Abstract
Epidermal growth factor (EGF) is a neurotrophic factor that plays an important role in Parkinson’s disease (PD). We measured plasma EGF level in PD, essential tremor (ET) and normal controls to investigate whether it changes in PD and whether it is associated with motor and non-motor symptoms of PD. 100 patients with PD, 40 patients with ET as disease control and 76 healthy persons were enrolled in the present study. Motor and non-motor symptoms were assessed by different scales. Plasma EGF levels of three groups were measured by enzyme-linked immunosorbent assay kit. Spearman test and linear logistics regression model were used to test the correlation of EGF with motor and non-motor symptoms of PD. Plasma EGF level was significantly decreased in early PD patients compared with normal control, but not in advanced PD patients. Interestingly, plasma EGF level was significantly increased in advanced PD and total PD patients compared with ET patients, but not in early PD patients. In addition, plasma EGF level was correlated with UPDRS-III scores in PD. Also plasma EGF level was correlated with UPDRS-III scores and NMS scores in early PD. Our results suggested that plasma EGF decreased in the early stage of PD and increased later on in the PD disease course. Also, plasma EGF level was increased significantly in PD compared with ET patients and correlated with motor and non-motor symptoms in early PD.
Keywords: epidermal growth factor, Parkinson’s disease, essential tremor
Parkinson’s disease (PD) is a neurodegenerative disease with a high prevalence in elderly. Clinically, it is characterized by motor symptoms including bradykinesia, rigidity, resting tremor and postural instability. However, non-motor symptoms are frequent and equally disabling which usually include autonomic dysfunction, depression, anxiety and sleep disturbance, olfactory and cognitive impairments [1].
Neurotrophic factors have been proposed to play a role in PD such as brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF) and epidermal growth factor (EGF) [2, 3]. EGF exhibits its neurotrophic activity on midbrain dopaminergic neurons. Expression of EGF/EGF receptor is found widespread in neocortex, limbic cortex, cerebellum, hippocampus and midbrain [4]. Many studies reported the change of EGF in PD. For example, EGF level was found decreased in PD brains [5]. Moreover, EGFR+ cells were significantly decreased in the SVZ of PD patients and that those remaining cells that did express the EGFR in the SVZ of PD patients demonstrated a very low expression profile [6]. In animal models, EGF supplement was shown to prevent dopaminergic neuron degeneration [5, 7]. Although these studies examined the change of EGF and its possible function in PD, it did not clearly demonstrate the change of EGF in the early and late stage of PD. Also, plasma level of EGF was reported to predict cognitive decline in PD [8], suggesting its association with non-motor symptom of PD. But apart from cognitive dysfunction, there was less study to investigate the change of EGF with other non-motor symptoms of PD, such as autonomic function, etc.
In addition, EGF pathway was also involved in essential tremor (ET), one of its “mimics”. For example, leucine-rich repeat and Ig domain containing 1 (LINGO-1) gene was a risk factor for ET. LINGO-1 protein expression was increased in ET brain [9,10]. As we known, LINGO-1 could bind EGFR to regulate the survival of dopaminergic neuron [11]. So it was interesting to investigate whether there was any difference of plasma EGF change between PD and ET.
So in the present study, we measured plasma levels of EGF in PD, essential tremor (ET) and healthy people, aiming to investigate whether there was any different change of plasma EGF in PD in comparison with normal control and ET. In addition, we examined the relationship of plasma EGF change with the severity of motor and non-motor symptoms in PD.
MATERIALS AND METHODS
Study population
One hundred patients with idiopathic PD were recruited from (1) outpatient clinics of Neurology department, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine (from 2013/1/28 to 2013/9/30) and (2) an epidemiological study (from 2012/10/9 to 2013/10/18). The epidemiological study was to investigate neurodegenerative diseases among people≥50-years-old in Malu suburb in Jia Ding district of Shanghai. Diagnosis of PD was based on United Kingdom Parkinson’s Disease Society Brain Bank Diagnostic Criteria. Secondary causes or other neurodegenerative diseases were excluded. 76 normal persons and 40 patients with essential tremor (ET) were enrolled from the epidemiologic study. ET was included as a disease control. All normal controls were examined by a neurologist to exclude neurological diseases. Diagnosis of ET was based on consensus statement of the movement disorders on Tremor [12]. Concomitant diseases, medication and family history were recorded in all groups. And we also calculated the total levodopa dosage for each patient who was on PD medications. All recruited people were informed of the study and signed the consent forms. The study was approved by ethnical committee of Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine.
