Abstract
Objective
To compare operative time and hemostasis of fiber-enabled CO2 laser (FECL) energy to that of the electrocautery (EC) technique for oral tongue resection, to compare return to oral intake and preoperative weight after FECL and EC resection, and to compare histologic changes in adjacent tissue after FECL and EC resection.
Study Design
Prospective animal study.
Setting
Research laboratory.
Subjects and Methods
The CO2 laser fiber and the Bovie cautery were each used to resect the anterior tongue in 15 adult rats. Fixative perfusion and killing were performed on postoperative day 0 (n = 10), 3 (n = 10), or 7 (n = 10). Body weight, food intake, and water intake were recorded daily for 3- and 7-day survival rats. After preparation for histologic analysis, the tongue tissue was graded with a mucosal wound-healing scale (MWHS).
Results
A higher incidence of intraoperative bleeding and shorter operative times were noted in the EC group. No statistically significant difference in postoperative food or water intake between the EC and FECL groups was noted. The FECL group returned to baseline weight by postoperative day 6. MWHS scores were lower in the EC group by postoperative day 3 and lower in the FECL group by postoperative day 7.
Conclusions
Both EC and FECL are effective for resection of the tongue in rats. EC has the advantage of shorter operative time and lower MWHS scores by postoperative day 3; FECL has the advantages of less intraoperative bleeding, faster return to baseline body weight, and lower MWHS score by postoperative day 7.
Keywords: CO2 laser, electrocautery, oral cavity, tongue, oral intake, weight loss, histology, mucosal injury
The line-of-slight CO2 laser was the subject of a multitude of studies involving the upper aerodigestive tract before the flexible fiber-optic CO2 laser carrier was developed in 2002.1–9 While research comparing the line-of-site CO2 laser to other accepted techniques (micro- debrider, KTP laser, electrocautery [EC], scalpel) has been published, comparison studies using the fiber-enabled CO2 laser (FECL) are not yet available.1,2,10,11
We compared the instrument’s usefulness to the already widely used EC method of resecting lesions of the oral cavity/oropharynx. The newly developed FECL has been proposed for use in resecting tumors of these areas in humans with a shorter operating time, comparable removal of tissue, less intraoperative bleeding, less postoperative bleeding, and faster return to normal mucosa. As the FECL is a considerably more expensive tool than conventional EC, efficacy data would be valuable.
Rats have been shown to be a useful model in evaluating the histologic and behavioral effects of surgical tools in the oral cavity.2,11 In our study, we used a rat model to compare the healing process and behavioral changes following tongue resection using 2 techniques. Specifically, we sought to compare the operative time and hemostasis of FECL to that of the EC technique, compare return to oral intake and preoperative weight after FECL and EC resection, and to compare histologic changes in adjacent tissue after FECL and EC resection.
Methods
Approval was obtained from the Institutional Animal Care and Use Committee, University of Tennessee Health Science Center (UTHSC). Thirty-two adult male rats (Sprague-Dawley) were obtained from the Jackson Laboratory (Bar Harbor, Maine). Animal care and housing were provided at an Association for Assessment and Accreditation of Laboratory Animal Care–approved facility at UTHSC. Food (Harlan Teklab 7012) and water were available ad libitum. One to 2 weeks prior to surgery, food and water consumption and body weight were measured for 2 days in the home cage.
Surgical Technique
Rats were anesthetized intraperitoneally with a mixture of ketamine hydrochloride (86 mg/kg) and zylazine hydrochloride (13 mg/kg). Surgery was performed using sterile techniques. The rats were laid supine and retained spontaneous ventilation throughout surgery. The tongue was retracted from the oral cavity with forceps. A ruler was used to measure 3 mm from the tip of the tongue, and the EC or FECL was then used to incise transversely at this level. A full-thickness incision was made to remove the anterior 3 mm of the tongue. Operative time was recorded with a handheld stopwatch. Operative time began when the EC or FECL initially touched the tongue tissue and ended when the tip of the tongue was completely transected or after any posttransection bleeding was effectively coagulated. Any blood loss was collected on a previously weighed cotton-tip applicator and weighed after use. Blood loss was recorded as change in weight of the cotton-tip applicator. For any bleeding during an FECL resection, the CO2 laser was withdrawn slightly from the operative site and used on the same power setting to coagulate the area. For any bleeding during an EC resection, the Bovie was used gently on a coagulation setting to stop the bleeding.
