Abstract
Background
The risk of subsequent infections in rheumatoid arthritis patients who receive biologic therapy after a serious infection is unclear.
Objective
To compare the subsequent risk of hospitalized infections associated with specific biologic agents among RA patients previously hospitalized for infection while receiving anti-TNF therapy.
Methods
Using 2006-2010 Medicare data for 100% of beneficiaries with rheumatoid arthritis enrolled in Medicare, we identified patients hospitalized with an infection while on anti-TNF agents. Follow-up began 61 days after hospital discharge and ended at the earliest of next infection, loss of Medicare coverage or 18 months after start of follow-up. We calculated the incidence rate of subsequent hospitalized infection for each biologic and used Cox regression to control for potential confounders.
Results
Following 10,794 eligible hospitalized infections contributed at least one day of biologic exposure during follow-up, we identified 7,807 person-years of exposure to selected biologics; 4% abatacept, 2% rituximab and 94% anti-TNFs (23% etanercept, 18% adalimumab, 53% infliximab) and 2,666 associated infections. Mean age across biologic exposure cohorts ranged from 64-69 years. The crude incidence rate of subsequent hospitalized infection ranged from 27.1 to 34.6 per 100 person years. After multivariable adjustment, abatacept (hazard ratio (HR): 0.80, 95% CI: 0.64-0.99) and etanercept (HR: 0.83, 95% CI: 0.72-0.96) users had significantly lower risks of a subsequent infection compared to infliximab users.
Conclusion
Among rheumatoid arthritis patients who experienced a hospitalized infection while on anti-TNF therapy, abatacept and etanercept were associated with the lowest risk of subsequent infection compared to other biologic therapies.
Keywords: infection, biologics, rheumatoid arthritis, anti-TNF therapy, abatacept, rituximab
INTRODUCTION
Many studies have evaluated the association between various biologic medications and an increased risk of serious infection in rheumatoid arthritis (RA) patients, with some but not all suggesting that anti-TNF therapy increases the risk for serious infections compared to non-biologic drugs.(1-13) Although the mechanisms of any increased risks remain unclear, the fact that biologic medications target key components of host immune defenses may result in an increased susceptibility to different types of infections.(14). Less is known about the comparative risk for anti-TNF medications versus biologics with other mechanisms of action (MOA).
Switching biologics is common in RA, and selecting a specific agent may be impacted not only by the expectation of efficacy but also safety considerations. Indeed, up to one-third of RA patients discontinue their first biologic within one year due to lack of efficacy and/or adverse events.(15) In the setting of a recent serious adverse event, such as a hospitalized infection, occurring while on anti-TNF therapy, the 2012 American College of Rheumatology (ACR) recommendations suggest to change to a non-anti-TNF biologic.(16) This recommendation was based on level C evidence (expert opinion). While clinicians and patients could choose to continue the same biologic or switch to a different anti-TNF, these options were not the preferred choices in the ACR recommendations.
Given limited evidence on the association between serious infection and biologic therapies in high risk RA patients, such as those experiencing a recent serious infection, the aim of this study was to evaluate whether the risks of subsequent hospitalized infections associated with specific biologic agents and associated with switching to a different MOA versus continuing anti-TNF therapy. The study population focused on RA patients recently hospitalized with an infection while receiving anti-TNF therapy.
METHODS
Study Design and Data Sources
This cohort study used 2006-2010 data for all Medicare beneficiaries with RA, obtained from the Centers for Medicare and Medicaid Services (CMS). Medicare is a national health insurance program in the U.S. that provides medical and pharmacy benefits to more than 50 million elderly (age >= 65), individuals under age 65 with disabilities, and individuals with end stage renal disease.(17) RA patients with significantly limitations in function are potentially eligible for disability benefits that include Medicare coverage after approximately two years.(18) Data included demographics, inpatient, outpatient, prescriptions, and claims for infusions given in provider offices and hospital-based outpatient infusion centers. CMS and the Institutional Review Board of the University of Alabama at Birmingham approved the study.
Eligible patient population and observation period
Patients eligible for this analysis had RA and an ‘index hospitalization’ with an infection while receiving an anti-TNF therapy (Appendix 1). To select this population, we identified patients who experienced a hospitalization with an infection discharge diagnosis in any position (primary or non-primary) on the hospital claim using diagnosis codes from the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). This approach has previously been shown to have high validity to identify confirmed hospitalized infections.(19)
To increase the homogeneity of patient characteristics and thereby reduce confounding, patients also had to meet these criteria: 1) had at least two ICD-9 codes for RA (ICD-9 714.x) from a physician office visit or hospitalization at any time preceding the hospital admission date; 2) had no diagnosis of cancer (excluding non-melanoma skin cancer) in the 6 months before the index hospitalization ( patients with cancer might have other infection-related risk factors compared patients without cancer); 3 ) were using anti-TNF therapy at the time of admission to the hospital; 4) had an index hospitalization with length of stay fewer than 14 days (to avoid excessive heterogeneity in the severity of infections); and 5) were not receiving nursing home care services during the first 60 days following hospital discharge. We used the 6 months before the index hospitalization discharge date as the baseline period to assess all covariates (e.g. demographics, comorbidities).