Plasma sampling and biomarker quantitation
Blood samples were collected from all participants after over-night fasting. Samples were immediately centrifuged and plasma aliquots were stored at -70°C until analysis. The plasma EGF levels were quantified by means of enzyme-linked immunosorbent assays (ELISA) with human EGF ELISA kit (BE101073, RB Corporation). First of all, Standard Solution were diluted and added to 10 standard wells (50μl in each well) whose densities were separately 3600, 2400, 1200, 600, 300 ng/L for each 2 wells. Secondly, set blank wells and testing sample wells (Sample Dilution 40μl and Testing Sample 10μl). Incubate for 30 min at 37° after closing plate with closure plate membrane. Uncover closure plate membrane, discard liquid, dry by swing, add washing buffer to each well, still for 30s then drain, repeat 5 times, dry by pat. Add HRP-Conjugate Reagent 50μl to each well, except blank well. Incubate and washing again. Add Chromogen Solution A 50μl and Chromogen Solution B 50μl to each well, evade the light preservation for 15 min at 37°C. Stop the reaction by adding Stop Solution 50μl to each well. Take blank well as zero, read absorbance at 450nm after stopping the reaction within 15min.
Assessment of motor and non-motor function of PD patients
Motor function was assessed in all PD patients in their “on” state by Part III of Unified Parkinson’s Disease Rating Scale (UPDRS-III) and Hohn-Yahr Staging Scale (H-Y). Non-motor Symptoms Questionnaire (NMS) was used to assess the overall non-motor impairments in PD patients. Cognitive function was assessed by Mini Mental State Examination (MMSE). Autonomic function was assessed by Scales for Outcomes in Parkinson’s disease-Autonomic (SCOPA-AUT). Depression symptom was assessed by Hamilton Depression Rating Scale. REM sleep disorder was assessed by Rapid Eye Movement Sleep Behavior Disorder Screening Questionnaire (RBDSQ). All these scales had been already used in the evaluation of motor and non-motor symptoms in Chinese PD patients [13].
Statistical analysis
Statistical analysis was performed by SPSS 19.0. Demographic data was compared in three groups (ANOVA test and Chi-square test). The level of serum EGF was compared among three groups by ANOVA test (LSD test for post hoc multiple comparison analysis). Student t test was used to compare the mean of plasma EGF concentration between drug-naïve or medication treated PD and controls. Spearman correlation test and linear logistics regression model were used to analyze the correlation between serum EGF level and age, age of onset, disease duration, motor and non-motor variables, and levodopa dosage in PD patients. All tests were 2-tailed and results were considered statistically significant at P<0.05.
RESULTS
One hundred PD patients (38 from epidemiological study and 62 from out-patients clinics), 40 ET patients and 76 normal controls were finally enrolled in our study. In PD group, 23 were drug-naive and 77 were on anti-Parkinson medications which include levodopa/benseraside or carbidopa, dopamine agonists, anti-cholinergic drug (trihexyphenidyl) and Amantadine. Also, 43 PD patients were in early stage (defined as patients with H-Y stage 1 or 1.5 and duration of PD disease no more than five years) and 57 were in advanced stage [14,15]. Demographic features were not statistically different among PD, ET and normal control groups (Table 1). Clinical features of PD were listed in detail (Table 2).
