37.7), allowed initial attachment of neurons but subsequent cell death, pointing to the importance of tight regulation of material charge density in obtaining functional neurons. The initial positive results in silk tropoelastin composites points to the potential utility of this system in controlling neuronal cell function and warrant further investigation into neural guide development.
5. Summary/outlook
Tunable multi-component protein systems are important for constructing three-dimensional substrates to mimic biological tissues with control of biochemical, structural, and mechanical properties. The physical interactions between the two proteins are currently under further investigation in order to underscore the mechanisms of stabilization by autoclaving and to control the protein system degradation lifetime. Autoclaved films at different silk-tropoelastin ratios are being studied in both accelerated (in vitro) and physiological (in vivo) degradation conditions.
Molecular interaction mechanisms between silk protein and recombinant human tropoelastin were based on charge interactions, and this feature was exploited to generate a new group of multifunctional protein biomaterials with different net charge. These new biomaterial designs provide tools to control biological outcomes via surface roughness, elasticity, and net charge for neuronal and mesenchymal stem cell-based tissue engineering. We are currently investigating the role of net charge tuning in three dimensional culture systems, through osteogenic and chondrogenic responses of bone marrow derived human mesenchymal stem cells encapsulated in silk-tropoelastin hydrogels. In addition, silk-tropoelastin systems are under development for nerve guides for peripheral nerve regeneration. We hypothesize that further improvements in nerve regeneration will result from control of the surface charge and stiffness of the nerve guide by ad hoc protein composition.
Figure 6. Modulation of neuron function through net charge differences in silk-tropoela stin protein systems.
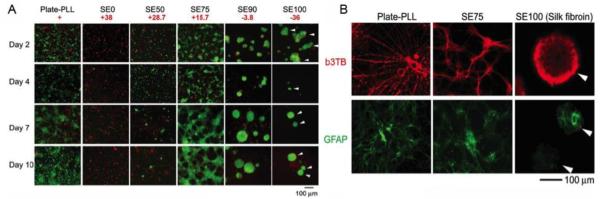
A) Primary co1tical neuronal cultures (rat El8) on autoclaved films with different silk-tropoelastin ratios (SEO (pure tropoelastin), SE50, SE75, SE90, SEIOO (pure silk)) compared to poly-lysine coated tissue culture plastic (Plate-PLL) over a 10 day period (green, live cells; red, dead cells). B) Prima1y cortical neuronal cultures (rat EIS) on SE75 and pure silk films compared with PLL control plate at day 10 in vitro. Sample were immunostained for neuronal processes with III tubulin (b3TB, red) and development of glial cell morphology with glial fibrillary acidic protein (GFAP, green). Scale bars 100 µm. Adapted from [15].
Acknowledgements
We thank funding agencies for support of these studies [NIH EB014283, AR005593, AR061988, P41 EB002520, Australian Research Council, National Health & Medical Research Council]. A.S.W. is the Scientific Founder of Elastagen Pty Ltd.
References
- [1].Multicomponent Polymer Materials. American Chemical Society; 1985. D. R P. Polymer Blends: Phase Behavior and Property Relationships; pp. 3–19. [Google Scholar]
- [2].Skopinska-Wisniewska J, Sionkowska A, Kaminska A, Kaznica A, Jachimiak R, Drewa T. Surface characterization of collagen/elastin based biomaterials for tissue regeneration. Applied Surface Science. 2009;255:8286–92. [Google Scholar]
- [3].Caves JM, Kumar VA, Martinez AW, Kim J, Ripberger CM, Haller CA, et al. The use of microfiber composites of elastin-like protein matrix reinforced with synthetic collagen in the design of vascular grafts. Biomaterials. 2010;31:7175–82. doi: 10.1016/j.biomaterials.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen JL, Yin Z, Shen WL, Chen X, Heng BC, Zou XH, et al. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials. 2010;31:9438–51. doi: 10.1016/j.biomaterials.2010.08.011. [DOI] [PubMed] [Google Scholar]
- [5].Ghezzi CE, Marelli B, Muja N, Hirota N, Martin JG, Barralet JE, et al. Mesenchymal stem cell-seeded multilayered dense collagen-silk fibroin hybrid for tissue engineering applications. Biotechnology Journal. 2011;6:1198–207. doi: 10.1002/biot.201100127. [DOI] [PubMed] [Google Scholar]