We desired greater certainty that patients subsequently hospitalized for an infection had experienced a new infection rather than simply a recurrence of the index infection. We also wanted to allow a ‘washout’ from anti-TNF drug exposures prior to the index hospitalization. Given these considerations, and in light of the usual dosing frequency of infliximab of 56 days, the “index date” for starting follow-up was 61 days after hospital discharge. Follow-up after each index hospitalization episode ended at the earliest time of admission for a new, subsequent hospitalized infection, cancer, death, or the end of the 18 month follow-up period. We censored all follow-up after 18 months because previous studies have reported that infection risk is higher earlier after initiation of biologics.(6, 8)
Eligible patients also must have been continuously enrolled with Medicare fee-for-service coverage with hospital, physician and prescription drug plans (i.e. part A, B, and D, excluding Medicare Advantage coverage) in the 6 months before the admission date of the index hospitalization and throughout follow-up.
Medication exposure
For each index hospitalization, we used pharmacy (for injected biologics, i.e. etanercept and adalimumab) and procedure claims (for infused biologics, i.e. abatacept, infliximab, rituximab) to determine the time-dependent medication exposure for each person day during follow-up. We determined etanercept or adalimumab exposure based upon the days of supply reported for filled prescriptions and assigned exposure as 30 days for abatacept, 56 days for infliximab and 180 days for rituximab based on recommended dosing frequency. For each biologic, we added a 30-day exposure ‘extension’.(3) The extension was added because patients who become ill often stop medications, and using an extension captures attributable events that occur shortly after biologic therapy is discontinued.(8)
We created biologic exposure groups defined by MOA as restarting the same anti-TNF biologic that the patient was treated with at the time of the index hospitalization, switched to a different anti-TNF biologic, and switched to a non-anti-TNF biologic. We classified days on which patients had overlapping biologic exposures (i.e. concurrent exposure) as exposed to the most recent biologic. There was insufficient use of golimumab, certolizumab and tocilizumab to study these agents independently.
Outcome
For each index hospitalization, the outcome was time to first subsequent hospitalized infection. We identified these infections by the use of hospital diagnosis codes for infections in any position using claims-based algorithms that have been validated.(4, 19) Types of hospitalized infections were categorized as pneumonia and respiratory tract, genitourinary tract, skin and soft tissue, sepsis/bacteremia and other.(3)
Confounder Control using an Infection Risk Score
Using previously described methods,(5, 20, 21) we derived an infection risk score for each index hospitalization that provided a composite risk to control for infection-related confounding for all factors unrelated to biological therapy using claims from the 6 month baseline (Appendix 2). Factors included were demographics, comorbidities, concurrent medications, and health service utilization. We categorized the infection risk score into deciles (21) and removed index hospitalization episodes with an infection risk score not overlapping between different biologics. Within deciles of the infection risk score, patients treated with each biologic were comparable with respect to their predicted risk for serious infection (Appendix 3).
Statistical Analysis
The unit of analysis was biologic-exposed person-days as a time-varying variable. All other covariates were evaluated during the 6 months baseline. We compared baseline characteristics as of the date of each index hospitalization between biologics. For each specific biologic, we calculated the crude incidence of subsequent hospitalized infections.
We used Cox regression to estimate the adjusted hazard ratio (HR) for subsequent hospitalized infection for each biologic compared to every other biologic. We applied the Huber-White “sandwich” variance estimator to control for correlations among the observations nested within the same person.(22) The regression models adjusted for the decile of the infection risk score, type of infection at the index hospitalization, number of previous index hospitalizations, specific anti-TNF used at the time of the index hospitalization, whether (for anti-TNFs) it was the same drug or a switch between baseline and follow-up, steroid use during baseline, non-biologic DMARD use during baseline, and concurrent (i.e. overlapping around the time of switch) biologic exposure.(22)
In order to examine time-varying hazard of infection, we produced a smoothed hazard plot using a publically-available R package “muhaz”.(23, 24) Within selected deciles of the infection risk score (lowest, median, and highest), we calculated the one year adjusted infection rate difference between each biologic and abatacept (referent) using the output from the Cox model (using the ‘Baseline’ statement in SAS).
