Abstract
Because aqueous liposomal formulations containing multiply unsaturated lipids are susceptible to chemical degradation, these formulations are often lyophilized. Despite their limited chemical stability, interest in the use of multiply unsaturated lipids to promote intracellular delivery has increased considerably in recent years. The goal of the current study was to examine the long term storage stability of lyophilized formulations containing lipids with increasing levels of unsaturation, and various strategies which can be employed to improve stability. Aqueous lipid-trehalose formulations containing 1,2-dilinolenoyl-sn-glycero-3-phosphocholine (DLPC), 1,2-dilinoleoyl-sn-glycero-3-phosphocholine (DLinPC) or 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) were lyophilized and stored at temperatures ranging from 4°C to 60°C. We observed that the lipid degradation rate increased as the storage temperature and unsaturation level were increased. Even the cleanest sugars which are available commercially contain iron contaminants, and it was observed that the chelation of these iron contaminants significantly improved the stability of DLPC during storage. However, the glass transition temperature of the sugar which was included in the formulation, the reduction of the oxygen in the aqueous sample prior to lyophilization, the inclusion of helper lipids (i.e., cholesterol), and the rate of freezing did not significantly improve stability.
Keywords: DLPC, lyophilization, liposomes, chemical stability, unsaturated lipids, oxidation, freeze-drying
Introduction
Liposomes are bilayer vesicles composed of cationic, anionic and/or neutral lipids. Liposomes are frequently used as delivery vehicles; currently, twelve liposomal products are marketed to deliver small molecule therapeutics.1,2 The commercially available products employing lipid-based delivery vehicles are typically composed of saturated, hydrogenated or singly unsaturated lipids; lipids primarily used due to their greater intrinsic chemical stability. Regarding liposomal formulations, hydrolysis and oxidation are the main degradation pathways. More specifically, the ester functionality connecting the fatty acid and glycerol backbone is susceptible to hydrolysis resulting in free fatty acids, lysophospholipids and/or glycerophospho compounds.3 It has been shown that hydrolytic degradation influences liposome size, bilayer rigidity and drug entrapment.4
In addition to hydrolysis, peroxidation is a serious concern with liposomal drug formulations. Peroxidation, a complex free radical mediated process, may be described via three distinct steps: i) initiation, ii) propagation, and iii) termination.5,6 Peroxidation initiation involves hydrogen atom abstraction (hemolytic cleavage) from the fatty acid to produce an initial radical. The number of unsaturated sites in the lipid fatty acid tail dictates the ease with which the hydrogen may be abstracted. The carbon-hydrogen bond energy progressively decreases from alkyl (101kcal/mol), to allylic (88 kcal/mol), and bis-allylic (75–80 kcal/mol).7 These lipid radicals may be resonance stabilized following the bond energy trend bis-allylic > allylic > alkyl; hence, the greater intrinsic chemical propensity of lipids containing two or more unsaturated sites to undergo greater peroxidation.5,8 During the propagation step, the lipid radical may react with oxygen to produce a peroxyl radical.8 Once formed, the peroxyl radical may abstract a hydrogen atom from another lipid molecule to produce additional lipid radicals and subsequent hydroperoxides.8 Additional reactions may also occur such as cyclization, fragmentation and rearrangement.8 The final lipid peroxidation step, termination, occurs when radical concentrations become very low and results in the coupling of two radicals to form a non-radical product.9 It has been demonstrated that the physical properties (i.e., permeability and fluidity) of liposome bilayers may be altered via peroxidation.10–12
Many lipid peroxidation studies have utilized aqueous formulations. More specifically, Vossen et al.13 investigated how increasing lipid unsaturation affected the ability of copper (II)/H2O2 to catalyze peroxidation. Lipid peroxidation was assessed by measuring conjugated diene accumulation; liposomes composed of PLinPC (16:0, 18:2) or PLPC (16:0, 18:3) were utilized. As liposomes composed of 16:0/16:1 lipids were observed to be insensitive to peroxidation, the formation of conjugated dienes resulting from peroxidation were attributed to linoleic acid (18:2) or linolenic acid (18:3). The observed peroxidation was much faster in PLPC samples compared to PLinPC samples; these data are consistent with the notion that increased peroxidation occurs more readily as the degree of unsaturation increases.13 Other studies have also demonstrated a correlation between the degree of unsaturation and the susceptibility to oxidative degradation for dissolved lipids and liposome suspensions.7,14,15
Lyophilization is a strategy often employed to improve liposomal formulation stability, due to reactivity being far less pronounced in the solid versus aqueous state. While dehydration would be expected to reduce chemical reactions involving water (i.e., hydrolysis), the specific elements that affect the chemical stability of lyophilized liposomal formulations (i.e., lipids in the dried state) are not well understood. Some studies have been conducted which utilized lyophilized liposomal systems, although these studies were primarily concerned with physical phenomena such as liposomal aggregation/fusion and the retention of small molecules.16–20 In an insightful study conducted by Mouradian et al.21, sarcoplasmic reticulum (SR) vesicles were prepared from isolated lobster tail muscle, lyophilized and stored for 49 days. The peroxidation of these SR vesicles was evaluated by monitoring the accumulation of lipid hydroperoxides and secondary oxidation products (i.e., aldehydes) using the thiobarbituric acid reactive substances (TBARS) assay. Due to the presence of polyunsaturated lipids in the sarcoplasmic reticulum membrane, peroxidation was expected to be a major degradation pathway. Exposure to light and moisture content present in the lyophilized samples dictated by the relative humidity to which the samples were exposed during storage (0% and 35%) significantly influenced SR vesicle stability. As one would expect, light exposure was conducive to increased hydroperoxide accumulation. Also, secondary oxidation product accumulation predominantly occurred in the samples with lower moisture content (exposed to 0% relative humidity). It was suggested that the secondary oxidation products were chemically less stable preventing their accumulation and subsequent detection in samples with the higher moisture content (exposed to 35% relative humidity).
Although liposomes have traditionally been used to deliver small molecules, more recent studies have utilized lipid nanoparticles to facilitate the delivery of nucleic acids (i.e., DNA, RNA) to the cell interior. Because polyunsaturated lipids have been shown to enhance intracellular delivery, there is increasing interest in developing stable formulations containing these types of lipids.22–24 The current study assesses the long term storage stability of lyophilized liposomal formulations. The chemical stability of freeze dried liposomal formulations containing a triply unsaturated lipid (DLPC, 18:3), a doubly unsaturated lipid (DLinPC, 18:2), or a singly unsaturated lipid (DOPC, 18:1) was evaluated. Additionally, the effect of temperature (4°C, 22°C room temperature, 37°C, 50°C and 60°C) on the rate of degradation of these formulations was assessed. To determine if formulations containing multiply unsaturated lipids could be stabilized sufficiently for successful commercial use, several stabilization strategies were evaluated including the use of helper lipids (i.e., DOPE and cholesterol), the use of different sugar excipients (e.g., trehalose, sucrose and sorbitol) and the use of iron chelators (e.g., diethylenetriaminepentaacetic acid (DTPA) and deferoxamine (DFO)). The effect of other processing parameters related to the preparation and lyophilization of samples (e.g., use of degassed buffer, rapid freezing) were also investigated. Although this study is primarily concerned with the chemical stability of lipids in relation to pharmaceutical applications, other studies have investigated the stability of lipids in relation to food and beverage products.25–31 In order to develop better lipid-based therapeutics for intracellular delivery, further investigation of the dried-state storage stability of lyophilized liposomal formulations is necessary, especially for polyunsaturated lipids.