- [6].Mithieux SM, Wise SG, Weiss AS. Tropoelastin - A multifaceted naturally smart material. Advanced Drug Delivery Reviews. 2013;65:421–8. doi: 10.1016/j.addr.2012.06.009. [DOI] [PubMed] [Google Scholar]
- [7].Rnjak-Kovacina J, Wise SG, Li Z, Maitz PKM, Young CJ, Wang Y, et al. Electrospun synthetic human elastin:collagen composite scaffolds for dermal tissue engineering. Acta Biomaterialia. 2012;8:3714–22. doi: 10.1016/j.actbio.2012.06.032. [DOI] [PubMed] [Google Scholar]
- [8].Mithieux SM, Wise SG, Weiss AS. Tropoelastin - a multifaceted naturally smart material. Adv. Drug Delivery Rev. 2013;65:421–8. doi: 10.1016/j.addr.2012.06.009. [DOI] [PubMed] [Google Scholar]
- [9].Omenetto FG, Kaplan DL. New Opportunities for an Ancient Material. Science. 2010;329:528–31. doi: 10.1126/science.1188936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Heinz A, Jung MC, Duca L, Sippl W, Taddese S, Ihling C, et al. Degradation of tropoelastin by matrix metalloproteinases - cleavage site specificities and release of matrikines. Febs Journal. 2010;277:1939–56. doi: 10.1111/j.1742-4658.2010.07616.x. [DOI] [PubMed] [Google Scholar]
- [11].Taddese S, Weiss AS, Jahreis G, Neubert RHH, Schmelzer CEH. In vitro degradation of human tropoelastin by MMP-12 and the generation of matrikines from domain 24. Matrix Biology. 2009;28:84–91. doi: 10.1016/j.matbio.2008.12.002. [DOI] [PubMed] [Google Scholar]
- [12].Qiu WG, Huang YD, Teng WB, Cohn CM, Cappello J, Wu XY. Complete Recombinant Silk-Elastinlike Protein-Based Tissue Scaffold. Biomacromolecules. 2010;11:3219–27. doi: 10.1021/bm100469w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rodriguez-Cabello CJ, Prieto S, Arias FJ, Reguera J, Ribeiro A. Nanobiotechnological approach to engineered biomaterial design: the example of elastin-like polymers. Nanomedicine. 2006;1:267–80. doi: 10.2217/17435889.1.3.267. [DOI] [PubMed] [Google Scholar]
- [14].Hu X, Wang X, Rnjak J, Weiss AS, Kaplan DL. Biomaterials derived from silk-tropoelastin protein systems. Biomaterials. 2010;31:8121–31. doi: 10.1016/j.biomaterials.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hu X, Tang-Schomer MD, Huang W, Xia X-X, Weiss AS, Kaplan DL. Charge-Tunable Autoclaved Silk-Tropoelastin Protein Alloys That Control Neuron Cell Responses. Adv Funct Mater. 2013 doi: 10.1002/adfm.201202685. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hu X, Park S-H, Gil ES, Xia X-X, Weiss AS, Kaplan DL. The influence of elasticity and surface roughness on myogenic and osteogenic-differentiation of cells on silk-elastin biomaterials. Biomaterials. 2011;32:8979–89. doi: 10.1016/j.biomaterials.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang SG. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–8. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- [18].Hudson SM. Silk polymers: material science and biotechnology. In: Kaplan D, Adams W, Farmer B, Viney C, editors. Polymers for Advanced Technologies. Vol. 6. American Chemical Society; 1995. p. 717. ACS Symposium Series 544. [Google Scholar]
- [19].Vollrath F, Edmonds DT. Modulation of the mechanical properties of spider silk by coating with water. Nature. 1989;340:305–7. [Google Scholar]
- [20].Jin HJ, Kaplan DL. Mechanism of silk processing in insects and spiders. Nature. 2003;424:1057–61. doi: 10.1038/nature01809. [DOI] [PubMed] [Google Scholar]
- [21].Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, et al. Silk-based biomaterials. Biomaterials. 2003;24:401–16. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- [22].Shao Z, Vollrath F. Materials: Surprising strength of silkworm silk. Nature. 2002;418:741. doi: 10.1038/418741a. [DOI] [PubMed] [Google Scholar]
- [23].Zhou C-Z, Confalonieri F, Jacquet M, Perasso R, Li Z-G, Janin J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins: Structure, Function, and Bioinformatics. 2001;44:119–22. doi: 10.1002/prot.1078. [DOI] [PubMed] [Google Scholar]
- [24].Vepari C, Kaplan DL. Silk as a biomaterial. Progress in Polymer Science. 2007;32:991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Keten S, Xu Z, Ihle B, Buehler MJ. Nanoconfinement controls stiffness, strength and mechanical toughness of [beta]-sheet crystals in silk. Nat Mater. 2010;9:359–67. doi: 10.1038/nmat2704. [DOI] [PubMed] [Google Scholar]