We conducted three sensitivity analyses including (1) defining the index hospitalization and subsequent hospitalized infection using only primary diagnosis codes, (2) using a 90-day extension to current exposure, (3) using a two year baseline lookback during which no other biologics were identified
Results
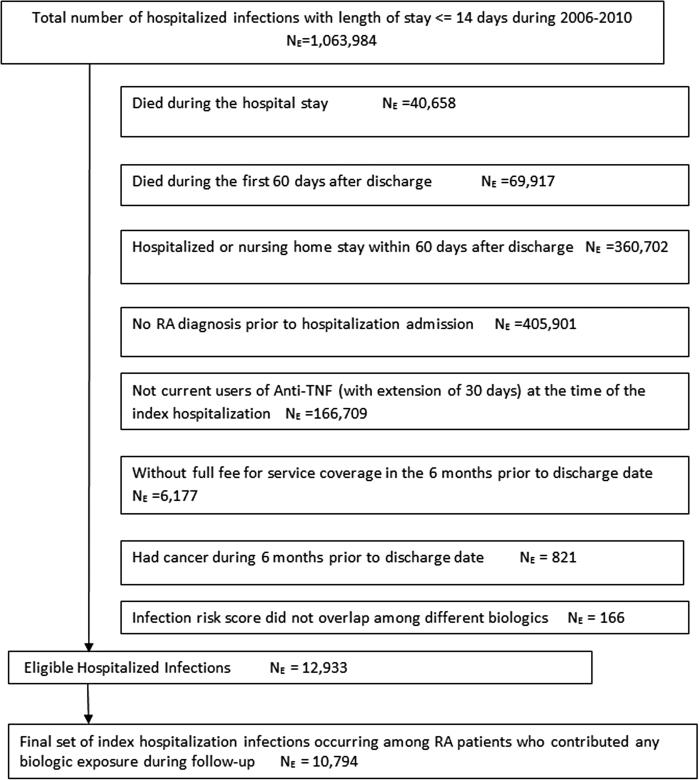
We identified 12,933 eligible index hospitalized infections occurring while patients were exposed to anti-TNF therapy (Figure 1). On the date of admission, 3,248 patients were receiving etanercept, 2,564 adalimumab, 6,990 infliximab, 72 certolizumab, and 59 golimumab. The most frequent types of index infections were pneumonia/respiratory tract (29.3 %), genitourinary tract (23.5%), skin and soft tissue (16.6%) and septicemia/bacteremia (11.3 %).
Figure 1.
Selection of eligible index hospitalized infections occurring among RA patients while on treatment with anti-TNF therapy
After discharge from the index hospitalization, most patients restarted the same anti-TNF medication (79%); 2% switched to another anti-TNF, 3% initiated a non-anti-TNF biologic and16% did not receive any biologic over 18 months. Of patients who initially restarted the same anti-TNF agent they had been on at the time of the index hospitalization, 10% switched to a different biologic during follow-up. Of patients who received any biologic during follow-up, we identified 10,794 index hospitalizations occurring among 10,183 unique RA patients, yielding 7,807 person-years of biologic exposure (Table 1). In terms of demographics, comorbidities, and common types of infections, we observed similar proportions across the medication exposure groups. Most biologic exposure time occurred within one year of drug initiation or restart.
Table 1.
Distribution of baseline characteristics by subsequent biologic exposure among RA patients hospitalized with an infection while taking anti-TNF drugs (n=10,794 index hospitalization episodes occurring among 10,183 unique patients)
| Baseline Characteristics | Biologic Exposure during Follow-up | |||
|---|---|---|---|---|
| Same TNF* | Abatacept | Rituximab | Different TNF* | |
| Total number of person years during follow up | 7,067 | 333 | 133 | 273 |
| Number of unique patients contributing to follow-up | 8,091 | 543 | 239 | 499 |
| Age, Years† | 69 (12) | 68 (11) | 69 (10) | 64(14) |
| Women, % | 82.4 | 84.6 | 78.8 | 82.9 |
| Comorbidities, % | ||||
| Diabetes | 27.8 | 25.0 | 25.0 | 29.4 |
| Chronic obstructive pulmonary disease | 36.7 | 34.1 | 47.5 | 43.6 |
| Heart failure | 14.5 | 15.5 | 12.5 | 15.2 |
| Angina | 2.6 | 1.4 | 1.3 | 3.3 |
| Renal disease | 9.2 | 8.6 | 1.3 | 5.7 |
| Any fracture | 10.2 | 8.2 | 8.8 | 8.1 |
| Hospitalized infections | ||||
| None | 8.0 | 5.0 | 5.0 | 10.0 |
| 1-2 episodes | 89.5 | 91.4 | 93.8 | 86.7 |
| ≥3 episodes | 2.5 | 3.6 | 1.2 | 3.3 |
| Ulcer | 1.9 | 1.8 | 1.3 | 2.4 |
| One Year predicted risk of infection (i.e. Infection Risk Score)** | 25 (17, 37) | 25 (18, 38) | 28 (17, 40) | 25 (18,35) |
| Medications, % | ||||
| Prednisone-equivalent, mg/day | ||||
| None | 41.1 | 32.3 | 40.0 | 31.3 |
| ≤7.5 | 44.9 | 48.6 | 40.0 | 49.8 |
| >7.5 | 14.0 | 19.1 | 20.0 | 19.0 |
| Non-steroidal anti-inflammatory drugs | 29.0 | 31.8 | 28.9 | 34.1 |
| Bisphosphonates | 26.4 | 25.0 | 33.8 | 29.9 |
| Narcotics | 76.1 | 76.4 | 78.9 | 86.3 |
| Hypertension medications | 54.8 | 52.3 | 55.0 | 50.2 |
| Antidepressants | 41.0 | 42.7 | 41.3 | 44.6 |
| Diagnosis or medication for hyperlipidemia | 11.7 | 10.9 | 11.3 | 9.5 |
| Thiazide diuretics | 20.9 | 23.6 | 26.3 | 20.9 |
| Health behaviors and health services utilization, % | ||||
| PSA screen (men only) | 25.1 | 25.5 | 15.8 | 19.6 |
| Mammography (women only) | 17.7 | 23.9 | 11.8 | 13.2 |
| All-cause hospitalization | ||||
| 0-1 hospitalization | 59.7 | 56.6 | 62.0 | 56.0 |