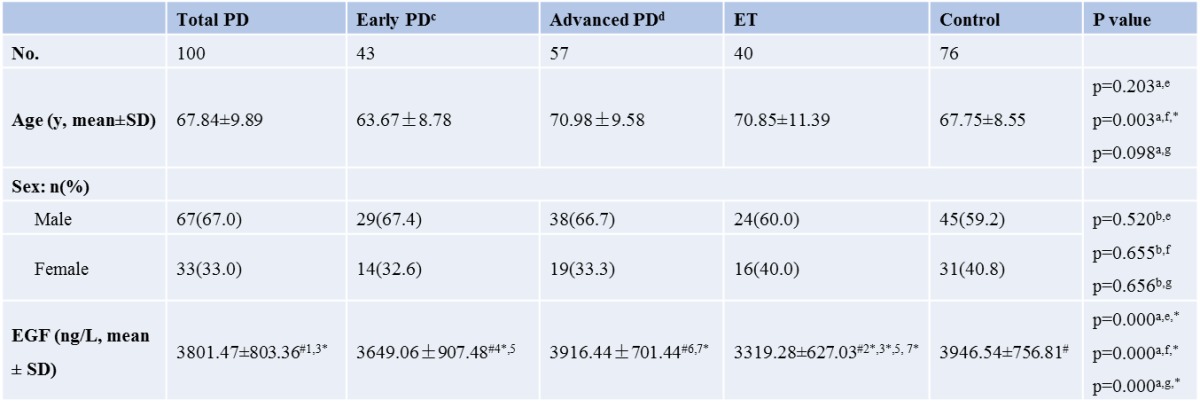
Table 1.
Demographic features and EGF level of PD, ET and controls
|
a ANOVA test; b chi-square test; c H-Y scale<=1.5 and duration of disease <=5 years; d H-Y scale >=2 or duration of disease >5 years; e comparison between total PD, ET and control; f comparison between early PD, ET and control; g comparison between advanced PD, ET and control; # post Hoc multiple comparison:(Least-significant difference, LSD); 1 p=0.210 (between total PD and Control); 2 p=0.000 (between ET and Control); 3 p=0.001 (between ET and total PD); 4 p=0.045 (between early PD and Control); 5 p=0.054 (between early PD and ET); 6 p=0.809 (between advanced PD and Control); 7 p=0.000 (between ET and advanced PD) ; * p<0.05
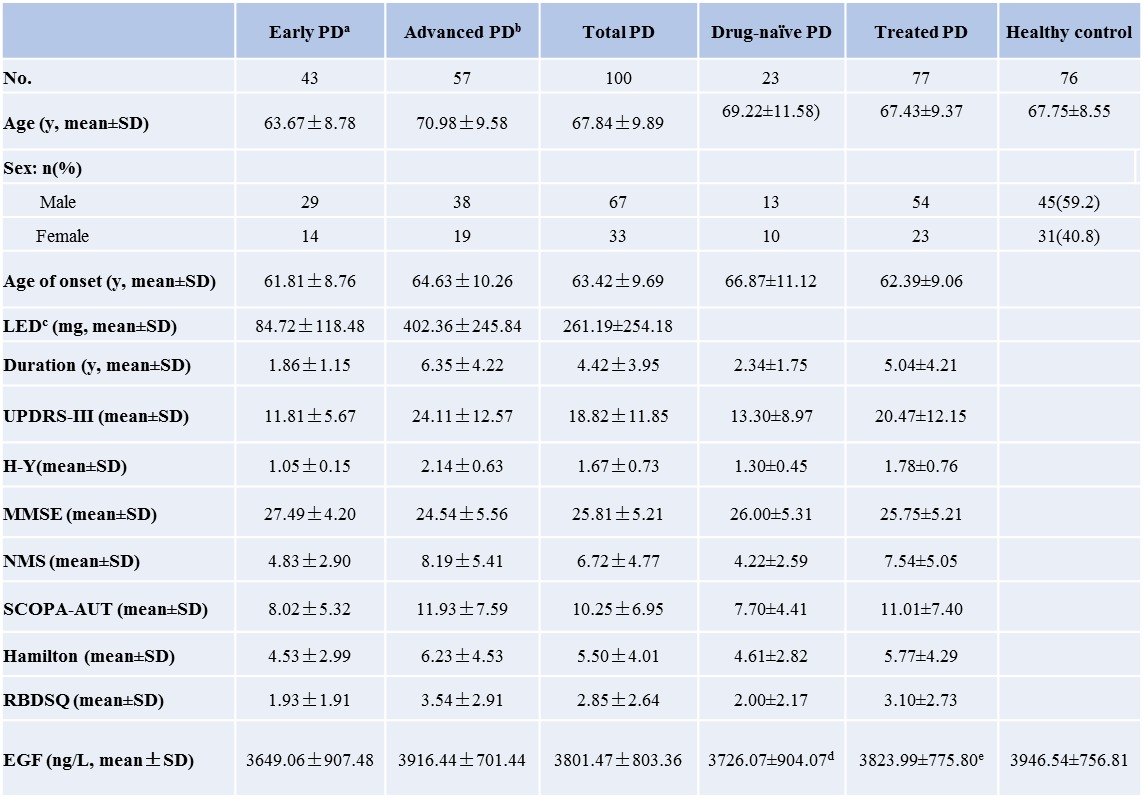
Table 2.