- [26].Marelli B, Ghezzi CE, Alessandrino A, Barralet JE, Freddi G, Nazhat SN. Silk fibroin derived polypeptide-induced biomineralization of collagen. Biomaterials. 2012;33:102–8. doi: 10.1016/j.biomaterials.2011.09.039. [DOI] [PubMed] [Google Scholar]
- [27].Rockwood DN, Preda RC, Yucel T, Wang XQ, Lovett ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nature Protocols. 2011;6:1612–31. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bini E, Knight DP, Kaplan DL. Mapping Domain Structures in Silks from Insects and Spiders Related to Protein Assembly. Journal of Molecular Biology. 2004;335:27–40. doi: 10.1016/j.jmb.2003.10.043. [DOI] [PubMed] [Google Scholar]
- [29].Pérez-Rigueiro J, Viney C, Llorca J, Elices M. Mechanical properties of single-brin silkworm silk. Journal of Applied Polymer Science. 2000;75:1270–7. [Google Scholar]
- [30].Cunniff PM, Fossey SA, Auerbach MA, Song JW, Kaplan DL, Adams WW, et al. Mechanical and thermal properties of dragline silk from the spider Nephila clavipes. Polymers for Advanced Technologies. 1994;5:401–10. [Google Scholar]
- [31].Hu X, Shmelev K, Sun L, Gil ES, Park SH, Cebe P, et al. Regulation of Silk Material Structure by Temperature-Controlled Water Vapor Annealing. Biomacromolecules. 2011;12:1686–96. doi: 10.1021/bm200062a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yucel T, Cebe P, Kaplan DL. Vortex-Induced Injectable Silk Fibroin Hydrogels. Biophysical Journal. 2009;97:2044–50. doi: 10.1016/j.bpj.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ramirez F. Pathophysiology of the microfibril/elastic fiber system: introduction. Matrix Biol. 2000;19:455–6. doi: 10.1016/s0945-053x(00)00098-6. [DOI] [PubMed] [Google Scholar]
- [34].Sandberg LB, Soskel NT, Leslie JG. Elastin structure, biosynthesis, and relation to disease states. N Engl J Med. 1981;304:566–79. doi: 10.1056/NEJM198103053041004. [DOI] [PubMed] [Google Scholar]
- [35].Almine JF, Bax DV, Mithieux SM, Nivison-Smith L, Rnjak J, Waterhouse A, et al. Elastin-based materials. Chem Soc Rev. 2010;39:3371–9. doi: 10.1039/b919452p. [DOI] [PubMed] [Google Scholar]
- [36].Uitto VJ, Larjava H. Extracellular matrix molecules and their receptors: an overview with special emphasis on periodontal tissues. Crit Rev Oral Biol Med. 1991;2:323–54. doi: 10.1177/10454411910020030301. [DOI] [PubMed] [Google Scholar]
- [37].Kielty CM. Elastic fibres in health and disease. Expert Rev Mol Med. 2006;8:1–23. doi: 10.1017/S146239940600007X. [DOI] [PubMed] [Google Scholar]
- [38].Wise SG, Mithieux SM, Weiss AS. Engineered tropoelastin and elastin-based biomaterials. Adv Protein Chem Struct Biol. 2009;78:1–24. doi: 10.1016/S1876-1623(08)78001-5. [DOI] [PubMed] [Google Scholar]
- [39].Mithieux SM, Weiss AS. Elastin. Advances in protein chemistry. 2005;70:437–61. doi: 10.1016/S0065-3233(05)70013-9. [DOI] [PubMed] [Google Scholar]
- [40].Baldock C, Oberhauser AF, Ma L, Lammie D, Siegler V, Mithieux SM, et al. Shape of tropoelastin, the highly extensible protein that controls human tissue elasticity. Proc Natl Acd Sci USA. 2011;108:4322–7. doi: 10.1073/pnas.1014280108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–28. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- [42].Wise SG, Weiss AS. Tropoelastin. Int J Biochem Cell Biol. 2009;41:494–7. doi: 10.1016/j.biocel.2008.03.017. [DOI] [PubMed] [Google Scholar]
- [43].Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: the elastic fiber. FASEB J. 1993;7:1208–18. [PubMed] [Google Scholar]
- [44].Cox BA, Starcher BC, Urry DW. Coacervation of alpha-elastin results in fiber formation. Biochim Biophys Acta. 1973;317:209–13. doi: 10.1016/0005-2795(73)90215-8. [DOI] [PubMed] [Google Scholar]
- [45].Kagan HM, Sullivan KA. Lysyl oxidase: preparation and role in elastin biosynthesis. Methods Enzymol. 1982;82:637–50. doi: 10.1016/0076-6879(82)82092-2. Pt A. [DOI] [PubMed] [Google Scholar]
- [46].Yeo GC, Keeley FW, Weiss AS. Coacervation of tropoelastin. Adv Colloid Interface Sci. 2011;167:94–103. doi: 10.1016/j.cis.2010.10.003. [DOI] [PubMed] [Google Scholar]
- [47].Tu Y, Wise SG, Weiss AS. Stages in tropoelastin coalescence during synthetic elastin hydrogel formation. Micron. 2010;41:268–72. doi: 10.1016/j.micron.2009.11.003. [DOI] [PubMed] [Google Scholar]