| 2 hospitalizations | 25.8 | 27.6 | 22.2 | 27.4 |
| ≥ 3 hospitalizations | 14.5 | 15.8 | 15.8 | 16.6 |
| Outpatient infection | 74.6 | 77.2 | 79.8 | 68.5 |
| Long-term care | 2.5 | 1.8 | 1.4 | 1.0 |
| Receiving Medicare for reasons other than age (e.g., disabled), % | 48.9 | 48.4 | 50.1 | 65.3 |
| Proportion of biologic exposure since drug initiation, % ‡ | ||||
| 0-6 months since drug initiation | 64 | 73 | 77 | 75 |
| 6-12 months since drug initiation | 27 | 25 | 22 | 21 |
| 12-18 months since drug initiation | 9 | 2 | 1 | 4 |
Except for the proportion of exposure time since drug initiation on the last row, all other factors were measured in the 6 month baseline prior to the index hospitalization discharge date.
Different TNF refers to a different anti-TNF therapy than the one that patients were using at the time of the index hospitalization; same TNF refers to re-starting the same anti-TNF therapy that the patients were using at the time of the index hospitalization.
Median (Interquartile Range)
Mean (standard deviation)
Measured during follow up based upon time since drug initiation.
During follow-up, we observed 2,666 subsequent hospitalized infection events (Table 2). The crude incidence rate of subsequent hospitalized infection ranged from 27.1 to 34.6 per 100 person years (pys). Compared to those who used the same anti-TNF after the index hospitalization, the adjusted HR for subsequent hospitalized infection was 0.86 (95% confidence interval (CI): 0.72-1.03) for non-anti-TNF biologics and 1.10 (95% CI: 0.89-1.35) for switch to a different anti-TNF biologic. Patients who did not receive any biologic during follow-up had a crude incidence of infection of 40.5 per 100 pys. The most frequent types of subsequent infections were similar to the most frequent types of index infection; pneumonia was the most common; the types of infection did not differ by specific drug (not shown).
Table 2.
Number of events, incidence rates and adjusted HRs for subsequent hospitalized infection by biologic DMARDS.
| Exposure Group | Events | PY | IR per 100 PY | Crude HR (95% CI) | Adjusted HR‡ (95% CI) |
|---|---|---|---|---|---|
| Switched to a non anti-TNF biologic | 126 | 466 | 27.1 | 0.96(0.80,1.16) | 0.86(0.72,1.03) |
| Switched to different anti-TNF* | 94 | 273 | 34.4 | 0.96(0.76,1.19) | 1.10(0.89,1.35) |
| Re-started the same anti-TNF* | 2446 | 7068 | 34.6 | 1.0 (ref) | 1.0 (ref) |
Abbreviations: HR = Hazard Ratio; IR = incidence rate; Person years
Different TNF refers to a different anti-TNF therapy than the one that patients were using at the time of the index hospitalization; same TNF refers to re-starting the same anti-TNF therapy that the patients were using at the time of the index hospitalization.
Adjusted for the decile of disease risk score, specific anti-TNF drug at the time of the index hospitalization, steroid use during baseline, methotrexate use during baseline, infection type for the index hospitalization and concurrent medication exposure during follow up.
In drug-specific analyses, abatacept had the lowest crude incidence rate of subsequent hospitalized infection, and etanercept had the highest (Table 3). After multivariable adjustment, abatacept (HR: 0.80, 95%CI: 0.64-0.99) and etanercept (HR: 0.83, 0.72-0.97) had significantly lower risks of infection compared to infliximab. The type of infection at the index hospitalization, biologic switch, and specific anti-TNF agent being used at the time of the index hospitalization were not significantly associated with subsequent hospitalized infection except for baseline etanercept (HR: 1.22, 95% CI: 1.08-1.38). Within patient risk groups defined as Lowest, Median, and Highest (Figure 2), the adjusted infection risk was lowest for abatacept (referent, y axis) and highest for infliximab in the Highest risk group, up to an 8/100py difference.