Materials and Methods
Materials
1, 2-Dilinolenoyl-sn-glycero-3-phosphocholine (DLPC; 18:3 (Cis) PC) (>99%, Lot # 183PC-12li), 1,2-dilinoleoyl-sn-glycero-3-phosphocholine (DLinPC; 18:2 (Cis) PC) (>99%, Lot # 182PC-183b), and 1, 2-di-(9Z-octadecenoyl)-sn-glycero-3-phosphocholine (DOPC 18:1 (Cis) PC) (>99%, Lot # 181PC-257) were purchased from Avanti Polar Lipids (Alabaster, AL). Sucrose (Ultrex© ultrapure, Lot # J25H00) was acquired from JT Baker (Center Valley, PA). Trehalose (100%, Lot #28549A) was obtained from Ferro Pfanstiehl (Waukegan, IL). Sorbitol (98.9%, Lot # B65414) was procured from Calbiochem (La Jolla, CA). Tris [hydroxymethyl] amino-methane, DFO (≥ 92.5%, Lot # 032K1210), hydranal water standard 1.0 (Fluka, Lot # SZBB2850V) and hydrochloric acid were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). 3-(2-Pyridyl)-5,6-diphenyl-1,2,4-triazine-p,p′-disulfonic acid, disodium salt hydrate (ferroZine iron reagent), dimethylformamide(extra dry 99.8%) and propionic acid were purchased from Acros Organics (NJ, USA). Chloroform (HPLC grade), water (HPLC grade), methanol,(LC/MS grade) acetone (LC/MS grade), freezer boxes, sodium hydroxide pellets (Lot # 110388) and Eppendorf tubes (1.5 mL) were procured from Fisher Scientific (Pittsburgh, PA). High grade nitrogen, helium and argon were obtained from Airgas (Radnor, PA). Amber glass vials (5 mL) were purchased from West Pharmaceutical Services (Lionville, PA). Solid phase extraction (SPE) tubes (Phenomenex Strata X, 1.0 mL) containing sorbent (30 mg; Lot # S300-147) were used (Torrance, CA). The aluminum pans and lids were purchased together as part of a kit from Perkin Elmer (Norwalk, NJ). Pyridine free vessel solution (Lot # C364746) and generator solution (Lot # 0135010) were purchased from Photovolt (Minneapolis, MN).
Sample Preparation
All lipid stocks were dissolved in chloroform and DLPC, DLinPC and DOPC lipid stocks concentrations were 10.0 mg/ml, 25.0 mg/ml and 20.0 mg/ml respectively. After transferring the appropriate lipid stock volume, the chloroform was evaporated under a slow stream of N2 gas. The lipid was subjected to vacuum (559 Torr; 1.0 hr) to remove residual chloroform. The lipid (DLPC, DLinPC or DOPC) was then suspended using an aqueous solution containing 2% sugar (trehalose, sucrose or sorbitol) and Tris buffer (0.5 mM, pH 7.4) to produce a final lipid concentration of 0.02 mg/ml. The rehydrated lipid (DLPC, DLinPC or DOPC) was then sonicated (2.0 min) and gently swirled. A 1.0 mL volume was aliquoted into amber glass vials (5 mL) and the final sample composition was lipid (0.02 mg/ml) and 2% sugar (sucrose, trehalose or sorbitol). After lyophilization and vials sealed, samples were then placed in boxes and stored at 4 ± 2°C, 22 ± 2°C (room temperature), 37 ± 2°C, 50 ± 2°C, or 60 ± 2°C. When DTPA or DFO were added to DLPC samples, DTPA or DFO were dissolved using an aqueous solution containing 2% trehalose, 0.5 mM Tris, and the final chelator concentration was 8.9 ppm. The pH of the chelator-containing solution was adjusted to 7.4 using 1.0 μL aliquots of 0.1 M NaOH. The resulting solution was used to rehydrate the lipid after chloroform removal as previously described.
Lyophilization Protocol
Sample vials and stoppers were typically washed with 0.1 M HCl water overnight and then rinsed three times with high-purity distilled water. Sample vials were placed directly on the shelf of a Lyostar 3 (SP Scientific, Gardiner, NY). The SMART lyophilization mode was utilized. During the freezing stage, the shelf temperature was progressively decreased from room temperature to 5°C, then to −5°C and finally to −40°C. The duration of time that the shelf temperature remained at these temperatures was related to the number of samples which were being lyophilized as well as the volume and composition of the samples. Next, for primary drying, the sample chamber pressure was reduced to 57 mTorr. After primary drying was complete (as indicated by a decrease in the pressure as measured by the pirani pressure gauge indicating the end of bulk water sublimation), the shelf temperature was increased at a rate of 0.1 °C/min, and secondary drying was conducted at 30°C (1800 min). Sample temperatures were monitored by inserting a thermocouple into a representative sample prior to starting the lyophilization cycle. After sample lyophilization was completed, the vials were stoppered while the chamber remained under vacuum. The samples comparing the effect of the degree of lipid unsaturation, which contained DLPC, DLinPC and DOPC, and the effect of different sugars were lyophilized in the same run. The samples containing sorbitol or sucrose collapsed during lyophilization and storage, respectively, whereas the structure of the trehalose samples was maintained throughout the duration of the experiments.
HPLC-UV Analysis
The DLPC remaining in stored samples were measured utilizing the HPLC/UV as previously described.32 To measure DLinPC and DOPC, the percent of methanol in the mobile phase was increased from 95% to 97% (19:1 methanol:water to 19.4:1 methanol:water). The duration of the method varied from 20 to 25 minutes depending on which lipid was being analyzed. For all analyses, a Shimadzu analytical HPLC system (LC-20AB, DGU-20A, CTO-20A, Sil-20A HT) equipped with SPD-20A UV-VIS detector was utilized (Shimadzu Scientific Instruments, Inc.; Columbia, MD). A zorbax extended-C18 50 × 4.6 mm (5 micron) column in combination with a guard column was used (Aligent, Santa Clara, CA). The flow-rate was 0.4 mL/min and the column temperature was maintained at 40°C. An isocratic method was used, and UV detection was monitored at 205 nm. For samples and standards (DLPC, DLinPC and DOPC), 10 μL sample volumes were injected.
Solid Phase Extraction Procedure
DLPC degradation product analysis was performed via GC/MS but required the removal of the sugar component; therefore we utilized a solid phase extraction (SPE) procedure. The SPE procedure was identical to that previously described by Payton et al.32 An internal standard solution, propionic acid (final concentration 10.0 μg/ml) diluted in acetone, was freshly prepared and spiked into samples.
GC/MS Analysis
To monitor DLPC degradation products, a Shimadzu GC/MS-QP2010 Plus (Shimadzu Scientific Instruments, Inc.; Columbia, MD) gas chromatograph mass spectrometer was utilized. A Phenomenex ZB-FFAP analytical column (30m × 0.25mm × 0.25 mm) was used in tandem with a 5 meter guard column (Phenomenex, Torrance, CA). The settings were as follows: column temperature was initially set at 50°C with an injector temperature of 250°C; the oven temperature was held at 50°C for 6 min, ramped at 8 °C/min to 200°C and held for 10 min and then the temperature was increased at 8 °C/min to 230°C and held for 10 min. The interface temperature was 230°C and the ion source temperature was 210°C. The column flow rate was 1.0 mL/min and helium was used as the carrier gas. The total flow was 14.1 mL/min and the purge flow was 3.0 mL/min. The pressure was 539 Torr. The splitless mode was used and the sampling time was 1.0 min after which the split mode was utilized (split ratio of 10). The detector delay was set to 5.0 min. The scanning mode was utilized and the event time was set to 0.5 sec from 5.0 min to 11.5 min (scan speed 1428) and 0.2 from 13.0 min to 47.5 min (scan speed 5000). The injection volume was 1.0 μL. Peaks corresponding to propanoic acid, the internal standard, and the main product detected in lyophilized DLPC samples were monitored.