Two rats served as pilots to define fiber and laser settings. In one rat, the CO2 laser fiber (150 cm length, 1.2 mm OD, 315 μm core size; OmniGuide, Inc, Cambridge, Massachusetts) was used to perform a transverse full-thickness excision of the anterior 3 mm of the oral tongue. The power setting of 7 W appeared to be both effective and delicate. In a second rat, the Colorado needle tip Bovie EC on a setting of 25 W spray was used to transversely resect the anterior 3 mm of the oral tongue. These settings were effective in cutting and coagulating and were chosen to be used on the 30 test rats.
Our study included a similar number of subjects as recent publications in the otolaryngology literature. Johnson et al10 included 12 canines in his comparison of microdebrider to EC, and Yamasaki et al11 included 30 rats as study animals in evaluating the CO2 laser. Carew et al2 included 40 rats, studying 4 different cutting modalities (CO2 laser, KTP laser, scalpel, and EC) with 10 rats per modality. Therefore, we chose to include 30 rodents: 15 in the FECL arm and 15 in the EC arm.
Rats 1 to 30 underwent resection of a 3-mm transverse area of the anterior tongue. For rats 1 to 15, this was performed using the fiber-enabled CO2 laser. For rats 16 to 30, each procedure was performed with Bovie cautery and a Colorado needle tip. The order resected with each surgical technique was randomized.
Following either surgical procedure, 3 mL of sterile NaCl and buprenorphine (0.005 mg/kg) were injected subcutaneously. Rats were monitored for 2 to 3 hours until fully awake and taking oral feeds.
Postoperatively, the rats were continued on a regular diet, weighed, and monitored daily. Food and water intake was measured by weighing food and water bottles every 24 hours. Water consumption was reported in milliliters. Rats were also weighed just before killing on postoperative day (POD) 3 or 7.
Procedural Analysis
We recorded the length of time required to resect each lesion (seconds), the amount of intraoperative bleeding (change in weight of cotton-tip applicators), and frequency of postoperative bleeding.
Histological Analysis
Samples of the healing excision sites were obtained for histologic evaluation at different postoperative intervals (POD 0, 3, and 7). Therefore, the 2 arms (15 animals in the FECL arm, 15 animals in the EC group) were divided into 3 groups of 5 depending on day of killing (POD 0, 3, and 7). The animals undergoing killing on POD 0 were taken directly from the operating table to the perfusion area, whereas the animals undergoing killing on POD 3 and 7 were anesthetized prior to perfusion. All rats were perfused transcardially with phosphate-buffered saline followed by 10% neutral-buffered formalin. The entire tongue was visualized and removed back to the vallecula. The entire remaining tongue was removed on POD 3 in 5 of the rats who underwent FECL excision and 5 of the rats who underwent EC resection. This was performed on POD 7 in the remaining 5 rats who underwent FECL excision and the remaining 5 rats who underwent EC resection.
Coded-labeled specimens were stored in formalin, embedded in paraffin, and sectioned longitudinally on a microtome, perpendicular to the incision. The specimens were mounted on slides and stained with hematoxylin and eosin (HE).
Johnson’s mucosal wound healing scale (MWHS) scores were used to compare healing rates between surgical groups. His team created this objective system in 2008 for quantifying observed mucosal wound healing in animals undergoing microdebrider and EC tonsillectomy.10 Interrater reliability and construct validity of this scale were verified.