Table 3.
Absolute incidence rates (IRs) and pairwise comparison of each biologic* to every other for subsequent hospitalized infection. Data shown are adjusted hazard ratios‡ with 95% confidence interval (CI).
| Biologies | Referent Group | ||||
|---|---|---|---|---|---|
| Infliximab | Adalimumab | Etanercept | Rituximab | Abatacept | |
| Crude IR Per 100 years (n/pys†) | 33.8 (1,382/4,087) | 34.9 (497/1,423) | 36.1 (661/1,831) | 28.5 (38/133) | 26.5 (88/333) |
| Adjusted HR (95% CI) ‡ | |||||
| Abatacept | 0.80 (0.64-0.99) | 0.88 (0.68-1.12) | 0.97 (0.76-1.23) | 0.93 (0.64-1.36) | 1.0 (Ref) |
| Rituximab | 0.87 (0.63-1.20) | 0.94 (0.67-1.32) | 1.04 (0.74-1.46) | 1.0 (Ref) | |
| Etanercept | 0.83 (0.72-0.97) | 0.91 (0.76-1.08) | 1.0 (Ref) | ||
| Adalimumab | 0.92 (0.79-1.09) | 1.0 (Ref) | |||
| Infliximab | 1.0 (Ref) | ||||
Biologic exposure was defined as the days' supply from filled prescriptions or assigned days' supply based on recommended dosing frequency, plus a 30-day 'extension' period to each exposure.
Person years
Adjusted for the decile of disease risk score, specific anti-TNF biologic at the time of the index hospitalization, steroid use during baseline, methotrexate use during baseline, infection type for the index hospitalization, and coexisting medication exposures during follow up.
Figure 2.
One year infection risk difference between various biologics referent to abatacept
Prediction infection risk deciles: Lowest = Decile 1; Median = Decile 5; Highest = Decile 10
Lowest refers to the subcohort of patients at the lowest risk for subsequent infection, from decile 1 of the infection risk score. Median represents the subcohort of patients from decile 5. Highest are the patients from decile 10.
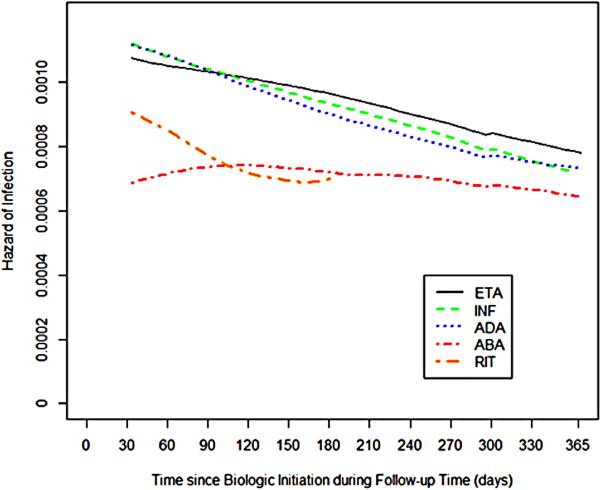
Smoothed, unadjusted hazard plots of subsequent hospitalized infection by specific biologic (Figure 3) showed that for three anti-TNF biologics, hazards peaked early, and then declined over time. In contrast, the hazard for abatacept was essentially flat and became similar to anti-TNF biologics at approximately 6-9 months. The hazard of rituximab was in-between abatacept and anti-TNF biologics and was truncated at 6 months due to the sparsity of data. The 95% confidence intervals for the associated hazard of infection between biologics overlapped one another (not shown).
Figure 3.
Hazard of subsequent hospitalized infection associated with various biologic therapies
ETA = etanercept; INF = infliximab; ADA = adalimumab; ABA = abatacept; RIT = rituximab
Note: the y-axis represents the hazard of infection at each day of follow-up.
Sensitivity analyses that defined hospitalized events using only primary diagnosis codes, and those using a two year look back period yielded results similar to the main analysis (not shown). The sensitivity analysis that used a 90-day extension to current exposure rather than 30 days yielded comparable results to those in table 3; the only additional significant differences were for abatacept (HR: 0.85, 0.73-0.98) and etanercept (HR: 0.88, 0.77-0.99) users, who had lower risks of subsequent infection compared to adalimumab users.