Demographic features, clinical characteristics and serum EGF level in PD patients and control
|
aH-Y scale<=1.5 and duration of disease <=5 years; bH-Y scale >=2 or duration of disease >5 years; c levodopa dosage; statistical analyze was performed between drug-naïve PD/treated PD and control by t test: d p=0.245;e p=0.324;
In our study, plasma EGF level was decreased in PD (3801.47±803.36 ng/L) compared to normal control individuals (3946.54±756.81 ng/L) (Table 1). The decrement of EGF reached statistical significance in early PD (3649.07±907.48 ng/L) vs normal control (p=0.045), but not in total PD group (p=0.210) and advanced PD patients (3916.44±701.44, p=0.809) (Table 1). Further comparison of EGF levels between drug-naïve and medication treated PD patients versus control did not find any significant difference either (Table 2). Interestingly, there was also significant different EGF level between total PD and ET (3801.47±803.36 vs 3319±803.36 ng/L, p=0.001). This difference was also found in advanced PD (p=0.000), but not in early PD (p=0.054).
In total PD cohort, plasma EGF level correlated positively with motor symptoms: UPDRS-III scores (r=0.260, p=0.009) and H-Y stage (r=0.225, p=0.024) (Figure 1). Further linear logistic regression model showed that plasma EGF was only correlated with UPDRS-III scores (p=0.02) (Figure 1). For non-motor symptoms, no correlation was found between plasma EGF level and MMSE scores (r=-0.030, p=0.769), even after adjusting educational background (r=0.048, p=0.634). No correlation was found between plasma EGF level with other non-motor symptom scales including Hamilton depression (r=0.171, p=0.089), SCOPA autonomic function (r=0.106, p=0.295), RBD (r=-0,019, p=0.851) and NMS score (r=0.177, p=0.078). Also, there was no correlation found between plasma EGF level and levodopa dosage (r=0.100, p=0.320).
Figure 1.
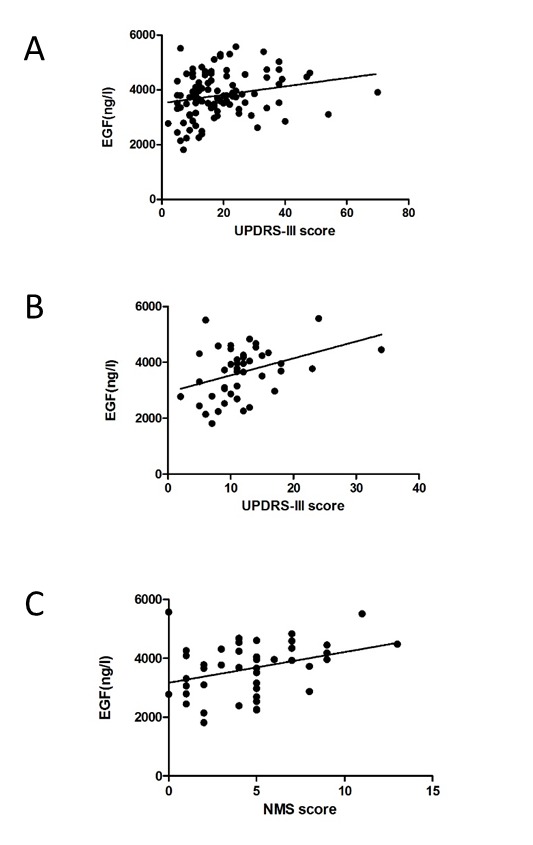
Correlation of plasma EGF with motor and non-motor symptoms of PD. A. Plasma EGF correlated with UPDRS-III scores in PD patients. B. Plasma EGF correlated with UPDRS-III scores in early PD. C. Plasma EGF correlated with NMS scores in early PD.