Moisture Content Analysis
The moisture content of lyophilized samples shown in Table I was determined using a DL37 coulometric moisture analyzer (Mettler Toledo, Columbus, OH) using pyridine free vessel solutions (Photovolt Instruments, Inc., St. Louis Park, MN). The samples were prepared in a glove box purged with dry nitrogen to avoid absorption of ambient air moisture. Anhydrous dimethylformamide was used to dissolve lyophilized samples. Samples were extracted from the lyophilization vials through the stopper, and the moisture content was subsequently analyzed as previously described.33,34 Sample moisture content was analyzed after lyophilization and after storage at the various storage temperatures utilized in this study.
Table 1.
Moisture Content and Glass Transition Temperatures (Tg) of Lyophilized Formulations before and after Storage
| Excipient | Before Storage
|
After Storage
|
||
|---|---|---|---|---|
| Moisture Contenta | Tgb | Moisture Contenta,c | Tgb,c | |
| Trehalose | 0.32 ± 0.07 | 122.0 ± 0.1 | 0.67 ± 0.05 | 114.5 ± 0.9 |
| Sucrose | 0.31 ± 0.11 | 60.6 ± 0.03 | 0.36 ± 0.10 | 59.2d |
| Sorbitol | 0.34 ± 0.08 | −5.4 ± 0.1 | 0.48 ± 0.15 | −5.8 ± 0.2 |
Values represent the mean ± SEM (n = 4 or 5). Moisture content expressed as weight percent.
Tg values reported are the onset temperature. Measurements represent the mean ± SEM (n = 3).
Moisture content and Tg values for samples stored at 60°C. Only trehalose samples were stored at lower temperature and the moisture content of trehalose samples stored at lower temperatures was comparable (data not shown).
n = 1.
Differential Scanning Calorimetry
Samples containing trehalose, sucrose or sorbitol were analyzed (thermal analysis) using a Perkin-Elmer Diamond DSC (Norwalk, CT). All samples were prepared in a glove box purged with dry nitrogen to prevent moisture absorption from the ambient air. The lyophilized cake (≈ 2 mg of powder) was removed from the lyophilization vial and the sample was placed in an aluminum pan and sealed. Trehalose samples were heated (from 25°C to 150°C) at 100 °C/min, held for 5 min, cooled to 25°C, and reheated again to 150°C. Sucrose samples were heated (from 0°C to 100°C) at 100 °C/min, held for 5 min, then cooled to 0°C, and reheated again to 100°C. Sorbitol samples were heated (from −40°C to 50°C) at 20 °C/min, held for 5 min, then cooled to −40°C, and reheated again to 50°C. The second scan was used to measure the glass transition temperature (Tg). The reported Tg values (Table I) refers to the onset temperature of the second-order change in heat capacity with temperature.
Ferrozine assay
Ferrozine and ascorbic acid were dissolved in 1.0 M HCl to afford final concentrations of 20 mM and 200 mM, respectively. The ascorbic acid/ferrozine solution (20 μl) was added to each sample (340 μl), mixed, and heated (60°C, 20 min). After cooling the reagent/sample to room temperature (12 h), the absorbance was measured at 562 nm. Ferric citrate standards were used to generate a standard curve covering a concentration range of 0–800 ppb (R2= 0.94).
Electron Spectroscopy for Chemical Analysis and Specific Surface Area
Previously published techniques were utilized to measure the lipid molecules on the surface of lyophilized trehalose samples; electron spectroscopy for chemical analysis (ESCA) and the specific surface area of lyophilized trehalose samples via Branauer, Emmett and Teller (BET) krypton adsorption.35 The mass of the lipid molecules on the surface of lyophilized trehalose cakes was calculated as previously described.35
Dynamic Light Scattering to Measure Hydrodynamic Diameter
A liposome suspension containing 0.02 μg/mL DLPC and 2% trehalose in Tris buffer was prepared as described above. An aliquot (200 μL) was transferred to a quartz cuvette and the size of DLPC liposomes was measured using dynamic light scattering (DLS) with a Zetasizer Nano-ZS (Malvern, UK). The reported diameter of DLPC liposomes (504 nm) was based on the size distribution by volume and the % volume of the main population was 85.3 %.
Results and Discussion
Lyophilized liposomal formulations have been utilized successfully in marketed pharmaceutical products, such as AmBisome® and Visudyne®; however, these formulations utilize saturated or singly unsaturated lipids.1 The use of multiply unsaturated lipids in lipid-based commercial products has been avoided due in part to their greater susceptibility to oxidative degradation despite their ability to improve intracellular delivery by enhancing endosomal escape.22 Although many studies have evaluated the degradation of aqueous liposomal formulations, few studies have investigated the stability of liposomal formulations in the dry state. The goal of the current study was to characterize the long term storage stability of lyophilized liposomal formulations and to evaluate the effect of liposome composition, formulation strategies, and processing parameters on the degradation of unsaturated lipids in the dried state.
Effect of liposome composition and degradation as a function of temperature
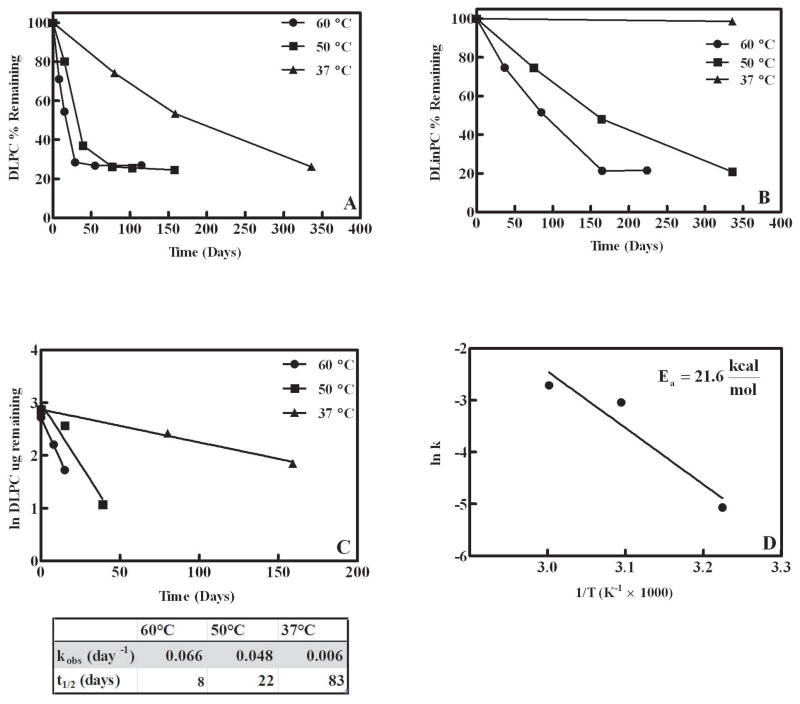
Significant DLPC degradation in samples occurred at 37°C, 50°C, and 60°C (Fig 1a) while significant degradation of DLinPC in samples was only observed at 50°C and 60°C (Fig 1b). Appreciable DLPC and DLinPC degradation did not occur at the lower storage temperatures, 4°C and 22°C (room temperature). DOPC samples were found to be stable at all storage temperatures over 48 weeks (data not shown). The degradation of DLPC samples occurred over a wider range of temperatures and at a faster rate relative to DLinPC samples (Fig. 1a+b). These results suggest that increased liposomal degradation in the dried state was dependent upon the degree of lipid unsaturation, and consistent with previous studies conducted using aqueous liposomal formulations, which concluded that multiply unsaturated lipids were particularly susceptible to oxidative degradation due the relative ease with which bis-allylic hydrogens may be abstracted.5,36
Figure 1.