DISCUSSION
Among high risk RA patients who experienced a hospitalized infection while on anti-TNF therapy, our results showed that abatacept and etanercept had a significantly lower rate of subsequent infection compared to infliximab. In analyses that grouped drugs by MOA, the subsequent hazard rate of hospitalized infections was not significantly different among patients who remained on the same anti-TNF agent, switched to a different anti-TNF medication, or who switched to a biologic with an alternate MOA, although trends suggested that switching to a non-anti-TNF agent might be preferable. Additionally, we observed that continued use of a previously prescribed anti-TNF agent after a hospitalized infection was common, accounting for 90% of observation time during the 18 months of follow-up.
The 2012 ACR recommendations (16) suggest that RA patients on anti-TNF therapy should switch to a non-anti-TNF biologic after a serious adverse event, including hospitalized infections. Evidence in support of this recommendation has been scant. We found that such switching happens infrequently, which is consistent with an earlier report in a younger RA population that most patients continued the same anti-TNF that they were treated with prior to hospitalization.(5) Our results suggest that the ACR recommendation may be appropriate, especially if switching to abatacept, but considering the grouped safety profile of medications defined by a common MOA may not be appropriate.
The absolute incidence rates for a subsequent hospitalized infection ranged from 26 -36 per 100 person years, which is appreciably higher than the typical range of hospitalized infections in RA cohorts (3-6/100pys) (3, 5, 7, 11, 25) and even older Medicare patients with RA (10-12/100pys).(20) Our findings are consistent with previous studies comparing anti-TNF therapies to one another and extend those observations by examining risk versus biologics with other MOAs. The observed higher absolute incidence rate but lower adjusted rate of infection for etanercept users probably reflects channeling of higher risk patients to this agent. All rates of infections were lower than the infection rates among patients not treated with biologics (40.5/100pys), which may indicate channeling of the highest risk patients away from all biologics or the possibility that higher-dose glucocorticoids were substituted for biologics, which increases infection risk.(26)
We found that abatacept had a lower hospitalized infection rate compared to infliximab, consistent with a trial that made a similar comparison.(27) The rate of infection for abatacept also was lower but of borderline significance compared to adalimumab in the main analysis, although did reach statistical significance in the sensitivity analysis. This result is consistent with a 2-year head-to-head clinical trial showing lower but non-significant serious infection risk for abatacept versus adalimumab.(28) Similarly, etanercept had a significantly lower adjusted infection rate compared to infliximab, and in a sensitivity analysis, a lower rate compared to adalimumab. Also concordant with our results, data from the Dutch RA registry and a network meta-analysis found the risk of serious infections in patients treated with etanercept to be lower than with adalimumab or infliximab(29, 30) although Europe use less infliximab. The range of absolute risk difference between abatacept and other therapies ranged from < 1 / 100py (etanercept) to a high of 8/100py (infliximab, Highest risk group), yielding a number needed to harm (NNH) of up to 13. This NNH and associated range of risk differences between specific drugs suggest that our results are important clinically. However, the differences between our results and infection rates from other, healthier RA cohorts suggest that drug-specific differences probably are outweighed by patient-related characteristics (e.g. age, comorbidities) and potentially modifiable factors (e.g. glucocorticoid dose).
The smoothed hazard plot indicated that the time-dependent risk of subsequent hospitalized infection for patients exposed to anti-TNFs were comparable to one another. These findings are compatible with studies suggesting an early increased risk for anti-TNF agents that declines over time.(6)(8) The pattern was different for abatacept, which was flat and eventually achieved parity with other biologics. Perhaps further accentuating these differences, the substantial majority of anti-TNF users in the analysis were re-starting therapy with a medication that had been on, whereas most abatacept and rituximab users were new users.
Strengths of our study include large number of high-risk RA patients that allowed us to inform the clinically-relevant question of how to best treat patients who experience a serious infection while receiving anti-TNF therapy. A limitation of our observational study is that patients were not randomly assigned to treatments; therefore, patients might have differed in their risk for serious infections at baseline. Specifically, patients at higher risk of subsequent infection might have been more likely to be changed to abatacept or rituximab rather than resume anti-TNF therapy. However, Table 1 and Appendix 2 did not provide much evidence for such channeling, although abatacept users were somewhat more likely to use prednisone; even if such channeling occurred, it would be expected to attenuate results towards the null. We used administrative data that lacked detailed information on RA disease severity and some clinical factors (e.g. smoking). Thus, misclassification and residual confounding are possible. Additionally, we had relatively modest amounts of exposure to rituximab and only 38 hospitalized infections, yielding some imprecision in the risks associated with this agent. Medical records were not available to confirm infections, although the claims-based algorithms used have been shown to have good positive predictive values.(19, 31, 32) Our multivariable adjustment for prior biologic use may be incomplete due to the short 6 months look-back period, although our sensitivity analysis addressed this. Finally, Medicare patients are generally older, and these results may not be generalizable to younger, healthier RA patients.