A blinded pathologist assigned an MWHS score to each of the samples. A second blinded pathologist verified the scores of the 30 specimens. No discrepancies in MWHS assignments between pathologists were noted. As described by Johnson, the variables of inflammatory surface crust and vessel wall necrosis were assigned a score of 0 if absent and a score of 2 if present.10 Surface epithelium was assigned a score of 2 if absent and 0 if present. Neutrophilic infiltrate was assigned values of 0 for no neutrophils, 1 for intermediate concentration, and 2 for thick sheets of neutrophils. Myofibroblast proliferation was labeled 0 for none appreciable, 1 for 1 to 50/ high-powered field (HPF), and 2 for more than 50/HPF. Values for each of these 5 categories ranging from 0 to 2 were totaled, for an MWHS from 0 to 10. Higher scores indicated greater injury, and lower scores indicated faster healing.10
Statistical Analysis
Mean values are provided with 95% confidence intervals (CIs). Body weight and behavioral data were analyzed using a general linear model: repeated measures (day) with a between-subjects factor (surgery type). Where appropriate, post hoc comparisons were made via t test with a Bonferroni correction for multiple comparisons. Other comparisons between surgical groups (eg, surgery time, MWHS) were made via t tests. The statistical rejection criterion was set a priori at the .01 level.
Results
Bleeding
No rat experienced postoperative bleeding after awakening from anesthesia. Intraoperative bleeding occurred more frequently in the rats undergoing EC resection (4 rats, 27%) than FECL resection (2 rats, 13%). However, among the rats that experienced intraoperative bleeding, the amount of blood loss per rat was greater in the FECL resections (mean change in cotton-tip applicator weight = 0.347 g for FECL, 0.152 g for EC).
Operative Time
The length of time required to resect the tongue was shorter when using the EC, with mean operative time for FECL resection of 10.63 seconds (CI, 9.11–12.14) and for EC resection 6.13 seconds (CI, 4.94–7.32). This difference was significant (P < .0001). The FECL also required more passes of the tool to perform complete transection than did the EC.
Food and Water Intake
Water intake
Over days 1 to 3, there was not a significant effect of surgical group (n = 10 per group), although there was a significant group × day interaction (P < .01). However, post hoc testing did not reveal significant differences on individual days 1 to 3. By day 3 in both the 3-day and 7-day experiments, mean water intake for both surgery types was 27.5 mL, with a CI of 22.5 to 32.5 (Figure 1). This was similar to the average daily presurgical intake (26.8 mL; CI, 23.6–30). Consumption on days 4 to 7 in the 7-day rats continued at about this same level. Over days 4 to 7, there was neither a significant main effect of surgical group (n = 5 group) nor a significant group × day interaction.
Figure 1.
Mean (SD) water consumption (mL) in laser- (FECL) or cautery-operated (EC) rats. Combined data from the 3- and 7-day groups (n = 10/surgery type) are shown for postsurgical days 1 to 3; consumption data from days 4 to 7 are also shown for the 7-day animals (n = 5/surgery type).
Food intake
Rats gradually increased their food intake across the test period(s) in both the 3-day and 7-day experiments (Figure 2), eventually exceeding the mean pretest intake value, which was 20.1 g per 24 hours (CI, 18.5–21.6). By day 3, mean food intake for all rats was 21.6 g (CI, 19.0–24.3). There was no significant main effect of surgery group over days 1 to 3 (n = 10 per group) or over days 4 to 7 (n = 5 per group).
Figure 2.
Mean (SD) food consumption (g) in fiber-enabled CO2 laser or electrocautery rats. Combined data from the 3- and 7-day groups (n = 10/surgery type) are shown for postsurgical days 1 to 3; consumption data from days 4 to 7 are also shown for the 7-day animals (n = 5/surgery type).