Lipid degradation during storage. A) DLPC loss as measured by HPLC in samples stored at 60°C, 50°C, and 37°C. B) DLinPC loss as measured by HPLC in samples stored at 60°C, 50°C and 37°C. C) Initial DLPC degradation rate (time points 0, 1 and 2) for samples stored at 60°C, 50°C, and 37°C. Rate constants (kobs) and the half-life (t1/2) were determined from the linear regression. For the samples stored at 60°C, 50°C, and 37°C, the R2 values for the linear regression trend lines are 0.9931, 0.9431 and 0.9762 respectively. D) Arrhenius graph plotted using the extrapolated rate constants versus 1/T (K−1 × 1000). The values represent the mean ± 1 SEM of triplicate determinations; errors are within the symbol size.
As expected, degradation rate was faster as the storage temperature was increased (Fig. 1a+b). The initial degradation rate was estimated by plotting the natural log of DLPC μgrams remaining at the first three time points. The pseudo first order rate constant, kobs, and the half-life (t1/2) were extrapolated from this plot (Fig 1c) which allowed for the construction of an Arrhenius plot (Fig 1d) to afford an estimation of the activation energy, Ea, at 21.6 kcal/mol. This value is comparable to other Ea values which have been reported previously for the chemical degradation of lipid molecules.37,38
Leveling off of the degradation rate
Curiously, in DLPC formulated in trehalose and stored at 50°C and 60°C, as well as in the DLinPC samples stored at 60°C, lipid levels remained constant after an initial phase of steady degradation (Fig 1a). The underlying cause of the decreased degradation rate (i.e., the observed plain) was considered from a variety of perspectives. We hypothesized that the decrease in the degradation rate may be related to lipid molecules on the surface of the trehalose cakes degrading more rapidly due to exposure to gases (e.g., O2) in the vial headspace as compared to lipid molecules that are located in the interior of the cake. To evaluate this potential explanation, the mass of the lipid molecules on the surface of the lyophilized trehalose cake was assessed, and it was determined that approximately 12% of the lipid molecules were located on the surface. Since approximately 75% (Fig 1a) of the lipid molecules degraded before the lipid concentrations appeared to level off (i.e., the plain), we concluded that surface exposure does not account for the marked decrease in degradation rate.
Alternatively, it was speculated that the accumulation of lipid hydroperoxides in the chloroform lipid stock might also be influencing the observed degradation profile. Each time the lipid stock was opened, it was briefly exposed to ambient atmosphere, potentially resulting in the introduction of oxygen and lipid hydroperoxide formation. Considering the iron contaminants known to be present in the sugars of these formulations, the hydroperoxides could potentially decompose as a result of reaction with iron and the subsequent formation of peroxyl radicals.39–41 To test if the age/handling of the lipid stock affected the amount of lipid remaining when the plain occurred, a freshly opened DLPC lipid stock as well as older lipid stocks opened 7 and 16 months earlier were used to prepare samples. After lyophilization, the DLPC-trehalose samples were stored at 60°C for 42 days (6 weeks); this storage duration was chosen because in previous experiments it was observed that the decreased degradation rate (i.e., plain) occurred after 29 days (time point 3, Fig 1a). Although we did observe some differences in the DLPC remaining after storage, they did not correlate with the age of the lipid stock (data not shown).
Another possible explanation for the plain is the depletion of a molecule (i.e., reactant) present in the sample vial which could be participating in the degradation reaction. The involvement of oxygen as a reactant is suspected because aldehyde products, which are characteristic products of lipid oxidation, were detected via the TBARS assay in DLPC samples stored at 37°C (data not shown). Oxygen could potentially originate from several different sources including gaseous oxygen in the headspace of the sample vials, leaking of the ambient atmosphere into the vials which were stoppered under vacuum, and dissolved oxygen present in the aqueous sample prior to lyophilization. However, since the removal of oxygen from the aqueous sample prior to lyophilization did not affect stability (Fig 6), and the variability between the samples was low (leaking would be expected to cause high variability among vials), we feel that it is unlikely that oxygen originated from these sources. Gaseous oxygen in the headspace of the vials is most likely the result of the residual atmospheric gases remaining after the pressure was decreased from atmospheric pressure to 57 mTorr. Even at the low pressures used during lyophilization (e.g. 57 mTorr), we calculate that approximately 5.1 nmoles of oxygen is present in the vial. Relative to the 25.7 nmoles of DLPC, the amount of gaseous oxygen may be sufficient to participate as a reactant in the DLPC degradation reactions. It is important to recognize that small molecules, such as oxygen, are capable of diffusing readily through the lyophilized cake (e.g., amorphous sugar matrix).42,43
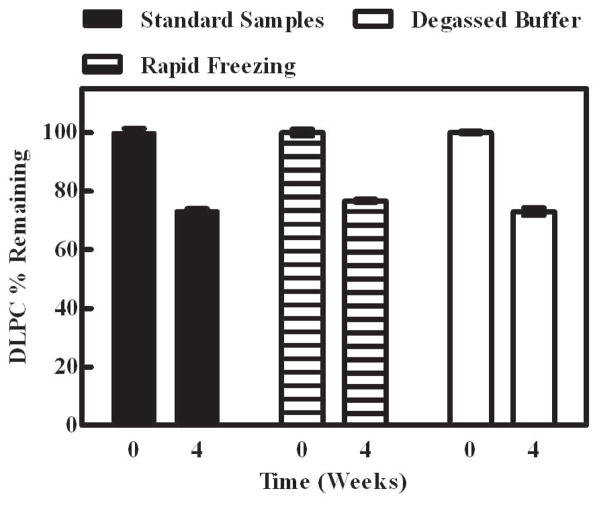
Figure 6.
Effect of processing conditions on the degradation of lyophilized DLPC samples during storage. Samples were stored at 60°C for 4 weeks. Degradation of DLPC in samples which were made with standard (i.e., not degassed) buffer and frozen slowly in the lyophilizer (black bar) or frozen rapidly by plunging vials into liquid nitrogen (hashed bar). Another set of samples were also frozen slowly in buffer degassed by nitrogen bubbling (white bar).
However, we believe that the relative number of iron atoms (i.e., iron contaminants) present within and outside of the liposome might contribute to the observed plain. During preparation, the liposomes were rehydrated with a Tris buffer solution containing trehalose, so both the inner and outer leaflet of the liposome would be exposed to the sugar excipient (e.g., trehalose) and also the iron contaminants in the sugar. Based on the average approximate size of the DLPC liposomes (504 nm), the interior liposome volume is quite small (i.e., 6.3 × 10−14 mL) relative to the aqueous volume outside of the liposome (i.e., 1.0 mL). Within liposomes, the iron-to-lipid mole ratio is approximately 1:4516. In contrast, the lipids in the outer liposome leaflets are exposed to significantly higher amounts of iron, a 1:40 iron-to-lipid mole ratio. This calculation suggests that the inner leaflet of the liposome would be less susceptible to iron-catalyzed degradation due to the lower iron-to-lipid ratio within the liposome; this could potentially contribute to the leveling-off of lipid degradation (plain) observed during storage.