In conclusion, we found that among RA patients who experienced a hospitalized infection while receiving anti-TNF therapy, most of them continued to use the same anti-TNF agent, and a small proportion switched to another biologic. Comparing individual biologics, abatacept and etanercept had the lowest rate of subsequent hospitalized infection. These findings provide new evidence for clinical management of high risk RA patients and suggest that drug-specific guidance may be more appropriate when making safety-based recommendations, rather than simply lumping drugs together based upon MOA. Further studies are needed to investigate the balance of harms and effectiveness simultaneously in key patient subgroups to optimize personalizing medication choices for individual patients.
Acknowledgments
Dr. Curtis received support from the Agency for Healthcare Research and Quality (R01 HS018517) and the National Institutes of Health (AR053351).
Dr. Yun was supported by grant 1 K12 HS021694 from the Agency for Healthcare Research and Quality, Rockville, MD, USA
Funding information
This work was supported by the Agency for Healthcare Research & Quality (R01 HS018517).
Footnotes
Author contributions
Conception and design: Yun, Xie, Delzell, Levitan, Curtis
Acquisition of Data: Yun, Delzell, Xie, Chen, Curtis
Analysis and interpretation of data: Yun, Delzell, Xie, Curtis
Drafting manuscript: Yun
Critical revision of manuscript for important intellectual content: Yun, Xie, Delzell, Chen, Levitan, Lewis, Saag, Beukelman, Winthrop, Baddley, Curtis
Statistical analysis: Yun, Xie, Curtis
Obtaining Funding: Curtis, Saag, Delzell
Administrative, technical, or material support: Yun, Xie, Delzell, Chen, Levitan, Lewis, Saag, Beukelman, Winthrop, Baddley, Curtis
Study supervision: Curtis
Competing interests
Disclosures for unrelated work
Curtis: research grants and/or consulting: Abbott, Amgen, BMS, Centocor, Crescendo, CORRONA, Pfizer, Roche/Genetech, UCB
Delzell: research grants: Amgen
Levitan: research grants: Amgen
Lewis: research grants: Pfizer, Prometheus, Lilly, Shire, Nestle, Janssen, AstraZeneca, Amgen, Consulting: Centocor, Shire, Takeda
Saag: research grants: Ardea, Regeneron, Svient, Takeda/Consulting fees: Ardea, Regeneron, Savient:Takeda
Beukelman: research grants: Genentech and Biogen IDEC/Consulting fees: Novartis Pharmaceutical Corporation, Pfizer Inc.
Winthrop: research grants: Pfizer, Inc/consulting fees: Pfizer, UCB, Genentech, Regeneron
Baddley: research grants: BMS, Pfizer
Reference
- 1.Doran MF, Crowson CS, Pond GR, et al. Predictors of infection in rheumatoid arthritis. Arthritis & Rheumatism. 2002;46(9):2294–300. doi: 10.1002/art.10529. [DOI] [PubMed] [Google Scholar]
- 2.Capell HA. Disease modifying antirheumatic drugs: longterm safety issues. J Rheumatol Suppl. 2001;62:10–5. Epub 2001/06/21. [PubMed] [Google Scholar]
- 3.Grijalva CG, Chen L, Delzell E, et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA : the journal of the American Medical Association. 2011;306(21):2331–9. doi: 10.1001/jama.2011.1692. Epub 2011/11/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56(4):1125–33. doi: 10.1002/art.22504. [DOI] [PubMed] [Google Scholar]
- 5.Curtis JR, Xie F, Chen L, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis. 2011;70(8):1401–6. doi: 10.1136/ard.2010.146365. Epub 2011/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis JR, Xi J, Patkar N, et al. Drug-specific and time-dependent risks of bacterial infection among patients with rheumatoid arthritis who were exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56(12):4226–7. doi: 10.1002/art.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon WG, Hyrich KL, Watson KD, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: Results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis. 2009 doi: 10.1136/ard.2009.118935. Epub 2009/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon WG, Symmons DP, Lunt M, et al. Serious infection following anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum. 2007;56(9):2896–904. doi: 10.1002/art.22808. Epub 2007/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston SS, Turpcu A, Shi N, et al. Risk of infections in rheumatoid arthritis patients switching from anti-TNF agents to rituximab, abatacept, or another anti-TNF agent, a retrospective administrative claims analysis. Seminars in arthritis and rheumatism. 2013 doi: 10.1016/j.semarthrit.2012.12.024. Epub 2013/03/05. [DOI] [PubMed] [Google Scholar]
- 10.Schneeweiss S, Setoguchi S, Weinblatt ME, et al. Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 2007;56(6):1754–64. doi: 10.1002/art.22600. [DOI] [PubMed] [Google Scholar]
- 11.Listing J, Strangfeld A, Kary S, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005;52(11):3403–12. doi: 10.1002/art.21386. [DOI] [PubMed] [Google Scholar]