Body Weight
Prior to presurgical testing, the mean body weight of all rats was 368.9 g (CI, 357.4-380.4). Weight loss following surgery was expressed as a percentage of the original presurgical body weight to control for potential group differences (Figure 3). Over days 1 to 3, there was not a significant main effect of surgery (n = 10 per group), but there was a significant effect of day (P <.0001) and a significant group × day interaction (P <.001). However, post hoc testing did not indicate significant effects on individual days 1 to 3. In the 7-day rats, there was (from day 4–7) a significant effect of day (P <.00001) and a significant day × group interaction (P <.05). There was a trend on days 5 to 7 for the FECL rats to gain more weight than the EC rats. However, when the groups were examined on individual days via post hoc tests, there were no significant differences (P’s < .12–.2).
Figure 3.
Mean (SD) percentage change in body weight in fiber-enabled CO2 laser or electrocautery rats. Combined data from the 3- and 7-day groups (n = 10/surgery type) are shown for postsurgical days 1 to 3; body weight data from days 4 to 7 are also shown for the 7-day animals (n = 5/surgery type).
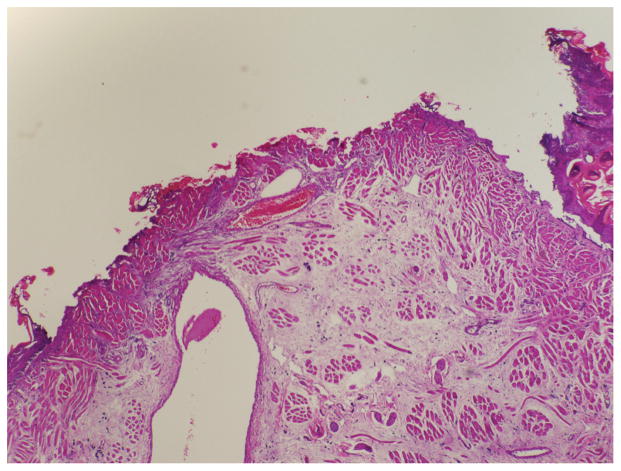
Histology
Mucosal wound-healing scale scores were recorded for each rat and averaged among the surgical groups. Photographs were taken of the histologic sections (Figures 4 and 5).
Figure 4.
Hematoxylin and eosin stain showing a high mucosal wound-healing scale (significant surface crusting, vessel wall necrosis, epithelium, neutrophils, and myofibroblasts).
Figure 5.
Hematoxylin and eosin stain showing a low mucosal wound-healing scale score (no surface crusting, vessel wall necrosis, neutrophils, or myofibroblasts). Surface epithelium is present.
POD 0 rats
No surface crusting was noted in any of the rats sacrificed immediately after EC or FECL resection. No epithelium was noted, indicating the fresh surgical incision. No vessel wall necrosis, neutrophils, or myofibroblasts were present yet.
POD 3 rats
The average MWHS scores of rats killed on POD 3 were 8.4 (FECL) and 6.6 (EC; not significantly different). Epithelium was not seen in any of the FECL rats and was seen in 1 of the 5 EC rats (20%). Crusting was now seen in all of the FECL rats (100%) and in 4 of 5 EC rats (80%). Incidences of vessel wall necrosis, neutrophilic infiltrate, and myofibroblast proliferation were similar between the 2 groups. However, the intensity of neutrophilic infiltrate was greater in the FECL group.
POD 7 rats
The average MWHS score of rats killed on POD 7 were 2.8 (FECL) and 3.4 (EC; not significantly different). Only 1 EC rat (20%) and 1 FECL rat (20%) showed surface crusting. No vessel wall necrosis was seen in either group. Three FECL rats (60%) showed surface epithelium, and 1 EC rat (20%) had epithelium. Neutrophilic infiltrate was seen in twice as many EC rats (80%) than FECL rats (40%). Myofibroblast proliferation was also seen in more EC rats (80%) than FECL rats (60%).