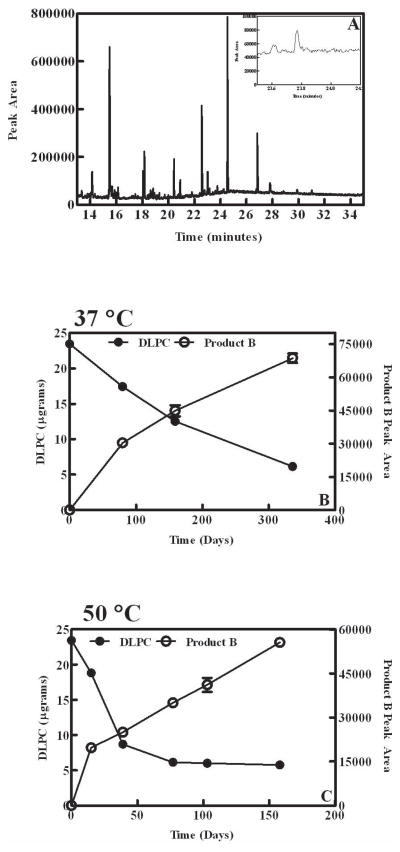
Analysis of DLPC degradation products
DLPC degradation products were measured via GC/MS and the accumulation of one distinct product, referred herein as Product B, was observed. A representative chromatogram illustrating the internal standard peak (propionic acid; tr=15.5 min) and Product B (tr= 23.75 min; inset) is presented in Figure 2a. The accumulation of Product B was observed and the relative amount of Product B increased as DLPC degradation occurred over time (Fig. 2 b+c). The rate of Product B accumulation was dependent upon the storage temperature, and Product B accumulation was most rapid in DLPC samples stored at 50°C. (Fig 2c) DLPC samples stored at 50°C displayed the accumulation of Product B, which continued even after the rate of DLPC loss decreased (e.g., after 75 days in Fig 2c); these observations support that notion that DLPC does not directly degrade to Product B and that intermediate species (e.g., lipid hydroperoxides) are most likely involved in Product B formation. A relatively slower accumulation of Product B was observed for samples stored at 37°C (Fig 2b). Product B was not detected in samples stored at 60°C, presumably due to instability at elevated temperatures.
Figure 2.
Product B accumulation in DPLC samples during storage. A) Representative gas chromatogram. Internal standard (propanoic acid) tR=15.5 minutes. Inset shows a chromatogram which has been rescaled to show the peak corresponding to the measured product (Product B) tR= 23.75. B) Loss of DLPC (closed circles) and the accumulation of Product B (open circles) in samples stored at 37°C. C) Loss of DLPC (closed circles) and the accumulation of Product B (open circles) in samples stored at 50°C. The values represent the mean ± 1 SEM of triplicate determinations.
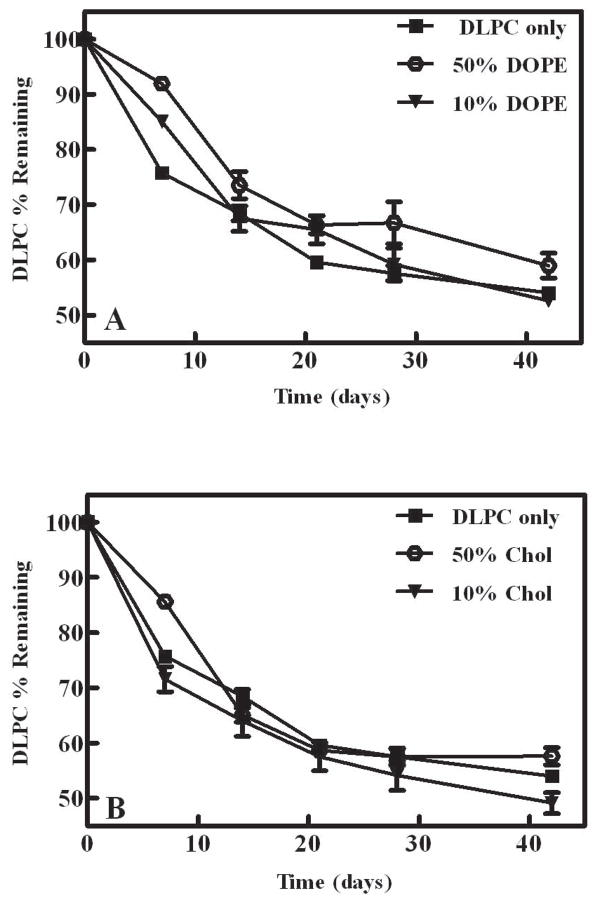
Effect of the inclusion of helper lipids
Cholesterol and DOPE, often referred to as helper lipids, are frequently included in liposomal formulations to improve the intracellular delivery of the cargo encapsulated or associated with the liposome.44,45 To assess the potential effect of helper lipids on storage stability, DOPE and cholesterol were each incorporated separately into the DLPC-trehalose formulation and stored at 60°C. Besides a DLPC-only formulation, two different helper lipid mole ratios were tested: 1:1 (DLPC: helper lipid) and 9:1 (DLPC: helper lipid). DLPC degradation was not mitigated by the inclusion of DOPE or cholesterol, and the mole fraction of each helper lipid also did not alter the observed rate or extent of DLPC degradation (Fig. 3a+b). Previous studies with hydrated formulations have reported inconsistent effects of cholesterol on the oxidative degradation of liposomal formulations, and results appear to depend on the composition of the liposomal formulation and the temperature at which the study was conducted.46 For example, a study by Mowri et al.47 reported a progressive decrease in oxidative degradation with increased cholesterol, and it was proposed that the effect of cholesterol on the fluidity of the lipid bilayer caused the reduced peroxidation which was observed. However, another study examining the effect of cholesterol on the stability of EPC liposomes reached somewhat different conclusions.48 In this latter study, the hydrophilic azoinitiator, 2,2′-azobis-2-methyl-propanimidamide, dihydrochloride (AAPH), was utilized and the cholesterol content was varied from 0% to 45% (mol %). Overall the presence of cholesterol did not alter the oxidation of egg PC liposomes except for the formulation which contained 10% cholesterol. Surprisingly, the oxidation detected in this formulation was greater than any of the formulations containing higher cholesterol contents.48 The authors suggested that increased peroxidation at 10 mol% cholesterol could be attributed to the coexistence of liquid disordered and ordered (LD and LO) phases, but it is unclear why such phases would not exist at the higher cholesterol concentrations that did not exhibit increased peroxidation. Although the studies mentioned above are clearly conflicted regarding the ability of cholesterol to affect peroxidation in aqueous formulations, our results strongly suggest that cholesterol does not affect DLPC degradation in the dried state.
Figure 3.
Degradation of DLPC and DLPC-helper lipid formulations as measured by HPLC. A) Degradation of DLPC and DLPC:DOPE formulations after 6 weeks of storage at 60°C. The DOPE formulations contain 50% DOPE (1:1), or 10% DOPE (9:1). B) Degradation of DLPC and DLPC: cholesterol formulations after 6 weeks of storage at 60°C. The cholesterol formulations contain 50% cholesterol (1:1), or 10% cholesterol (9:1). The values represent the mean ± 1 SEM of triplicate determinations.