- 12.Dewedar AM, Shalaby MA, Al-Homaid S, et al. Lack of adverse effect of anti-tumor necrosis factor-alpha biologics in treatment of rheumatoid arthritis: 5 years follow-up. International journal of rheumatic diseases. 2012;15(3):330–5. doi: 10.1111/j.1756-185X.2012.01715.x. Epub 2012/06/20. [DOI] [PubMed] [Google Scholar]
- 13.Keyser FD. Choice of Biologic Therapy for Patients with Rheumatoid Arthritis: The Infection Perspective. Current rheumatology reviews. 2011;7(1):77–87. doi: 10.2174/157339711794474620. Epub 2011/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furst DE. The risk of infections with biologic therapies for rheumatoid arthritis. Seminars in arthritis and rheumatism. 2010;39(5):327–46. doi: 10.1016/j.semarthrit.2008.10.002. Epub 2009/01/02. [DOI] [PubMed] [Google Scholar]
- 15.Hyrich KL, Lunt M, Watson KD, et al. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum. 2007;56(1):13–20. doi: 10.1002/art.22331. Epub 2006/12/30. [DOI] [PubMed] [Google Scholar]
- 16.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64(5):625–39. doi: 10.1002/acr.21641. Epub 2012/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medicare at a Glance fact sheet. The Henry J. Kaiser Family Foundation; [Sep 19, 2013]. http://kff.org/medicare/fact-sheet/medicare-at-a-glance-fact-sheet/ [Google Scholar]
- 18.Social Security [Jan 31, 2014];Disability Evaluation Under Social Secuity. http://www.ssa.gov/disability/professionals/bluebook/14.00-Immune-Adult.htm#14_09.
- 19.Patkar NM, Curtis JR, Teng GG, et al. Administrative codes combined with medical records based criteria accurately identified bacterial infections among rheumatoid arthritis patients. Journal of clinical epidemiology. 2009;62(3):321–7, 7 e1-7. doi: 10.1016/j.jclinepi.2008.06.006. Epub 2008/10/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtis JR, Xie F, Chen L, et al. Use of a disease risk score to compare serious infections associated with anti-tumor necrosis factor therapy among high-versus lower-risk rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2012;64(10):1480–9. doi: 10.1002/acr.21805. Epub 2012/07/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glynn RJ, Gayne JJ, Schneeweiss S. Role of disease risk scores in comparative effectiveness research with emerging therapies. pharmacoepidemiology and drug safety. 2012;21(S2):238–47. doi: 10.1002/pds.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074–8. [Google Scholar]
- 23.Gentleman R, Hess KR. [05/17/2013];Hazard Function Estimation in Survival Analysis. 2010 Available at http://cran.r-project.org/web/packages/muhaz/muhaz.pdf.
- 24.Hess KR, Serachitopol DM, Brown BW. Hazard function estimators: a simulation study. Stat Med. 1999;18(22):3075–88. doi: 10.1002/(sici)1097-0258(19991130)18:22<3075::aid-sim244>3.0.co;2-6. Epub 1999/11/02. [DOI] [PubMed] [Google Scholar]
- 25.Dixon WG, Hyrich KL, Watson KD, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis. 2010;69(3):522–8. doi: 10.1136/ard.2009.118935. Epub 2009/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strangfeld A, Eveslage M, Schneider M, et al. Treatment benefit or survival of the fittest: what drives the time-dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient? Ann Rheum Dis. 2011;70(11):1914–20. doi: 10.1136/ard.2011.151043. Epub 2011/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiff M, Keiserman M, Codding C, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67(8):1096–103. doi: 10.1136/ard.2007.080002. Epub 2007/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiff M, Weinblatt ME, Valente R, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203843. Epub 2013/08/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Dartel SA, Fransen J, Kievit W, et al. Difference in the risk of serious infections in patients with rheumatoid arthritis treated with adalimumab, infliximab and etanercept: results from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Ann Rheum Dis. 2013;72(6):895–900. doi: 10.1136/annrheumdis-2012-201338. Epub 2012/08/14. [DOI] [PubMed] [Google Scholar]
- 30.Singh JA, Christensen R, Wells GA, et al. A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: a Cochrane overview. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2009;181(11):787–96. doi: 10.1503/cmaj.091391. Epub 2009/11/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curtis JR, Martin C, Saag KG, et al. Confirmation of administrative claims-identified opportunistic infections and other serious potential adverse events associated with tumor necrosis factor alpha antagonists and disease-modifying antirheumatic drugs. Arthritis Rheum. 2007;57(2):343–6. doi: 10.1002/art.22544. [DOI] [PubMed] [Google Scholar]
- 32.Curtis J, Saag K, Martin C, et al. Validation of Claims-Identified Serious Adverse Events in Rheumatoid Arthritis and Crohn's Disease Patients. Arthritis & Rheumatism. 2005;52(12):4085. [Google Scholar]