Discussion
In our study, the incidence of intraoperative bleeding was higher during EC resection than FECL resection. Given that the resection was slower when using the FECL, perhaps this resulted in more extensive cauterization of vessels in this group. Other reports support the fact that the laser results in increased coagulation.1,3
When using the pulse or super pulse mode of the CO2 laser, thermal injury to surrounding tissue is minimized. However, the disadvantage of using either of these modes is longer operative time.1,3 This was evident in our study, as resection using EC took 43% less time than resection using FECL. When using the instrument in larger subjects (humans), the difference in procedure time may become a greater concern.
In a previous study, rats were weighed daily to assess oral intake.2 In our study, we directly measured oral intake (food and water). Remarkably, all rats in our study drank a significant amount of water on the first POD but relatively little on the second day. This could be due to increased thirst following surgery. After repletion, the rats did not need to consume as much water the following day. In addition, the effects of the analgesia may have dissipated by POD 2. Food intake was slightly more stable. Rats initially showed decreased food intake and then gradually increased their food intake across the test period independent of type of surgery.
Traditional electrosurgical techniques (EC) have been presumed to cause more pain and inflammation.10 Similarly, we found an earlier return to baseline weight in rats undergoing FECL resection, which may have been related to less postoperative pain or swelling caused by the FECL.
EC has been postulated to result in greater crusting than FECL. However, we noticed no increase in crusting with EC. In fact, no crusting was seen in either group until POD 3 and resolved in all but 2 rats (1 in each surgical group) by POD 7. Therefore, the low rate of crusting was identical in the 2 surgical groups and seemed to reach a peak at POD 3.
We examined the specimens on POD 3 and 7 to allow comparison with previous studies.10,11 Johnson et al10 reported improved healing by POD 3 when comparing the microdebrider to EC. However, we found similar healing between the FECL and EC groups on POD 3 (Figure 6).
Figure 6.
Mean (SD) mucosal wound-healing scale scores in laser-or cautery-operated rats in the 0-day groups, 3-day groups, and 7-day groups.
By POD 7, neutrophilic infiltrate was seen in twice as many EC rats, the opposite of the POD 3 results. Greater epithelialization and myofibroblast proliferation were seen in more EC rats, supporting the theory that EC results in greater scarring. Johnson et al10 also found greater rates of myofibroblast and neutrophil infiltration on POD 3 and 9 in dogs undergoing EC resection.
Average MWHS were higher in the FECL group on POD 0 and POD 3. However, by POD 7, the FECL had slightly lower MWHS than the EC group and therefore evidence of better recovery. Despite the trends and differences of MWHS scores and weight loss between the surgical groups, there were no statistically significant differences. With greater number of subjects in our study, perhaps statistical significance would have been reached to confirm these apparent trends.
The incidence of mucosal malignancies related to the human papillomavirus is rising and subsequently so is the need for scientific investigations of various treatment options, including resection using the FECL. With greater costs for newer, more sophisticated surgical tools, it is important to compare the benefits of the tools with the cost to the patient, hospital, and payer before justifying use in human subjects. Multiple studies have been performed with each laser as it is introduced, and now attention has been turned to the new fiber-enabled carrier of the CO2 laser. This study accentuates advantages and disadvantages of this tool when considering histologic and behavioral aspects.
Conclusions
EC and FECL are both effective for resection of the tongue in rats. There was no effect of surgery on body weight in the first 3 days postoperatively, with both groups having equal changes in weight. Although EC has the advantage of shorter operative time and lower MWHS scores by POD 3, FECL has the advantages of lower incidence of intraoperative bleeding, faster return to baseline body weight, and lower MWHS score by POD 7. Despite definite trends, no statistical significance was noted in MWHS, weight loss, or return to oral intake between the FECL and EC methods. This is in contrast to studies with the line-of-site CO2 laser, which showed notable benefits of the laser in comparison to the EC.