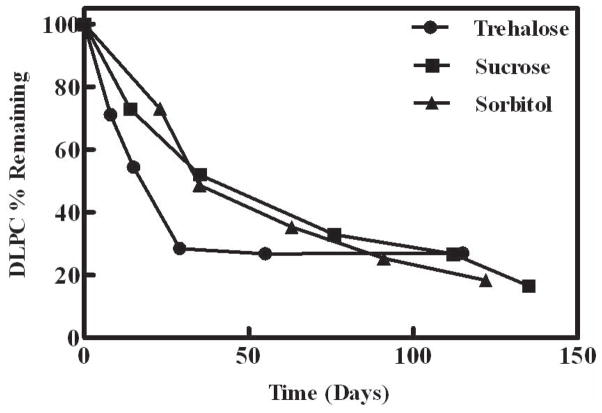
Effect of the lyoprotectant (trehalose, sucrose or sorbitol)
DLPC samples formulated with trehalose, sucrose or sorbitol were lyophilized and stored at 60°C. These non-reducing sugars were selected based upon their distinctly different glass transition temperatures, thereby allowing us to evaluate the role, if any, of glassy dynamics. We hypothesized that the rate of degradation for the DLPC-sugar samples would correlate with the glass transition temperature of the sugars, and that the samples lyophilized in trehalose (Tg = 122.0 ± 0.1 °C), sucrose (Tg = 60.6 ± 0.03 °C) and sorbitol (Tg = −5.4 ± 0.1 °C) (Table I) would exhibit relatively slow, intermediate and fast degradation rates, respectively.49,50 In contrast, the DLPC-trehalose samples exhibited a slightly faster degradation rate relative to the DLPC-sucrose and DLPC-sorbitol samples (Fig. 4). Also, the rates of DLPC degradation for the samples formulated in sucrose and sorbitol were comparable. These data demonstrate that DLPC degradation rate in the different sugar formulations does not correlate with state (glass or rubber) of the sugar amorphous matrix. Considering that the diffusion of lipids would likely be affected by the physical state of the matrix, this result suggests that the translational mobility of the lipid molecules was not dictating the rate of degradation, but it does not rule out a role for small molecules (e.g., O2) that are able to diffuse through the amorphous matrix, 42,51,52 and are less affected by the physical state of the matrix.
Figure 4.
Degradation of DLPC samples formulated with different sugar excipients as measured by HPLC. Samples were stored at 60°C for up to 135 days (≈ 19 weeks). Closed circles – trehalose; closed squares – sucrose; and closed triangles- sorbitol. The values represent the mean ± 1 SEM of triplicate determinations; errors are within the symbol size.
Effect of chelators
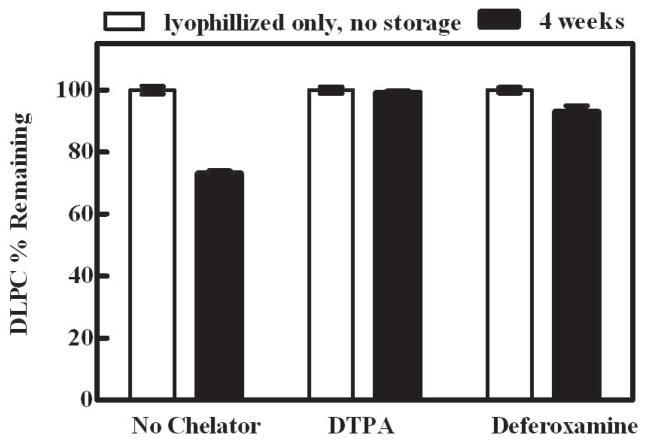
It is known that pharmaceutical grade sugars inherently contain trace metal contaminants (e.g., iron) 32,53 and the trehalose which was utilized in this experiment contained approximately 1.78 ± 0.01ppm iron (n =3, average ± SEM data not shown). To assess the influence of the iron contaminants on the stability of lipids in the dried state, DTPA and DFO were added to DLPC samples prior to lyophilization and storage at 60°C (4 weeks). The addition of DTPA or DFO to DLPC samples significantly (p < 0.0001) decreased the observed DLPC degradation that occurred during storage (Fig. 5). The decreased DLPC degradation in samples containing DTPA or DFO supports the notion that iron (and potentially other trace metals, e.g., Cu+2) deleteriously impacts the overall lipid stability in the dried state. Although the exact mechanism(s) by which iron catalyzes DLPC degradation was not fully elucidated in the current study, this data clearly illustrates that a method that circumvents iron exposure results in a more stable lipid formulation. It is important to note that DTPA and DFO are particularly effective iron chelators because they occupy all of the iron coordination sites.54,55
Figure 5. Effect of iron contaminant chelation on DLPC stability.
Degradation of DLPC with and without DTPA or deferoxamine as measured by HPLC in samples lyophilized and stored at 60 °C for 4 weeks. Chelator concentration was 8.9 ppm which is 5 times greater than the concentration of the iron contaminants (1.78 ppm). White bars - after lyophilization, no storage. Black bars - after 4 weeks of storage at 60°C. The values represent the mean ± 1 SEM of triplicate determinations. Degradation of stored DLPC samples containing chelator compared to stored DLPC samples containing no chelator. *, p < 0.0001
Effects of freezing method and degassing
The effects of freezing method and buffer degassing on the storage stability were evaluated in samples formulated with trehalose. Because slow versus fast freezing rates have been shown to influence the gas content (O2 and N2) of aqueous formulations56, we assessed the effect of employing different freezing methods. For example, DLPC samples were frozen utilizing two different protocols: plunging vials directly into liquid nitrogen or placing on the lyophilizer shelf which was subsequently cooled to −40°C. The observed DLPC degradation was not dramatically altered by the freezing method and 76.6 ± 0.9 % and 73.2 ± 0.9 % (average ± SEM) of the initial DLPC remained in samples frozen in liquid nitrogen and on the lyophilizer shelf, respectively, after 4 weeks of storage at 60°C (Fig. 6). Because we speculated that oxygen may be playing an important role in the observed DLPC degradation, degradation was assessed in samples prepared with buffer degassed via nitrogen bubbling. Our data show that DLPC degradation was comparable to samples prepared with the standard buffer, and no significant differences were observed. More specifically, samples prepared with standard and degassed Tris buffer and stored at 60°C for 4 weeks, 73.2 ± 0.9 % and 73.0 ± 2.2 % (average ± SEM) DLPC remained, respectively (Fig. 6).
Conclusions
This study examined the effect of liposome composition as well as a variety of formulation strategies and processing parameters on the long term stability of unsaturated lipids in the dried state. The degradation of lyophilized liposome samples increased as the degree of lipid unsaturation increased. The rate of lipid degradation occurred at a greater rate at higher storage temperatures, but the state of the lyophilized samples (glass or rubber) did not correlate with observed degradation rates. The incorporation of helper lipids (cholesterol and DOPE) into the liposome formulation did not improve the chemical stability of the triply unsaturated lipid, DLPC. The addition of chelators, DTPA and DFO, significantly improved the stability of DLPC, indicating that trace levels of metal contaminants contribute to the observed degradation during storage. We feel that this is a significant finding considering that our earlier work concluded that trace levels of iron contaminants do not facilitate degradation during acute lyophilization unless reducing agents are present in the formulation. In this context, it is possible that even fairly inert molecules (e.g., sugars) may be capable of reducing iron during prolonged storage, thereby converting iron to a more reactive form. This suggestion is consistent with previous studies in our laboratory wherein lyophilized sugar-only controls generate reactive oxygen species during prolonged storage.57 Although a specific mechanism of lipid degradation in the dry state was not definitively elucidated, the results from this study which indicated that the degradation rate increased with greater lipid unsaturation, that iron contaminants enhanced the rate of lipid degradation, and that aldehydes formed in the lyophilized samples during storage are all consistent with oxidative degradation. The results from this study may be useful for developing stable formulations of pharmaceuticals containing polyunsaturated lipids.