- [48].Mithieux SM, Rasko JEJ, Weiss AS. Synthetic elastin hydrogels derived from massive elastic assemblies of self-organized human protein monomers. Biomaterials. 2004;25:4921–7. doi: 10.1016/j.biomaterials.2004.01.055. [DOI] [PubMed] [Google Scholar]
- [49].Hardingham TE, Fosang AJ. Proteoglycans - many forms and many functions. FASEB Journal. 1992;6:861–70. [PubMed] [Google Scholar]
- [50].Vanderrest M, Garrone R. Collagen family of proteins. FASEB Journal. 1991;5:2814–23. [PubMed] [Google Scholar]
- [51].Herbert FV, Daniel JS. Biomedical Engineering Fundamentals. CRC Press; 2006. An Outline of Cardiovascular Structure and Function; pp. 39814–25. [Google Scholar]
- [52].Thomas JT, Ayad S, Grant ME. Cartilage collagens - Strategies for the study of their organization and expression in the extracellular-matrix. Ann Rheum Dis. 1994;53:488–96. doi: 10.1136/ard.53.8.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yu J, Urban JPG. The elastic network of articular cartilage: an immunohistochemical study of elastin fibres and microfibrils. J Anat. 2010;216:533–41. doi: 10.1111/j.1469-7580.2009.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Guidry C, Grinnell F. Heparin Modulates the Organization of Hydrated Collagen Gels and Inhibits Gel Contraction by Fibroblasts. J Cell Biol. 1987;104:1097–103. doi: 10.1083/jcb.104.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Aubin M, Bédard Y, Morrissette M-F, Prud'homme RE. Miscible blends prepared from two crystalline polymers. Journal of Polymer Science: Polymer Physics Edition. 1983;21:233–40. [Google Scholar]
- [56].Miwa Y, Usami K, Yamamoto K, Sakaguchi M, Sakai M, Shimada S. Direct Detection of Effective Glass Transitions in Miscible Polymer Blends by Temperature-Modulated Differential Scanning Calorimetry. Macromolecules. 2005;38:2355–61. [Google Scholar]
- [57].Lammel AS, Hu X, Park S-H, Kaplan DL, Scheibel TR. Controlling silk fibroin particle features for drug delivery. Biomaterials. 2010;31:4583–91. doi: 10.1016/j.biomaterials.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Foo CWP, Bini E, Hensman J, Knight DP, Lewis RV, Kaplan DL. Role of pH and charge on silk protein assembly in insects and spiders. Appl Phys A. 2006;82:223–33. [Google Scholar]
- [59].Reguera J, Fahmi A, Moriarty P, Girotti A, Rodríguez-Cabello JC. Nanopore Formation by Self-Assembly of the Model Genetically Engineered Elastin-like Polymer [(VPGVG)2(VPGEG)(VPGVG)2]15. J Am Chem Soc. 2004;126:13212–3. doi: 10.1021/ja047417f. [DOI] [PubMed] [Google Scholar]
- [60].Park S-H, Gil ES, Shi H, Kim HJ, Lee K, Kaplan DL. Relationships between degradability of silk scaffolds and osteogenesis. Biomaterials. 2010;31:6162–72. doi: 10.1016/j.biomaterials.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, et al. Synthesis and properties of crosslinked recombinant pro-resilin. Nature. 2005;437:999–1002. doi: 10.1038/nature04085. [DOI] [PubMed] [Google Scholar]
- [62].Domke J, Radmacher M. Measuring the elastic properties of thin polymer films with the atomic force microscope. Langmuir. 1998;14:3320–5. [Google Scholar]
- [63].Domke J, Dannohl S, Parak WJ, Muller O, Aicher WK, Radmacher M. Substrate dependent differences in morphology and elasticity of living osteoblasts investigated by atomic force microscopy. Colloids and Surfaces B-Biointerfaces. 2000;19:367–79. doi: 10.1016/s0927-7765(00)00145-4. [DOI] [PubMed] [Google Scholar]
- [64].Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- [66].Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- [67].Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-Smith LB, et al. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat Biotechnol. 2010;28:1123–U168. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]
- [68].Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Ann Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- [69].Schmidt CE, Shastri VR, Vacanti JP, Langer R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc Natl Acad Sci USA. 1997;94:8948–53. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Valentini RF, Vargo TG, Gardella JA, Jr., Aebischer P. Patterned neuronal attachment and outgrowth on surface modified, electrically charged fluoropolymer substrates. J Biomater Sci Polym Ed. 1993;5:13–36. doi: 10.1163/156856294x00626. [DOI] [PubMed] [Google Scholar]