Acknowledgments
The project received a restricted educational grant from OmniGuide, Inc Company. Representatives were involved with calibrating the CO2 laser and quality assurance but had no role in the study design, data collection, analysis, interpretation, manuscript preparation, or decision to submit for publication. The first author of this study had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding source: OmniGuide, Inc Company. Representatives were involved with calibrating the CO2 laser and quality assurance but had no role in the study design, data collection, analysis, interpretation, manuscript preparation, or decision to submit for publication.
Footnotes
Presented at the American Head & Neck Society 2011 Annual Meeting, Combined Otolaryngological Spring Meeting; April 27-28, 2011; Chicago, Illinois.
Author Contributions
Courtney Brooke Shires, design of project, acquisition of data, analysis and interpretation of data, writing the article, approving the article; Jennifer Marie Saputra, acquisition of data, revising the article, and approving the article; Lauren King, design of project, analysis of histology slides, revising the article, approving the article; Jerome W. Thompson, design of project, revising the article, approving the article; Detlef H. Heck, creation of equipment to analyze animal behavior, data analysis, revision of article, approval of article; Merry Ellen Sebelik, design, acquisition of data, approval of article; John Dudley Boughter Jr, design of project, statistical analysis, animal care, animal anesthesia, revising the article, approving the article.
Disclosures
Competing interests: None.
Sponsorships: None.
References
- 1.Evans PH, Frame JW, Brandrick J. A review of carbon dioxide laser surgery in the oral cavity and pharynx. J Laryngol Otol. 1986;100:69–77. doi: 10.1017/s0022215100098765. [DOI] [PubMed] [Google Scholar]
- 2.Carew JF, Ward RF, LaBruna A, et al. Effects of scalpel, electrocautery, and CO2 and KTP lasers on wound healing in rat tongues. Laryngoscope. 1998;108:373–380. doi: 10.1097/00005537-199803000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Carruth JA. Resection of the tongue with the carbon dioxide laser. J Laryngol Otol. 1982;96:529–543. doi: 10.1017/s002221510009280x. [DOI] [PubMed] [Google Scholar]
- 4.Jako GJ. Laser surgery of the vocal cords. Laryngoscope. 1972;82:2204–2216. doi: 10.1288/00005537-197212000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Lesinski SG, Palmer A. Lasers in otosclerosis: CO2 vs. argon and KTP-532. Laryngoscope. 1989;99:1–8. [PubMed] [Google Scholar]
- 6.Kay SL, Mehmet CO, Haber M, et al. Soft tissue effects of the THC:YAG laser on canine vocal cords. Otolaryngol Head Neck Surg. 1992;107:438–443. doi: 10.1177/019459989210700317. [DOI] [PubMed] [Google Scholar]
- 7.Mihashi S, Jako GJ, Incze J, et al. Laser surgery in otolaryngology: interaction of CO2 laser and soft tissue. Ann N Y Acad Sci. 1976;267:263–294. doi: 10.1111/j.1749-6632.1976.tb41614.x. [DOI] [PubMed] [Google Scholar]
- 8.Strong MS, Vaughan CW, Healey GB, et al. Transoral management of localized carcinoma of the oral cavity using the CO2 laser. Laryngoscope. 1979;89:897–905. doi: 10.1288/00005537-197906000-00005. [DOI] [PubMed] [Google Scholar]
- 9.The OmniGuide® BeamPath™ flexible CO2 laser system: A reality now. Omniguide; 2008. [Accessed April 10 2011]. http://www.omni-guide.com/about-omniguide-the-OG-story.htm. [Google Scholar]
- 10.Johnson K, Vaughan A, Derkay C, et al. Microdebrider vs electrocautery: a comparison of tonsillar wound healing histopathology in a canine model. Otolaryngol Head Neck Surg. 2008;138:486–491. doi: 10.1016/j.otohns.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Yamasaki A, Tamamura K, Sakurai Y, et al. Remodeling of the rat gingival induced by CO2 laser coagulation mode. Lasers Surg Med. 2008;40:695–703. doi: 10.1002/lsm.20712. [DOI] [PubMed] [Google Scholar]