Acknowledgments
This work was partially supported by NIH/NIBIB grant 1R1EB016378and NIH/NIGMS grant RO1GM093287. This research utilized services of the Medicinal Chemistry Core Facility (MCCF) housed within the Department of Pharmaceutical Sciences (DOPS) at the University of Colorado Anschutz Medical Campus. The MCCF receives funding via Colorado Clinical and Translational Sciences Institute grant NIH-NCATS, UL1TR001082. We would also like to acknowledge Drs. Theodore W. Randolph and Alan MacKenzie for extremely helpful discussions and critical insight as well as Dr. Pawel Grobelny for assisting with the ESCA measurements.
Contributor Information
N.M. Payton, Email: Nicole.payton@ucdenver.edu.
M.F. Wempe, Email: Michael.wempe@ucdenver.edu.
Y. Xu, Email: Yemin.Xu@bms.com.
References
- 1.Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. International Journal of Nanomedicine. 2012;7:49–60. doi: 10.2147/IJN.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Advanced Drug Delivery Reviews. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 3.Grit M, Zuidam NJ, Underberg WJ, Crommelin DJ. Hydrolysis of partially saturated egg phosphatidylcholine in aqueous liposome dispersions and the effect of cholesterol incorporation on hydrolysis kinetics. J Pharm Pharmacol. 1993;45(6):490–495. doi: 10.1111/j.2042-7158.1993.tb05585.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang JA, Xuan T, Parmar M, Ma L, Ugwu S, Ali S, Ahmad I. Development and characterization of a novel liposome-based formulation of SN-38. International journal of Pharmaceutics. 2004;270(1):93–107. doi: 10.1016/j.ijpharm.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Porter NA. Mechanisms for the autoxidation of polyunsaturated lipids. Accounts of Chemical Research. 1986;19(9):262–268. [Google Scholar]
- 6.Frankel E. Lipid oxidation: mechanisms, products and biological significance. Journal of the American Oil Chemists’ Society. 1984;61(12):1908–1917. [Google Scholar]
- 7.Wagner BA, Buettner GR, Burns CP. Free radical-mediated lipid peroxidation in cells: oxidizability is a function of cell lipid bis-allylic hydrogen content. Biochemistry. 1994;33(15):4449–4453. doi: 10.1021/bi00181a003. [DOI] [PubMed] [Google Scholar]
- 8.Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30(4):277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 9.Yin H, Porter NA. New insights regarding the autoxidation of polyunsaturated fatty acids. Antioxidants & Redox Signaling. 2005;7(1–2):170–184. doi: 10.1089/ars.2005.7.170. [DOI] [PubMed] [Google Scholar]
- 10.Smolen JE, Shohet SB. Permeability changes induced by peroxidation in liposomes prepared from human erythrocyte lipids. Journal of Lipid Research. 1974;15(3):273–280. [PubMed] [Google Scholar]
- 11.Kunimoto M, Inoue K, Nojima S. Effect of ferrous ion and ascorbate-induced lipid peroxidation on liposomal membranes. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1981;646(1):169–178. doi: 10.1016/0005-2736(81)90284-4. [DOI] [PubMed] [Google Scholar]
- 12.Bruch RC, Thayer WS. Differential effect of lipid peroxidation on membrane fluidity as determined by electron spin resonance probes. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1983;733(2):216–222. doi: 10.1016/0005-2736(83)90525-4. [DOI] [PubMed] [Google Scholar]
- 13.Vossen RC, van Dam-Mieras MC, Hornstra G, Zwaal RF. Continuous monitoring of lipid peroxidation by measuring conjugated diene formation in an aqueous liposome suspension. Lipids. 1993;28(9):857–861. doi: 10.1007/BF02536243. [DOI] [PubMed] [Google Scholar]
- 14.Xu L, Davis TA, Porter NA. Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J Am Chem Soc. 2009;131(36):13037–13044. doi: 10.1021/ja9029076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosgrove JP, Church DF, Pryor WA. The kinetics of the autoxidation of polyunsaturated fatty acids. Lipids. 1987;22(5):299–304. doi: 10.1007/BF02533996. [DOI] [PubMed] [Google Scholar]
- 16.Sun WQ, Leopold AC, Crowe LM, Crowe JH. Stability of dry liposomes in sugar glasses. Biophys J. 1996;70(4):1769–1776. doi: 10.1016/S0006-3495(96)79740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crowe LM, Womersley C, Crowe JH, Reid D, Appel L, Rudolph A. Prevention of fusion and leakage in freeze-dried liposomes by carbohydrates. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1986;861:131–140. [Google Scholar]
- 18.Crommelin D, Van Bommel E. Stability of liposomes on storage: freeze dried, frozen or as an aqueous dispersion. Pharmaceutical Research. 1984;1(4):159–163. doi: 10.1023/A:1016344523988. [DOI] [PubMed] [Google Scholar]
- 19.Van Winden E, Crommelin D. Long term stability of freeze-dried, lyoprotected doxorubicin liposomes. European Journal of Pharmaceutics and Biopharmaceutics. 1997;43(3):295–307. [Google Scholar]
- 20.Stark B, Pabst G, Prassl R. Long-term stability of sterically stabilized liposomes by freezing and freeze-drying: Effects of cryoprotectants on structure. European Journal of Pharmaceutical Sciences. 2010;41(3):546–555. doi: 10.1016/j.ejps.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Mouradian R, Womersley C, Crowe LM, Crowe JH. Degradation of functional integrity during long-term storage of a freeze-dried biological membrane. Cryobiology. 1985;22(2):119–127. doi: 10.1016/0011-2240(85)90166-x. [DOI] [PubMed] [Google Scholar]
- 22.Heyes J, Palmer L, Bremner K, MacLachlan I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J Control Release. 2005;107(2):276–287. doi: 10.1016/j.jconrel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Sun S, Alberti KA, Xu QB. A Combinatorial Library of Unsaturated Lipidoids for Efficient Intracellular Gene Delivery. Acs Synthetic Biology. 2012;1(9):403–407. doi: 10.1021/sb300023h. [DOI] [PubMed] [Google Scholar]
- 24.Zhi DF, Zhang SB, Wang B, Zhao YN, Yang BL, Yu SJ. Transfection Efficiency of Cationic Lipids with Different Hydrophobic Domains in Gene Delivery. Bioconjugate Chem. 2010;21(4):563–577. doi: 10.1021/bc900393r. [DOI] [PubMed] [Google Scholar]
- 25.Maloney JF, Labuza TP, Wallace DH, Karel M. Autoxidation of Methyl Linoleate in Freeze-Dried Model Systems. I. Effect of Water on the Autocatalyzed Oxidation. Journal of Food Science. 1966;31(6):878–884. [Google Scholar]
- 26.Labuza TP, Maloney JF, Karel M. Autoxidation of Methyl Linoleate in Freeze-Dried Model Systems. II. Effect of Water on Cobalt-Catalyzed Oxidation. Journal of Food Science. 1966;31(6):885–891. [Google Scholar]