In early PD subgroup, plasma EGF level was correlated with motor and non-motor symptoms: UPDRS-III scores (r=0.369, p=0.015), H-Y stage (r=0.310, p=0.043) and NMS score (r=0.308, p=0.044). Further linear logistic regression model showed that plasma EGF was correlated with UPDRS-III scores (p=0.02) and NMS scores (p=0.04) (Figure 1). However in advanced PD subgroup, no significant correlation was found between plasma EGF levels with any motor or non-motor symptom.
DISCUSSION
Decrement of EGF has already been detected in PD brain [5]. Our results suggested that this change of EGF was more prominent in early PD (HY<2). Our further study suggested that EGF increased later on during PD disease course by showing a positive correlation of plasma EGF level with motor (UPDRS-III score) and non-motor symptoms (NMS score) of PD. This change of plasma EGF was only existed in the early stage but not in the advanced stage. As we know, EGF exerts neurotrophic effects to prevent dopaminergic degeneration [5] and EGF-EGFR pathway regulates the survival of midbrain dopaminergic neurons [11]. Therefore, our finding suggested that the decrement of plasma EGF in early PD might be the response to the neurodegeneration in the early stage and raised back later on in PD as compensation. When neurons continued to degenerate in PD, this compensation became less effective than before. Certainly, this assumption needs to be tested in further studies in the future.
We further use ET as a disease control model to investigate plasma EGF change between PD and its “mimics”. Interestingly, we found a significant different level of plasma EGF between advanced PD and ET in our study. To the best of our knowledge, this is the first paper to report a different plasma EGF level between PD and ET. Although EGF/EGF-R is both involved in PD and ET [11,16-19], our results suggested that there may be different underlying mechanisms which results in the significant different level of plasma EGF between PD and ET. The disease duration of ET in our cohort was 15 years in average. Unfortunately, we were unable to obtain the sample of ET in their early years. So it is not clear whether plasma EGF remain steady low along the disease course in ET, or drops first and elevate later on. Further well-designed studies are needed to answer this question.
Unfortunately, we did not find any association between EGF and cognitive impairment in PD which has been reported in many studies [7, 20]. The probable reason could be that we had included less PD patients with cognitive impairment in our study. Only 13% (13/100) of PD patients in our study had cognitive impairment defined by MMSE score according to educational background, which was much less than PD with normal cognitive functions (87/100). Also, we used MMSE to evaluate cognitive impairment and MMSE was less sensitive to assess the cognitive impairment in PD because cognitive changes in PD are thought to be involved in other domains like frontal functions more than memory [21]. More sensitive scales on other cognitive functions will be needed to investigate the relationship between EGF and cognitive function in PD in the future studies.
In conclusion, we found a low level of plasma EGF level in early PD. Moreover, plasma EGF level was associated with motor and non-motor symptoms of early PD. Most importantly, we also firstly reported a significant difference of plasma EGF level between advanced PD and ET which need to be investigated in the future as well. These results indicated that plasma EGF could be a potential biomarker for diagnosis and differential diagnosis of PD.
Acknowledgements
This study was supported by grants from the National Program of Basic Research (2010CB945200, 2011CB504104) of China, National Natural Science Fund (81371407), and National Nature Science Fund for Youth (81200979). We thank all the physicians, nurses and social workers of Malu medical center and graduate students for their support to our study.