- 27.Karel M, Labuza TP, Maloney JF. Chemical changes in freeze-dried foods and model systems. Cryobiology. 1967;3(4):288–296. [Google Scholar]
- 28.Labuza TP, Tannenbaum SR, Karel M. Water content and stability of low-moisture & intermediate-moisture foods. Food Technology. 1970;24:35–42. [Google Scholar]
- 29.Labuza T, Heidelbaugh N, Silver M, Karel M. Oxidation at intermediate moisture contents. Journal of the American Oil Chemists Society. 1971;48(2):86–90. doi: 10.1007/BF02544555. [DOI] [PubMed] [Google Scholar]
- 30.Labuza T, Silver M, Cohn M, Heidelbaugh N, Karel M. Metal-catalyzed oxidation in the presence of water in foods. Journal of the American Oil Chemists’ Society. 1971;48(10):527–531. doi: 10.1007/BF02544555. [DOI] [PubMed] [Google Scholar]
- 31.Labuza T, Chou Y. Decrease of linoleate oxidation rate due to water at intermediate water activity. Journal of Food Science. 1974;39(1):112–113. [Google Scholar]
- 32.Payton NM, Wempe MF, Betker JL, Randolph TW, Anchordoquy TJ. Lyophilization of a triply unsaturated phospholipid: Effects of trace metal contaminants. European Journal of Pharmaceutics and Biopharmaceutics. 2013;85:306–313. doi: 10.1016/j.ejpb.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May JC, Grim E, Wheeler RM, West J. Determination of residual moisture in freeze-dried viral vaccines: Karl Fischer, gravimetric and thermogravimetric methodologies. Journal of Biological Standardization. 1982;10(3):249–259. doi: 10.1016/s0092-1157(82)80026-7. [DOI] [PubMed] [Google Scholar]
- 34.Molina MD, Anchordoquy TJ. Formulation strategies to minimize oxidative damage in lyophilized lipid/DNA complexes during storage. J Pharm Sci. 2008;97(12):5089–5105. doi: 10.1002/jps.21365. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y, Grobelny P, Von Allmen A, Knudson K, Pikal M, Carpenter JF, Randolph TW. Protein Quantity on the Air–Solid Interface Determines Degradation Rates of Human Growth Hormone in Lyophilized Samples. J Pharm Sci. 2014;103(5):1356–1366. doi: 10.1002/jps.23926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner BA, Buettner GR, Burns CP. Free Radical-Mediated Lipid-Peroxidation in Cells - Oxidizability Is a Function of Cell Lipid Bis-Allylic Hydrogen Content. Biochemistry. 1994;33(15):4449–4453. doi: 10.1021/bi00181a003. [DOI] [PubMed] [Google Scholar]
- 37.Carvajal AK, Rustad T, Mozuraityte R, Storrø I. Kinetic studies of lipid oxidation induced by hemoglobin measured by consumption of dissolved oxygen in a liposome model system. Journal of Agricultural and Food Chemistry. 2009;57(17):7826–7833. doi: 10.1021/jf9013394. [DOI] [PubMed] [Google Scholar]
- 38.Mozuraityte R, Rustad T, Storrø I. Pro-oxidant activity of Fe2+ in oxidation of cod phospholipids in liposomes. European Journal of Lipid Science and Technology. 2006;108(3):218–226. [Google Scholar]
- 39.Schaich KM. Metals and lipid oxidation. Contemporary issues. Lipids. 1992;27(3):209–218. doi: 10.1007/BF02536181. [DOI] [PubMed] [Google Scholar]
- 40.Girotti AW. Mechanisms of lipid peroxidation. Journal of Free Radicals in Biology & Medicine. 1985;1(2):87–95. doi: 10.1016/0748-5514(85)90011-x. [DOI] [PubMed] [Google Scholar]
- 41.Frankel EN. Review. Recent advances in lipid oxidation. Journal of the Science of Food and Agriculture. 1991;54(4):495–511. [Google Scholar]
- 42.Orlien V, Andersen AB, Sinkko T, Skibsted LH. Hydroperoxide formation in rapeseed oil encapsulated in a glassy food model as influenced by hydrophilic and lipophilic radicals. Food Chemistry. 2000;68(2):191–199. [Google Scholar]
- 43.Andersen AB, Risbo J, Andersen ML, Skibsted LH. Oxygen permeation through an oil-encapsulating glassy food matrix studied by ESR line broadening using a nitroxyl spin probe. Food chemistry. 2000;70(4):499–508. [Google Scholar]
- 44.Hafez I, Maurer N, Cullis P. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Therapy. 2001;8(15):1188–1196. doi: 10.1038/sj.gt.3301506. [DOI] [PubMed] [Google Scholar]
- 45.Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacological Reviews. 2006;58(1):32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 46.McLean LR, Hagaman KA. Effect of lipid physical state on the rate of peroxidation of liposomes. Free Radical Biology and Medicine. 1992;12(2):113–119. doi: 10.1016/0891-5849(92)90004-z. [DOI] [PubMed] [Google Scholar]
- 47.Mowri H-o, Nojima S, Inoue K. Effect of lipid composition of liposomes on their sensitivity to peroxidation. Journal of Biochemistry. 1984;95(2):551–558. doi: 10.1093/oxfordjournals.jbchem.a134638. [DOI] [PubMed] [Google Scholar]
- 48.Tirosh O, Kohen R, Katzhendler J, Alon A, Barenholz Y. Oxidative stress effect on the integrity of lipid bilayers is modulated by cholesterol level of bilayers. Chemistry and Physics of Lipids. 1997;87(1):17–22. doi: 10.1016/s0009-3084(97)00019-4. [DOI] [PubMed] [Google Scholar]
- 49.Armstrong TK, Zhang Y, Patel MM, Lentz YK, Anchordoquy TJ. The stability of lyophilized lipid/DNA complexes during prolonged storage. J Pharm Sci. 2004;93(9):2259–2273. doi: 10.1002/jps.20138. [DOI] [PubMed] [Google Scholar]
- 50.Franks F. Freeze drying: From empiricism to predictability. Cryo Letters. 1990;11:91–110. [Google Scholar]
- 51.Hans Tromp R, Parker R, Ring SG. Water diffusion in glasses of carbohydrates. Carbohydrate Research. 1997;303(2):199–205. [Google Scholar]
- 52.Schoonman A, Ubbink J, Bisperink C, Le Meste M, Karel M. Solubility and diffusion of nitrogen in maltodextrin/protein tablets. Biotechnology Progress. 2002;18(1):139–154. doi: 10.1021/bp010126f. [DOI] [PubMed] [Google Scholar]
- 53.Molina M, Anchordoquy T. Metal contaminants promote degradation of lipid/DNA complexes during lyophilization. Biochimica et Biophysica Acta. 2007;1768(3):669–677. doi: 10.1016/j.bbamem.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graf E, Mahoney JR, Bryant RG, Eaton JW. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984;259(6):3620–3624. [PubMed] [Google Scholar]
- 55.Liu ZD, Hider RC. Design of clinically useful iron (III)-selective chelators. Medicinal Research Reviews. 2002;22(1):26–64. doi: 10.1002/med.1027. [DOI] [PubMed] [Google Scholar]
- 56.Swartz HM. Effect of oxygen on freezing damage: II. Physicalchemical effects. Cryobiology. 1971;8(3):255–264. doi: 10.1016/0011-2240(71)90048-4. [DOI] [PubMed] [Google Scholar]
- 57.Molina MD, Anchordoquy TJ. Formulation strategies to minimize oxidative damage in lyophilized lipid/DNA complexes during storage. J Pharm Sci. 2008;97(12):5089–5105. doi: 10.1002/jps.21365. [DOI] [PubMed] [Google Scholar]