References
- [1].Lees AJ,Hardy J,Revesz T (2009). Parkinson’s disease. Lancet, 373(9680):2055-2066. [DOI] [PubMed] [Google Scholar]
- [2].Weissmiller AM,Wu C (2012). Current advances in using neurotrophic factors to treat neurodegenerative disorders. Translational neurodegeneration, 1(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Allen SJ,Watson JJ,Shoemark DK,Barua NU,Patel NK (2013). GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacology & therapeutics, 138(2):155-175. [DOI] [PubMed] [Google Scholar]
- [4].Gomez-Pinilla F,Knauer DJ,Nieto-Sampedro M (1998). Epidermal growth factor receptor immunoreactivity in rat brain. Development and cellular localization. Brain research, 438(1-2):385-390. [DOI] [PubMed] [Google Scholar]
- [5].Iwakura Y,Piao YS,Mizuno M, et al. (2005). Influences of dopaminergic lesion on epidermal growth factor-ErbB signals in Parkinson’s disease and its model: neurotrophic implication in nigrostriatal neurons. Journal of neurochemistry, 93(4):974-983. [DOI] [PubMed] [Google Scholar]
- [6].Keeffe Grainne C.O,Tyers Pam,Aarsland Dag et al. (2009). Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. PNAS, 106: 8754-8759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pezzoli G,Zecchinelli A,Ricciardi S, et al. (1991). Intraventricular infusion of epidermal growth factor restores dopaminergic pathway in hemiparkinsonian rats. Movement disorders : official journal of the Movement Disorder Society, 6(4):281-287. [DOI] [PubMed] [Google Scholar]
- [8].Chen-Plotkin AS,Hu WT,Siderowf A, et al. (2011). Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Annals of neurology, 69(4):655-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kuhlenbäumer G,Hopfner F,Deuschl G (2014). Genetics of essential tremor: meta-analysis and review. Neurology, 82(11):1000-7. [DOI] [PubMed] [Google Scholar]
- [10].Delay C,Tremblay C,Brochu E, et al. (2014). Increased LINGO1 in the cerebellum of essential tremor patients. Mov Disord. February 14 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [11].Inoue H,Lin L,Lee X, et al. (2007). Inhibition of the leucine-rich repeat protein LINGO-1 enhances survival, structure, and function of dopaminergic neurons in Parkinson’s disease models. PNAS, 104(36):14430-14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Deuschl G,Bain P,Brin M (1998). Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Movement disorders : official journal of the Movement Disorder Society, 13 Suppl 3:2-23. [DOI] [PubMed] [Google Scholar]
- [13].Chen W,Chen S,Kang WY, et al. (2012). Application of odor identification test in Parkinson’s disease in China: a matched case-control study. Journal of the neurological sciences, 316(1-2):47-50. [DOI] [PubMed] [Google Scholar]
- [14].Booij J,Tissingh G,Boer GJ, et al. (1997). [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson’s disease. Journal of neurology, neurosurgery, and psychiatry, 62(2):133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brusa L,Pavino V,Massimetti MC,Bove R,Iani C,Stanzione P (2013). The effect of dopamine agonists on cognitive functions in non-demented early-mild Parkinson’s disease patients. Functional neurology, 28(1):13-17. [PMC free article] [PubMed] [Google Scholar]
- [16].Babij R,Lee M,Cortes E,Vonsattel JP,Faust PL,Louis ED (2013). Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain : a journal of neurology, 136(Pt 10):3051-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Birecree E,King LE Jr.,Nanney LB (1991). Epidermal growth factor and its receptor in the developing human nervous system. Brain research Developmental brain research, 60(2):145-154. [DOI] [PubMed] [Google Scholar]
- [18].Kuo SH,Tang G,Louis ED, et al. (2013). Lingo-1 expression is increased in essential tremor cerebellum and is present in the basket cell pinceau. Acta neuropathologica, 125(6):879-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhou ZD,Sathiyamoorthy S,Tan EK (2012). LINGO-1 and Neurodegeneration: Pathophysiologic Clues for Essential Tremor. Tremor and other hyperkinetic movements, 2 pii: tre-02-51-249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pellecchia MT,Santangelo G,Picillo M, et al. (2013). Serum epidermal growth factor predicts cognitive functions in early, drug-naive Parkinson’s disease patients. Journal of neurology, 260(2):438-444. [DOI] [PubMed] [Google Scholar]
- [21].Rocha NP,Teixeira AL,Scalzo PL, et al. (2014). Plasma levels of soluble tumor necrosis factor receptors are associated with cognitive performance in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society, 29(4):527-31. [DOI] [PubMed] [Google Scholar]


